Abstract
Given salvage treatment for recurrent nasopharyngeal carcinoma (NPC) remains a clinical dilemma, immunotherapy targeting NPC-specific immunosuppression may bring new hope. We analyzed the expression of CD8, CD4, Foxp3 and Tim-3 in lymphocytes, and of Galectin-9 in tumour cells between paired primary and recurrent NPC from 95 patients and we noted that there was significant increase in the expression of Galectin-9+ tumour cells (p < 0.001) and Foxp3+ lymphocytes (p < 0.001) but a significant decrease in the expression of CD8+ lymphocytes (p = 0.01) between paired primary and recurrent NPC. Of all patients, 53 patients (55.79%) and 57 patients (60%) had increased percentages of Galectin-9+ tumour cells and of Foxp3+ lymphocytes, respectively. Conversely, 42 patients (44.21%) had decreased percentages of CD8+ lymphocytes. The patients with high Galectin-9 expression in recurrent NPC frequently also had high Tim-3 (p = 0.04) and Foxp3 (p = 0.01), and low CD8 (p = 0.04) expression in lymphocytes. After multivariate analyses, low CD8 expression in lymphocytes was an independent risk factor for relapse-free survival (p = 0.002) and overall survival (p = 0.02). Our data suggests that recurrent NPC may had more immunologic advantage than primary NPC, especially the Galectin-9/Tim-3 pathway. The immunotherapies targeting Galectin-9/Tim-3/Foxp3 interaction may serve as a potential salvage treatment for recurrent NPC.
Introduction
In contrast to other head and neck squamous cell carcinomas, nasopharyngeal carcinoma (NPC) is characterized by its close association with Epstein-Barr virus (EBV) infection, poor differentiation of tumour cells and high susceptibility to radiotherapy and chemotherapy1, 2. Due to its high sensitivity to radiation, the mainstream treatment for primary NPC is always radiotherapy with or without chemotherapy3. However, some patients may experience recurrent disease and must undergo salvage treatment4–6. In general, recurrent NPC responds to treatment differently than primary NPC. First, it is easy to speculate that recurrent NPC might become more radioresistant than primary NPC7. Second, the current choices of salvage treatment such as reirradiation, salvage nasopharyngectomy or other multiple modalities frequently result in severe delayed toxicities, such as cranial nerve damage, radionecrosis or temporal lobe necrosis, which can seriously affect quality of life in patients with recurrent NPC8. Most importantly, there has not been a specific consensus regarding the salvage treatment for recurrent NPC. Therefore, recurrent NPC still represents a clinical dilemma and a challenging problem for clinicians.
In recent years, the progress in immunotherapy has opened new fields in cancer treatment. For example, multiple immunosuppressive mechanisms in NPC have been proposed, including interleukin-10, transforming growth factor β1, Fas ligand, Foxp3+ regulatory T cells and Galectin-99–14. The Galectin-9/Tim-3 axis had been reported to be one of the NPC-specific immunosuppression pathway13. The immunotherapeutic drug targeting this axis are also available now15. Therefore, it is interesting and important to discover the changes of Galectin-9/Tim-3 pathway and generalized immunologic status (CD8+ , CD4+ and Foxp3+ lymphocytes in tumour microenvironment) between primary and recurrent NPC. The change in immunologic status between primary and recurrent NPC may have clinical implication in understanding the possibility of immunotherapy for the recurrent NPC patients. Therefore, we evaluated the paired immunologic expression of CD8+ lymphocytes, CD4+ lymphocytes, Foxp3+ lymphocytes, and the Galectin-9/Tim-3 pathway in the tumour microenvironment between primary and recurrent NPC from 95 patients.
Immunotherapy targeting NPC-specific immunosuppression may bring new hope for patients with recurrent NPC. For clinicians, the question of whether immunotherapy could be the choice of salvage treatment is imperative to answer. To answer this question, we analyzed the immunologic changes in the tumour microenvironment between primary and recurrent NPC. The scientific aim of our study is to evaluate the consequential changes in tumour immunology from chemotherapy and radiotherapy of primary NPC and understand the possible clinical implication of immunotherapeutic potential for patients with recurrent NPC in the future.
Results
Patient demographics
A total of 95 eligible patients with recurrent NPC were enrolled in this study, including 70 male and 25 female patients. Their ages ranged from 27 to 77 years, with a mean age of 50 ± 11 years at diagnosis. The length of time between when primary treatment for NPC ended and diagnosis of recurrent NPC occurred ranged from 1 to 163 months, with a mean of 26 ± 26 months. The overall follow-up period was from 12 to 174 months, with a mean of 59 ± 27 months. The recurrent NPC profile of the 97 patients are as follows: 43 patients had local recurrence in nasopharynx, 12 patients had local and regional neck recurrence, 11 patients had regional neck recurrence, 11 patients had distant Lung metastases, 5 patients had distant Bone metastases, 5 patients had multiple (≥2) distant metastases, 3 patients had distant Liver metastases, 3 patients had distant Spinal metastases and 2 patients had recurrence over parotid gland. The basic characteristics, clinical characteristics and changes of immunologic markers between primary and recurrent NPC are listed in Table 1.
