ABSTRACT
Despite the widespread use of antiretrovirals (ARV), more than 150,000 pediatric HIV-1 infections continue to occur annually. Supplemental strategies are necessary to eliminate pediatric HIV infections. We previously reported that maternal HIV envelope-specific anti-V3 IgG and CD4 binding site-directed antibodies, as well as tier 1 virus neutralization, predicted a reduced risk of mother-to-child transmission (MTCT) of HIV-1 in the pre-ARV era U.S.-based Women and Infants Transmission Study (WITS) cohort. As the majority of ongoing pediatric HIV infections occur in sub-Saharan Africa, we sought to determine if the same maternal humoral immune correlates predicted MTCT in a subset of the Malawian Breastfeeding, Antiretrovirals, and Nutrition (BAN) cohort of HIV-infected mothers (n = 88, with 45 transmitting and 43 nontransmitting). Women and infants received ARV at delivery; thus, the majority of MTCT was in utero (91%). In a multivariable logistic regression model, neither maternal anti-V3 IgG nor clade C tier 1 virus neutralization was associated with MTCT. Unexpectedly, maternal CD4 binding-site antibodies and anti-variable loop 1 and 2 (V1V2) IgG were associated with increased MTCT, independent of maternal viral load. Neither infant envelope (Env)-specific IgG levels nor maternal IgG transplacental transfer efficiency was associated with transmission. Distinct humoral immune correlates of MTCT in the BAN and WITS cohorts could be due to differences between transmission modes, virus clades, or maternal antiretroviral use. The association between specific maternal antibody responses and in utero transmission, which is distinct from potentially protective maternal antibodies in the WITS cohort, underlines the importance of investigating additional cohorts with well-defined transmission modes to understand the role of antibodies during HIV-1 MTCT.
KEYWORDS: antiretrovirals, clade C HIV-1, humoral immunity, mother-to-child transmission, peripartum transmission
INTRODUCTION
The wide availability of maternal antiretroviral (ARV) therapy and infant prophylaxis has reduced the number of new HIV infections in infants by 70% between 2000 and 2015, making the rate of mother-to-child transmission (MTCT) of HIV less than 5% (1). Nevertheless, more than 150,000 pediatric HIV-1 infections still occur annually due to MTCT (1). Difficulties in adherence to ARV treatment, breakthrough transmission, fetal/infant toxicities of ARVs, ARV-resistant HIV strains, and acute HIV infection of mothers late in pregnancy and during the breastfeeding period all prevent the complete elimination of MTCT of HIV with ARVs. Additional prevention strategies, such as maternal and/or pediatric vaccines, are needed to end the pediatric HIV epidemic.
Current HIV vaccination efforts have demonstrated the possible role of nonneutralizing antibodies in protection against HIV acquisition. For instance, the immune correlate analysis of the RV144 vaccine trial in Thailand revealed that higher levels of antibodies against the variable loop 1 and 2 (V1V2) region of the glycoprotein gp120 (gp120) envelope protein, but not broad neutralization, were associated with protection against HIV-1 heterosexual transmission (2). The protective role of antibodies against HIV-1 acquisition can also be studied in the setting of MTCT. In fact, there is evidence that maternal and/or infant immune factors contribute to the relatively low rate of MTCT, as the overall rate of MTCT in the absence of ARVs is between 30 and 40%, with different transmission modes disparately contributing to this rate (20 to 30% during pregnancy and delivery [perinatal transmission] and 10 to 15% via breastfeeding [postnatal transmission]) (3).
To identify maternal immune responses responsible for this natural protection, our group previously conducted a maternal humoral immune correlate analysis of MTCT risk in a large cohort of U.S. clade B HIV-1-infected women (n = 248) enrolled in the pre-ARV era Women and Infants Transmission Study (WITS). After controlling for well-known risk factors of infant HIV-1 acquisition, such as maternal virus load and CD4+ T cell counts, we observed an association between the neutralization of tier 1 (easy-to-neutralize) viruses, IgG antibodies against the envelope (Env) variable loop 3 (V3), and IgG antibodies against the CD4 binding site (bs) and decreased risk of MTCT. Moreover, maternal V3-specific monoclonal antibodies were able to neutralize and apply immune pressure on autologous virus strains, suggesting neutralization of autologous viruses as a possible mechanism of this potential protection (4). Importantly, previous studies have established that vaccination of HIV-1-infected individuals can increase V3-specific and tier 1 virus-neutralizing responses and that V3-specific antibodies can neutralize autologous virus strains, supporting the potential for maternal vaccination to enhance protective maternal antibody responses as a way to prevent MTCT of HIV (4–7).
Maternal antibodies are transferred to the fetus across the placenta, and fetal plasma IgG levels at term can even exceed those of their mothers (4, 5). HIV Env-specific antibodies could partially protect against HIV-1 transmission either by neutralizing/impeding virus in maternal plasma prior to infant virus exposure or by protecting infants upon virus exposure via passively acquired maternal antibodies. It is therefore critical to assess the role of both maternal and infant transplacentally acquired antibodies during HIV vertical transmission.
