Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder in which a significant proportion of patients do not respond to treatment. The objective of this study was to examine the role of genetic risk variants in the response to treatment with methylphenidate (MPH). The effectiveness of MPH was evaluated based on variations in the CGI-S and CGAS scales over a 12-month treatment period using linear mixed effects models. A total of 208 ADHD patients and 34 polymorphisms were included in the analysis. For both scales, the response was associated with time, extended-release MPH/both formulations, and previous MPH treatment. For the CGI-S scale, response was associated with SLC6A3 rs2550948, DRD4 promoter duplication, SNAP25 rs3746544, and ADGRL3 rs1868790. Interactions between the response over time and SLC6A3 and DRD2 were found in the CGI-S and CGAS scales, respectively. The proportion of the variance explained by the models was 18% for the CGI-S and 22% for the CGAS. In this long-term study, the effects of SLC6A3, DRD4, SNAP25, and ADGRL3 on response to treatment reflect those observed in previous studies. In addition, 2 previously unreported interactions with response to treatment over a 12-month period were found (SLC6A3 and DRD2).
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a very common neurodevelopment condition in children, involving about 5% of children and adolescents1. About 65% of ADHD children are also symptomatic in adulthood, thus suggesting that the disease is chronic2, 3. The symptoms of ADHD include inappropriate levels of attention and/or hyperactivity and impulsivity. In addition, over 65% of ADHD patients present psychiatric comorbidities, such as depression, anxiety, and learning disorders4, 5, all of which affect academic performance and family life, with huge social and economic repercussions6, 7.
Stimulants are the most effective medications for improvement of ADHD symptoms, and methylphenidate (MPH) is often the first choice owing to its effectiveness and safety, as demonstrated in several studies8–10. However, although the clinical condition of most patients treated with MPH improves, a considerable proportion (35%) do not respond to treatment or present adverse effects, thereby making response to MPH variable and difficult to predict11, 12. As a result, clinicians often use a trial-and-error approach based on different types of medication or on titration of dosages to find the best fit for each patient13. It seems clear that identifying accurate predictors of response to medication would be beneficial for clinical practice14–16.
ADHD is a heterogeneous and complex disorder involving environmental and genetic risk factors. The strong genetic component of ADHD is supported by family, twin, and adoption studies, which have found a mean estimated heritability of 76%17, suggesting that ADHD is among the most heritable neuropsychiatric disorders.
Predictions based on genetic factors are the basis of pharmacogenetic testing. Numerous candidate genes have been associated with an increased risk of ADHD18. As many of these genes play a role in the mechanisms of action of psychostimulants, there is a high probability that they are also associated with response to treatment19. The results of pharmacogenetic studies of ADHD are variable and inconclusive19. The objective of this study was to examine the role of risk genes in the response to MPH in children with ADHD and to evaluate the effectiveness of the drug over 12 months of follow-up.
Results
A total of 238 Caucasian ADHD patients were included in the initial step of the study. After the quality control procedure, 208 patients remained in the final analysis, and 176 completed the 12-month follow-up. Fifty-seven (27%) were treatment-naive. Among the MPH-experienced patients (151), 41% reported a poor or partial response at the time they entered in the study. The demographic and clinical characteristics of the cohort are shown in Table 1.
Table 1.
Demographic and clinical characteristics of ADHD patients.
