Abstract
Recent position statements and guidelines have raised the distinction between a true and false, age-related hypogonadism (HG) or late-onset hypogonadism (LOH). The former is the consequence of congenital or acquired “organic” damage of the brain centers or of the testis. The latter is mainly secondary to age-related comorbidities and does not require testosterone (T) therapy (TTh). In addition, concerns related to cardiovascular (CV) safety have further increased the scepticism related to TTh. In this paper, we reviewed the available evidence supporting the efficacy of TTh in non-organic HG and its long term safety. A large amount of evidence has documented that sexual symptoms are the most specific correlates of T deficiency. TTh is able to improve all aspects of sexual function independent of the pathogenetic origin of the disease supporting the scientific demonstration that LOH does exist according to an “ex-juvantibus” criterion. Although the presence of metabolic derangements could mitigate the efficacy of TTh on erectile dysfunction, the positive effect of TTh on body composition and insulin sensitivity might counterbalance the lower efficacy. CV safety concerns related to TTh are essentially based on a limited number of observational and randomized controlled trials which present important methodological flaws. When HG is properly diagnosed and TTh correctly performed no CV and prostate risk have been documented.
Keywords: Erectile dysfunction, Hypogonadism, Prostate, Safety, Testosterone
INTRODUCTION
Male hypogonadism (HG) is a medical condition characterized by a failure of the testis to produce and release adequate concentrations of its products, i.e., sperms and sex steroids. It can be congenital or acquired. The latter form is far more common than the former and it is usually apparent during adulthood [1]. Adult-onset HG is mainly related to aging itself and to the presence of comorbid conditions, increasingly more prevalent in ageing men, which-might impair the regular functioning of the testis and of its controllers, such as the pituitary (follicular stimulating hormone [FSH] and luteinizing hormone [LH]) and hypothalamus (gonadotropin-releasing hormone [GnRH]) [2,3,4]. Among these potentially impairing conditions, there are metabolic disturbances which are very common in the general population, such as obesity, metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM) [1,4,5,6]. The mechanisms by which an altered metabolism could impair the hypothalamic-pituitary-gonadal axis is still unclear, but the possibility that a low-grade hypothalamic inflammation can cause a disruption in GnRH formation or action is growing [7]. Adult-onset HG- or more commonly defined late-onset hypogonadism (LOH) is a condition characterized by a constellation of unspecific symptoms and signs, often superimposable to those of the aging process per se. Among those, sexual dysfunctions appear as the mostly specific symptoms characterizing the androgen deficiency. In fact, there is considerable evidence that testosterone (T) plays a crucial role in regulating male sexual function [8,9,10,11]. The European Male Aging Study (EMAS), a population based survey performed on more than 3,400 men recruited from 8 European centres, has clearly shown that sexual symptoms—i.e., decreased frequency of sexual thoughts, decreased morning erections, and erectile dysfunction (ED)—are the most sensitive and specific symptoms in identifying patients with low T [12]. Similar results have been more recently reported by the T Trials [13], a randomized controlled trial performed in 788 community-dwelling men recruited from 12 sites in the United States, and by Rastrelli et al [14] in a large sample of men seeking medical care for sexual dysfunction. It has been proposed that when the presence of sexual symptoms is associated with a biochemical deficiency of circulating T a diagnosis of symptomatic LOH can be made. In the European adult population, 2% of the sample satisfy these criteria, whereas 17% demonstrated an isolated T deficiency (total T <11 nmol/L) [12].
As in all endocrine disorders, the goal of treatment of HG is to restore the deficient glandular function. If fertility is the issue and the testis is under-stimulated because of a gonadotropin deficiency, FSH and LH administration is recommended. In all the other cases, testosterone therapy (TTh) is the most convenient choice [15]. Quite surprisingly, in the United States, after a 2015 Food and Drug Administration (FDA) position statement (http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm), TTh is approved only for congenital HG or for acquired forms due to an “organic” damage to the brain centres or to the testis. Hence, either comorbidity-associated or age-related HG are not recognized as true pathological conditions needing a pharmacological intervention. The recently published Guidelines of the Australian Endocrine Society [16] fully endorsed the FDA's position, suggesting that removing the underlying metabolic conditions, such as losing weight, must be the optimal therapeutic strategy for treating obesity-associated HG, and that TTh is not justified in such conditions. Hence, the concept that a distinction between a true HG (classicor organic HG) and a false, age-related, HG (the so-called low T syndrome or andropause or pseudohypogonadism) is raising [16]. It is interesting to note that among more than 4,000 subjects seeking medical care at an endocrine unit for sexual dysfunction, we found a large proportion of HG subjects (i.e., 20%, defined as total T below 10.4 nmoles/L [17]). Of this, only 15% of all causes of HG fulfil the criteria of “classic HG”, whereas the large majority presents comorbidities, including obesity, MetS and T2DM [17]. According to the FDA's position and the Australian recommendations, all these symptomatic HG subjects should be left untreated, with a pharisaic advice of conducting a healthier lifestyle. Scepticism and even reluctance to start an androgen supplementation is essentially based on two kinds of considerations regarding TTh: 1) lack of efficacy in non-organic HG and 2) side effects. In this review we will analyse evidence underlying these believes.