While studying the WITS cohort allowed the identification of immune factors associated with reduced MTCT risk, it is important to note that this cohort is not the most representative of current MTCT because (i) the WITS cohort was enrolled prior to the availability of ARVs that are now widely used to prevent MTCT, and (ii) the study was done in U.S. HIV-1-infected women who were infected with clade B strains of the virus, whereas the overwhelming majority of infant HIV-1 infections occur in African populations infected with clade C variants. Therefore, in this study, we sought to determine the applicability of the maternal humoral immune correlates of MTCT risk identified in the WITS to other MTCT settings, namely, in clade C virus-infected African mother-infant pairs representative of the majority of ongoing pediatric HIV infections. Using samples from the Malawian Breastfeeding, Antiretrovirals and Nutrition (BAN) study (8), we investigated if commonly elicited Env-specific antibodies are associated with reduced MTCT risk in this large cohort of clade C-infected women who received ARVs around the time of delivery. This study offered a unique opportunity to study how distinct MTCT transmission modes, HIV-1 clade, and ARV administration during delivery could influence immune correlates of peripartum transmission of HIV-1.
RESULTS
Plasma samples from 45 transmitting and 43 nontransmitting HIV-infected mothers collected before delivery and from their corresponding infants from the Malawian BAN study were studied. Table 1 provides clinical information regarding the mothers and infants studied, including maternal viral load, CD4+ T cell count, the timing of the visit for the mother and infant pairs, and samples studied. Transmitting and nontransmitting mothers were well matched by CD4+ T cell count yet differed in median plasma viral load (64,263 copies/ml for transmitting and 20,612 copies/ml for nontransmitting mothers, P = 0.02, Mann-Whitney U test; Table 1) and visit time points (antenatal versus during labor/delivery) (P = 0.03, Fisher's exact test; Table 1). However, the previous WITS established that the overwhelming majority of maternal antibody levels are stable over several months and are not statistically significantly different among peripartum time points (4). Meanwhile, infected and uninfected infant samples were similar by visit number (i.e., at delivery, 2 weeks postdelivery, or 6 weeks postdelivery).
TABLE 1.
Clinical characteristics of postmatching cohort of transmitting and nontransmitting HIV-1-infected mothers and corresponding uninfected and infected infants from the BAN study
| Variable | Transmission status | n | Median (interquartile range) or percent | SD | P value |
|---|---|---|---|---|---|
| Mothers | |||||
| Plasma viral load (copies/ml) | Nontransmitters | 43 | 20,612 (8,345–109,635) | 115,457 | 0.02 (Mann-Whitney U test) |
| Transmitters | 45 | 64,263 (20,036–196,620) | 258,630 | ||
| CD4 count (cells/μl) | Nontransmitters | 43 | 367 (281–499) | 166.6 | 0.90 (Mann-Whitney U test) |
| Transmitters | 45 | 381 (278–497) | 179.3 | ||
| Visit | |||||
| Antenatal | Nontransmitters | 43 | 100 | 0.03 (Fisher's exact test) | |
| Transmitters | 39 | 86.7 | |||
| Labor/delivery | Nontransmitters | 0 | 0 | ||
| Transmitters | 6 | 13.3 | |||
| Infants | |||||
| Visit | |||||
| Delivery | Uninfected | 15 | 34.9 | 0.96 (chi-square test) | |
| Infected | 17 | 37.8 | |||
| 2 wk postdelivery | Uninfected | 24 | 55.8 | ||
| Infected | 24 | 53.3 | |||
| 6 wk postdelivery | Uninfected | 4 | 9.3 | ||
| Infected | 4 | 8.9 | |||
Primary analysis of maternal viral load and Env-specific maternal humoral immune responses and their association with MTCT risk.
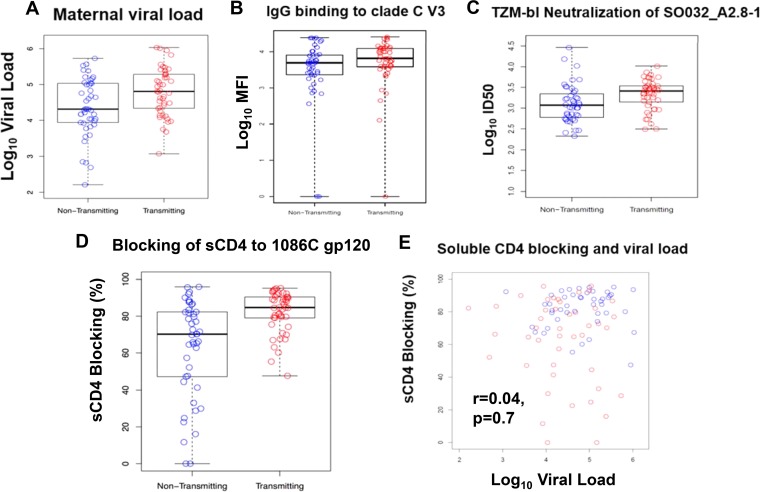
We first assessed if maternal viral load was predictive of MTCT risk, given that the transmitting mothers in our cohort had a significantly higher maternal plasma viral load than the nontransmitting mothers (P = 0.02, Mann-Whitney U test) even after matching on maternal CD4+ T cell count. As previously observed (9–11), maternal plasma viral load significantly predicted peripartum MTCT risk (odds ratio [OR], 2.66; 95% confidence interval [CI], 1.25 to 5.66; P = 0.03) (Fig. 1A). Thus, all subsequent analyses and multivariable logistics regression models were corrected for maternal plasma viral load as well as for CD4+ T cell count.
FIG 1.
