Abstract
Introduction
A large number of different cells including embryonic and adult stem cells have been transplanted into animal models of spinal cord injury, and in many cases these procedures have resulted in modest sensorimotor benefits. In October 2010 the world’s first clinical trial using human embryonic stem cells began, using stem cells converted into oligodendrocyte precursor cells.
Sources of data
In this review we examine some of the publically-available pre-clinical evidence that some of these cell types improve outcome in animal models of spinal cord injury. Much evidence is not available for public scrutiny, however, being private commercial property of various stem cell companies.
Areas of agreement
Transplantation of many different types of stem and progenitor cell enhances spontaneous recovery of function when transplanted acutely after spinal cord injury in animal models.
Areas of disagreement
The common mechanism(s) whereby the generic procedure of cellular transplantation enhances recovery of function are not well understood, although a range of possibilities are usually cited (including preservation of tissue, remyelination, axon sprouting, glial cell replacement). Only in exceptional cases has it been shown that functional recovery depends causally on the survival and differentiation of the transplanted cells. There is no agreement about the optimal cell type for transplantation: candidate stem cells have not yet been compared with each other or with other cell types (e.g., autologous Schwann cells) in a single study.
Areas timely for developing research
Transplantation of cells into animals with a long lifespan is important to determine whether or not tumours will eventually form. It will also be important to determine whether long-term survival of cells is required for functional recovery, and if so, how many are optimal.
Keywords: Geron, spinal cord injury, stem cells, transplant, endogenous, paralysis
Introduction
Note: this article represents our personal scientific view: neither of us are clinicians. Spinal cord injured people should consult a wide variety of sources of information before making decisions concerning treatment or enrolment into a clinical trial. The following organizations provide a wealth of relevant information concerning spinal cord injury (SCI): the Christopher Reeve Foundation, Spinal Research, IRME (France), the Miami Project to Cure Paralysis (USA), the Neil Sacshe Foundation (Australia), the Rick Hansen Foundation (Canada), Wings for Life (Austria), the Japan Spinal Cord Foundation and the Paralyzed Veterans of America. The ICCP (International Campaign for Cures of spinal cord injury Paralysis) is an umbrella consortium comprised of these organizations. We highly recommend their guide to “Experimental treatments for spinal cord injury: what you should know if you are considering participation in a clinical trial”. This downloadable guide 1 provides a broad overview of the issues relating to experimental therapies for SCI. Readers unfamiliar with terms such as “blinding”, “randomized” and “control” should read this guide first as it contains an excellent introduction to the subject with a comprehensive glossary. Our review which follows will attempt to consider some of these issues as they relate specifically to stem cell transplantation.