LACK OF EFFICACY OF TESTOSTERONE THERAPY IN NON-ORGANIC HYPOGONADISM
There is no controversy about the fact that losing weight is an effective strategy to overcome T deficiency in obese subjects. In a previous meta-analysis we demonstrated that either lifestyle changes or bariatric surgery are effective in raising gonadotropin and T secretion [1,18]. Besides male HG, T2DM is another obesity-associated medical condition with a growing prevalence in the general population worldwide [19]. Also for T2DM, weight reduction and change in lifestyle are the frontline therapy, but it is inconceivable to negate effectiveness of pharmacological antidiabetic intervention just because the diabetic patient is also obese [19]. In fact, metformin administration is strongly suggested in newly diagnosed T2DM, regardless of any lifestyle intervention [19]. Why, along with lifestyle changes, is pharmacological treatment with metformin advised in T2DM and TTh is not in HG? Maybe because T2DM is a more “organic” condition than HG? It is interesting to note that the well-defined organic conditions leading to diabetes represent less than 1% of all cases of diabetes [19]. All the other cases are multi-factorial conditions that may or may not have a genetic background, still largely unidentified. This does not mean that diabetes exists in all cases or that it should not be promptly treated. Similarities between T2DM and male HG are schematized in Table 1.
Table 1. Similarities between T2DM and LOH.
| Variable | T2DM | LOH |
|---|---|---|
| Prevalence (%) | 2~15 | 2~15 |
| Association with aging | Yes | Yes |
| Association with obesity | Yes | Yes |
| Association with sexual dysfunction | Yes | Yes |
| Symptom specificity | No/mild | No/mild |
| Biochemical threshold for definition | Yes | Yes |
| Well-known (genetic or organic) etiological factors (%) | 1 | 15 |
| Improved by weight loss or lifestyle changes | Yes | Yes |
| Improved by a specific therapy | Yes | Yes |
T2DM: type 2 diabetes mellitus, LOH: late-onset hypogonadism.
The fact that symptoms of androgen deficiency in adulthood often overlap with those characteristics of the aging process (and its associated comorbidities) further complicates the debate. However, T2DM also is a condition characterized by unspecific symptoms, often overlapping with those of the aging process (Table 1). Interestingly, criteria for diagnosis of diabetes are based only on well-defined alterations of biochemical parameters, such as increased glycaemia or HbA1c, independently of symptoms [19]. This is not the case of HG. In fact, in LOH, all the Scientific Societies indicate that a reduction of circulating T is not enough for defining the disease. Specific symptoms must be present along with T deficiency. Symptoms of androgen deficiency include psychological (i.e., depression), physical (i.e., fatigue) and sexual concerns [20,21,22]. Recently, Millar et al [23], reported a review of available literature to estimate the accuracy and operating characteristics of signs and symptoms for predicting low T in aging men. They found a weak correlation between signs, symptoms and T levels, uncertainty about what threshold T levels should be considered low for aging men and wide variation in estimated prevalence of the condition [23]. In addition, all relationships with symptoms are relatively poor in terms of sensitivity and specificity. However, as stated before, the EMAS study has clearly shown that sexual symptoms, and in particular their syndromic association, allow a better identification of the so-called LOH [12,24]. Similar results were recently reported by us in a large cohort (n=4,890) of subjects consulting for ED at the University of Florence (UNIFI) [14]. However, in both the EMAS [12] and UNIFI [14] cohorts, Loess curve analysis did not indicate a clear-cut threshold where the association between sexual symptoms and hormonal deficiency is apparent, with a total T below 11 and 10.4 nmoles/L being the best indicators of sexual symptomatology, respectively. In contrast, in the EMAS study [12], the relationship between T deficiency and psychological and physical symptoms was rather poor or even absent.
Considering that sexual symptoms are the most genuine correlates of T deficiency, it is interesting to evaluate whether or not treating this deficiency is able to restore sexuality. If this is the case, we have the scientific demonstration that the so-called LOH does exist according to an “ex-juvantibus” criterion. A recent systematic qualitative review on the effect of TTh on several outcomes concluded that T did not show consistent benefit for sexual function [25]. However, the same Authors declared that sexual function was evaluated using different tools [25]. In addition, they included both eugonadal and hypogonadal subjects in the analysis [25]. Hence, their conclusions (based only on a personal perspective) are not derived from validated statistical methods. In contrast with the last report, we previously documented, using a meta-analytic method, that TTh is superior, when compared to placebo, in improving all aspects of sexual function [26]. Table 2 summarizes the main results of the meta-analysis. The outcomes observed were independent of age but indirectly related to the levels of T at enrolment. Furthermore, as expected, the effects of TTh were lower in the presence of vascular damage, such as in the case of T2DM [26]. Considering that metabolic conditions are often associated with relatively milder forms of T deficiency, it is possible that a lower apparent efficacy of TTh in T2DM is due to higher T baseline levels [27]. In addition, the mitigated effect of TTh in T2DM is also in line with the lower efficacy of all available treatment for ED in subjects with diabetes, due to the presence of the diabetes-associated chronic complications, which are not necessarily due to an androgen deficiency [21,22,28,29]. Although the presence of metabolic derangements could limit the efficacy of TTh on ED, the positive effect of TTh on body composition and insulin sensitivity [30,31,32] might counterbalance the lower efficacy. Our meta-analysis confirmed epidemiological observation showing that sexual dysfunctions are a hallmark of HG, because TTh can significantly improve them. The vast majority of the subjects enrolled in the included RCTs are not those with “classic” HG. For example, in the T trial [33] 63% of the participants were obese and all were 65 years or older without apparent reason for HG other than age. In conclusion, there is robust evidence that all the forms of symptomatic HG should be treated irrespective to their “organic” or “non-organic” (metabolic) origin because most specific symptoms (sexual ones) are improved.