Maternal viral load and Env-specific maternal humoral immune responses and their association with MTCT risk. (A) Maternal viral load (OR, 2.66; 95% CI, 1.25 to 5.66; P = 0.03) significantly predicted peripartum MTCT risk. (B and C) IgG-specific response in mothers to clade C V3 (V3.C) (OR, 0.57; 95% CI, 0.19 to 1.71; P = 0.42) (B) and the magnitude of maternal tier 1 neutralization (SO032_A2.8-1) (OR, 1.37; 95% CI, 0.28 to 6.65; P = 0.70) (C) were not associated with peripartum MTCT risk. (D) Plasma blocking of soluble CD4-gp120 interactions (OR, 1.06; 95% CI, 1.02 to 1.10; P = 0.03) was significantly associated with peripartum MTCT risk. (E) Soluble CD4 plasma blocking and viral load are not correlated (r = 0.04, P = 0.7). Nontransmitting mothers are displayed in blue and transmitting mothers in red.
Our analysis to determine if maternal anti-clade C V3 IgG CD4 binding-site (bs)-blocking antibodies and clade C tier 1 neutralization were associated with risk of peripartum MTCT in the clade C-infected BAN cohort revealed that neither anti-clade C V3 IgG (OR, 0.57; 95% CI, 0.19 to 1.71; P = 0.42) nor maternal plasma tier 1 virus neutralization (OR, 1.37; 95% CI, 0.28 to 6.65; P = 0.70) was associated with peripartum MTCT risk (Table 2 and Fig. 1B and C). However, maternal plasma antibodies blocking the CD4 bs were associated with an increased risk of MTCT, independent of maternal plasma viral load (OR, 1.06; 95% CI, 1.02 to 1.10; P = 0.03) (Table 2 and Fig. 1D and E).
TABLE 2.
ORs of peripartum HIV-1 MTCT in primary immune correlates analysis using multivariable logistic regression model
| Humoral immune variable | Multivariable logistic regression |
||
|---|---|---|---|
| OR (95% CI)a | Uncorrected P valueb | Corrected P valueb | |
| IgG binding to V3.C | 0.57 (0.19–1.71) | 0.32 | 0.42 |
| Neutralization of tier 1 clade C virus SO032_A2.8-1 | 1.37 (0.28–6.65) | 0.70 | 0.70 |
| % CD4 bs blocking on clade C gp120 (1086C gp120) | 1.06 (1.02–1.10) | 0.003 | 0.03 |
Adjusted for maternal CD4+ T cell count and maternal viral load. 95% CI, 95% confidence interval.
Humoral immune variable interactions with corrected P value of <0.05 are in bold font.
Secondary analysis of maternal Env-specific maternal and infant humoral immune responses and mother-infant transfer efficiency of IgG antibodies and their association with MTCT risk.
A secondary analysis was conducted to determine if any of the other measured maternal or infant Env-specific antibody responses were associated with MTCT risk. These included maternal and infant binding antibodies against a multiclade panel of Env antigens measured by binding antibody multiplex assay (BAMA) and infant CD4 bs antibodies measured by blocking enzyme-linked immunosorbent assay (ELISA). Maternal clade C V1V2-specific IgG level was significantly associated with increased odds of peripartum MTCT, independent of maternal plasma viral load (OR, 1.62; 95% CI, 1.08 to 2.44; P = 0.04) (Table 3 and Fig. 2). The other tested maternal antibody specificities (Table 3), infant Env-specific antibodies measured against the same antigen panel used to measure maternal antibodies, infant clade C tier 1 neutralization, and infant CD4 bs-blocking antibodies were all not significantly associated with peripartum MTCT. As most transmitted strains of HIV have a tier 2 (more difficult to neutralize) phenotype, we also measured maternal neutralization of two clade C tier 2 viruses (CE1176_A3 and CE703010217_B6). There was no association between maternal tier 2 virus neutralization and peripartum MTCT (OR, 1.20; 95% CI, 0.56 to 2.55; P = 0.64 against CE1176_A3; and OR, 0.79; 95% CI, 0.33 to 1.91; P = 0.64 against CE703010217_B6).
TABLE 3.
ORs of peripartum HIV-1 MTCT for remaining maternal Env-specific humoral immune responses (secondary analysis)
| Antigen | OR (95% CI)a | Uncorrected P valueb | Corrected P valueb |
|---|---|---|---|
| 1086Cgp140 | 1.12 (0.55–2.28) | 0.75 | 0.86 |
| 1086D7 gp120K160N | 1.21 (0.58–2.52) | 0.62 | 0.79 |
| 4403 BMC5 gp120 | 7.20 (0.96–54.07) | 0.06 | 0.10 |
| A244 gp120 gDneg/293F | 1.93 (0.85–4.42) | 0.12 | 0.19 |
| B.con env03 gp140_CF | 1.25 (0.57–2.73) | 0.58 | 0.79 |
| Bio-MPER656 | 1.27 (0.84–1.93) | 0.26 | 0.40 |
| Bio-V2.1086C | 1.03 (0.71–1.51) | 0.87 | 0.90 |
| Bio-V2.B | 1.07 (0.67–1.73) | 0.78 | 0.86 |
| Bio-V3.B | 1.10 (0.60–2.01) | 0.76 | 0.86 |
| Con6gp120/B | 3.04 (0.76–12.18) | 0.12 | 0.19 |
| ConC_gp120_WT | 2.12 (0.83–5.39) | 0.12 | 0.19 |
| gp70 MNV3 | 0.90 (0.59–1.36) | 0.61 | 0.79 |
| gp70B.caseA V1V2/293F | 1.51 (1.00–2.28) | 0.05 | 0.10 |
| gp70_B.caseA2_V1V2/169K | 1.37 (0.94–2.00) | 0.10 | 0.18 |
| gp70_C.1086CV1/V2 | 1.62 (1.08–2.44) | 0.02 | 0.04 |
Adjusted for maternal CD4+ T cell count and maternal viral load. 95% CI, 95% confidence interval.
Humoral immune variable interactions with corrected P value of <0.05 are in bold font.