Background
The neurological consequences of a spinal cord injury
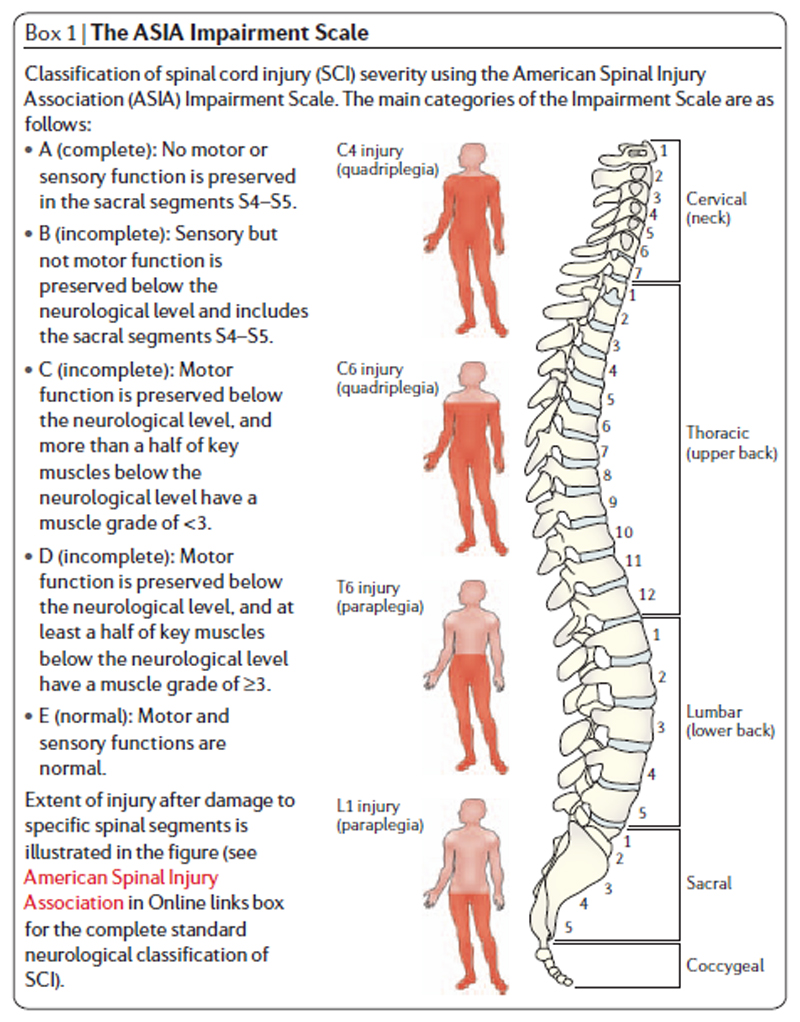
SCI in humans can be devastating and often leads to permanent loss of bodily functions affecting bladder and bowel, reproductive organs, somatosensation and limb movement. It is estimated that more than 130,000 people receive new spinal cord injuries each year. Disabilities can result from various types of injury including contusion (bruising) or compression of the spinal cord 2. The severity of a human SCI is determined by neurological assessment often using the ASIA impairment scale (American Spinal Injury Association). This scale divides SCIs into 5 categories: A, B, C, D or E (Figure 1). There is some spontaneous recovery of function after human SCI although for initially severe injuries this is rarely complete 3. Accordingly there is an urgent need for therapies that improve outcome after SCI.
Figure 1.
The ASIA impairment scale, taken from Thuret, Moon & Gage (2006).
The cellular and molecular consequences of a spinal cord injury
The majority of our information about pathological changes after human SCI depends on inferences drawn from animal models, particularly rats and mice, although we do have some information from human autopsy material about cellular changes (and in some cases, molecular changes) after SCI. SCI causes marked loss of neurons and other cell types at the injury site, both immediately after injury, and progressively with time. Communication between one neuron and its target is lost when its nerve fiber (the axon) is interrupted. Communication is also impaired when surviving nerve fibers lose their insulating sheaths comprised of “myelin” (generated by oligodendrocytes). Neurons and myelinating cells also die progressively 4 and there is evidence that some axons remain demyelinated in the long term 4–7. We have reviewed these changes in detail elsewhere 2. These pathological events indicate a number of different opportunities for SCI repair: 1) to minimise progressive cell death (by “neuroprotection”), 2) to replace lost cells (by transplantation) or to stimulate the injured cord to produce new cells (“neurogenesis”), 3) to reconnect injured nerve fibers with their original targets or with substitute targets (“axon regeneration and sprouting”), 4) to maximize the function of spared pathways by altering connectivity (“synaptic plasticity”) 5) to maximize the function of spared nerve fibers by repairing their myelin sheath (“re-myelination”), 6) to rehabilitate muscle and function, and 7) to use prostheses, robotics or other technologies to restore function. Stem cells may eventually play a role in many or all of these goals.
Areas of controversy: What cell type will be best?