Table 2. Effect size (with 95% confidence interval) in several sexual parameters across randomized controlled trials evaluating the effect of testosterone treatment vs. placebo.
| Sexual parameter | No. of trials included | No. of patients included | Outcome |
|---|---|---|---|
| Erectile function component | |||
| Overall erectile function componenta | 24 | 1,473 | 0.82 [0.47~1.17]* |
| Overall sexual-related function componentb | 19 | 1,341 | 0.75 [0.37~1.12]** |
| Sleep-related erections | 10 | 436 | 0.87 [0.47~1.27]** |
| Libido component | |||
| Overall libido component | 17 | 1,111 | 0.81 [0.47~1.17]** |
| Orgasm component | |||
| Overall orgasmic component | 10 | 677 | 0.68 [0.34~1.02]** |
| Other sexual parameters | |||
| Frequency of intercourse | 10 | 327 | 0.75 [0.33~1.16]** |
| Overall sexual satisfaction | 9 | 618 | 0.80 [0.41~1.20]** |
| Overall sexual function | 10 | 990 | 0.67 [0.22~1.12]** |
*p<0.001, **p<0.0001. aIncluding coital and non coital erections; bOnly coital erections considered.
Adapted from Corona G et al (J Sex Med 2014;11:1577-92) [26].
LONG TERM SAFETY OF TESTOSTERONE THERAPY
T esters have been available for almost 80 years; however, only over the past two decades has the introduction and use of newer, user-friendly preparations dramatically expanded the market [4]. Since their introduction to the market, the risk of prostate cancer (PC) was considered the most feared side effect related to the use of T preparations. In addition, quite recently,conflicting data have raised the possible relationship between TTh and increased cardiovascular (CV) risk. In the following section, these two aspects will be analyzed in detail.
1. Cardiovascular events during testosterone therapy
Over the last four years, there have been mounting concerns that TTh may increase the risk for CV events, such as heart attacks and strokes. These concerns were essentially based on two retrospective pharmaco-epidemiological papers published between the end of 2013 and the beginning of 2014 [34,35]. Salient pitfalls related to these reports have been commented elsewhere [17,36,37]. Although these studies presented many flaws, due to their retrospective design and to the little information on the databases, they received much attention in the lay media, including The New York Times. Other studies report that low T levels in older men are associated with increased CV risk and that TTh may have CV benefits [17,36,37]. In particular, besides the two aforementioned observational studies, several other reports were published either previously or thereafter [34,35,38,39,40,41,42,43,44,45,46,47,48]. Overall, 13 studies have been published so far, including 194,609 cases and 1,195,582 controls (Table 3). Among them, six studies compared the effect of TTh in HG men vs. untreated HG men. In the other seven studies, the effect of T prescription was compared to subjects who were not prescribed T. In the latter cases limited information regarding the T levels was available (Table 3). Overall, an increased CV risk was reported only in the Vigen et al [34] and Finkle et al [35] whereas in all other reports a neutral or beneficial effect of TTh was found (Table 3). In particular, in 2017 the largest observational study published so far became available [48]. The study evaluated 44,335 male patients at Kaiser Permanente medical centers in Northern and Southern California who had been diagnosed with androgen deficiency between January 1, 1999 and December 31, 2010. Of these, 8,808 men were treated with TTh, whereas 35,527 were never dispensed T. Among men with androgen deficiency, those who were dispensed T prescriptions were associated with a lower risk of CV outcomes overa median follow-up of 3.4 years. Despite their strengths, observational studies present important limitations including: selection, information, and confounding biases. In addition, usually limited information regarding the level of T before and during TTh are available. Hence, caution is needed when evaluating results reporting small differences.
Table 3. Descriptive characteristics of the available pharmaco-epidemiological studies evaluating the impact of testosterone treatment on forthcoming overall mortality or myocardial infarction.
| Study | Age (y)a | Number (case/control) | Follow-up (wk) | Diagnosis of hypogonadism | Study design | Adjusted overall mortality | Adjusted acute mycardial infarction |
|---|---|---|---|---|---|---|---|
| Shores et al (2012) [38] | 62.1 | 398/633 | 81 | TT <250 ng/dL (8.7 nmol/L) | T treatment vs. no treatment | ↓ | NA |
| Finkle et al (2014) [35] | 54.3 | 55,593/141,031 | 140 | NA | TTh prescription vs. PDE5i prescription | NA | ↑ |
| Muraleedharan et al (2013) [39] | 45~59 | 16.5 | TT <300 ng/dL (10.4 nmol/L) | T treatment vs. no treatment | ↓ | NA | |
| Vigen et al (2013) [34] | 63.4 | 1,223/7,486 | 110 | TT <300 ng/dL (10.4 nmol/L) | T treatment vs. no treatment | ↑b | ↑b |
| Baillargeon et al (2014) [40] | ≥66 | 6,355/19,065 | 182 | NA | TTh prescription vs. no TTh prescription | NA | ↔ |
| Eisenberg et al (2015) [41] | 54.4 | 284/225 | 520 | NA | TTh prescription vs. no TTh prescription | ↔ | NA |
| Etminan et al (2015) [42] | 70.4 | 30.066/120.264 | 145 | NA | TTh prescription vs. no TTh prescription | NA | ↔ |
| Ramasamy et al (2015) [43] | 74.3 | 153/64 | 191 | TT <300 ng/dL (10.4 nmol/L) | T treatment vs. no treatment | ↔ | NA |
| Sharma et al (2015) [44] | 66.2 | 60.632/21.380 | 304 | TT lower than the local laboratory reference range | T treatment vs. no treatment | ↓c | ↓c |
| Tan et al (2015) [45] | 20~86 | 19.968/821.725 | 72 | TT <350 ng/dL (12.0 nmol/L) | TTh prescription vs. no TTh prescription | NA | ↓ |
| Maggi et al (2016) [46] | 59.1 | 759/249 | 156 | TT lower than the local laboratory reference range | T treatment vs. no treatment | ↔ | ↔ |
| Wallis et al (2016) [47] | ≥65 | 10.311/28.029 | 260 | NA | TTh prescription vs. no TTh prescription | ↓ | NA |
| Cheetham et al (2017) [48] | 58.4 | 8,808/35,527 | 158 (median) | Mixed hypogonadanal and eugonaladl subjcts | T treatment vs. no treatment | ↓d | ↓d |
TT: total testosterone, NA: not available, T: testosterone, TTh: testosterone therapy, PDE5i: phosphodiesterase type 5 inhibitor, ↓: reduced risk, ↑: increased risk, ↔: unchanged risk.