| Baseline | 3 months | 6 months | 12 months | |
|---|---|---|---|---|
| N | 208 | 172 | 183 | 176 |
| Age | ||||
| Mean (SD) | 10.6 (2.9) | 10.7 (2.9) | 10.5 (2.8) | 10.5 (2.8) |
| Range | 6–18 | 6–18 | 6–18 | 6–18 |
| Gender | ||||
| Male (%) | 163 (78.3) | 136 (79.1) | 145 (79.2) | 138 (78.4) |
| Female (%) | 45 (21.7) | 36 (20.9) | 38 (20.8) | 38 (21.6) |
| ADHD diagnosis | ||||
| Combined type (%) | 121 (58.2) | 99 (57.7) | 106 (57.9) | 105 (59.6) |
| Inattentive type (%) | 78 (37.5) | 66 (38.4) | 69 (37.7) | 65 (36.9) |
| Hyperactive type (%) | 9 (4.3) | 7 (4.1) | 8 (4.4) | 6 (3.5) |
| Previous treatment | ||||
| Naive patients (%) | 57 (27.4) | 47 (27.3) | 46 (25.1) | 47 (26.7) |
| Experienced patients (%) | 151 (72.6) | 125 (72.7) | 137 (74.9) | 129 (73.3) |
| Methylphenidate | ||||
| Immediate-release (%) | 17 (8.2) | 13 (7.6) | 8 (4.4) | 8 (4.5) |
| Extended-release (%) | 173 (83.1) | 144 (83.7) | 155 (84.7) | 143 (81.3) |
| Both at the same time (%) | 18 (8.7) | 15 (8.7) | 20 (10.9) | 25 (14.2) |
| Doses mg/day | ||||
| Mean (SD) | 35.5 (15) | 39.5 (15.7) | 39.4 (15.8) | 40.5 (15.5 |
| Cases with at least one side effect (%) | 51 (29.6) | 72 (39.3) | 47 (26.7) | |
| CGI-S score | ||||
| Mean (SD) | 3.24 (0.58) | 3.10 (0.56) | 3.06 (0.55) | 3.07 (0.57) |
| CGAS score | ||||
| Mean (SD) | 69.15 (11.67) | 74.62 (9.26) | 75.26 (9.69) | 75.90 (9.29) |
For all variants, the call rates of genotype per-marker were higher than 96%; therefore, no polymorphisms were excluded from the analysis. The fixed effects of the models are summarized in Table 2 with the point estimate, 95% confidence intervals (95% CI), and p values.
Table 2.
Significant results of fixed effects from linear mixed-effect models to evaluate the association between covariates/genetic variants and response according to the CGI-S and CGAS scales.
| Variable | Genotype | β (95% CI) | Model | P |
|---|---|---|---|---|
| CGI-S | ||||
| Time | — | −0.01 (−0.02 to −0.01) | — | <0.001 |
| Previously treated patients | — | −0.25 (−0.40 to −0.12) | — | 0.001 |
| Extended-release MPH or both formulations | — | −0.38 (−0.58 to −0.20) | — | <0.001 |
| SLC6A3 rs2550948 | A/G o G/G | −0.14 (−0.27 to 0.02) | Dominant | 0.011 |
| DRD4 promoter duplication | S/S | 0.74 (0.40–1.18) | Recessive | 0.001 |
| SNAP25 rs3746544 | A/C o C/C | −0.16 (−0.27 to −0.03) | Dominant | 0.018 |
| ADGRL3 rs1868790 | A/A | 0.23 (0.02–0.45) | Recessive | 0.026 |
| Interaction | ||||
| SLC6A3 intron 8 VNTR *Time | 6/− o −/− | −0.02 (−0.03 to −0.01) | Dominant | 0.010 |
| CGAS | ||||
| Time | — | 0.51 (0.39–0.60) | — | <0.001 |
| Treatment-experienced patients | — | 5.15 (2.57–7.73) | — | <0.001 |
| Extended-release MPH or both formulation | — | 7.74 (4.54–10.94) | — | <0.001 |
| Dosage | — | 0.03 (0.02–0.09) | — | 0.008 |
| Interaction | ||||
| DRD2 rs1800497*Time | T/T | −0.63 (−1.17 to −0.08) | Recessive | 0.024 |
Sex, age, and ADHD subtype were not significant as covariates for any of the efficacy models. We found a significant improvement in the response over time in the CGI-S and CGAS scales. In addition, previous MPH treatment and extended-release MPH alone/both formulations were positive predictors of response according to CGI-S and CGAS. Dosage was a significant factor only in the CGAS effectiveness model.