FIG 2.
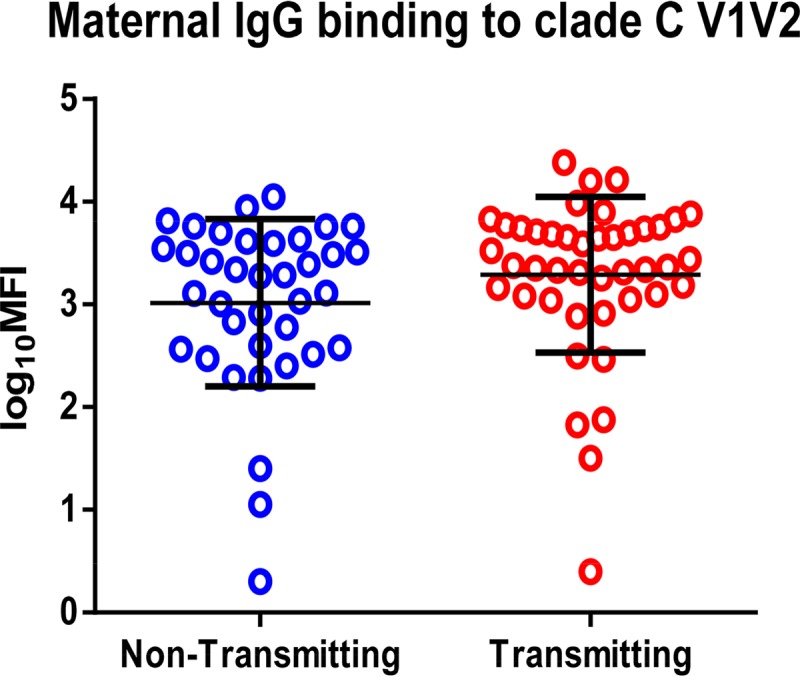
Comparison of anti-clade C V1V2 responses in HIV-1-infected transmitting and nontransmitting mothers. IgG-specific response in mothers to clade C V1V2 (OR, 1.62; 95% CI, 1.08 to 2.44; P = 0.04) was significantly associated with peripartum MTCT risk. Nontransmitting women are displayed in blue (left) and transmitting women in red (right).
Association between maternal transplacental IgG transfer and MTCT risk.
As maternal HIV infection can interfere with the transplacental transfer of antibodies (12, 13), we measured the transfer efficiency of clade C gp120 (ConC gp120)-, V1V2-, and V3-specific IgG in mother-infant pairs. We then sought to determine if transplacental IgG transfer efficiency was associated with MTCT risk. For ConC gp120-, V1V2-, and V3-specific IgG, infant IgG concentrations were significantly lower than their matched maternal IgG levels (for ConC gp120, infant median, 577.4 μg/ml versus maternal median, 1,334 μg/ml; for V1V2, infant median, 0.2 μg/ml versus maternal median, 0.7 μg/ml; for V3, infant median, 96.1 μg/ml versus maternal median, 951.6 μg/ml; P < 0.0001, Wilcoxon matched-pairs signed-rank test). Importantly, placental transfer efficiency of IgG against consensus clade C gp120 (OR, 1.00; 95% CI, 0.98 to 1.01; P = 0.79) and against clade C V3 (OR, 0.99; 95% CI, 0.98 to 1.00; P = 0.41) was not associated with transmission risk. Interestingly, although there was a strong correlation between maternal and infant anti-V1V2-specific IgG concentration (r = 0.93, P < 0.0001, Spearman's rank correlation), anti-gp120-specific IgG concentration (r = 0.81, P < 0.0001), and anti-V3-specific IgG concentration (r = 0.81, P < 0.0001), the transfer efficiency of clade C V1V2-specific IgG did not predict transmission risk (OR, 1.00; 95% CI, 0.99 to 1.00; P = 0.67) despite the association between maternal V1V2-specific IgG and increased MTCT risk. Thus, degree of HIV Env-specific IgG transfer from mother to infant did not predict the risk of MTCT in this cohort of ARV-exposed clade C virus-infected mothers and their infants.
DISCUSSION
In a previous humoral immune correlate analysis of MTCT risk using the WITS cohort, common maternal clade B V3-specific IgG and CD4 bs antibody responses predicted a decreased risk of peripartum MTCT (in utero plus intrapartum), while maternal tier 1 neutralization predicted a decreased risk of MTCT in the intrapartum cohort alone. We therefore sought to determine if the same humoral immune factors predicted perinatal MTCT risk in the African clade C HIV-1-infected BAN cohort of mothers and infants who were given ARV prophylaxis around the time of delivery. Our results demonstrated that clade C V3-specific IgG and maternal tier 1 virus neutralization were not associated with peripartum transmission risk in the BAN cohort, although we acknowledge the confidence interval for the odds ratio of maternal tier 1 virus neutralization is large, and that confidence for this immune correlate is limited given the sample size. More surprisingly, however, maternal anti-CD4 binding-site responses and clade C V1V2-specific IgG were associated with increased odds of peripartum transmission independent of maternal viral load.