aValues are presented as mean only or mean range
bComposite of all-cause mortality, acute myocardial infarction, and ischemic stroke.
cComposite cardiac events= myocardial infarction, coronary revascularization, unstable angina, and sudden cardiac death.
dNormalized treated vs. untreated.
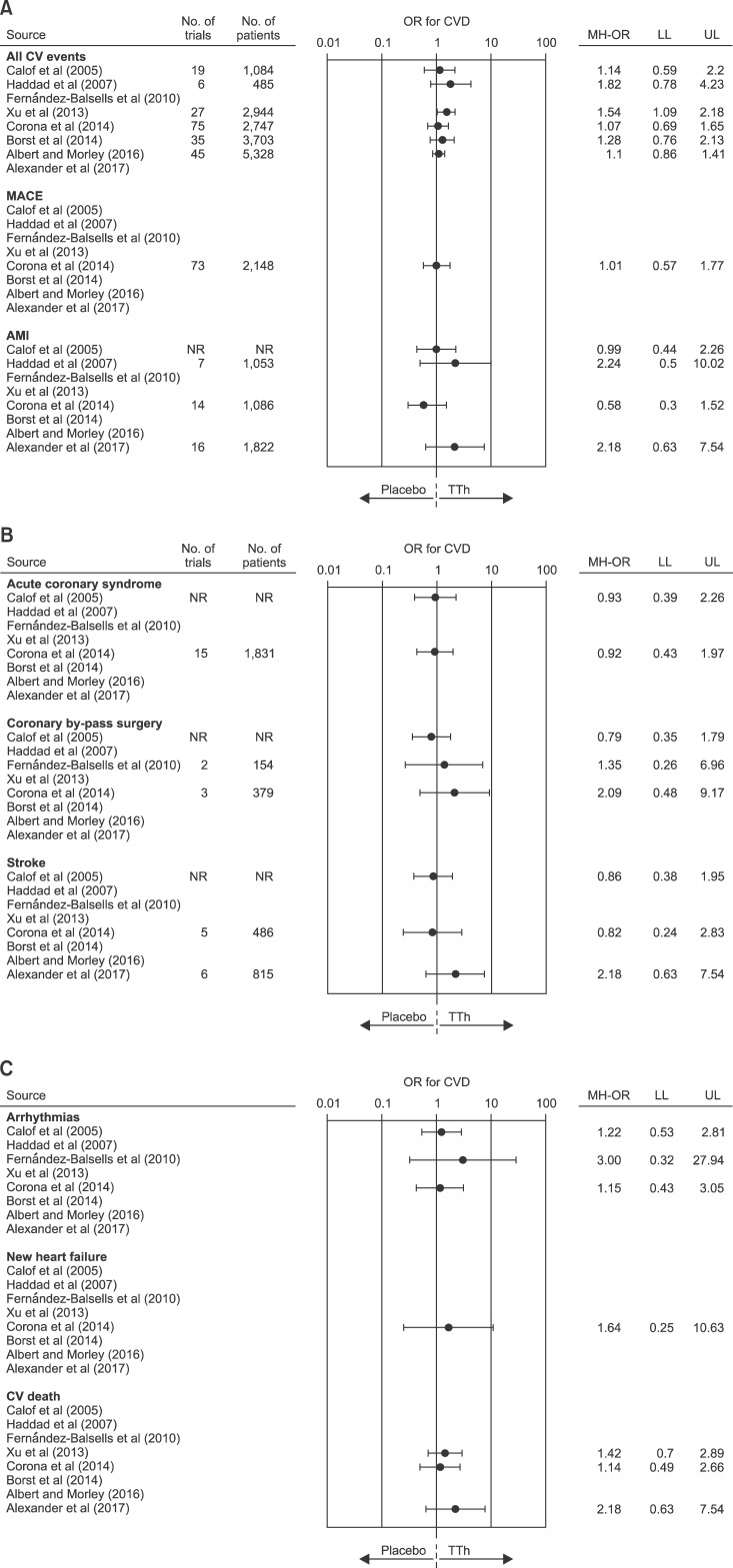
Systematic reviews and meta-analyses are often considered as the highest level of evidence for evaluating interventions in healthcare and as a particularly useful tool to address questions for which multiple data sources are conflicting. Eight systematic meta-analyses on the association between TTh and CV risk have been published so far (Table 4) [49,50,51,52,53,54,55,56]. The number of included trials ranged from 19 to 75 including from 1,084 to 5,464 subjects. Seven meta-analyses [49,50,52,53,54,55,56] reported outcomes on aggregate CV events, whereas one [51] investigated disaggregate events. Forest plots of estimated odds ratio (95% confidence intervals) for aggregate or disaggregate CV events, as derived from available meta-analyses investigating the effects of TTh on CV risk, are summarized in Fig. 1. Only Xu et al [52] reported an increased CV risk related to TTh. However, it is important to emphasize that the latter authors used, for their analysis, “composite CV-related events”, including all investigator-reported adverse events assigned to the CV system. Hence, cases of “peripheral edema” and “self-reported syncope” were also allocated to the category CV events, leading to an artificial increase of the overall number of events [52]. Conversely, all other meta-analyses published so far did not report an increased CV risk related to TTh either when aggregated or disaggregate CV were considered (Fig. 1). It is important to recognize that all available trials included in the meta-analyses have a relatively short duration, lasting atmost three years. Therefore, although there is no clear sign of risk in the short term, no information is available on possible long-term effects.