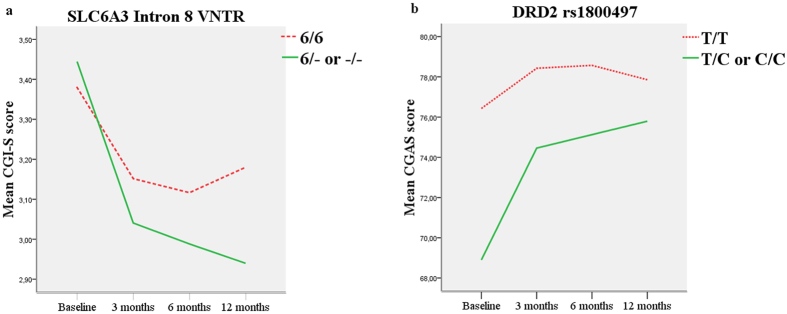
As for the genetic component, in the CGI-S model, we found recessive effects in DRD4 promoter duplication, and ADGRL3 rs1868790, which were associated with significant impairment, and a dominant effect in SLC6A3 rs2550948 and SNAP25 rs3746544, with a significant improvement in the symptoms. Moreover, an interaction was found between the SLC6A3 intron 8 VNTR and treatment over time (Table 2 and Fig. 1).
Figure 1.
Significant interactions between response over time and genetic variants in the CGI-S model (a) and CGAS model (b).
Finally, no significant association was observed between CGAS scores and genetic variants, although an interaction was found between DRD2 rs1800497 and treatment over time (Table 2 and Fig. 1).
The proportion of the variance explained by the model was 18% for CGI-S and 22% for CGAS. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Discussion
We investigated the role of genetic factors that were associated with ADHD as predictors of the clinical response to MPH in a Caucasian population; the interaction between genetic variants and treatment over time was also assessed.
The values for CGI-S and CGAS improved significantly over time, although with a modest effect size, maybe because 73% of patients were on treatment before the beginning of the study. ADHD subtype was not a significant factor in response to treatment, according to previously studies20. In addition, treatment-experienced patients and patients who took extended-release MPH or both formulations had a better response in all the efficacy parameters considered (CGI-S and CGAS scales). Treatment-experienced patients were better responders, possibly because of selection bias: they continued on treatment because they were responding to the treatment, with no safety issues. Adjusting models by treatment status, we controlled which polymorphisms were associated with the response regardless of the confounding variable. The resulting data suggest that the extended-release formulation could have led to an improvement in the response to MPH, for example, by improving adherence to treatment21. Dosage was only a significant factor in the CGAS model. In CGI-S, statistical significance may not have been reached because of insufficient sample size.
As for the genetic component, our results showed the implication of the genes SLC6A3 and DRD4, which have been widely associated with changes in response to MPH19, 22. On the other hand, we failed to replicate the most studied variant in SLC6A3 (VNTR 3′ UTR), thus reflecting previous negative results on the implication of this variant and the pharmacogenetics of ADHD19, 23. Consequently, other polymorphisms of this gene must be explored. In this context, we found a previously unreported association between a promoter variant (rs2550948) and response to MPH. In the case of DRD4, once again, we did not replicate the most studied association, which was with the VNTR exon 3 variant19. The results of studies that investigate the implication of this variant in response to MPH are also conflicting19. However, we did find an association between the DRD4 tandem duplication polymorphism in the promoter region and response to MPH. No significant effect on response to MPH was found with this variant in 2 small-scale studies of children24 and adults25.
A statistically significant association with response to MPH was found in the neurodevelopmental genes SNAP25 and ADGRL3. Although the role of these genes in susceptibility to ADHD is widely studied, they have been less largely studied in terms of the effectiveness of MPH. Contini et al.26 evaluated the same polymorphism in SNAP25 (rs3746544) but identified no effect in adults with ADHD. Elsewhere in the literature, inconsistent findings were found between ADGRL3 and response to MPH. The marker rs6551665 had previously been associated with the response to MPH27, 28. Arcos-Burgos et al.27 reported that the G allele was associated with a good response, whereas Labbe et al.25 found that it was associated with a poor response. The divergent results could be explained by differences in sample subtypes or outcome measures29. In addition, the marker rs6858066 was associated with a better response28, 30. In the present study, the associations between rs6551665 and rs6858066 and response to MPH were not statistically significant. In contrast, we established a previously unreported statistically significant association between rs1868790 and response to MPH.