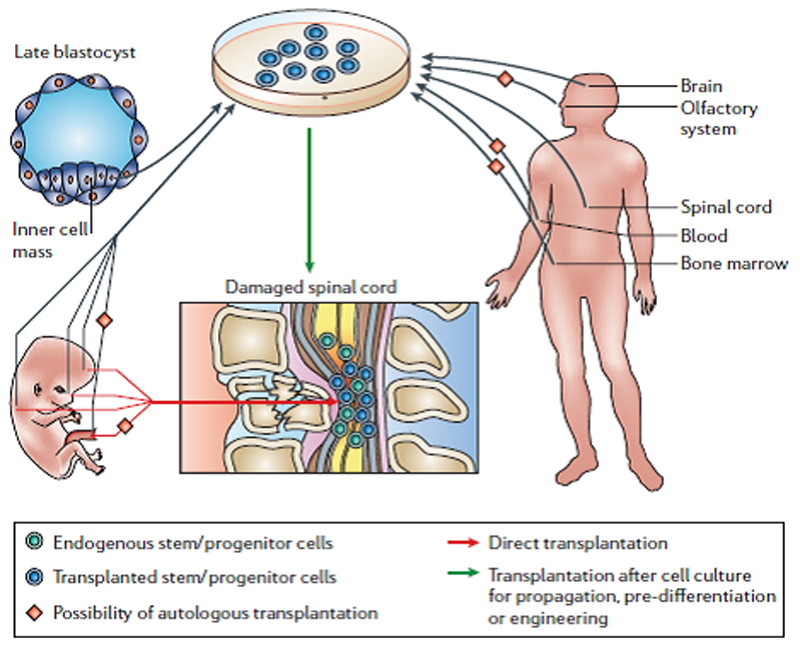
There are a wide variety of stem cells. Human embryonic stem (HES) cells are self-renewing cells that are “totipotent”: i.e., they give rise to all the cells in the body, including the brain and spinal cord. Stem cells become other cell types through the process of “differentiation” (i.e., maturation). ES cells were first isolated from the inner mass of late blastocysts in mice. Later, it became possible to obtain ES cells from non-human primates and humans. Using various biological reagents (e.g., growth factors), ES cells can be differentiated in the laboratory into a range of different cell types, including neural progenitors and glial-restricted precursors (e.g., oligodendrocyte precursor cells). In turn, progenitor/precursor cells differentiate into a restricted set of other cell types (e.g., neurons or glia). Stem cells are also present within adult tissues: in the CNS, adult stem cells can be found in neurogenic zones including the sub-ventricular zone and the hippocampus, and these give rise to small numbers of neurons throughout adulthood. In the adult, stem cells are also present in areas outside the CNS (e.g., in bone marrow or blood). All of these cell types have been transplanted into animal models of SCI (see below).
The ideal cell for transplantation would be one which does not require the person with SCI to be immunosuppressed, as this involves some clinical risk. Some stem cells may be obtained in autologous fashion from the patient; others, heterologous (likely requiring at least transient immunosuppression). Stem cells that may be suited to autologous transplantation include bone marrow stromal cells, haematopoetic stem cells (from blood), cells from the olfactory system and cells from umbilical cord blood (which can be cryogenically frozen at birth for potential later use in life). In contrast, neural stem cells are usually obtained from other donors and therefore will likely require immunosuppression. An alternative source of autologous stem cells comes from inducible pluripotent stem cells (iPSCs). These are generated by re-engineering mature, fully differentiated cells (e.g., human skin fibroblasts) by modifying the cells with a set of transgenes (typically 4). The theoretical advantage of iPSCs is that a person with SCI might provide his/her own pluripotent or mature cells for transplantation without need for immunosuppression. One goal for researchers is to transform iPSCs from a spinally injured patient into neural cells prior to autologous transplantation. iPSCs can retain an epigenetic memory from their mature parent cell 8 and it will be important to ensure that the resulting cells are phenotypically appropriate for transplantation.
An alternative to autologous stem cells would be a bank of stem cell lines that can be mass-produced in uniform batches and distributed so that it can be administered wherever the patient lies. If a variety of these cells were available then they could be matched to a person’s immunological type. It will be important to ensure that cell lines do not change in genotype and phenotype over time due to random mutation and selection after many rounds of division.
It will be essential to avoid transplantation of cells which result in cancers (i.e., where cells divide uncontrollably) especially if the cells also migrate away from the site of transplantation. Already in humans, transplanted neural stem cells have caused a brain tumour in at least one patient 9. Further, some types of iPSC-derived neural cells have an increased likelihood of tumour formation after transplantation into the brain or spinal cord: selection of safe iPSC-derived clones may overcome this issue 10, 11. Finally, it will be important to make sure that transplanted cells do not induce neuropathic pain or autonomic dysreflexia: notably, one study found that one type of stem cell was found to cause pain after transplantation into a SCI 12.
Which cell therapies reproducibly promote recovery of function after SCI?