Table 4. Comparisons of the available meta-analyses evaluating the relationship between testosterone therapy and CV risk.
| Variable | Study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calof et al (2005) [49] | Haddad et al (2007) [50] | Fernández-Balsells et al (2010) [51] | Xu et al (2013) [52] | Corona et al (2014) [53] | Borst et al (2014) [54] | Albert and Morley (2016) [55] | Alexander et al (2017) [56] | |||||||||
| No. of trials included | 19 | 30 | 51 | 27 | 75 | 35 | 45 | 39 | ||||||||
| No. of patients analyzed | 1,084 | 1,642 | 2,679 | 2,944 | 5,464 | 3,703 | 5,328 | 5,441 | ||||||||
| Inclusion criteria | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| Primary end point MACE incidence | × | × | × | × | × | × | × | × | ||||||||
| Primary end point CV event other than MACE | × | × | × | × | × | × | × | × | ||||||||
| Time restriction (>12 wk) | × | × | × | × | × | × | × | × | ||||||||
| Age restriction (≥45 y) | × | × | × | × | × | × | × | × | ||||||||
| CV event analysis | ||||||||||||||||
| All CV events | × | × | × | × | × | × | × | × | ||||||||
| Serious adverse events | × | × | × | × | × | × | × | × | ||||||||
| (including MACE) | ||||||||||||||||
| MACE | × | × | × | × | × | × | × | × | ||||||||
| AMI | × | × | × | × | × | × | × | × | ||||||||
| Acute coronary syndrome | × | × | × | × | × | × | × | × | ||||||||
| Coronary by-pass surgery | × | × | × | × | × | × | × | × | ||||||||
| Stroke | × | × | × | × | × | × | × | × | ||||||||
| New heart failure | × | × | × | × | × | × | × | × | ||||||||
| Arrhythmias | × | × | × | × | × | × | × | × | ||||||||
| CV mortality | × | × | × | × | × | × | × | |||||||||
| Overall mortality | × | × | × | × | × | × | × | × | ||||||||
X indicates if Yes or No. CV: cardiovascular, MACE: major adverse cardiovascular events, AMI: acute myocardial infarction.
Fig. 1. Forest plot of estimated odds ratio (OR) (95% confidence intervals) for aggregate or disaggregate cardiovascular disease (CVD) events as derived from available meta-analyses of randomized controlled trials on the effect of testosterone therapy (TTh) vs. placebo. MACE: major adverse cardiovascular events, AMI: acute myocardial infarction, NR: not reported, MH-OR: Mantel-Haenszed odds ratio, LL: lower limits, UL: upper limits.
2. Prostate safety
Prostate safety remains one of the most important controversial issues of TTh since, historically, PC has been associated with an increased androgenicity [57,58]. Hence, clinicians and regulatory agencies are all concerned by the fact that TTh could cause or promote PC.
Much evidence published in the two last decades has substantially better clarified the role of T in regulating prostate growth and differentiation [57,58,59,60,61]. Epidemiological studies have failed to find an association between endogenous T levels and risk of PC. In particular, the most recent meta-analysis—that pooled worldwide data from 20 prospective studies including 5,623 PC cases and 14,604 controls and mean follow-up of 10 years—reports a relative risk of PC of 0.99 [0.96;1.02] for an increase of 5 nmol/L of T [62].
In line with these data it has been demonstrated that androgen-dependency of prostate growth is evident only in the hypogonadal condition, but not in the eugonadal state. In fact, according to Morgentaler and Traish's “saturation hypothesis”, the human prostate androgen receptors are “saturated” by the circulating androgens and therefore rather insensitive to further T increase, such as those derived from TTh in cases of mild HG [63]. Accordingly, in vitro studies have documented that prostate cell proliferation increased at low T concentration but at higher concentrations even logarithmic increases in T could not enhance growth [58]. This observation can explain the positive effect of androgen deprivation therapy (ADT) in androgen sensitive advanced PC and the possible recurrence as the androgen levels normalize from castrated levels after treatment [58].
