Abstract
Background
Diabetic cardiac autonomic neuropathy (CAN) is one of the important complications of diabetes. It is characterized by reduced heart rate variability (HRV).
Methods
In this randomized, double-blind, placebo-controlled, multicenter trial, 75 patients were randomly assigned to one of two groups. One group (n=41) received α-lipoic acid (ALA) at an oral dose of 600 mg/day for the first 12 weeks and then 1,200 mg/day for the next 12 weeks. The other group (n=34) received placebo treatment for 24 weeks. CAN was assessed by measuring HRVs in people with diabetes.
Results
Most of the baseline measures for HRVs were similar between the ALA and placebo groups. Although there were no statistically significant HRV changes in the ALA group compared to the placebo group after 24 weeks of trial, we found a positive tendency in some of the HRV parameters of the ALA group. The standard deviations of normal-to-normal RR intervals in the standing position increased by 1.87 ms in the ALA group but decreased by −3.97 ms in the placebo group (P=0.06). The power spectrum of the low frequency (LF) band in the standing position increased by 15.77 ms2 in the ALA group, whereas it declined by −15.04 ms2 in the placebo group (P=0.08). The high frequency/LF ratio in the upright position increased by 0.35 in the ALA group, whereas it declined by −0.42 in the placebo group (P=0.06). There were no differences between the two groups regarding rates of adverse events.
Conclusion
Although a slight improvement tendency was seen in HRV in the ALA group, there were no statistically significant HRV changes in the ALA group compared to the placebo group after 24 weeks of trial. However, the high oral dose of ALA was well-tolerated.
Keywords: Cardiac autonomic neuropathy, Diabetes, Heart rate variability, Thioctic acid
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is associated with many complications, such as retinopathy, nephropathy, neuropathy, and cardiovascular diseases. Hyperglycemia has an effect on the progression of autonomic neural dysfunction and can result in cardiac autonomic neuropathy (CAN) [1].
CAN has many implications. It is correlated with increased mortality [2,3,4,5] and associated with autonomic cardiomyopathy [6,7,8]. Furthermore, it is one of the potential causes of sudden cardiac death [9,10,11]. Autonomic innervation is the main extrinsic control mechanism regulating heart rate variability (HRV) [1]. Therefore, diabetic cardiac autonomic dysfunction is inevitably associated with a decrement of HRV [12]. HRVs can be assessed with noninvasive methods. These facts make HRV an important index of CAN. According to a recent study, the prevalence of CAN in Korean patients with diabetes mellitus (DM) was 54.7% [13]. This means that diabetic CAN is a relatively common diabetic complication in Korea, so it is important to pay attention to diabetic CAN as a major health issue. However, there are only a few randomized studies about the treatment of diabetic CAN in Korea.
Like other diabetic complications, the progress of diabetic CAN is related to the duration of DM and degree of glycemic control [14], so control of hyperglycemia is regarded as a standard of treatment for CAN [14]. However, the Diabetes Control and Complications Trial has shown that CANs, represented by HRVs, were not completely prevented by focusing only on intensive blood glucose control [15].
The Deutsche Kardiale Autonome-Neurophathie (DEKAN) study showed that a daily oral dose of 800 mg α-lipoic acid (ALA), a free-radical scavenger, may be associated with positive clinical effects in the treatment of patients with diabetic CAN [16].
In this randomized, placebo-controlled trial, we assessed the effectiveness and safety of ALA (600 to 1,200 mg/day) orally administered to Korean patients with diabetic CAN for 24 weeks.
METHODS
Study oversight
This randomized, double-blind, placebo-controlled trial was conducted at three centers. A list of participating centers is provided in the Appendix 1. This study was approved by the Institutional Review Board and was done in accordance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Helsinki Declaration in 1975.
Study population
Eligible patients were aged between 20 and 80 years and retroactively assessed to have had T2DM based on medical records. CAN was defined as two or more abnormal results determined according to the time and frequency domains of HRV and five-standard cardiovascular reflex tests [17] according to the Ewing's protocol [18]. If there were no other clinical explanations for CAN than DM, patients were classified as having diabetic CAN. All enrolled patients provided written informed consent.