An enormous number of studies have now been conducted using animal models of SCI to evaluate the safety and efficacy of cell transplantation therapies. The vast majority use rodents and a smaller number have used non-human primates. The enormous variety of cells (stem or otherwise) that has been transplanted includes embryonic stem cells, embryonic stem cells differentiated into OPCs, adult neural precursor cells, glial-restricted precursors, Schwann cells, mixed preparations from olfactory tissues that contain stem cells, bone marrow-derived stem cells and stem cells from umbilical cord. Each of these cell types has different properties and the rationale for transplantation differs widely (including neuroprotection, cell replacement and re-myelination). We have reviewed some of these studies elsewhere2. At this stage it is difficult to judge which of the many competing cell types is the best at restoring function after SCI, because competing cell types are rarely compared side-by-side in a single experiment, although human fibroblasts or “unsafe” iPSCs are sometimes used as a negative control 13, 14.
When considering whether to enroll in a trial for a SCI therapy, an important consideration is whether the positive results obtained in animal models has been reproduced by an independent laboratory 1. It may surprise clinicians and patients that this is not a requirement for a clinical trial (although the FDA do usually require that positive results obtained have been obtained in more than one animal model: e.g., in rats and in non-human primates). It may also surprise clinicians and patients that the vast majority of positive findings obtained using animal models of SCI have not been reproduced by a second laboratory. This is partly because there is little glory in reproducing someone else’s work: publication in a high-impact journal requires the work to be novel. However, work from another laboratory is sometimes reproduced when the original therapy is examined with or without a second therapy of interest. To encourage attempts to replicate novel experimental therapeutic strategies in animal models of SCI, the National Institute of Neurological Disorders and Stroke in the USA provided funding via a program entitled “Facilities of Research Excellent in Spinal Cord Injury” (FORE-SCI). Advisory panels select therapies for reproduction and the work is conducted, where possible, with the full co-operation of the original laboratory, using materials and information provided by them. Publications exist for attempted replications of five different promising therapies and these are very thorough in their treatment of the data and in the quality of reporting: indeed, in many ways, these are model scientific papers 15–19. It is striking and discouraging that these careful re-assessments of previous positive findings have largely failed to reproduce the findings of functional recovery reported in the original papers. At the very least, it seems that we do not yet have a robust, independently verified pre-clinical therapy for SCI. People with SCI and their clinicians should bear this in mind when considering potential therapies for SCI. To date, the FORE-SCI teams have not reported any attempted replication of any of the high-profile findings obtained using stem cells.
In the absence of independent replication of positive findings obtained using stem cells, how should clinicians and SCI patients evaluate pre-clinical work using animals? Recently, a consortium of scientists have advocated the use of standardized reporting of experiments that use animals 20 and they provide a 20-point checklist which allows studies of high methodological quality to be identified. Improvements in the reporting and performance of experiments using animals is vital: a survey conducted in 2009 found that of 271 papers using animals, 86% did not report the use of blinding and 87% did not report randomisation when allocating treatments 21. These aspects of experimental design are essential to avoid bias and a lack of reporting of these issues leads to the suspicion that many experiments using animals are being designed and implemented poorly. If this is the case, it is not surprising that many studies are never replicated.
Notwithstanding this caveat, it is remarkable that transplantation of a large variety of cell types early after SCI leads to a moderate functional recovery in the hands of many different groups 2. It is usually claimed that recovery of function is due to a specific reparative property of the particular cells that are transplanted. For example, most papers will claim that specific anatomical or morphological changes correlate with the recovery of function: however, in very few cases are these changes linked causally to the recovery of function. There are notable exceptions: for example, one study subsequently ablated transplanted neural stem cells using a targeted toxin and showed that this led to loss of recovered function, with the implication that the recovered function depended on the transplanted cell integrity 22.
There may be an alternative (or additional) explanation for the fact that many cell types promote functional recovery after SCI: there may be a mechanism common to the procedure of transplantation of different cell types that causes functional recovery. For example, many cells die during transplantation, and it may be that the host response to the transplantation and cell death results in neuroprotection or functional recovery. We have shown that after transplanting cells into a site of SCI, large numbers of endogenous Schwann cells invade the injury site: these support regeneration and re-myelination of axons23. Remarkably, this reparative process occurs even when the “transplant” consists of cells that have been deliberately killed prior to transplantation. Accordingly, we recommend that transplant studies include a “dead cells” control group to verify whether or not any improvements depend on transplantation of viable cells. This is important because it will reduce the number of claims that a particular cell type has a unique reparative property independent of the procedure of cell transplantation itself.