The distinct humoral immune correlates of MTCT risk in the WITS and the BAN mother-infant cohorts could be due to a number of differences between the two cohorts. One of the most striking differences is the breakdown of mother-to-infant HIV transmission modes. In the WITS cohort, which was conducted in the pre-ARV era, 13% of MTCT occurred in utero, 52% occurred in the peripartum period, and 35% were unknown due to the unavailability of nucleic acid HIV detection. In contrast, the majority of MTCT in the BAN cohort was due to in utero transmission (91%). The high proportion of in utero transmission in the BAN cohort is likely due to the fact that women standardly received ARVs at delivery, which considerably reduced the proportion of intrapartum transmission events. Another difference between the two cohorts is the smaller available sample size for the BAN immune correlate analysis than that of the WITS analysis, which is a limitation of our current study. Finally, the clade of HIV-1 infection is a major difference between the studies. While the WITS cohort consisted of clade B HIV-1-infected women from the United States, the BAN cohort of women from Malawi were infected with clade C HIV-1 strains. Interclade amino acid differences in HIV Env glycoproteins can be as high as 35% and influence the genetic selection of different viral strains and their interactions with their host's immune response (14).
Importantly, previous studies have suggested that the mechanisms of in utero versus intrapartum transmission differ in a number of ways. While in utero transmission occurs transplacentally, intrapartum transmission occurs through infant exposure to maternal secretions and blood, followed by infection of CD4+ CCR5+ T cells in the neonatal gut during the delivery process (15). Dickover et al. described that mothers who transmitted HIV-1 to their infants in utero were more likely to transmit one or multiple maternal variants of HIV-1 that were dominant in maternal plasma, while intrapartum transmitters were more likely to transmit minor maternal viral variants (16). This difference in HIV-1 variants transmitted through the genetic bottleneck from mother to infant during in utero versus intrapartum transmission might contribute to the difference in humoral immune correlates associated with MTCT risk in the WITS and BAN cohorts.
The direct association between maternal V1V2-specific IgG antibodies and soluble CD4 (sCD4)-blocking responses and MTCT risk in the BAN cohort was unexpected. Although our small sample size might have contributed to these unexpected associations, tight confidence intervals increase the support for these correlates. Future studies on larger populations will be important to confirm these findings and elucidate whether these correlates have a mechanistic basis. It is possible that high levels of some Env-specific antibodies may simply be a biomarker for increased viral infectivity of the dominant maternal variant. It is also possible that these nonneutralizing antibodies bind to the HIV Env in vivo, change its conformation, and enhance its infectivity across the placenta. However, this hypothesis needs to be tested with structural analysis to address whether or not maternal antibodies may enhance placental transmission of HIV-1 virions, and if so, how such antibody enhancement may occur. In addition, although BAN participants were screened for serious infections prior to enrollment in the study (8), it is possible that immune activation associated with coinfections, such as cytomegalovirus (CMV), or comorbidities in mothers and infants that were not accounted for could impact levels of maternal and/or infant plasma antibodies and/or the risk of MTCT of HIV-1 and possibly influence the findings of this study. The immune activation of placental membranes and secretion of proinflammatory cytokines into the amniotic fluid during chorioamnionitis can increase localized HIV-1 replication in the placenta and may increase in utero MTCT risk (17–20). In addition, maternal sexually transmitted diseases (STDs) and cervical inflammation are associated with increased intrapartum MTCT of HIV-1 (17, 21, 22). Unfortunately, peripheral blood mononuclear cells (PBMCs) from this subset of patients were not available to test the possible role of immune activation in MTCT in our study.
Interestingly, all significant humoral immune correlates of increased risk of MTCT were observed in maternal plasma but not in infant plasma and were not associated with transfer efficiency of Env-specific antibodies. Despite the association between high maternal anti-V1V2 IgG and increased MTCT risk, the efficiency of the transplacental transfer of anti-V1V2 IgG from mother to infant was not associated with increased MTCT risk. This suggests that transplacental transfer of nonneutralizing IgG is not enhancing MTCT by assisting with viral transmission across the placenta. Although some studies have suggested that CMV virions may be transported across the placenta by coopting receptors responsible for antibody transport across the placenta (23), our results do not seem to support this type of scenario for peripartum mother-to-child transmission of HIV-1. Despite the significantly lower infant Env-specific IgG levels than those of their mothers, maternal gp120-, V1V2-, and V3-specific IgG levels were all strongly correlated with the matched infant Env-specific IgG levels. Despite this correlation, infant IgG responses at birth did not fully reflect maternal antibody responses, suggesting that humoral immune correlate analyses of MTCT should be conducted in both mothers and infants. Further studies of the infant immune correlates of MTCT may be important to define which types of immune responses a potential pediatric HIV vaccine should elicit.
In the clade B virus-infected WITS cohort, maternal plasma clade B V3-specific IgG was associated with transmission risk, whereas this association was not observed between maternal plasma clade C V3-specific IgG in the clade C virus-infected BAN cohort. One limitation of our study is that heterologous peptides, including the V3 loop peptide, were used in binding assays to measure plasma antibody responses. However, we attempted to select a clade C consensus V3 peptide that would be appropriate for binding assays among a large collection of clade C HIV-1 strains from Malawian mothers and infants. This approach is similar to the one used in the WITS in which IgG binding to a clade B consensus V3 peptide was found to be associated with a reduced risk of MTCT (4). Overall, using viral epidemiology signature pattern analysis (VESPA), 16/23 amino acids of the consensus clade C V3 peptide used in our assays were similar to >90% of 697 HIV envelope V3 sequences generated through single genome amplification (SGA) from 19 clade C HIV-1-infected Malawian mother-infant pairs in a study by Russell et al. (24) (see Fig. S1 in the supplemental material). Furthermore, studies of V3 among different clades have revealed that the clade C V3 domain is relatively more conserved than that of clade B, as measured by the ratio of nonsynonymous to synonymous substitutions in V3 (14). In addition, the amino acid motifs of a highly conserved turn region of V3 differ between clades B and C (14). Clade C appears to be less susceptible to anti-V3-mediated neutralization, suggesting that perhaps clade C V3 is less exposed on the Env trimer (14). Greater exposure of V3 on the Env trimer in clade B viruses might explain why V3-specific antibodies are associated with decreased peripartum transmission risk in a clade B virus-infected cohort but not in a clade C virus-infected cohort.