Patients were excluded if their CAN was secondary to diseases other than diabetes: if they had nervous system disorders and/or severe concomitant disease such as liver or renal dysfunction; if there were changes in oral antidiabetic medications or insulin dosages during the preceding 3 months; if there was a change in glycosylated hemoglobin (HbA1c) level of more than 0.5% during the preceding 3 months; if they had a history of uncontrolled hypertension and/or heart disease unsuitable for this study, such as myocardial infarction, arrhythmia, or severe heart failure; if they had an episode of ketoacidosis within 4 weeks before the study; if HbA1c (%) was greater than 11% at baseline; if they were pregnant or breastfeeding or in childbearing years without contraception; if they had taken medications that had influence on the autonomic nervous system, such as α-blockers, β-blockers, or anti-tuberculosis drugs; or if they had taken ALA during the preceding 3 months.
Study design
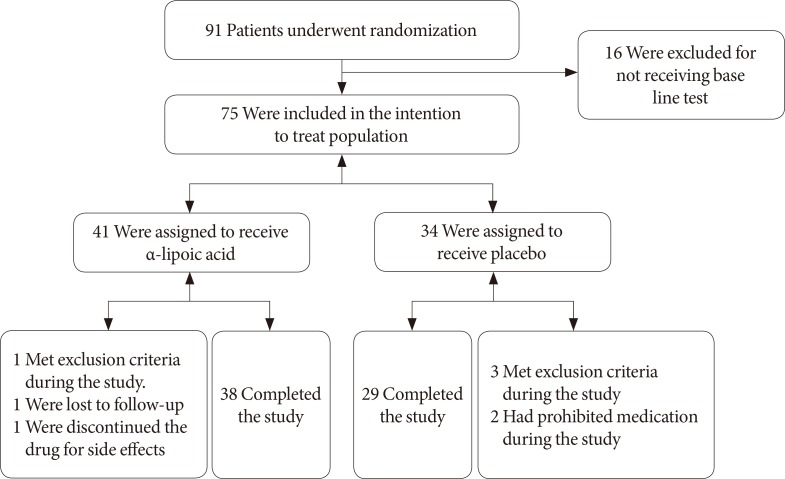
A total of 75 patients were randomly assigned to one of the two groups (Fig. 1). One group (n=41) received ALA at an oral dose of 600 mg per day for the first 12 weeks and then 1,200 mg per day for the next 12 weeks. The other group (n=34) received matching placebo medication administered on the same dosing schedule for 24 weeks.
Fig. 1. Study enrollment and follow-up.
Evaluation of HRV and other autonomic nerve function tests were carried out at baseline and after 24 weeks. The evaluation of HRV and other autonomic function tests were conducted in a quiet room. All patients were requested to abstain from caffeine-containing food and beverages on the day of their assessment. After 15 minutes of supine rest with a regular and calm breathing pattern, a 5-minute electrocardiogram recording was performed using a DICAN (Medicore Co., Seoul, Korea) machine.
Assessment of cardiac autonomic neuropathy
There are time and frequency domains of HRV. As a time domain variable, the standard deviation of normal-to-normal RR intervals (SDNN; unit, ms; correlated to total autonomic activity) and the square root of the mean of the squares of differences between successive RR intervals (RMSSD; unit, ms; correlated to parasympathetic activity) were calculated. To measure frequency domain variables, total power in the frequency range (0 to 0.40 Hz) can be divided by very low frequency (<0.04 Hz), low frequency (LF; 0.04 to 0.15 Hz; modulated by the sympathetic nervous system [SNS]), and high frequency (HF; 0.15 to 0.4 Hz; modulated by the parasympathetic nervous system) measures. The LF/HF ratio, regarded as an index of cardiac sympathetic/parasympathetic tone balance, was also calculated in this study [19]. A decrease in HRV was defined as SDNN <28.2 ms, RMSSD <18.8 ms, LF <132.6 ms2, HF <75.1 ms2, and/or LF/HF <0.9. Other autonomic function tests according to the Ewing's protocol [18] were estimated by using beat-to-beat variation with deep breathing, 30:15 heart rate ratio while standing, Valsalva ratio, hand grip blood pressure, and orthostatic blood pressure. The criteria between normal and abnormal response for the five autonomic function tests are as follows. In beat-to-beat variation with deep breathing, a difference in heart rate should be more than 15 beats/minute normally. The 30:15 heart rate ratio while standing should be more than 1.03 normally. Valsalva ratio should be more than 1.2 normally. The normal response in orthostatic blood pressure is a fall of systolic blood pressure below 10 mm Hg; an increase in diastolic blood pressure, more than 16 mm Hg, can be regarded as a normal hand grip response [20].