Furthermore, our pharmacogenetic study suggested that SLC6A3 and DRD2 genotypes were associated with different degrees of improvement in ADHD symptoms. For both genes, a faster response effect was observed during the first 3 months, respectively, in patients with the genotypes 6/− or −/− and T/C or C/C. To our knowledge, this is the first report of differences in the response to MPH over time in these genes.
Our study reflects the considerable difficulty in replicating pharmacogenetic association studies in ADHD. The results reported are conditioned by polymorphisms analyzed per gene or model of inheritance evaluated. Furthermore, they depend on factors that influence the response, such as phenotype, concomitant treatment, and sample characteristics (age, sex, ADHD subtype), which are not always taken into account19, 31, 32. Results are also difficult to reproduce because of the definition of response in assessment scales. In fact, there is no clear consensus on the best approach to find objective and reliable measures of response to treatment33.
The strengths of our study are that we evaluated response using 2 scales that provide more detailed information and thus reveal the heterogeneity in response effect. Moreover, we evaluated the response to MPH under conditions of routine clinical practice, thus highlighting the role of genetic factors in real-world situations34. Ours is the first 12-month study of the pharmacogenetic of response to MPH in children, and we provide much more relevant clinical information than short-term studies. The literature contains little evidence of the long-term effects of medication owing to the difficulty in follow-up and the low persistence on therapy rate35.
Some limitations of this study should be considered. First, the determination of the scores for the clinical response through the scales CGI-S and CGAS, which are recorded by doctors, despite are based on what parents and children report, is not free of subjectivity risk of the doctor. However, having scales assessing what parents and patients believe with regards to the overall functioning of patients would be of interest in the assessment of the treatment effectiveness, since it would be free of evaluator’s bias36. By contrast, this approach has to cope with the risk of serious discrepancies between informants, which ultimately will hinder patient’s assessment37.
Another limitation of the study to be considered is that 73% of patients were on treatment before beginning the study. Although we have adjusted the models by previous treatment status, experienced patients started with better response at baseline visit, and for that reason they had limited clinical improvement at the end of the study period.
In conclusion, we report moderate effects of the genes SLC6A3, DRD4, SNAP25, and ADGRL3 in the response to MPH, thereby supporting several previous studies of these genes. We also found interactions between response to treatment over 12 months and genotypes of SLC6A3 and DRD2. When all the covariates are taken into account, the models explain around 20% of the response to MPH. Therefore, other genetic or non-genetic factors must be involved in the variability of response to MPH. More research is required to find pharmacogenetic variants that could help to establish the best treatment regimen.
Method
Patients, clinical assessment, and ethical review
We performed a prospective, observational study of unrelated Spanish Caucasian patients with ADHD aged 6 to 18 years who were enrolled and clinically assessed by psychiatrists and pediatricians at Fundación Jiménez Díaz University Hospital. ADHD was diagnosed following the Diagnostic and Statistical Manual of Mental Disorders38. All patients were evaluated, taking into account different sources of information (parents, children and clinicians).
To be included, patients had to have ADHD, be Spanish and Caucasian, be treatment-naive or have been treated with only MPH at baseline, and have been receiving MPH at least from baseline onwards. Patients could receive one of 2 formulations: (a) the immediate-release formulation (Rubifen) or (b) the extended-release formulation (Medikinet and Concerta)/ both formulations. Patients treated with medication (in addition or instead of) other than MPH were excluded.
Doses and type of MPH formulation were individually prescribed according to the summary of product characteristics and the clinical criteria of the psychiatrist and were adjusted during follow-up visits until the desired therapeutic effects were obtained.
Clinical effectiveness was evaluated using the Clinical Global Impression-Severity (CGI-S) scale39 and the Children’s Global Assessment Scale (CGAS)40. CGI-S provides a global evaluation of the severity of illness at the time of evaluation using a 7-point scale ranging from 1 (no impairment, normal) to 7 (maximum impairment). CGAS is used to rate the general functional status in children and adolescents using a numerical scale, with values ranging from 1 (need for constant supervision) to 100 (superior functioning).