Table 1.
Clinical characteristics, Histopathological characteristics and Immunologic expression in primary NPC and recurrent NPC in our series.
| Characteristics | Primary NPC (n = 95) | Recurrent NPC (n = 95) | p value |
|---|---|---|---|
| Age (years) | 50 ± 11 | ||
| Gender | 70 males, 25 females | ||
| Histological types | |||
| Keratinizing squamous cell carcinoma | 4/95 (4.21%) | ||
| Non-keratinizing carcinoma, differentiated | 20/95 (21.05%) | ||
| Non-keratinizing carcinoma, undifferentiated | 71/95 (74.74%) | ||
| Tumour stages | |||
| Early stages (I/II/III) | 45/95 (47.37%) | 52/95 (54.74%) | 0.38 |
| Advanced stages (IVA/IVB) | 50/95 (52.63%) | 43/95 (45.26%) | |
| Changes of Galectin-9+ tumour cells | |||
| Increased | 53/95 (55.79%) | ||
| Same | 22/95 (23.16%) | ||
| Decreased | 20/95 (21.05%) | ||
| Changes of CD8+ lymphocytes in TM | |||
| Increased | 24/95 (25.26%) | ||
| Same | 29/95 (30.53%) | ||
| Decreased | 42/95 (44.21%) | ||
| Changes in Foxp3+ lymphocytes in TM | |||
| Increased | 57/95 (60%) | ||
| Same | 15/95 (15.79%) | ||
| Decreased | 23/95 (24.21%) | ||
| Changes of CD4+ lymphocytes in TM | |||
| Increased | 38/95 (40%) | ||
| Same | 24/95 (25.26%) | ||
| Decreased | 33/95 (34.74%) | ||
| Changes of Tim-3+ lymphocytes in TM | |||
| Increased | 34/95 (35.79%) | ||
| Same | 25/95 (26.32%) | ||
| Decreased | 36/95 (37.89%) | ||
Abbreviation: NPC, nasopharyngeal carcinoma; TM: tumour microenvironment.
Changes in immunologic marker expression from primary to recurrent NPC
When we compared the expression of all immunologic markers (Fig. 1) in the tumour microenvironment between primary and recurrent NPC, we found significant differences in the mean percentages of Galectin-9+ tumour cells, Foxp3+ lymphocytes and CD8+ lymphocytes. The means and standard deviations of the percentages of Galectin-9+ tumour cells in primary and recurrent NPC were 29.41 ± 27.87% and 41.14 ± 29.60%, respectively (p = 0.006). The means and standard deviations of the percentages of Foxp3+ lymphocytes in primary and recurrent NPC were 25.65 ± 21.70% and 37.54 ± 28.23%, respectively (p = 0.01). The means and standard deviations of the percentages of CD8+ lymphocytes in primary and recurrent NPC were 17.85 ± 18.99% and 11.61 ± 15.11%, respectively (p = 0.02). On the contrary, there were no significant difference in the mean percentages of CD4+ lymphocytes and Tim-3+ lymphocytes. The means and standard deviations of the percentages of CD4+ lymphocytes in primary and recurrent NPC were 14.91 ± 19.09% and 17.12 ± 21.27%, respectively (p = .74). The means and standard deviations of the percentages of Tim-3+ lymphocytes in primary and recurrent NPC were 15.88 ± 15.52% and 14.38 ± 14.50%, respectively (p = .43).
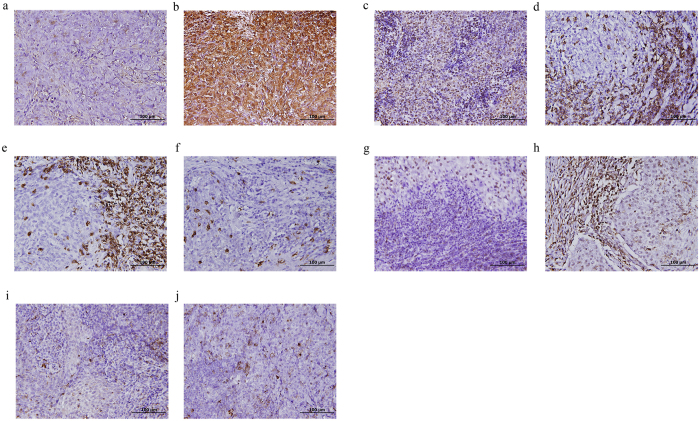
Figure 1.