Outcome
Primary endpoints were changes of parameters in HRV before and after treatment of ALA compared with placebo. Secondary endpoints were changes in the five autonomic function tests (beat-to-beat variation with deep breathing, 30:15 heart rate ratio while standing, Valsalva ratio, hand grip blood pressure, and orthostatic blood pressure) from the beginning to the end of study.
Statistical analysis
Continuous data were analyzed by Student t-test, and the changes in the parameters of HRV from baseline and other autonomic function tests after 24 weeks were analyzed by paired t-test. Each one of the parameters of HRV and autonomic nerve function tests had normal distribution. In that case, a parametric statistical test was used. Comparisons of the treatment groups were based on the intent to treat analysis because this approach is recommended to avoid any bias in superiority trials, like this study. All measures of upper 5% and lower 5% of the groups were excluded from the analysis to avoid deviation. All statistical computations were performed using the SPSS version 21.0 (IBM Co., Armonk, NY, USA). Data with a P value of less than 0.05 were considered significant.
RESULTS
Baseline characteristics
A total of 91 patients underwent randomization from January 2010 through August 2012. Sixteen subjects were excluded because of lack of results of parameters of HRV. The remaining 75 subjects were included in the intent-to-treat population, with 41 assigned to receive ALA and 34 assigned to receive the placebo. Overall, 38 patients (92.7%) in the ALA group and 29 patients (86.3%) in the placebo group completed the study. Body mass index, blood pressure, HbA1c level, and lipid profiles were similar between the two groups at the baseline and at the end of the study. The demographic and clinical data of the patients at baseline and after 24 weeks of the study are shown in Tables 1 and 2.
Table 1. Demographic and clinical baseline characteristics of the patients.
| Characteristic | α-Lipoic acid | Placebo | P value |
|---|---|---|---|
| No. of patients | 46 | 45 | - |
| Sex, male/female | 27/19 | 20/25 | - |
| Age, yr | 64.37±7.80 | 62.40±9.10 | 0.270 |
| BMI, kg/m2 | 25.38±3.29 | 25.64±2.86 | 0.723 |
| Duration of diabetes, yr | 13.84±9.11 | 11.37±8.82 | 0.240 |
| HbA1c, % | 7.54±1.12 | 7.35±1.04 | 0.393 |
| SBP, mm Hg | 124.26±12.45 | 125.76±13.89 | 0.590 |
| DBP, mm Hg | 73.24±8.57 | 75.09±8.52 | 0.305 |
| Mean HR, beat/min | 72.54±7.91 | 70.60±7.35 | 0.228 |
| Lipid profile, mg/dL | |||
| Total cholesterol | 162.33±36.69 | 163.42±42.65 | 0.896 |
| Triglyceride | 145.67±100.67 | 133.56±79.97 | 0.527 |
| HDL-C | 48.09±12.52 | 45.51±11.03 | 0.301 |
| LDL-C | 84.03±35.22 | 87.01±31.99 | 0.705 |
| No. of patients on the medication | |||
| Oral anti-hypertensives | 33 | 26 | - |
| Statin | 27 | 26 | - |
| Insulin | 13 | 10 | - |
| Oral anti-diabetes | 31 | 24 | - |
Values are presented as mean±standard deviation.