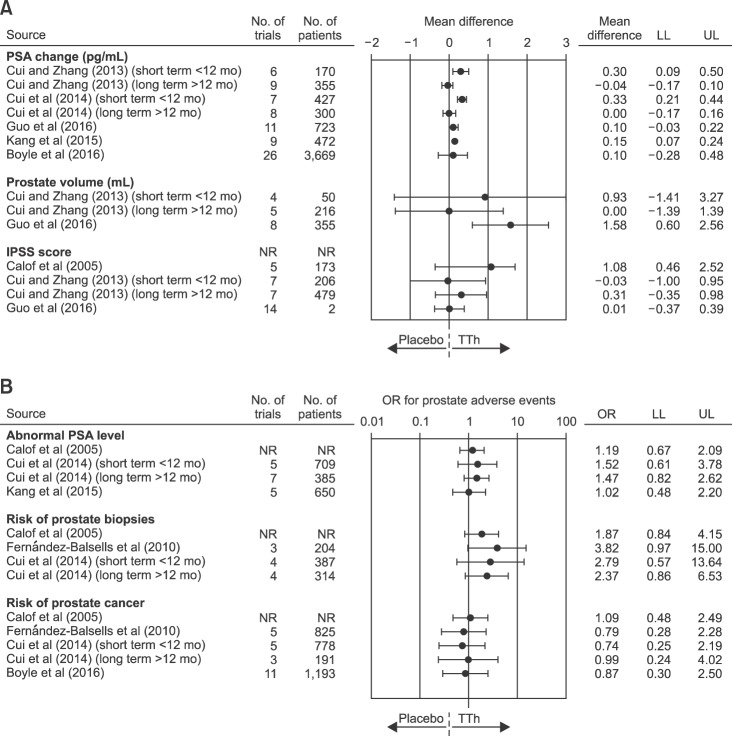
The aforementioned evidence was further confirmed by the analysis of interventional trials. Since 2005, seven systematic meta-analyses on the association between TTh and prostate safety are available and their results are summarized in Table 5 [49,51,62,64,65,66,67]. The number of trials considered ranged from 4 to 26 including from 1,084 to 5,464 subjects. As expected, TTh was associated with a significant short term increase in prostate-specific antigen (PSA) levels [64,65] or prostate volume (Fig. 2) [67]. Accordingly, we previously demonstrated that low PSA could be considered a reliable biochemical marker of reduced bioactive circulating androgens in subjects consulting for sexual dysfunction, providing an index of low androgenization, which includes T-activated post-receptor downstream events [68,69]. No increased risk in International prostate symptom score (IPSS) worsening, in detection of abnormal PSA levels or in developing PC was observed (Fig. 2). Interestingly, by evaluating data from observational studies based on the use of long-term injectable T undecanoate, we previously observed an improvement of IPSS, particularly when the analysis was restricted to those studies enrolling only hypogonadal subjects at enrolment [32]. In the last few years, we have produced both experimental and clinical data suggesting a link between reduced T levels, prostate inflammation and benign prostatic hyperplasia (BPH) [59,60,61]. The underlying pathogenetic mechanisms are not completely understood, but an intriguing hypothesis indicates a close link between obesity and its related metabolic derangement (particularly dyslipidemia), reduced T and an increased risk of BPH and its related inflammatory component [59,60,61,70].
Table 5. Comparisons of the available meta-analyses evaluating the relationship between testosterone therapy and prostate safety.
| Variable | Calof et al (2005) [49] | Fernández-Balsells et al (2010) [51] | Cui and Zhang (2013) [64] | Cui et al (2014) [65] | Guo et al (2016) [67] | Kang et al (2015) [66] | Boyle et al (2016) [62] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of trials included | 19 | 51 | 16 | 22 | 16 | 15 | 27 | |||||||
| No. of patients analyzed | 1,084 | 2,679 | 1,030 | 2,351 | 1,921 | 1,124 | 2,213 | |||||||
| Inclusion criteria | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| Primary end point prostate safety | × | × | × | × | × | × | × | |||||||
| Data presented according to trial duration | × | × | × | × | × | × | × | |||||||
| Time restriction (>12 wk) | × | × | × | × | × | × | × | |||||||
| Time restriction (>24 wk) | × | × | × | × | × | × | × | |||||||
| Age restriction (≥45 yr) | × | × | × | × | × | × | × | |||||||
| Prostate adverse event analysis | ||||||||||||||
| PSA change | × | × | × | × | × | × | × | |||||||
| Prostate volume | × | × | × | × | × | × | × | |||||||
| IPSS | × | × | × | × | × | × | × | |||||||
| Abnormal PSA | × | × | × | × | × | × | × | |||||||
| Prostate biopsy | × | × | × | × | × | × | × | |||||||
| Prostate cancer | × | × | × | × | × | × | × | |||||||
X indicates if Yes or No. PSA: prostatic specific antigen, IPSS: international prostatic symptoms score.
Fig. 2. Forest plot of estimated odds ratio (OR) (95% confidence intervals) for prostate adverse events as derived from available meta-analyses of randomized controlled trials on the effect of testosterone therapy (TTh) vs. placebo. PSA: prostatic specific antigen, IPSS: international prostatic symptoms score, NR: not reported, LL: lower limits, UL: upper limits.