The immunohistochemical staining of different immunologic markers in paired initial and recurrent nasopharyngeal carcinoma (NPC) tissue. (a) Initial non-keratinizing NPC, undifferentiated with low level of Galectin-9 expression in tumour cells (0%). (b) The liver metastatic carcinoma, undifferentiated with high level of Galectin-9 expression in tumour cells (80%). (c) Initial non-keratinizing NPC, undifferentiated with low level of Foxp3 expression in stromal infiltrating lymphocytes (5%). (d) The contralateral neck nodal recurrent carcinoma with high level of Foxp3 expression in stromal infiltrating lymphocytes (70%). (e) Initial non-keratinizing NPC, differentiated with high level of CD8 expression in stromal infiltrating lymphocytes (80%). (f) The ipsilateral neck nodal recurrent carcinoma with low level of CD8 expression in stromal infiltrating lymphocytes (15%). (g) Initial non-keratinizing NPC, undifferentiated with low level of CD4 expression in stromal infiltrating lymphocytes (10%). (h) The lung metastatic carcinoma, undifferentiated with high level of CD4 expression in stromal infiltrating lymphocytes (80%). (i) Initial non-keratinizing NPC, undifferentiated with modest Tim-3 expression in stromal infiltrating lymphocytes (20%). (j) The lung metastatic carcinoma, undifferentiated with mildly increased Tim-3 expression in stromal infiltrating lymphocytes (30%).
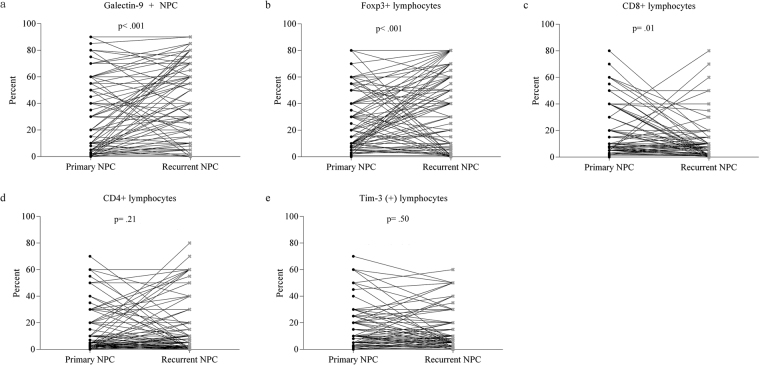
Furthermore, the differences in the expression of these immunologic markers between primary and recurrent NPC were verified by Wilcoxon signed-rank test. All changes in the expression of the tested immunologic markers in the paired samples are shown in Fig. 2. Again, there were significant differences in the percentages of Galectin-9+ tumour cells, Foxp3+ lymphocytes and CD8+ lymphocytes between the paired primary and recurrent NPC. The percentages of Galectin-9+ tumour cells and Foxp3+ lymphocytes increased significantly (p < 0.001 and p < 0.001, respectively) in recurrent NPC. Conversely, the percentage of CD8+ lymphocytes decreased significantly in recurrent NPC (p = 0.01).
Figure 2.
The changes in the percentages of (a) Galectin-9+ tumour cells (significantly increased), (b) Foxp3+ lymphocytes (significantly increased), (c) CD8+ lymphocytes (significantly decreased), (d) CD4+ lymphocytes (increased) and (e) Tim-3+ lymphocytes (decreased) in the paired primary and recurrent nasopharyngeal carcinoma tissues.
The different patterns of immunologic markers between patients with low and high Galectin-9 expression levels in recurrent NPC
The basic characteristics and clinicopathological data of the patients with high and low Galectin-9 expression levels in recurrent NPC are shown in Table 2. There were no other significant differences between the groups with low and high Galectin-9 expression. However, the expression patterns of specific immunologic markers including CD8, Tim-3 and Foxp3 in lymphocytes differed in the group with high Galectin-9 expression (Table 3) from those with low Galectin-9 expression. In summary, the patients with high Galectin-9 expression in recurrent NPC was associated with a higher percentage of Tim-3+ lymphocytes and a more immunocompromised status (a lower percentage of CD8+ and higher percentage of Foxp3+ lymphocytes) in the tumour microenvironment.
Table 2.
The characteristics and clinicopathological data of all patients with nasopharyngeal carcinoma in our series.