During the assessment period, the following side effects were evaluated: loss of appetite, insomnia, gastrointestinal problems, headaches, cognitive, emotional and behavioral disturbances.
Clinical assessments were performed at baseline and after 3, 6, and 12 months of treatment with MPH.
The study protocol was reviewed and authorized by the Research Ethics Committee of the IIS-Fundación Jiménez Díaz University Hospital. The study was carried out in accordance with the ethical principles that are reflected in the Declaration of Helsinki. Before recruitment, once the study objectives and procedures had been detailed, parents or legal guardians signed a written informed consent.
DNA extraction and genotyping
Peripheral blood lymphocytes or saliva were used to obtain genomic DNA, employing an automatic DNA extractor (BioRobot EZ1, Qiagen, Hilden, Germany) or the Oragene DNA self-collection kit (DNA Genotek, Kanata, Ontario, Canada), respectively, according to the manufacturer’s recommendations. DNA concentration and sample quality were evaluated through a spectrophotometer (NanoDrop® ND-1000 Spectrophotometer, Wilmington, DE, USA).
Thirty-four polymorphisms from 18 genes were chosen according to their significance in the literature. All genes were previously associated with ADHD (Table 3).
Table 3.
Description of the genes and polymorphisms analyzed.
| Gene | Description | Variant | Reference |
|---|---|---|---|
| SLC6A2 | Norepinephrine transporter | rs28386840a | 44 |
| r5569c | 18 | ||
| ADRA2A | Adrenergic receptor alpha 2A | rs1800544a | 18 |
| rs553668e | 18 | ||
| SLC6A3 | Dopamine transporter | rs2550948b | 45 |
| rs2652511b | 45 | ||
| rs11564750a | 45 | ||
| 3’UTR VNTRe | 18 | ||
| Intron8 VNTRd | 18 | ||
| DRD2 | Dopamine receptor D2 | rs1800497f | 46 |
| DRD4 | Dopamine receptor D4 | rs3758653a | 47 |
| Exon3 VNTRc | 46 | ||
| Promoter duplicationb | 46 | ||
| SLC6A4 | Serotonin transporter | Promoter VNTRb | 18 |
| Intron2 VNTRd | 18 | ||
| HTR2A | Serotonin-2A receptor | rs7322347d | 48 |
| HTR2C | Serotonin-2C receptor | rs6318c | 49 |
| GRM7 | Glutamate receptor metabotropic 7 | rs3792452d | 50 |
| SLC9A9 | Glycine transporter | rs9810857f | 51 |
| COMT | Catechol-O-methyltransferase | rs4680c | 18 |
| rs4818c | 52 | ||
| SNAP25 | Synaptosomal-associated protein 25kDA | rs3746544e | 18 |
| DDC | Dopa decarboxylase | rs6592961d | 48 |
| STS | Steroid sulfatase | rs12861247d | 53 |
| rs17268988d | 53 | ||
| FADS2 | Fatty acid desaturase 2 | rs498793d | 54 |
| ADGRL3 | Adhesion G protein-coupled receptor L3 | rs1397548c | 27 |
| rs2305339d | 27 | ||
| rs6551655d | 27 | ||
| rs1868790d | 30 | ||
| rs6813183d | 30 | ||
| rs6858066d | 30 | ||
| CDH13 | Cadherin 13 | rs6565113d | 47 |
| GFOD1 | Glucose-fructose oxidoreductase domain containing 1 | rs552655d | 47 |
Position in the gene: aUpstream variant, bPromoter variant, cExon variant, dIntron variant, e3′UTR variant, fDownstream variant.
Single-nucleotide polymorphisms (SNPs) were genotyped through the TaqMan on-demand or pre-designed SNP genotyping assays system, according to the company’s instructions (Applied Biosystems, Foster City, CA, USA). We run PCR and allelic discrimination assays in a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) and we analyzed them using the LightCycler® 480 software, version 1.5. (Roche Diagnostics, Mannheim, Germany).