CONCLUSIONS
A large body of evidence has documented that sexual symptoms are the most specific correlates of T deficiency. TTh is able to improve all aspects of sexual function independent of the pathogenetic origin of the disease supporting the scientific demonstration that the so-called LOH does exist according to an “ex-juvantibus” criterion. Although the presence of metabolic derangements could mitigate the efficacy of TTh on ED, the positive effect of TTh on body composition and insulin sensitivity might counterbalance the lower efficacy. CV safety concerns related to TTh are essentially based on a limited number of observational and randomized controlled trials which present important methodological flaws. When HG is properly diagnosed and TTh correctly performed no CV and prostate risk have beendocumented.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Corona G, Vignozzi L, Sforza A, Mannucci E, Maggi M. Obesity and late-onset hypogonadism. Mol Cell Endocrinol. 2015;418(Pt 2):120–133. doi: 10.1016/j.mce.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Corona G, Bianchini S, Sforza A, Vignozzi L, Maggi M. Hypogonadism as a possible link between metabolic diseases and erectile dysfunction in aging men. Hormones (Athens) 2015;14:569–578. doi: 10.14310/horm.2002.1635. [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013;31:103–125. doi: 10.5534/wjmh.2013.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, et al. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016;41:196–210. [PubMed] [Google Scholar]
- 5.Brand JS, Rovers MM, Yeap BB, Schneider HJ, Tuomainen TP, Haring R, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One. 2014;9:e100409. doi: 10.1371/journal.pone.0100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamm S, Chidakel A, Bansal R. Obesity and hypogonadism. Urol Clin North Am. 2016;43:239–245. doi: 10.1016/j.ucl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Morelli A, Vignozzi L, Maggi M. Hypogonadotropic hypogonadism and metabolic syndrome: insights from the high-fat diet experimental rabbit animal model. Minerva Endocrinol. 2016;41:240–249. [PubMed] [Google Scholar]
- 8.Corona G, Isidori AM, Aversa A, Burnett AL, Maggi M. Endocrinologic control of men's sexual desire and arousal/erection. J Sex Med. 2016;13:317–337. doi: 10.1016/j.jsxm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Isidori AM, Balercia G, Calogero AE, Corona G, Ferlin A, Francavilla S, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38:103–112. doi: 10.1007/s40618-014-0155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona G, Rastrelli G, Ricca V, Jannini EA, Vignozzi L, Monami M, et al. Risk factors associated with primary and secondary reduced libido in male patients with sexual dysfunction. J Sex Med. 2013;10:1074–1089. doi: 10.1111/jsm.12043. [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Mannucci E, Lotti F, Boddi V, Jannini EA, Fisher AD, et al. Impairment of couple relationship in male patients with sexual dysfunction is associated with overt hypogonadism. J Sex Med. 2009;6:2591–2600. doi: 10.1111/j.1743-6109.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Ellenberg SS, Matsumoto AM, et al. Association of sex hormones with sexual function, vitality, and physical function of symptomatic older men with low testosterone levels at baseline in the testosterone trials. J Clin Endocrinol Metab. 2015;100:1146–1155. doi: 10.1210/jc.2014-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39:473–484. doi: 10.1007/s40618-015-0425-1. [DOI] [PubMed] [Google Scholar]
- 15.Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2012;17:239–259. doi: 10.1517/14728214.2012.683411. [DOI] [PubMed] [Google Scholar]
- 16.Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, Conway AJ, et al. Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations. Med J Aust. 2016;205:228–231. doi: 10.5694/mja16.00448. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Maggi M. Perspective: regulatory agencies' changes to testosterone product labeling. J Sex Med. 2015;12:1690–1693. doi: 10.1111/jsm.12951. [DOI] [PubMed] [Google Scholar]
- 18.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–843. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 19.Authors/Task Force Members. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 20.Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Giorda CB, Cucinotta D, Guida P, Nada E SUBITO-DE study group. The SUBITO-DE study: sexual dysfunction in newly diagnosed type 2 diabetes male patients. J Endocrinol Invest. 2013;36:864–868. doi: 10.3275/8969. [DOI] [PubMed] [Google Scholar]
- 22.Corona G, Giorda CB, Cucinotta D, Guida P, Nada E Gruppo di studio SUBITO-DE. Sexual dysfunction at the onset of type 2 diabetes: the interplay of depression, hormonal and cardiovascular factors. J Sex Med. 2014;11:2065–2073. doi: 10.1111/jsm.12601. [DOI] [PubMed] [Google Scholar]
- 23.Millar AC, Lau AN, Tomlinson G, Kraguljac A, Simel DL, Detsky AS, et al. Predicting low testosterone in aging men: a systematic review. CMAJ. 2016;188:E321–E330. doi: 10.1503/cmaj.150262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor DB, Lee DM, Corona G, Forti G, Tajar A, O'Neill TW, et al. The relationships between sex hormones and sexual function in middle-aged and older European men. J Clin Endocrinol Metab. 2011;96:E1577–E1587. doi: 10.1210/jc.2010-2216. [DOI] [PubMed] [Google Scholar]
- 25.Huo S, Scialli AR, McGarvey S, Hill E, Tügertimur B, Hogenmiller A, et al. Treatment of men for “low testosterone”: a systematic review. PLoS One. 2016;11:e0162480. doi: 10.1371/journal.pone.0162480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11:1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 27.Corona G, Rastrelli G, Filippi S, Vignozzi L, Mannucci E, Maggi M. Erectile dysfunction and central obesity: an Italian perspective. Asian J Androl. 2014;16:581–591. doi: 10.4103/1008-682X.126386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kizilay F, Gali HE, Serefoglu EC. Diabetes and sexuality. Sex Med Rev. 2017;5:45–51. doi: 10.1016/j.sxmr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Corona G, Giorda CB, Cucinotta D, Guida P, Nada E SUBITO-DE Study Group. Sexual dysfunction in type 2 diabetes at diagnosis: progression over time and drug and non-drug correlated factors. PLoS One. 2016;11:e0157915. doi: 10.1371/journal.pone.0157915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest. 2016;39:967–981. doi: 10.1007/s40618-016-0480-2. [DOI] [PubMed] [Google Scholar]