BMI, body mass index; HbA1c, glycosylated hemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Table 2. Demographic and clinical characteristics of the patients after 24 weeks of the study.
| Characteristic | α-Lipoic acid | Placebo | P value |
|---|---|---|---|
| No. of patients | 41 | 34 | - |
| Sex, male/female | 24/17 | 18/16 | - |
| Age, yr | 63.76±7.84 | 62.09±9.43 | 0.405 |
| BMI, kg/m2 | 25.17±3.17 | 25.84±2.75 | 0.512 |
| HbA1c, % | 7.64±1.02 | 7.65±1.04 | 0.262 |
| SBP, mm Hg | 120.70±12.84 | 123.71±15.06 | 0.307 |
| DBP, mm Hg | 78.02±10.14 | 76.78±10.69 | 0.570 |
| Mean HR, beat/min | 74.30±9.58 | 73.71±9.72 | 0.770 |
| Lipid profiles, mg/dL | |||
| Total cholesterol | 161.23±32.49 | 164.32±41.55 | 0.685 |
| Triglyceride | 146.46±86.74 | 141.64±82.47 | 0.314 |
| HDL-C | 48.48±11.47 | 46.41±10.24 | 0.218 |
| LDL-C | 82.43±34.82 | 88.41±36.48 | 0.642 |
Values are presented as mean±standard deviation.
BMI, body mass index; HbA1c, glycosylated hemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Primary outcomes
The primary outcomes are shown in Table 3. All the baseline measures of HRV were similar between the two groups, except for the LF band; the measures of the LF band were greater in the placebo group than in the ALA group. In the upright position, the LF was 39.44±42.05 ms2 in the ALA group and 75.92±57.58 ms2 in the placebo group. In the supine position, the LF was 58.36±59.79 ms2 in the ALA group and 93.60±89.99 ms2 in the placebo group.
Table 3. Heart rate variability at baseline and changes after 24 weeks.
| Position | Index | Baseline | Changes after 24 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | Placebo | P value | ALA | Placebo | P value | ||||||||
| No. of patient | Mean±SD | No. of patient | Mean±SD | t-test | No. of patient | Mean±SD | Paired t-test | No. of patient | Mean±SD | Paired t-test | t-test | ||
| Up-right | Mean HR, beat/min | 36 | 78.94±8.88 | 30 | 77.20±8.66 | 0.425 | 36 | 1.31±9.04 | 0.392 | 28 | 2.43±7.06 | 0.080 | 0.591 |
| SDNN, ms | 37 | 19.59±7.54 | 29 | 24.72±9.14 | 0.015 | 36 | 1.87±11.79 | 0.347 | 28 | –3.97±12.64 | 0.108 | 0.061 | |
| RMSSD, ms | 38 | 14.05±8.23 | 32 | 15.95±9.13 | 0.362 | 34 | 0.87±8.44 | 0.553 | 31 | 0.47±10.17 | 0.799 | 0.864 | |
| LF, ms2 | 39 | 39.44±42.05 | 31 | 75.92±57.58 | 0.003 | 37 | 15.77±58.87 | 0.112 | 28 | –15.04±82.26 | 0.342 | 0.083 | |
| HF, ms2 | 39 | 31.47±34.19 | 33 | 38.78±35.44 | 0.377 | 39 | –1.58±32.44 | 0.763 | 28 | 8.14±49.87 | 0.395 | 0.371 | |
| LF/HF ratio | 38 | 1.98±1.53 | 31 | 2.19±1.69 | 0.587 | 37 | 0.35±1.60 | 0.195 | 29 | –0.42±1.69 | 0.191 | 0.063 | |
| Supine | Mean HR, beat/min | 35 | 72.37±7.22 | 31 | 71.42±8.37 | 0.621 | 36 | 1.31±9.04 | 0.392 | 28 | 2.43±7.06 | 0.080 | 0.591 |
| SDNN, ms | 37 | 25.80±12.03 | 29 | 23.77±6.64 | 0.389 | 37 | –0.61±9.93 | 0.710 | 28 | –0.11±9.01 | 0.947 | 0.836 | |
| RMSSD, ms | 36 | 16.08±9.46 | 31 | 16.98±9.25 | 0.694 | 34 | 0.80±12.38 | 0.709 | 31 | –3.18±9.50 | 0.072 | 0.154 | |
| LF, ms2 | 38 | 58.36±59.79 | 31 | 93.60±89.99 | 0.067 | 37 | 26.75±97.70 | 0.105 | 28 | 2.57±75.99 | 0.859 | 0.282 | |
| HF, ms2 | 38 | 50.94±58.93 | 31 | 46.37±34.42 | 0.690 | 38 | 22.16±85.75 | 0.120 | 31 | –6.40±50.90 | 0.489 | 0.091 | |
| LF/HF ratio | 38 | 1.74±1.20 | 30 | 2.02±1.81 | 0.470 | 38 | 0.71±2.31 | 0.066 | 28 | 0.49±2.20 | 0.248 | 0.700 | |
ALA, α-lipoic acid; SD, standard deviation; HR, heart rate; SDNN, standard deviation of RR intervals; RMSSD, root mean square of successive differences; LF, low-frequency component; HF, high-frequency component.