Variable number tandem repeat (VNTR) polymorphisms were identified using fragment analysis. PCR products were displayed on an ABI Prism 3130xl DNA sequencer (Applied Biosystems Foster City, CA, USA), and we analyzed the results by means of GeneMapper software, version 4.0 (Applied Biosystems, Foster City, CA, USA). Primer sequences and PCR conditions can be provided upon request.
For each VNTR polymorphism, subjects were categorized into 3 genotypes according to the previously described risk allele18, as follows: SLC6A3 3′UTR VNTR (10/10, 10/−, −/−), SLC6A3 intron8 VNTR (6/6, 6/−, −/−), DRD4 promoter duplication (L/L, L/S, S/S), DRD4 exon3 VNTR (7/7, 7/−, −/−), SLC6A4 promoter VNTR (L/L, L/S, S/S), and SLC6A4 intron2 VNTR (10/10, 10/−, −/−).
Statistical analysis
During the quality control procedure, genotype call rates per sample and per polymorphism < 80% were excluded from the analysis. The outcome measures of treatment with MPH were evaluated as quantitative data according to CGI-S and CGAS. Analyses of the effects of different genotypes on response to treatment over time were performed using linear mixed-effects models. These mixed-models are useful for repeated-measures analyses where follow-up times are not uniform across all subjects41. Models were constructed using the lme function from the nlme package in R.
As in other genetically complex diseases in which the model of inheritance is uncertain, the analyses were performed under the assumption of dominant, recessive, codominant, and additive models. The best model was selected based on the one with the lowest Akaike information criterion (AIC). Data for variants located on chromosome X (HTR2C and STS genes) were analyzed taking X inactivation into account according to Clayton’s approach42.
Age, sex, ADHD subtype, previous treatment, type of MPH (immediate-release vs. extended-release/both formulations), and dosage were also entered into the models as potential explanatory covariates. Statistical significance for main effects and interactions was assessed using the ANOVA F-test and set at a 2-tailed p value of 0.05. In these multivariable models, the effect size of the associations was measured by the coefficients of the models “β”.
The proportion of the variance explained by the model was assessed using Omega Squared43.
Acknowledgements
We are grateful to patients and controls, for their participation in this study. This study was supported by several grants from the Instituto de Salud Carlos III (ISCIII) of the Spanish Ministry of Health, including CIBERER (06/07/0036), FEDER (European Regional Development Fund) and IIS-FJD Biobank (PT13/0010/0012). This research was also funded by grants from Fundación Alicia Koplowitz (4019-004IIS) and the IIS-Fundación Jiménez Díaz UAM Genome Medicine Chair. CG-S was supported by CIBERER. We thank Thomas O’Boyle for his assistance in editing the English version.
Author Contributions
C.I.G.S. contributed to the design of the study, data collection process, data analysis and wrote the first drafts of the manuscript. J.J.C. contributed to the design of the study, recruitment of patients, data collection and revisions of the manuscript. R.R.A. contributed to the design of the study and revision of the manuscript. V.S.I. was involved in the recruitment of patients, data collection and revisions of the manuscript. M.R. was involved in the recruitment of patients, data collection and revisions of the manuscript. I.M.F. contributed to the statistical analysis. F.A.S. contributed to the revision of the manuscript. R.D.R. contributed to the design of the study, data analysis and revision of the manuscript. C.A. was involved in the design of the study, data analysis, intellectual content and revisions of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polanczyk G, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6) doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2) doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 3.Sibley, M. H. et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry (2016). [DOI] [PMC free article] [PubMed]
- 4.Spencer TJ. ADHD and comorbidity in childhood. J Clin Psychiatry. 2006;67(Suppl 8) [PubMed] [Google Scholar]
- 5.Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148(5) doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(Suppl 12) [PubMed] [Google Scholar]
- 7.Le HH, et al. Economic impact of childhood/adolescent ADHD in a European setting: the Netherlands as a reference case. Eur Child Adolesc Psychiatry. 2014;23(7) doi: 10.1007/s00787-013-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8(4) [PMC free article] [PubMed] [Google Scholar]