The surprising finding of an association of high levels of certain maternal plasma Env-specific antibodies and in utero MTCT risk in the BAN cohort adds to the complexity of humoral immune correlates of protection against HIV-1 MTCT. In particular, this study shows that there are differences in immune correlates associated either with risk of peripartum MTCT between various transmission modes (in utero versus intrapartum) and perhaps among different clades of HIV infection. Notably, further investigations into interclade differences in the Env V1V2 loops and the CD4 binding site may aid in explaining the distinct association between antibodies against these regions and MTCT risk in the WITS and BAN cohorts. As over half of current infant HIV-1 infections occur in sub-Saharan populations infected with clade C HIV (25), it is critical to study additional clade C virus-infected cohorts to confirm the association we observed in the BAN cohort. A study of clade C HIV-1-infected cohorts with a higher proportion of intrapartum transmission cases would be useful to further shed light on humoral immune correlates of distinct modes of MTCT. Further characterization of the role and epitope specificity of maternal nonneutralizing antibodies associated with MTCT risk, specifically the maternal clade C V1V2-specific IgG and sCD4 binding-site antibodies found to be positively associated with peripartum MTCT risk in our study, would also be useful. A clear definition of immune factors associated with protection against peripartum MTCT risk will help define which immune responses should be elicited by a vaccine that can assist in the elimination of pediatric HIV-1 worldwide.
MATERIALS AND METHODS
Study design.
The BAN study conducted between 2004 and 2010 enrolled clade C HIV-1-infected pregnant women from Malawi with a CD4+ T cell count of >200 cells/μl (8). All women and infants received a single dose of nevirapine at the time of delivery, followed by 1 week of zidovudine (AZT) and lamivudine. Women then breastfed for 24 to 28 weeks in the presence or absence of ARVs, depending on randomization to the treatment or standard-of-care group.
Criteria for the selection of HIV-1-infected women from the BAN study for investigating humoral immune correlates of peripartum transmission in the ARV era (Table 1) included nonheparin maternal plasma samples available for immune assays from the third trimester of pregnancy, before delivery and ARV administration, and corresponding infant plasma samples from infected infants (n = 45). The control group (n = 45) of nontransmitting HIV-infected mothers and their corresponding uninfected infants were selected based on similar characteristics and were matched only for maternal blood CD4+ T cell count with case samples, as maternal viral load was unavailable prior to study initiation. Infant plasma samples were collected at delivery (n = 34), 2 weeks postdelivery (n = 48), or 6 weeks postdelivery (n = 8). Two samples from the nontransmitting group (one maternal and one infant) and their paired infant and maternal data were excluded from analysis due to lack of adequate sample. Postmatching analysis of the clinical characteristics of the transmitting and nontransmitting cohort revealed no significant difference in peripheral CD4+ T cell count (P = 0.90, Mann-Whitney U test; Table 1) but a significant difference in maternal plasma viral load (P = 0.02, Mann-Whitney U test; Table 1). Because ARV administration occurred per protocol around the time of delivery, the majority of MTCT in this subset of the BAN cohort studied occurred in utero and not intrapartum (91% of HIV-infected infants in the HIV transmitting group were infected in utero). Following the delivery window, all mother-infant pairs breastfed for 24 to 28 weeks and were assigned to one of three prophylaxis strategies: (i) maternal ARVs (zidovudine, lamivudine, lopinavir, and ritonavir), (ii) daily infant ARV (nevirapine), or (iii) no ARV (control). In the cohort used in this study, mothers from 38 mother-infant pairs received ARVs, infants from 27 pairs received nevirapine, and in 25 pairs, neither the mother nor the infant received ARVs during breastfeeding.
The laboratory analyst performing all assays was blinded to the transmission status of maternal samples and infection status of infant samples at the time of assays and data analysis.
Ethics statement.
All adult study participants provided written informed consent for study participation of themselves and their infants. Ethical approval for the BAN study was provided by the Malawi National Health Science Research Committee and the institutional review boards at the University of North Carolina at Chapel Hill and the U.S. Centers for Disease Control and Prevention.
HIV Env-specific IgG BAMA.