| Low Galectin-9 in recurrent NPC (n = 45) | High Galectin-9 in recurrent NPC (n = 50) | p value | |
|---|---|---|---|
| Age (years) | |||
| >50 | 23/45 (51.11%) | 22/50 (44%) | 0.54 |
| ≤50 | 22/45 (48.89%) | 28/50 (56%) | |
| Gender | |||
| Female | 8/45 (17.78%) | 17/50 (34%) | 0.10 |
| Male | 37/45 (82.22%) | 33/50 (66%) | |
| Histological types | |||
| Keratinizing squamous cell carcinoma | 2/45 (4.44%) | 2/50 (4%) | 0.25* |
| Non-keratinizing carcinoma, differentiated | 6/45 (13.33%) | 14/50 (28%) | |
| Non-keratinizing carcinoma, undifferentiated | 37/45 (82.22%) | 34/50 (68%) | |
| Type of recurrence | |||
| Primary sites (local nasopharynx) | 22/45 (48.89%) | 21/50 (42%) | 0.92 |
| Regional sites (Neck lymph node) | 5/45 (11.11%) | 6/50 (12%) | |
| Distant metastases | 13/45 (28.89%) | 16/50 (32%) | |
| Both Primary and Regional sites | 5/45 (11.11%) | 7/50 (14%) | |
| Initial stages | |||
| Early stages (I/II/III) | 24/45 (53.33%) | 21/50 (42%) | 0.31 |
| Advanced stages (IVA/IVB) | 21/45 (46.67%) | 29/50 (58%) | |
| Recurrent stages | |||
| Early stages (rI/rII/rIII) | 27/45 (60%) | 27/50 (54%) | 0.41 |
| Advanced stages (rIVA/rIVB/rIVC) | 18/45 (40%) | 23/50 (46%) | |
| Initial treatment modalities | |||
| CCRT | 32/45 (71.11%) | 28/50 (56%) | 0.14 |
| Induction C/T + CCRT | 13/45 (28.89%) | 22/50 (44%) | |
| Salvage treatment modalities | |||
| Surgery only | 14/45 (31.11%) | 17/50 (34%) | 0.08* |
| Re-CCRT | 13/45 (28.89%) | 5/50 (10%) | |
| Salvage C/T only | 14/45 (31.11%) | 25/50 (50%) | |
| Multiple modalities | 4/45 (8.89%) | 3/50 (6%) | |
Abbreviation: CCRT, concurrent chemoradiotherapy; C/T: chemotherapy; NPC, nasopharyngeal carcinoma; *using Fisher’s exact test.
Table 3.
The distribution and association of different immunologic factors in recurrent nasopharyngeal carcinoma and tumour-infiltrating lymphocytes.
| Low Galectin-9 in recurrent NPC (n = 45) | High Galectin-9 in recurrent NPC (n = 50) | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| Expression of CD8+ lymphocytes | ||||
| Low | 19/45 (42.22%) | 32/50 (64%) | 2.43 (1.06~5.56) | 0.04 |
| High | 26/45 (57.78%) | 18/50 (36%) | ||
| Expression of Foxp3+ lymphocytes | ||||
| Low | 27/45 (60%) | 17/50 (34%) | 2.91 (1.26~6.71) | 0.01 |
| High | 18/45 (40%) | 33/50 (66%) | ||
| Expression of Tim-3+ lymphocytes | ||||
| Low | 28/45 (62.22%) | 20/50 (40%) | 2.47 (1.08~5.65) | 0.04 |
| High | 17/45 (37.78%) | 30/50 (60%) | ||
| Expression of CD4+ lymphocytes | ||||
| Low | 24/45 (53.33%) | 24/50 (48%) | 1.24 (0.55~2.77) | 0.68 |
| High | 21/45 (46.67%) | 26/50 (52%) | ||
Abbreviation: NPC, nasopharyngeal carcinoma; CI, confidence interval.
Immunologic expression had a significant association with relapse-free survival and overall survival
In the univariate analysis (Table 4), a high percentage of Galectin-9+ tumour cells (p = 0.004), a low percentage of CD8+ lymphocytes (p = 0.005) and a high percentage of Foxp3+ lymphocytes (p = 0.004) in the recurrent tumour microenvironment as well as in the advanced stages of recurrent NPC (p < 0.001) were identified as significant risk factors for relapse-free survival. For overall survival, only an advanced stage of recurrent NPC (p < 0.001) was a significant risk factor. The relapse-free survival and overall survival curves with significant difference (p < 0.05) are shown in Fig. 3.
Table 4.
Univariate analysis of possible risk factors for survival.
| Characteristics | Relapse-free survival | Overall survivals | ||
|---|---|---|---|---|
| Rates | p values | Rates | p value | |
| Ages (years) | ||||
| >50 | 41.7% | 0.97 | 58.8% | 0.36 |
| ≤50 | 31.9% | 62.8% | ||
| Gender | ||||
| Male | 40.4% | 0.21 | 60.2% | 0.43 |
| Female | 22.5% | 62.4% | ||
| Histological types | ||||
| Nonkeratinizing carcinoma, undifferentiated | 29.5% | 0.26 | 75% | 0.48 |
| Nonkeratinizing carcinoma, differentiated | 59.7% | 66.9% | ||
| Keratinizing squamous cell carcinoma | 75% | 57.9% | ||
| Recurrent NPC Galectin-9 expression | ||||
| Low | 45.4% | 0.004 | 69.4% | 0.06 |
| High | 29.7% | 53.2% | ||
| TILs CD8+ lymphocytes | ||||
| Low | 26.1% | 0.005 | 53.5% | 0.06 |
| High | 46.6% | 69.1% | ||
| TILs CD4+ lymphocytes | ||||
| Low | 44.6% | 0.21 | 65.1% | 0.20 |
| 56.5% | ||||
| High | 29.5% | |||
| TILs Foxp3+ lymphocytes | ||||
| Low | 50.2% | 0.004 | 68.9% | 0.08 |
| 53.8% | ||||
| High | 25.8% | |||
| TILs Tim-3+ lymphocytes | ||||
| Low | 42.2% | 0.32 | 61.8% | 0.65 |
| High | 31.0% | 59.7% | ||
| Initial stage | ||||
| Early stages (I/II/III) | 35% | 0.33 | 58.4% | 0.36 |
| 63.1% | ||||
| Advanced stages (IVA/IVB) | 39.1% | |||
| Recurrent stage | ||||
| Early stages (rI/rII/rIII) | 58.4% | <0.001 | 79.8% | <0.001 |
| Advanced stages (rIVA/rIVB/rIVC) | 10.9% | 36.9% | ||
Abbreviation: NPC, nasopharyngeal carcinoma; TILs, tumour-infiltrating lymphocytes.