Although there were no statistically significant (P<0.05) changes in the ALA group compared to the placebo group after 24 weeks of trial, we found a positive tendency in some HRV indexes of the ALA group as follows. The SDNN in the standing position increased from the baseline to 24 weeks by 1.87 ms in the ALA group but decreased by −3.97 ms in the placebo group (P=0.06 for ALA vs. placebo). The power spectrum of the LF band in the standing position increased by 15.77 ms2 in the ALA group, whereas it declined by −15.04 ms2 in the placebo group (P=0.08 for ALA vs. placebo). The HF/LF ratio in the upright position increased by 0.35 in the ALA group, whereas it declined by −0.42 in the placebo group (P=0.06 for ALA vs. placebo). Furthermore, there was a trend toward a favorable effect of ALA versus placebo for the HF band power spectrum in the supine position (P=0.091 for ALA vs. placebo).
Secondary outcomes
The secondary outcomes are shown in Table 4. All the baseline measures of autonomic nerve function tests were similar between the groups, and after 24 weeks of treatment with ALA, there was no statistically significant difference of autonomic nerve function tests (beat-to-beat variation with deep breathing, 30:15 heart rate ratio while standing, Valsalva ratio, hand-grip blood pressure, and orthostatic blood pressure) in either group.
Table 4. Autonomic nerve functions at baseline and changes after 24 weeks.
| Index | Baseline | Changes after 24 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALA | Placebo | P value | ALA | Placebo | P value | |||||||
| No. of patient | Mean±SD | No. of patient | Mean±SD | t-test | No. of patient | Mean±SD | Paired t-test | No. of patient | Mean±SD | Paired t-test | t-test | |
| 30:15 Heart rate ratio with standing | 39 | 1.02±0.04 | 32 | 1.04±0.08 | 0.088 | 39 | 0.02±0.07 | 0.036 | 28 | 0.04±0.08 | 0.014 | 0.359 |
| Valsalva ratio | 36 | 1.11±0.12 | 33 | 1.16±0.14 | 0.150 | 34 | 0.07±0.14 | 0.009 | 31 | 0.05±0.18 | 0.140 | 0.611 |
| Beat to beat variation with deep breathing, ms | 38 | 10.32±4.59 | 29 | 10.83±6.54 | 0.721 | 38 | 4.53±8.70 | 0.003 | 24 | 2.13±6.96 | 0.148 | 0.259 |
| Orthostasis blood pressure (SBP), mm Hg | 37 | 2.38±10.21 | 29 | 3.59±8.11 | 0.604 | 36 | –4.39±10.03 | 0.013 | 27 | 0.63±9.44 | 0.732 | 0.048 |
| Orthostasis blood pressure (DBP), mm Hg | 34 | 4.26±5.33 | 30 | 4.83±5.58 | 0.678 | 34 | –2.35±5.62 | 0.020 | 25 | –0.76±5.61 | 0.504 | 0.286 |
| Hand grip blood pressure (SBP), mm Hg | 38 | 149.37±18.45 | 31 | 150.71±22.56 | 0.787 | 36 | –1.17±21.09 | 0.742 | 28 | 3.36±19.89 | 0.380 | 0.386 |
| Hand grip blood pressure (DBP), mm Hg | 38 | 94.53±13.28 | 31 | 92.84±16.39 | 0.638 | 36 | –3.22±13.11 | 0.149 | 28 | 3.57±16.94 | 0.274 | 0.075 |
ALA, α-lipoic acid; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Safety outcomes
Overall incidences of abnormal reactions were similar to each other (ALA 39.13% vs. placebo 37.78%) except in any events that were probably or definitely not related to ALA. In the ALA group, there were three minor abnormal reactions: gastrointestinal problem, urological problem, and nervous problem. However, there were no abnormal reactions in the placebo group. In terms of severe adverse events, there were three: one (gastric cancer) in the ALA group and two (duodenitis, injury of cartilage) in the placebo group. In short, there were no differences between the groups regarding the rates of adverse events.