HIV Env-specific binding IgG antibodies were measured by BAMA, as previously described (2, 26), in maternal and infant plasma samples. A multiclade panel of primary and consensus Env gp120 and gp140 antigens, V1V2 constructs, clade C V2 (KKKTELKDKKHKVHALFYKLDVVP), V3 peptide (KKKNNTRKSIRIGPGQTFYATGDIIGDIRQAHC), and the membrane-proximal external region (MPER) region of gp41 (KKKNEQELLELDKWASLWNWFNITNWLW) was tested (Table S1). Purified HIV-1 antigens were covalently coupled to carboxylated fluorescent beads (Bio-Rad, Hercules, CA). Antigen-coupled beads were incubated with plasma at predetermined dilutions of the antigens listed in Table S1: 1:250 for 4403 BMC5 gp120, A244 gp120 gDneg/293F, 1086D7 gp120K160N, ConC_gp120_WT, Con6gp120/B, 1086Cgp140, B.con env03 gp140_CF, gp70_C.1086CV1/V2, gp70B.caseA V1V2/293F, gp70_B.caseA2_V1V2/169K, Bio-V2.1086C, Bio-V2.B, Bio-V3.C, Bio-V3.B, gp70 MNV3, and Bio-MPER656 and 1:2,500 for clade C mutated gp120 (1086D7gp120K160N), clade C gp140, V3 peptide, and consensus gp120. These dilutions were found to be within the linear range of the assay based on preliminary testing of serial dilutions of five samples. IgG binding was detected with a phycoerythrin (PE)-conjugated mouse anti-human IgG antibody (SouthernBiotech, Birmingham, AL) at 2 μg/ml. Assay plates were read on a Bio-Plex instrument (Bio-Rad, Hercules, CA), and the readout was expressed in mean fluorescence intensity (MFI) units. Polyclonal HIV immunoglobulin (HIVIG) was used as a positive control for each assay to ensure assay consistency, specificity, and reproducibility among assays on different dates. Blank beads were included in every assay. The positivity cutoff for each antigen was determined as the average + 3 standard deviations of MFIs of 30 HIV-seronegative samples tested at a 1:250 or 1:2,500 dilution. Preset assay quality control criteria for reporting data included percent coefficient of variation (%CV) for duplicate values <20%, ≥100 beads counted per well, and positive-control HIVIG titer within ±3 standard deviations of the mean for each antigen, tracked with Levey-Jennings plots. The concentrations of V3-, V1V2-, and consensus gp120-specific IgG antibodies were measured using specific antibodies as standards (V1V2-specific monoclonal antibody CH58, V3-specific monoclonal antibody CH22, and gp120-specific monoclonal antibody B12, generously provided by Barton Haynes).
HIV-1 neutralization assay.
Neutralization assays were performed using heat-inactivated maternal and infant plasma samples, as previously described (27), using Tat-regulated Luc reporter gene expression to quantify the reduction in viral infection of TZM-bl cells (NIH AIDS Reagent Program; contributed by John Kappes and Xiaoyun Wu). Neutralization was measured against one clade C tier 1 “easy-to-neutralize” Env-pseudotyped virus, SO032_A2.8-1 (GenBank accession no. KF114894), for both maternal and infant samples. In addition, maternal samples were tested against two clade C tier 2 “difficult-to-neutralize” Env-pseudotyped viruses, CE1176_A3 (GenBank accession no. FJ444437) and CE703010217_B6 (GenBank accession no. FJ443575). These clade C tier 2 viruses were selected from a global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralization antibodies to approximate the neutralizing activity seen against other clade C tier 2 subtype-matched viruses as best as possible (28). Plasma was tested at eight 3-fold dilutions starting at 1:20. The broadly neutralizing monoclonal antibodies B12 and VRC01 were used as positive controls. Neutralization titer is reported as the dilution at which relative luminescence units (RLU) were reduced by 50% compared to the RLU in the virus control well (50% inhibitory dose [ID50]). Plasma samples were also tested for neutralization against a nonspecific murine retrovirus (MLV) to account for any nonspecific neutralization activity. A plasma sample was considered positive for neutralization if the ID50 against SO032_A2.8-1, CE1176_A3, or CE703010217_B6 was at least three times higher than the ID50 of MLV.
Soluble CD4 plasma-blocking assay.
Blocking of the binding of soluble CD4 on the HIV Env by IgG was measured as described previously (2). Briefly, 384-well ELISA plates (VWR, Radnor PA) were coated with 15 μl of 2 μg/ml HIV-1 Env 1086C gp120 (generously provided by the Duke Human Vaccine Institute [DHVI] Protein Production Facility) diluted in 0.1 M sodium bicarbonate (30 ng/well) overnight. The plate was washed once with phosphate-buffered saline (PBS)–0.1% Tween 20 prior to being blocked with 40 μl of SuperBlock (PBS containing 4% whey protein–15% normal goat serum, 0.5% Tween 20) for at least 1 h at room temperature (RT). The plate was washed one time, and then 10 μl of plasma samples diluted 1:50 in SuperBlock was incubated for 1 h at RT. The CD4 binding-site monoclonal antibody VRC01 was used as a positive control. VRC01 was plated in a 2-fold dilution series starting at 32 μg/ml. The plate was washed 2 times, and then 10 μl of soluble CD4 (sCD4; Progenics Pharmaceuticals, Inc.) was added at a predetermined saturating concentration of 0.64 μg/ml, incubated for 1 h at RT, and then washed 2 times. The plate was then incubated with biotinylated anti-CD4 monoclonal antibody OKT4 (0.03 μg/ml) for 1 h at RT to detect sCD4 binding. The plate was washed 2 times and incubated with streptavidin horseradish peroxidase (HRP; SouthernBiotech, Birmingham, AL) diluted at 1:30,000 in the dark for 1 h at RT. After a 4 washes, substrate (SureBlue Reserve microwell substrate; VWR, Radnor, PA) was added to the plate. TMB (3,3′,5,5′-tetramethylbenzidine) stop solution (VWR) was added after a 10- to 15-min exposure time, and the plate was immediately read at 450 nm with a plate reader. Background was subtracted from all of the well absorbance readings and averaged for triplicate wells. Percent sCD4 plasma blocking was calculated as: 100 − (plasma triplicate mean/no plasma control mean) × 100.
Statistical methods.