Which stem cells have been shown to improve outcome after SCI in non-human primates?
Human spinal cords are substantially different to rodent spinal cords: for example, there are major differences in the physiology and anatomy of the corticospinal tract 24. Damage to the corticospinal tract in humans and non-human primates causes permanent deficits during stepping whereas in rodents the deficits in stepping are subtle. Accordingly, for many stem cell therapies it may be important to evaluate outcome in animals more closely related to humans. Humans recover some function following SCI: for instance, humans suffering from Brown-Séquard Syndrome resulting from a lateral hemisection of the spinal cord, can exhibit spontaneous recovery, such as a recovery of voluntary motor strength. Interestingly, recent research shows that a greater amount of corticospinal plasticity exists in non-human primates than previously thought. In that study, several groups of anaesthetized rhesus monkeys underwent lateral hemisection of the cervical level 7 spinal cord. Initially, no detectable function was found in the right arm and leg. However, after 4 weeks post-lesion, the monkeys began to recover the ability to retrieve food and to step on a treadmill with their affected limbs. Subsequently, the density of corticospinal axons was found to be 60% of the density of normal monkeys 25. This indicates that it may be difficult to detect distinguish spontaneous improvements in function after SCI in non-human primates from therapy-induced improvements: large numbers of primates with very consistent injuries may be required.
Embryonic stem cells have been isolated from non-human primates 26. However, for clinical relevance, a small number of researchers have transplanted human stem cells into non-human primates. In one experiment, 10 adult common marmosets received a C5 contusive SCI. 9 days afterwards, injections of transplant media or of human neural/stem progenitor cells (NSPCs; derived as neurospheres from 8 week fetuses) were injected into the spinal lesion epicenter. Motor functions were assessed by measuring the marmosets’ forearms’ grip strength. Spontaneous movement was recorded through the use of an infrared sensor placed within the cage. The study reported a significant difference in grip strength between the two groups, but there appears to have been a trend towards a difference in performance prior to transplantation which was not taken into account in the analysis (e.g., as a covariate in a repeated measures analysis of variance). BrdU staining suggested that the transplanted NSPCs differentiated into neuronal and glial lineage cells 27. However, the group recently reported that “the observed functional recovery … was not sufficient to qualify the procedure for a clinical trial in patients with complete SCI” 28.
A recent experiment by the same group focused on the transplantation of human NSPCs engineered to produce galectin-1 (a protein that has been reported to have reparative properties of its own). Again, adult common marmosets received a C5 contusive SCI and 9 days later, injections of transplant media or cells expressing galectin-1 (plus GFP) or GFP alone were transplanted into the lesion epicenter. Three functional outcomes were measured: grip strength, spontaneous locomotion in the home cage, and treadmill locomotion. In this study, the performance levels of the groups appeared similar prior to treatment. Following treatment, grip strength recovered to a significantly higher level in the marmosets that received galectin-1 expressing NSPCs relative to NSPCs that expressed GFP only. Both groups performed better than the group that received only transplant medium. Again, histological analysis suggested that the NSPCs survived and differentiated into neuronal and glial lineages 28. These two studies did not report whether treatments were randomly allocated or whether animals were assessed by observers blind to treatment.
There are now reports in the media that this group has also transplanted neurospheres derived from iPSCs (from human skin) into marmosets nine days after SCIi (following positive results in mice after SCI11). However, there are, as yet, no peer-reviewed publications describing the results of this primate work.
Stem cell transplantation into humans with SCI
Clinical trials proceed in Phases (typically 1 to 4). In Phase 1 or 2 clinical trials, the primary concern is with safety 1. There is an emerging consensus that Phase 1 trials of cell transplantation are best conducted in people with thoracic SCI because if the procedure exacerbated local cell death, say over one segment, then the additional loss of function might not be life threatening, whereas this might be extremely disabling or fatal for a person with a cervical injury. Accordingly, people with thoracic SCI may more often be enrolled in Phase 1 clinical trials for cell transplantation than people with other injury types (see Geron and StemCells Inc. trials below). Pre-clinical work and Phase 1 and 2 trials will be very important for ensuring that stem cell therapies do not induce pain 12, spasticity, autonomic dysreflexia, tumours or other negative outcomes. In Phase 3 clinical trials, the primary concern is with efficacy of the therapy. At the present time, we have relatively insensitive measures for assessing recovery of function after thoracic SCI. In contrast, various sensitive tests are available for assessing recovery of upper limb function after cervical SCI. Accordingly, Phase 3 clinical trials for cell transplantation may address cervical SCI.