- 31.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 32.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15:1903–1926. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 33.Snyder PJ, Ellenberg SS, Farrar JT. Testosterone treatment in older men. N Engl J Med. 2016;375:90. doi: 10.1056/NEJMc1603665. [DOI] [PubMed] [Google Scholar]
- 34.Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 35.Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corona GG, Rastrelli G, Maseroli E, Sforza A, Maggi M. Testosterone replacement therapy and cardiovascular risk: a review. World J Mens Health. 2015;33:130–142. doi: 10.5534/wjmh.2015.33.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90:224–251. doi: 10.1016/j.mayocp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 39.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 40.Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, et al. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–1144. doi: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenberg ML, Li S, Herder D, Lamb DJ, Lipshultz LI. Testosterone therapy and mortality risk. Int J Impot Res. 2015;27:46–48. doi: 10.1038/ijir.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015;35:72–78. doi: 10.1002/phar.1534. [DOI] [PubMed] [Google Scholar]
- 43.Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association between testosterone supplementation therapy and thrombotic events in elderly men. Urology. 2015;86:283–285. doi: 10.1016/j.urology.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 45.Tan RS, Cook KR, Reilly WG. Myocardial infarction and stroke risk in young healthy men treated with injectable testosterone. Int J Endocrinol. 2015;2015:970750. doi: 10.1155/2015/970750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maggi M, Wu FC, Jones TH, Jackson G, Behre HM, Hackett G, et al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: results from the Registry of Hypogonadism in Men (RHYME) Int J Clin Pract. 2016;70:843–852. doi: 10.1111/ijcp.12876. [DOI] [PubMed] [Google Scholar]
- 47.Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. doi: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 48.Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, et al. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499. doi: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 49.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 50.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corona G, Maseroli E, Rastrelli G, Isidori AM, Sforza A, Mannucci E, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13:1327–1351. doi: 10.1517/14740338.2014.950653. [DOI] [PubMed] [Google Scholar]
- 54.Borst SE, Shuster JJ, Zou B, Ye F, Jia H, Wokhlu A, et al. Cardiovascular risks and elevation of serum DHT vary by route of testosterone administration: a systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf) 2016;85:436–443. doi: 10.1111/cen.13084. [DOI] [PubMed] [Google Scholar]
- 56.Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017;130:293–305. doi: 10.1016/j.amjmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Pastuszak AW, Rodriguez KM, Nguyen TM, Khera M. Testosterone therapy and prostate cancer. Transl Androl Urol. 2016;5:909–920. doi: 10.21037/tau.2016.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: where are we now? J Endocrinol Invest. 2011;34:232–243. doi: 10.1007/BF03347072. [DOI] [PubMed] [Google Scholar]
- 59.Corona G, Vignozzi L, Rastrelli G, Lotti F, Cipriani S, Maggi M. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int J Endocrinol. 2014;2014:329456. doi: 10.1155/2014/329456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014;37:313–322. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 61.Vignozzi L, Gacci M, Cellai I, Santi R, Corona G, Morelli A, et al. Fat boosts, while androgen receptor activation counteracts, BPH-associated prostate inflammation. Prostate. 2013;73:789–800. doi: 10.1002/pros.22623. [DOI] [PubMed] [Google Scholar]
- 62.Boyle P, Koechlin A, Bota M, d'Onofrio A, Zaridze DG, Perrin P, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 2016;118:731–741. doi: 10.1111/bju.13417. [DOI] [PubMed] [Google Scholar]
- 63.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 64.Cui Y, Zhang Y. The effect of androgen-replacement therapy on prostate growth: a systematic review and meta-analysis. Eur Urol. 2013;64:811–822. doi: 10.1016/j.eururo.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 65.Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17:132–143. doi: 10.1038/pcan.2013.60. [DOI] [PubMed] [Google Scholar]
- 66.Kang DY, Li HJ. The effect of testosterone replacement therapy on prostate-specific antigen (PSA) levels in men being treated for hypogonadism: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e410. doi: 10.1097/MD.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo C, Gu W, Liu M, Peng BO, Yao X, Yang B, et al. Efficacy and safety of testosterone replacement therapy in men with hypogonadism: a meta-analysis study of placebo-controlled trials. Exp Ther Med. 2016;11:853–863. doi: 10.3892/etm.2015.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rastrelli G, Corona G, Vignozzi L, Maseroli E, Silverii A, Monami M, et al. Serum PSA as a predictor of testosterone deficiency. J Sex Med. 2013;10:2518–2528. doi: 10.1111/jsm.12266. [DOI] [PubMed] [Google Scholar]
- 69.Corona G, Boddi V, Lotti F, Gacci M, Carini M, De Vita G, et al. The relationship of testosterone to prostate-specific antigen in men with sexual dysfunction. J Sex Med. 2010;7:284–292. doi: 10.1111/j.1743-6109.2009.01549.x. [DOI] [PubMed] [Google Scholar]
- 70.Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. 2015;115:24–31. doi: 10.1111/bju.12728. [DOI] [PubMed] [Google Scholar]