Figure 3.
The relapse-free survival and overall survival curves with significant difference (p < 0.05). The median percentages of all immunologic markers were used as the cut-off points for grouping high or low level of expression groups. (a) Recurrent patients with low or high Galectin-9+ tumour, relapse-free survival curves (b) Recurrent patients with low or high CD8+ lymphocytes, relapse-free survival curves. (c) recurrent patients with low or high Foxp3+ lymphocytes, relapse-free survival curves. (d) Patients with early or advanced recurrent stages, relapse-free survival curves. (e) patients with early or advanced recurrent stages, overall survival curves
All significant risk factors for both relapse-free survival and overall survival were further examined by multivariate analysis (Table 5). This analysis revealed that an advanced stage of recurrent disease (hazard ratio (HR): 2.58, p = 0.001) and a low percentage of CD8+ lymphocytes (HR: 2.40, p = 0.002) were independent risk factors for worse relapse-free survival. For overall survival, the analysis also revealed that an advanced stage of recurrent disease (HR: 3.92, p < 0.001) and a low percentage of CD8+ lymphocytes (HR: 2.16, p = 0.02) were independent risk factors. In short, the patients with recurrent NPC who had an advanced stage of disease or a low percentage of CD8+ lymphocytes in the tumour microenvironment had significantly worse relapse-free survival and overall survival.
Table 5.
Multivariate analysis of possible risk factors using the Cox logistic regression method.
| Characteristics | Relapse-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | p value | |
| Recurrent Stages | ||||
| Advanced stage (rIVA/rIVB/rIVC) | 2.58 (1.47~4.54) | 0.001 | 3.92 (2.01~7.64) | < 0.001 |
| Early stage (rI/rII/rIII) | 1.0 (reference) | 1.0 (reference) | ||
| TILs Foxp3+ lymphocytes | ||||
| High | 1.72 (0.96~3.05) | 0.07 | 1.30 (0.68~2.50) | 0.43 |
| Low | 1.0 (reference) | 1.0 (reference) | ||
| TILs CD8+ lymphocytes | ||||
| Low | 2.40 (1.37~4.18) | 0.002 | 2.16 (1.14~4.08) | 0.02 |
| High | 1.0 (reference) | 1.0 (reference) | ||
| Recurrent NPC Galectin-9 expression | ||||
| High | 1.72 (0.96~3.06) | 0.07 | 1.46 (0.78~2.73) | 0.23 |
| Low | 1.0 (reference) | 1.0 (reference) | ||
Abbreviation: NPC, nasopharyngeal carcinoma; TILs, tumour-infiltrating lymphocytes, HR: hazard ratio.
Discussion
After we compared immunologic marker expression in the tumour microenvironment between primary and recurrent NPC, we report two interesting findings from our study. The first finding primarily focuses on the immunologic changes between primary and recurrent NPC. In contrast to primary NPC, more than half of the patients with recurrent NPC had increased percentages of Galectin-9+ tumour cells and of Foxp3+ lymphocytes. More than one-third of the patients with recurrent NPC had increased percentages of Tim-3+ and CD4+ lymphocytes. Conversely, nearly half of the patients with recurrent NPC had a decrease in the percentage of CD8+ lymphocytes. Furthermore, the increased percentages of Galectin-9+ tumour cells and Foxp3+ lymphocytes and the decreased percentage of CD8+ lymphocytes significantly differed between primary and recurrent NPC. Finally, the patients with recurrent NPC had a high percentage of Galectin-9+ tumour cells accompanied by a low percentage of CD8+ lymphocytes and high percentages of Foxp3+ and Tim-3+ lymphocytes.
Theoretically, NPC is an EBV-related malignancy. During the process of carcinogenesis, NPC cells still retain functional antigen-presenting machinery. Therefore, the immune escape has been regarded as a specific feature of the tumour microenvironment in NPC16, 17. Among several possible immune escape mechanisms, the immune checkpoint Galectin-9/Tim-3 interaction has been reported as one of the most common types in NPC13, 14, 18, 19. This immunologic interaction could promote the formation of Foxp3+ lymphocytes and then inhibit the population of CD8+ lymphocytes20, 21. Moreover, it has been shown that Galectin-9 was extremely abundant in NPC cells in either xenografted tumours or clinical specimens19. Because patients with recurrent NPC in our series had a significantly higher percentage of Galectin-9+ tumour cells, we speculate that the initial treatment may have an important effect on tumour selection. It has been reported that radiation induces dying cells to express significantly more tumour-specific antigens on their surface, resulting in CD8+ lymphocytes being recruited to perform tumour-specific killing22. This finding suggests that Galectin-9+ tumour cells in primary NPC may be more resistant to this radiation-induced cytotoxic killing by CD8+ lymphocytes. Subsequently, these Galectin-9+ tumour cells had selective growth advantage in recurrent NPC. In recurrent NPC, to maintain immune escape, these high Galectin-9 expressing NPC cells maintains their ability to inactivate the survival and growth of tumour-infiltrating CD8+ lymphocytes. In addition, this inhibitory effect of CD8+ lymphocytes may be related to the presence of more Foxp3+ lymphocytes in the tumour microenvironment of recurrent NPC23.