A range of clinical experiments involving administration of stem cells for SCI have already taken place. For example, a recent review has described that transplantation of bone marrow stromal cells by various groups into patients was “relatively safe but the effect was limited” 29. In the USA, small pieces of human embryonic foetal tissue were transplanted into a small number of patients with progressively expanding cavities (“syrinxes”) after SCI and this appeared to be safe 30. However, the restricted availability of human embryonic foetal tissue for grafting and the associated ethical issues mean that this procedure has rarely been performed in Europe or the USA after SCI. In contrast, many hundreds of patients have received transplants of human embryonic foetal tissue in China for SCI. However, the consensus view of many clinicians and researchers is that physicians should not recommend this procedure to patients 31 because the procedures do not meet the international standards for a clinical trial: for example, controls have not been included 32. Independent observation of patients has also indicated complications in some patients including meningitis. Without controls, it is difficult to determine whether any improvements are consistent with the degree of spontaneous recovery that occurs after SCI 3. The Chinese Ministry of Health has now set in place legislation that requires providers of stem-cell therapies to have conducted clinical trials for safety and efficacy before treating patients 33. The ICCP have advised “very strongly that you should only participate in properly designed and conducted clinical trials of treatments for which there is compelling evidence of efficacy from animal experiments”.
Transplantation of HES-OPCs after SCI
A trial that has recently generated excitement within both the scientific community and the wider world is the Geron trial. This landmark trial is attempting to discern the safety of stem cell therapy in SCI on humans by using OPCs derived from HES cells to re-myelinate demyelinated axons within the injured spinal cord. The stem cells themselves come from surplus in vitro fertilised embryosii and the product is known as “GRNOPC1”. To be eligible for the Phase 1 clinical trial, patients must be treated within 7 to 14 days of their injury and must have had a thoracic injury resulting in an ASIA grade A injury with neurological level of T3 to T10.
This clinical trial is the result of studies showing that these cells can improve recovery after SCI in rats. Some of these studies are in the public domain 13, 34, 35 and others have been conducted privately by Geron. In the first study 13, cells were transplanted into female adult rats either 7 days (n=8) or 10 months (n=6) following a thoracic (T10) contusion (bruising) injury to the spinal cord made under anaesthesia with a 200kdyn force, which severely impaired hindlimb function. Rats received either OPCs, human fibroblasts or media. Eight weeks after transplantation, a significant improvement was detected in the rats which received OPCs relative to the other two control groups. In the histological analysis, a 136% increase in re-myelination (by oligodendrocytes and SCs) was seen in the 7d group; approximately 55% of the re-myelination was due to the OPCs. When treatment was initiated after 10 months, no significant re-myelination was seen and no significant improvement in locomotion was detected.
In a second study 35, HES-OPCs were injected seven days after midline contusion (200 kdyn) of the C5 cervical spinal cord of rats. Forelimb scores were determined by videotapes of the animals crossing a clear Plexiglass walkway. The transplanted group showed significantly longer forelimb stride lengths than the control group. Histological analysis using anti-human nuclear staining detected that in SCI rats, the OPCs had survived, migrated and differentiated into oligodendrocyte-like cells. Control rats exhibited substantial white and grey matter loss and cavitation but with extensive endogenous re-myelination. Transplanted rats had more spared white and grey matter and, strikingly, essentially no cavities were reported. Strangely, however, rats transplanted with OPCs appeared to have fewer axons remyelinated by oligodendrocytes (Figure 5G in 35), which undermines the original rationale for this therapeutic approach. Transplanted rats also had fewer axons remyelinated by Schwann cells than non-transplanted rats. In the Discussion, the authors stated that “no significant difference in re-myelination was detected between the transplanted and non-transplanted groups” whereas it appears that rats transplanted with OPCs had fewer remyelinated axons in total (sum of bars in Figure 5G in 35). The authors conclude that the better functional outcome following OPC transplantation relates to neuroprotection rather than re-myelination.