DISCUSSION
The results of this trial failed to show any statistically significant (P<0.05) differences of HRV or other autonomic nerve function tests between the ALA group and the placebo group after 24 weeks of experiment. However, a slight trend toward improvement in some HRV indexes of the ALA group was noticed, but the rates of overall abnormal reactions or severe adverse events between the both groups were similar, indicating that the treatment with high-dose ALA is safe.
As a treatment for diabetic neuropathy, ALA has been studied for many years. In the DEKAN study, ALA improved the HRV index of patients with diabetic CAN [16]. In the Alpha-Lipoic Acid in Diabetic Neuropathy (ALADIN) study, it showed that ALA could improve neuropathic symptoms such as pain, burning, paresthesia, and numbness [21]. Based on previous studies, we thought that ALA can be used to reduce symptoms of diabetic peripheral neuropathy and to improve cardiac autonomic dysfunction. In the DEKAN study, the oral dose of 800 mg/day was used, and that dose was well tolerated. In the ALADIN study, an intravenous dose of 600 mg/day was more effective than an intravenous dose of 100 mg/day. It is generally believed that 600 mg of intravenous administration corresponds to 1,200 to 1,800 mg of oral administration. We thought the oral dose of 1,200 mg/day could be well tolerated and might be more effective. This presumption led us to use ALA at an oral dose of 1,200 mg/day in this study. The pathophysiology of diabetic CAN is complex and multifactorial. One of the possible explanations is that a hyperglycemia-induced increment of oxidative stress can cause direct neuronal damage and dysfunction [12,22]. ALA, which is a free-radical scavenger, could reduce this oxidative stress [23]. The antioxidant effect of ALA may explain how it improved symptoms of diabetic polyneuropathy and cardiac autonomic dysfunction in the previous studies, such as the DEKAN study and the ALADIN study.
HRV is a function of sympathetic and parasympathetic nerve activities on the sinus nodes, which can be seen as an oscillation of RR intervals between each heartbeat [19,24]. There are time domain methods and frequency domain methods by which to analyze HRV [19]. In this study, we used SDNN and RMSSD, which are time domain methods, and LF, HF, and LF/HF ratio as frequency domain methods. The HF component is known to be modulated by parasympathetic activity, whereas the LF component is considered by some studies to be modulated by sympathetic activity. Other studies say that the LF/HF ratio reflects both sympathetic and parasympathetic activities [19]. In normal individuals, the heart has a high degree of HRV, but as cardiac autonomic nerve dysfunction progresses, HRV decreases from an early stage [1]. Because a decrease in HRV reflects the progression of CAN, improvement in HRV could be a sign of recovery of autonomic nerve function.
The DEKAN study showed that an oral dose of 800 mg/day of ALA for 4 months can improve the HRV index (RMSSD, LF band, HF band) [16], indicating that ALA may also improve CAN. In our study, we used ALA at a dose of 1,200 mg/day, higher than the dose in the DEKAN study, and our 6-month observation period was longer than the duration of the DEKAN study. Even though a higher dosage and a longer duration of ALA were used, there was no significant adverse effect in either group. Although we conducted this study with an Asian population, we failed to show statistically significant changes of any HRV indexes between the placebo group and the ALA group, but we could see a trend toward a favorable effect of ALA versus placebo for some HRV indexes, such as SDNN in the standing position, the LF band in the standing position, the HF band in the supine position, and the HF/LF ratio in the upright position.
Our study has certain limitations. First, the study had a small sample size. Second, we did not evaluate symptoms and signs of diabetic CAN. Because of this fact, we could not analyze the effects of ALA on symptoms and signs of diabetic CAN. Third, we did not adjust for confounding factors such as use of antihypertensive agents and statin. Further studies are needed to answer whether some improvement of HRV can be realized by treatment with ALA.