Our second finding primarily focuses on the impact of immunologic markers in the tumour microenvironment, specifically how a low percentage of CD8+ lymphocytes, high percentage of Galectin-9+ NPC cells and high percentage of Foxp3+ lymphocytes affect the outcome of recurrent NPC. Although a high percentage of Galectin-9+ tumour cells and a high percentage of Foxp3+ lymphocytes in the tumour microenvironment were only marginally significant, a low percentage of CD8+ lymphocytes was a significant risk factor for relapse-free survival and overall survival. Therefore, it is reasonable to speculate that the Galectin-9/Tim-3/Foxp3+ immunologic interaction and its subsequent suppression in CD8+ lymphocytes may play an important role in determining the outcome of salvage treatment. In fact, the association between CD8+ lymphocytes in the tumour microenvironment and treatment outcomes have been reported in NPC and other malignancies24–27. Recurrent NPC cells with high Galectin-9 expression had the ability to maintain immune escape by interacting with Tim-3+ lymphocytes13, 18, 28. The low percentage of CD8+ lymphocytes in the tumour microenvironment, which is a sign of an immunocompromised status, could serve as an effective biomarker to predict the response to salvage treatment in recurrent NPC.
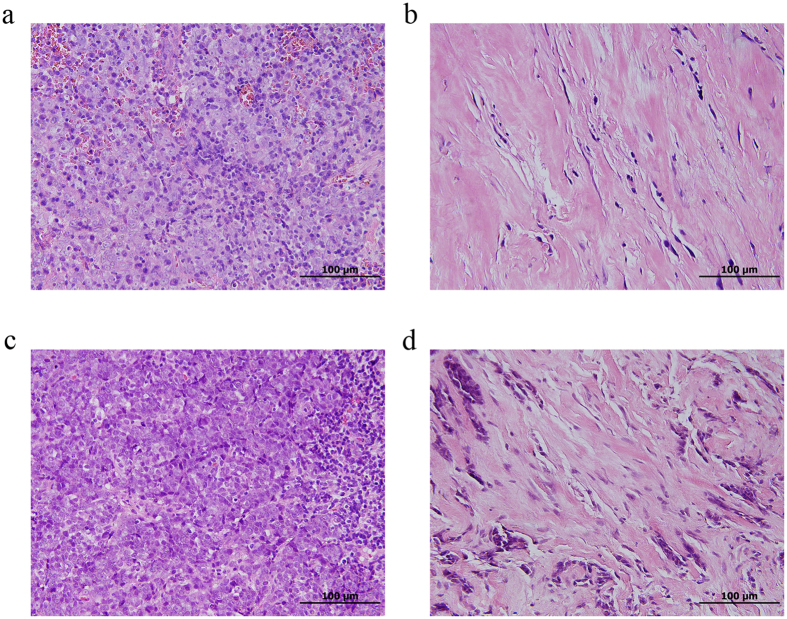
In recurrent NPC, previous radiation could alter the tumour microenvironment and induce tissue fibrosis and microvasculature damage. These changes in physical barriers might play a role in tumour-mediated immune clearance because cells of the adaptive immune system must be able to infiltrate or invade into the tumour to eradicate it. In our series, we frequently identified fibrotic tissue changes (Fig. 4) in the previous radiation field. This radiation-associated physical barrier may partially account for the decreased percentage of CD8+ lymphocytes in recurrent NPC. However, this radiation-associated fibrosis did not prevent the infiltration of Tim-3+ and Foxp3+ lymphocytes in the tumour microenvironment. A recent breakthrough in the treatment of melanoma and other cancers with immune checkpoint inhibitors has rejuvenated enthusiasm in immunotherapy for cancer treatment29–32. Currently, the choice of salvage treatment is limited for recurrent NPC. Immunotherapeutic strategies, especially those targeting the Galectin-9/Tim-3/Foxp3 interaction, may serve as the potential salvage treatments for recurrent NPC33 in recurrent NPC.
Figure 4.
The radiation-associated changes in histopathologic examination. (a) Primary nasopharyngeal carcinoma (NPC) without fibrosis. (b) Recurrent NPC with marked fibrosis in local nasopharyngeal tissue. (c) Primary NPC without fibrosis. (d) Recurrent NPC with marked fibrosis in regional neck tissue.