The rat studies mention that behavioural assessments were conducted blind but it is not stated whether (or how) treatments were randomized to subjects. Evidence that HES-OPC transplantation does not cause gross side-effects comes from work showing a lack of any loss (or gain) in locomotor function after a mild thoracic contusive SCI 34. However, it should be noted that this paper did not comprehensively profile locomotor behaviour and did not formally assess other negative outcomes such as pain-related behaviours or autonomic dysreflexia and really should not be quoted by itself as evidence that the procedure is “safe” 35. To our knowledge, there is no data in the public domain relating to the safety or efficacy of GRNOPC1 in non-human primates after SCI.
Subsequent (unpublished) preclinical data showing that SCI animals treated with Geron’s cell line developed small spinal cysts at the treatment site caused the FDA (Food and Drug Administration) regulatory authority to put a hold on the clinical trialiii. The company subsequently reported having identified batches of GRNOPC1 that do not cause cyst formation in animal models. This allowed the Phase 1 clinical trial to be initiated and the first patient received a transplant in October 2010.
The future: who will be first-to-market?
A number of other biotech companies have now received regulatory approval to proceed with clinical trials using stem cells, including ReNeuron (UK) for stroke and StemCells Inc. (USA) for SCI 36. StemCells Inc. has Swiss regulatory approval for a Phase 1/2 clinical trial of its adult neural stem cell product “HuCNS-SC” in chronic SCI patients: it aims to enroll about a dozen patients with thoracic injuries (T2 to T11) of grade ASIA A with the goal of transplanting within 3 to 12 months after injuryiv. Transient immunosuppression will be given. All being well, subsequent cohorts of ASIA B and C patients will be recruited.
The StemCell Inc trial is based on a promising series of rodent studies 5, 14, 22, 37. In the most recent study, transplantation of HuCNS-SC cells into mice 30 days after thoracic SCI was reported to produce a functional improvement relative to transplantation of human fibroblast cells. However, the authors do not state whether the mice treated with HuCNS-SC cells improved after transplantation relative to the immediate pre-transplant baseline. Rather, they note that the HuCNS-SC group showed a greater linear increase from 1 week to 16 weeks post-transplantation than controls. This is not the same thing as a transplant-mediated improvement: the transplant procedure led to an apparent drop in locomotor function over the first week in all the groups, and the difference appears to be in the extent of the drop and the trajectories of performance from this point onwards. The authors note that there is a progressive loss of function in the vehicle control group after the surgical procedure at 30 days whereas the fibroblast-treated group neither recover nor deteriorate. This indeed leads to significant differences in performance at the end-point (16 weeks) but it might have been equally efficacious not to intervene at all. Otherwise, the quality of experimental design and reporting was very high in this study: mice were randomized to treatments and assessment was carried out by observers blinded to treatment, exclusion criteria were transparent, and sensory testing was carried out to exclude induction of mechanical sensitivity as a side-effect of the transplantation procedure. To our knowledge, this cell type has not yet been evaluated in a non-human primate model of SCI.
It remains to be seen whether cell transplantation will become a “gold standard” of care for people with spinal cord injuries. If this is the case, then there is a considerable advantage to being the first-to-market, because all following treatments may have to be compared to or combined with the “gold standard”.
Milestones that need to be achieved to bring therapy from bench to bed
The Geron trial has shown that it is possible to bring a therapy from bench to bed within 15 years. Geron first started working with HES in 1999v. The original preclinical data from the Keirstead lab was published in 2005 and the clinical trial began in late 2010. Subsequent trials of this or other cell types will benefit from the experiences obtained and should be even more rapid. It has been estimated that this work has cost more than 170 million dollars to date and Phase 3 trials will be very expensive.