Although a slight tendency toward improvement was seen in HRV in the ALA group, there were no statistically significant HRV changes in the ALA group compared to the placebo group after 24 weeks of trial. However, a high oral dose of ALA was well tolerated.
Appendix 1
A list of participating centers

Footnotes
CONFLICTS OF INTEREST: This trial is supported by grants from the Chong Kun Dang Pharmaceuticals Korea Co., Ltd., Seoul, Republic of Korea.
References
- 1.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33:434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orchard TJ, LLoyd CE, Maser RE, Kuller LH. Why does diabetic autonomic neuropathy predict IDDM mortality? An analysis from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Res Clin Pract. 1996;34:S165–S171. doi: 10.1016/s0168-8227(96)90025-x. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Jang HJ, Kim YH, Lee EJ, Choe YS, Choi Y, Lee MG, Lee SH, Kim BT. Prognostic value of cardiac autonomic neuropathy independent and incremental to perfusion defects in patients with diabetes and suspected coronary artery disease. Am J Cardiol. 2003;92:1458–1461. doi: 10.1016/j.amjcard.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 4.Astrup AS, Tarnow L, Rossing P, Hansen BV, Hilsted J, Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:334–339. doi: 10.2337/diacare.29.02.06.dc05-1242. [DOI] [PubMed] [Google Scholar]
- 5.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 6.Mustonen J, Uusitupa M, Lansimies E, Vainio P, Laakso M, Pyorala K. Autonomic nervous function and its relationship to cardiac performance in middle-aged diabetic patients without clinically evident cardiovascular disease. J Intern Med. 1992;232:65–72. doi: 10.1111/j.1365-2796.1992.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 7.Didangelos TP, Arsos GA, Karamitsos DT, Athyros VG, Karatzas ND. Left ventricular systolic and diastolic function in normotensive type 1 diabetic patients with or without autonomic neuropathy: a radionuclide ventriculography study. Diabetes Care. 2003;26:1955–1960. doi: 10.2337/diacare.26.7.1955. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JK, Zola B, Juni JE, Vinik AI. Radionuclide assessment of left ventricular diastolic filling in diabetes mellitus with and without cardiac autonomic neuropathy. J Am Coll Cardiol. 1986;7:1303–1309. doi: 10.1016/s0735-1097(86)80150-4. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JK, Sisson JC, Vinik AI. Prediction of sudden cardiac death in diabetic autonomic neuropathy. J Nucl Med. 1988;29:1605–1606. [PubMed] [Google Scholar]
- 10.Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, Wieland DM, Corbett J, Schwaiger M. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575–1584. doi: 10.1016/s0735-1097(98)00128-4. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka M, Ito C, Sasaki H, Yamane K, Kohno N. Low heart rate variability is a risk factor for sudden cardiac death in type 2 diabetes. Diabetes Res Clin Pract. 2004;64:51–58. doi: 10.1016/j.diabres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17–39. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko SH, Kwon HS, Lee JM, Kim SR, Cho JH, Yoo KD, Park YM, Lee WC, Song KH, Yoon KH, Cha BY, Son HY, Ahn YB. Cardiovascular autonomic neuropathy in patients with type 2 diabetes mellitus. J Korean Diabetes Assoc. 2006;30:226–235. [Google Scholar]
- 14.Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison's principles of internal medicine. 19th ed. New York: McGraw-Hill; 2015. Chapter 419, Diabetes mellitus: complications; pp. 2426–2427. [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G Deutsche Kardiale Autonome Neuropathie. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study) Diabetes Care. 1997;20:369–373. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 18.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 20.Kempler P. Autonomic neuropathy: a marker of cardiovascular risk. Br J Diabetes Vasc Dis. 2003;3:84–90. [Google Scholar]
- 21.Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–1433. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 22.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 23.Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995;18:1160–1167. doi: 10.2337/diacare.18.8.1160. [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI. The conductor of the autonomic orchestra. Front Endocrinol (Lausanne) 2012;3:71. doi: 10.3389/fendo.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]