There were some limitations in our study. First, this retrospective study may be vulnerable to selection bias. Although we try to statistically control for this bias using Cox regression model, residual bias may remain. Second, the immunologic characteristics in different histological types, local nasopharynx, regional neck and distant sites, may have originally had different immunologic profiles34. These differences may reflect underlying mechanisms in the effect of immunologic changes in recurrent NPC. The strength of this study is that we succeeded in comparing all tissue samples between primary and recurrent NPC in 95 patients. Well-designed prospective case series studies with more patients are necessary in the future to clarify the changes in and the roles of immunologic profiles for recurrent NPC.
Patients and Methods
Ethics Statement
This study was approved by the Institutional Review Board of National Taiwan University Hospital. As this was a retrospective analysis of routine data, we requested and were granted a waiver of individual informed consent from the ethics committee. All methods were performed in accordance with the relevant guidelines and regulations. Patient records/information were anonymized and de-identified prior to analyses.
Patient population
We retrospectively reviewed the medical records of patients who were diagnosed with recurrent NPC (defined as persistent tumour after primary treatment, local/regional neck recurrence or distant metastasis) at the National Taiwan University Hospital between October 2007 and October 2015. The exclusion criteria included patients who had no history of curative treatment for primary NPC in our hospital, patients with primary and/or recurrent NPC diagnosis but without tissue for definite pathologic confirmation, and patients with malignancies other than NPC. Therefore, all the patients in our series had complete primary and recurrent NPC tissue for pathologic comparison. The TNM status of primary and recurrent NPC was classified according to the 2010 criteria of the American Joint Committee on Cancer (AJCC)35.
Immunohistochemical analyses of Galectin-9+ tumour cells and Tim-3+ , CD4+ , CD8+ and Foxp3+ lymphocytes in the tumour microenvironment of primary and recurrent NPC
For immunohistochemical (IHC) staining of Tim-3, Galectin-9, CD4, CD8 and Foxp3, 4-μm-thick sections from each formalin-fixed, paraffin-embedded tissue block were de-waxed with xylene and rehydrated through a graded series of ethanol. Antibodies against Tim-3 (Clone D5D5R, Cell Signalling Technology, Inc., Danvers, MA, USA), Galectin-9 (GeneTex, Inc., Hsinchu City, Taiwan, R.O.C.), CD4 (clone SP35, Spring Bioscience, Inc., Pleasanton, CA, USA), CD8 (clone SP16, Spring Bioscience, Inc., Pleasanton, CA, USA), and Foxp3 (clone 2A11G9, Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) were used for immunohistochemistry. Sections were incubated with anti-Tim-3 antibody (1:300 dilution) for 1 hour; antigen retrieval was performed by autoclaving the sections in Novocastra Epitope Retrieval Solution pH 9.0 (Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, UK) for 10 min at 121 °C. Sections were incubated with anti-Galectin 9 antibody (1:300 dilution) for 1 hour; antigen retrieval was performed by incubating the sections with Protease 1 (Ventana Medical Systems, Inc., Tucson, Arizona, USA) for 10 min. Sections were incubated with anti-CD4 antibody (1:50 dilution) for 1 hour; antigen retrieval was performed by autoclaving the sections in citrate buffer (pH 6.0) for 10 min at 121 °C. Sections were incubated with anti-CD8 antibody (1:100 dilution) for 1 hour; antigen retrieval was performed by autoclaving the sections in citrate buffer (pH 6.0) for 10 min at 121 °C. Sections were incubated with anti-Foxp3 antibody (1:500 dilution) for 1.5 hour; antigen retrieval was performed by autoclaving the sections in citrate buffer (pH 6.0) for 10 min at 121 °C. The UltraVision Quanto Detection System HRP DAB (Thermo Fisher Scientific, Fremont, CA, USA) was used according to the manufacturer’s instructions. The sections were counterstained with haematoxylin and then mounted. For each stromal infiltrating lymphocyte subgroups, the percentages of lymphocytes compared to the total amount of nucleated cells in the stromal compartment were examined. The scoring of positive lymphocytes and Galectin 9+ tumour cells were estimated by two independent pathologists (Y.-L.C. and C.-T.W.) using a dual-handed microscope by screening the whole immunohistochemical staing sections. High expression of CD4+, CD8+, Tim-3+, Fop3+ lymphocytes and Galectin-9+ tumour cells was defined as values greater than the median percentages.
Statistical analysis
All immunologic factors were tested for normal distribution by Kolmogorov-Smirnov Test. The Wilcoxon signed-rank test was used to compare the changes in percentages of all immunologic markers between primary and recurrent NPC. The median percentages of all immunologic markers were used as the cut-off points for grouping the patients into high or low level of expression groups. Chi-square tests and Fisher’s exact tests (only in cell number less than five) were used to determine differences in the clinical and immunological characteristics (low or high expression) between the patients with low and high Galectin 9 expression as appropriate. The starting point of the follow-up period was defined as the completion of the curative treatment of primary NPC for each patient. The end point of the follow-up period was either June 2016 or the time when the patient died. The primary outcomes were relapse-free survival and overall survival. All sites of persistent, residual or recurrent tumours after salvage treatment were recorded as failure in terms of the relapse-free survival metric, and all deaths were recorded against the overall survival parameter. The 5-year survival rates of relapse-free and overall survival were calculated by using the Kaplan–Meier product limit method. Significance levels among the curves were determined using the log-rank test. Potential risk factors and pathological characteristics were further analyzed using a multivariate Cox regression model. All statistical analyses were performed using the SPSS software package, version 18.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed and corresponding p values < 0.05 were interpreted as statistically significant.