There are several milestones that need to be achieved. First, more sensitive outcome measures may be required if Phase 3 clinical trials are to detect improvements in function (particularly after thoracic SCI). Second, it seems likely that transplantation of any cell type by itself will only lead to modest benefits: co-treatments are likely to be required 2. Third, if the predominant common mechanism of action of cell transplantation is tissue preservation, then methods will need to be discovered for using stem cells to treat patients with long-standing injuries, where the majority of cell death has already happened. Finally, it will be very important to monitor patients in the long-term to exclude the possibility that transplantation of stem cells or their progeny leads to tumour formation or other negative side effects. No number of experiments in animals with short life spans can provide this information about the ultimate safety of stem cell transplantation for SCI. For a person with a recent spinal injury, the cost/benefit analysis is very difficult to make because there is insufficient information with which to make a decision. This very difficult dilemma unfortunately can only be answered in several decades time, with the advantage of hindsight. It seems prudent to assume that there will be unforeseen issues of safety and efficacy to address ahead but, at this stage, the long-term prospects for stem cell transplantation for SCI appear very promising.
Figure 2.
Potential sources of stem cells for treatment of SCI. Taken from Thuret, Moon and Gage (2006).
Footnotes
References
- 1.Steeves JD, Fawcett JW, Tuszynski MH, Lammertse DP, Curt AEP, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, et al. Experimental treatments for spinal cord injury: what you should know if you are considering participation in a clinical trial. 2007 [Google Scholar]
- 2.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 3.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 4.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Medicine. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 5.Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS ONE. 2009;4:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–93. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. The Journal of comparative neurology. 2005;486:373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–9. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–53. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 13.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp K, Flanagan L, Yee KM, Steward O. A re-assessment of a combinatorial treatment involving Schwann cell transplants and elevation of cyclic AMP on recovery of motor function following thoracic spinal cord injury in rats. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–68. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steward O, Sharp K, Selvan G, Hadden A, Hofstadter M, Au E, Roskams J. A re-assessment of the consequences of delayed transplantation of olfactory lamina propria following complete spinal cord transection in rats. Exp Neurol. 2006;198:483–99. doi: 10.1016/j.expneurol.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 18.Pinzon A, Marcillo A, Quintana A, Stamler S, Bunge MB, Bramlett HM, Dietrich WD. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008;1243:146–51. doi: 10.1016/j.brainres.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinzon A, Marcillo A, Pabon D, Bramlett HM, Bunge MB, Dietrich WD. A re-assessment of erythropoietin as a neuroprotective agent following rat spinal cord compression or contusion injury. Exp Neurol. 2008;213:129–36. doi: 10.1016/j.expneurol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, Hutton J, Altman DG. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4:e7824. doi: 10.1371/journal.pone.0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–74. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–43. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 24.Lemon RN. Descending pathways in motor control. Ann Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 25.Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–10. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf DP. Nonhuman primate embryonic stem cells: an underutilized resource. Regen Med. 2008;3:129–31. doi: 10.2217/17460751.3.2.129. [DOI] [PubMed] [Google Scholar]
- 27.Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–90. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 28.Yamane J, Nakamura M, Iwanami A, Sakaguchi M, Katoh H, Yamada M, Momoshima S, Miyao S, Ishii K, Tamaoki N, Nomura T, et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 29.Hyun JK, Kim HW. Clinical and experimental advances in regeneration of spinal cord injury. J Tissue Eng. 2010;2010:650857. doi: 10.4061/2010/650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth ED, 3rd, Reier PJ, Fessler RG, Thompson FJ, Uthman B, Behrman A, Beard J, Vierck CJ, Anderson DK. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:911–29. doi: 10.1089/089771501750451839. [DOI] [PubMed] [Google Scholar]
- 31.Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyranoski D. Chinese network to start trials of spinal surgery. Nature. 2007;446:476–7. doi: 10.1038/446476b. [DOI] [PubMed] [Google Scholar]
- 33.Cyranoski D. Stem-cell therapy faces more scrutiny in China. Nature. 2009;459:146–7. doi: 10.1038/459146a. [DOI] [PubMed] [Google Scholar]
- 34.Cloutier F, Siegenthaler MM, Nistor G, Keirstead HS. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regenerative medicine. 2006;1:469–79. doi: 10.2217/17460751.1.4.469. [DOI] [PubMed] [Google Scholar]
- 35.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–63. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker M. Tumours spark stem-cell review. Nature. 2009;457:941. doi: 10.1038/457941a. [DOI] [PubMed] [Google Scholar]
- 37.Cummings BJ, Uchida N, Tamaki SJ, Anderson AJ. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol Res. 2006;28:474–81. doi: 10.1179/016164106X115116. [DOI] [PubMed] [Google Scholar]