- 9.Faraone SV. Using Meta-analysis to Compare the Efficacy of Medications for Attention-Deficit/Hyperactivity Disorder in Youths. P T. 2009;34(12) [PMC free article] [PubMed] [Google Scholar]
- 10.Maia, C. R. et al. Long-Term Efficacy of Methylphenidate Immediate-Release for the Treatment of Childhood ADHD: A Systematic Review and Meta-Analysis. J Atten Disord (2014). [DOI] [PubMed]
- 11.Hodgkins P, Shaw M, Coghill D, Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012;21(9) doi: 10.1007/s00787-012-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston BA, Coghill D, Matthews K, Steele JD. Predicting methylphenidate response in attention deficit hyperactivity disorder: a preliminary study. J Psychopharmacol. 2015;29(1) doi: 10.1177/0269881114548438. [DOI] [PubMed] [Google Scholar]
- 13.Greenhill L, et al. Guidelines and algorithms for the use of methylphenidate in children with Attention-Deficit/ Hyperactivity Disorder. J Atten Disord. 2002;6(Suppl 1) doi: 10.1177/070674370200601S11. [DOI] [PubMed] [Google Scholar]
- 14.Pagerols M, et al. Pharmacogenetics of methylphenidate response and tolerability in attention-deficit/hyperactivity disorder. Pharmacogenomics J. 2016;17(1) doi: 10.1038/tpj.2015.89. [DOI] [PubMed] [Google Scholar]
- 15.Wong HK, et al. Personalized Medication Response Prediction for Attention-Deficit Hyperactivity Disorder: Learning in the Model Space vs. Learning in the Data Space. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegvik TA, et al. A candidate gene investigation of methylphenidate response in adult attention-deficit/hyperactivity disorder patients: results from a naturalistic study. J Neural Transm (Vienna) 2016;123(8) doi: 10.1007/s00702-016-1540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraone SV, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11) doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1) doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 19.Bruxel EM, et al. ADHD pharmacogenetics across the life cycle: New findings and perspectives. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(4) doi: 10.1002/ajmg.b.32240. [DOI] [PubMed] [Google Scholar]
- 20.Unal D, Unal MF, Alikasifoglu M, Cetinkaya A. Genetic Variations in Attention Deficit Hyperactivity Disorder Subtypes and Treatment Resistant Cases. Psychiatry Investig. 2016;13(4) doi: 10.4306/pi.2016.13.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Schans J, et al. Cost-effectiveness of extended-release methylphenidate in children and adolescents with attention-deficit/hyperactivity disorder sub-optimally treated with immediate release methylphenidate. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, et al. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12(6) doi: 10.1016/S0924-977X(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 23.Kambeitz J, Romanos M, Ettinger U. Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics J. 2014;14(1) doi: 10.1038/tpj.2013.9. [DOI] [PubMed] [Google Scholar]
- 24.McGough J, et al. Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(11) doi: 10.1097/01.chi.0000235083.40285.08. [DOI] [PubMed] [Google Scholar]
- 25.Kooij JS, et al. Response to methylphenidate in adults with ADHD is associated with a polymorphism in SLC6A3 (DAT1) Am J Med Genet B Neuropsychiatr Genet. 2008;147B(2) doi: 10.1002/ajmg.b.30586. [DOI] [PubMed] [Google Scholar]
- 26.Contini V, et al. No significant association between genetic variants in 7 candidate genes and response to methylphenidate treatment in adult patients with ADHD. J Clin Psychopharmacol. 2012;32(6) doi: 10.1097/JCP.0b013e318270e727. [DOI] [PubMed] [Google Scholar]
- 27.Arcos-Burgos M, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010;15(11) doi: 10.1038/mp.2010.6. [DOI] [PubMed] [Google Scholar]
- 28.Labbe A, et al. Refining psychiatric phenotypes for response to treatment: contribution of LPHN3 in ADHD. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(7) doi: 10.1002/ajmg.b.32083. [DOI] [PubMed] [Google Scholar]
- 29.Bruxel EM, et al. LPHN3 and attention-deficit/hyperactivity disorder: a susceptibility and pharmacogenetic study. Genes Brain Behav. 2015;14(5) doi: 10.1111/gbb.12224. [DOI] [PubMed] [Google Scholar]