Author Contributions
Conceived and designed the research: T.-C.C., C.-T.W. and Y.-L.C.; Collected the data: T.-C.C., C.-P.W. and P.-H.L.; Analyzed the data: T.-C.C., C.-H.C. and C.-P.W.; Reviewed and pictured the pathologic slide: C.-T.W. and Y.-L.C.; Wrote and edited the paper: T.-C.C., C.-H.C. and C.-P.W.; Supervised all research: C.-P.W., T.-L.Y., P.-J.L., and J.-Y.K.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Chen-Tu Wu and Yih-Leong Chang contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chien YC, et al. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, et al. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015;51:1041–1046. doi: 10.1016/j.oraloncology.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Wee J, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 4.Chang JT, et al. Locally recurrent nasopharyngeal carcinoma. Radiother Oncol. 2000;54:135–142. doi: 10.1016/S0167-8140(99)00177-2. [DOI] [PubMed] [Google Scholar]
- 5.Xu T, et al. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 2013;20:e406–419. doi: 10.3747/co.20.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AW, Fee WE, Jr., Ng WT, Chan LK. Nasopharyngeal carcinoma: salvage of local recurrence. Oral Oncol. 2012;48:768–774. doi: 10.1016/j.oraloncology.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Lo WC, et al. Salvage treatment for isolated regional failure of nasopharyngeal carcinoma after primary radiotherapy. Ann Surg Oncol. 2012;19:1001–1008. doi: 10.1245/s10434-011-2018-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22:233–244. doi: 10.1016/j.semradonc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Yao M, et al. Interleukin-10 expression and cytotoxic-T-cell response in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer. 1997;72:398–402. doi: 10.1002/(SICI)1097-0215(19970729)72:3<398::AID-IJC4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Menezes J, Prasad U, Ahmad A. Elevated serum levels of transforming growth factor beta1 in Epstein-Barr virus-associated nasopharyngeal carcinoma patients. Int J Cancer. 1999;84:396–399. doi: 10.1002/(SICI)1097-0215(19990820)84:4<396::AID-IJC11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Ho SY, et al. Prognostic implications of Fas-ligand expression in nasopharyngeal carcinoma. Head Neck. 2004;26:977–983. doi: 10.1002/hed.20090. [DOI] [PubMed] [Google Scholar]
- 12.Lau KM, et al. Increase in circulating Foxp3+ CD4+ CD25(high) regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer. 2007;96:617–622. doi: 10.1038/sj.bjc.6603580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klibi J, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 14.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22:127–136. doi: 10.1016/j.semcancer.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2:393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 16.Khanna R, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58:310–314. [PubMed] [Google Scholar]
- 17.Lee SP, et al. CTL control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen-processing function of the tumor cells. J Immunol. 2000;165:573–582. doi: 10.4049/jimmunol.165.1.573. [DOI] [PubMed] [Google Scholar]
- 18.Keryer-Bibens C, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6 doi: 10.1186/1471-2407-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pioche-Durieu C, et al. In nasopharyngeal carcinoma cells, Epstein-Barr virus LMP1 interacts with galectin 9 in membrane raft elements resistant to simvastatin. J Virol. 2005;79:13326–13337. doi: 10.1128/JVI.79.21.13326-13337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Fueyo A, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 21.Seki M, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2 doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, et al. Tim-3-Galectin-9 pathway involves the suppression induced by CD4+ CD25+ regulatory T cells. Immunobiology. 2009;214:342–349. doi: 10.1016/j.imbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Hsu MC, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–1403. doi: 10.1038/modpathol.2010.130. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 26.Naito Y, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer research. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 27.Sharma P, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 29.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGray AJ, et al. Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther. 2014;22:206–218. doi: 10.1038/mt.2013.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero D. Immunotherapy: PD-1 says goodbye, TIM-3 says hello. Nat Rev Clin Oncol. 2016;13:202–203. doi: 10.1038/nrclinonc.2016.40. [DOI] [PubMed] [Google Scholar]
- 34.Yip WK, Abdullah MA, Yusoff SM, Seow HF. Increase in tumour-infiltrating lymphocytes with regulatory T cell immunophenotypes and reduced zeta-chain expression in nasopharyngeal carcinoma patients. Clin Exp Immunol. 2009;155:412–422. doi: 10.1111/j.1365-2249.2008.03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edge, S. B. et al. AJCC cancer staging manual (7th ed.), Springer, New York D.R. (2010).