- 30.Ribases M, et al. Contribution of LPHN3 to the genetic susceptibility to ADHD in adulthood: a replication study. Genes Brain Behav. 2011;10(2) doi: 10.1111/j.1601-183X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- 31.Bonvicini C, Faraone SV, Scassellati C. Attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry. 2016;21(7) doi: 10.1038/mp.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polanczyk G, et al. The impact of individual and methodological factors in the variability of response to methylphenidate in ADHD pharmacogenetic studies from four different continents. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8) doi: 10.1002/ajmg.b.30855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamo N, Seth S, Coghill D. Pharmacological treatment of attention-deficit/hyperactivity disorder: assessing outcomes. Expert Rev Clin Pharmacol. 2015;8(4) doi: 10.1586/17512433.2015.1050379. [DOI] [PubMed] [Google Scholar]
- 34.Weisz JR, Donenberg GR, Han SS, Kauneckis D. Child and adolescent psychotherapy outcomes in experiments versus clinics: why the disparity? J Abnorm Child Psychol. 1995;23(1) doi: 10.1007/BF01447046. [DOI] [PubMed] [Google Scholar]
- 35.Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5) doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Banaschewski T, et al. Health-related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs. 2013;27(10) doi: 10.1007/s40263-013-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matza LS, et al. Challenges of Developing an Observable Parent-Reported Measure: A Qualitative Study of Functional Impact of ADHD in Children. Value Health. 2017;20(6) doi: 10.1016/j.jval.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Du Paul GJ. Parent and Teacher Ratings of ADHD Symptoms: Psychometric Properties in a Community-Based Sample. J Clin Child Psychol. 1991;20(3) doi: 10.1207/s15374424jccp2003_3. [DOI] [Google Scholar]
- 39.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7) [PMC free article] [PubMed] [Google Scholar]
- 40.Shaffer D, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11) doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 41.Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2011;6 doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clayton DG. Sex chromosomes and genetic association studies. Genome Med. 2009;1(11) doi: 10.1186/gm110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu R. Measuring explained variation in linear mixed effects models. Stat Med. 2003;22(22) doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- 44.Kim CH, et al. A polymorphism in the norepinephrine transporter gene alters promoter activity and is associated with attention-deficit hyperactivity disorder. Proc Natl Acad Sci U S A. 2006;103(50) doi: 10.1073/pnas.0510836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genro JP, et al. A common haplotype at the dopamine transporter gene 5’ region is associated with attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8) doi: 10.1002/ajmg.b.30863. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, et al. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45(3) doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- 47.Lasky-Su J, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8) doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- 48.Ribases M, et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry. 2009;14(1) doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- 49.Bobb AJ, et al. Support for association between ADHD and two candidate genes: NET1 and DRD1. Am J Med Genet B Neuropsychiatr Genet. 2005;134B(1) doi: 10.1002/ajmg.b.30142. [DOI] [PubMed] [Google Scholar]
- 50.Park S, et al. Association between the GRM7 rs3792452 polymorphism and attention deficit hyperacitiveity disorder in a Korean sample. Behav Brain Funct. 2013;9 doi: 10.1186/1744-9081-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mick E, et al. Family-based genome-wide association scan of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9) doi: 10.1016/j.jaac.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaelovsky E, et al. Association between a common haplotype in the COMT gene region and psychiatric disorders in individuals with 22q11.2DS. Int J Neuropsychopharmacol. 2008;11(3) doi: 10.1017/S1461145707008085. [DOI] [PubMed] [Google Scholar]
- 53.Brookes KJ, et al. Association of the steroid sulfatase (STS) gene with attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8) doi: 10.1002/ajmg.b.30873. [DOI] [PubMed] [Google Scholar]
- 54.Brookes K, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11(10) doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]