The statistical analysis plan was finalized prior to data analysis. We used a multivariable conditional logistic regression model that was adjusted for maternal plasma viral load and peripheral CD4+ T cell count, with transmission status as the dependent variable. Our primary analysis applied multivariable logistic regression models for maternal clade C anti-V3 IgG response, tier 1 virus neutralization, and sCD4 blocking percent inhibition. Secondary analyses included using logistic regression models for each individual humoral immune response measured in the cohort, with the dependent variable being transmission status for maternal samples or infection status for infant samples. To account for alpha inflation from multiple comparisons, we applied the Benjamini-Hochberg false-discovery rate correction (29) and reported the corrected P values. Corrected and uncorrected P values can be found in Tables 1 to 3. We conducted a post hoc analysis to study the impact of transplacental transfer from mother to infant of IgG antibodies against clade C V3, clade C V1V2, and clade C consensus gp120 on MTCT. Transfer efficiency (%) was calculated according to the following formula, where concentration refers to concentration of Env-specific IgG antibody as determined from monoclonal antibody standards (concentrations are reported in micrograms per milliliter): (infant concentration/maternal concentration) × 100.
Wilcoxon two-sample tests (Mann-Whitney U tests) were used to compare maternal viral load and CD4+ T cell count between transmitting and nontransmitting mothers. The correlation between infant and maternal antibody concentrations was determined using Spearman's rank correlation.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network, with overall support provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award no. UM1AI106716 (IMPAACT LC), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), the Duke University Center for AIDS Research (CFAR), an NIH-funded program (grant 5P30AI064518), and the Doris Duke Charitable Foundation (DDCF) Clinical Research Mentorship Award. The BAN study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (grants SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01), the National Institute of Allergy and Infectious Diseases, the University of North Carolina CFAR (grant P30-AI50410), the NIH Fogarty AIDS International Training and Research Program (grant DHHS/NIH/FIC 2-D43 Tw01039-06), the Fogarty International Clinical Research Scholars Program (grant R24 Tw00798), the American Recovery and Reinvestment Act, and the Bill and Melinda Gates Foundation (grant OPP5310). The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children's Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention, the NIH, or the DDCF.
We thank Amit Kumar and David Martinez for their help in manuscript development and sequence analysis. We thank the Breastfeeding, Antiretrovirals and Nutrition (BAN) study participants and the staff involved in these studies.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00062-17.
REFERENCES
- 1.UNAIDS. 2016. Children and HIV: fact sheet. UNAIDS, Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf. [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braibant M, Barin F. 2013. The role of neutralizing antibodies in prevention of HIV-1 infection: what can we learn from the mother-to-child transmission context? Retrovirology 10:103. doi: 10.1186/1742-4690-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, Jaeger FH, Pollara J, Martelli A, Liebl BE, Lloyd K, Yates NL, Overman RG, Shen X, Whitaker K, Chen H, Pritchett J, Solomon E, Friberg E, Marshall DJ, Whitesides JF, Gurley TC, Von Holle T, Martinez DR, Cai F, Kumar A, Xia SM, Lu X, Louzao R, Wilkes S, Datta S, Sarzotti-Kelsoe M, Liao HX, Ferrari G, Alam SM, Montefiori DC, Denny TN, Moody MA, Tomaras GD, Gao F, Haynes BF. 2015. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest 125:2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein F, Nogueira L, Nishimura Y, Phad G, West AP Jr, Halper-Stromberg A, Horwitz JA, Gazumyan A, Liu C, Eisenreich TR, Lehmann C, Fatkenheuer G, Williams C, Shingai M, Martin MA, Bjorkman PJ, Seaman MS, Zolla-Pazner S, Karlsson Hedestam GB, Nussenzweig MC. 2014. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J Exp Med 211:2361–2372. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moody MA, Gao F, Gurley TC, Amos JD, Kumar A, Hora B, Marshall DJ, Whitesides JF, Xia SM, Parks R, Lloyd KE, Hwang KK, Lu X, Bonsignori M, Finzi A, Vandergrift NA, Alam SM, Ferrari G, Shen X, Tomaras GD, Kamanga G, Cohen MS, Sam NE, Kapiga S, Gray ES, Tumba NL, Morris L, Zolla-Pazner S, Gorny MK, Mascola JR, Hahn BH, Shaw GM, Sodroski JG, Liao HX, Montefiori DC, Hraber PT, Korber BT, Haynes BF. 2015. Strain-specific V3 and CD4 binding site autologous HIV-1 neutralizing antibodies select neutralization-resistant viruses. Cell Host Microbe 18:354–362. doi: 10.1016/j.chom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett JA, Wasserman SS, Hicks CB, Dodge RT, Weinhold KJ, Tacket CO, Ketter N, Wittek AE, Palker TJ, Haynes BF. 1998. Safety and immunogenicity of an HLA-based HIV envelope polyvalent synthetic peptide immunogen. DATRI 010 Study Group. Division of AIDS Treatment Research Initiative. AIDS 12:1291–1300. [DOI] [PubMed] [Google Scholar]
- 8.van der Horst C, Fiscus S, Piwoz E, Corenli A, Moses A, Jones D, Adair L, Bentley M, Hoffman I, Kashuba A, Shugars D, Bandiwala K, Tien H. 2003. Prevention of mother to infant transmission of HIV through breastfeeding and reduction of morbidity and mortality of the breastfeeding mothers: a study in Malawi. Antivir Ther 8(Suppl 1):S477. [Google Scholar]
- 9.Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA III, Whitehouse J, Moye J Jr, Reichelderfer P, Harris DR, Fowler MG, Mathieson BJ, Nemo GJ. 1999. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med 341:385–393. [DOI] [PubMed] [Google Scholar]
- 10.Mayaux MJ, Dussaix E, Isopet J, Rekacewicz C, Mandelbrot L, Ciraru-Vigneron N, Allemon MC, Chambrin V, Katlama C, Delfraissy JF, Puel J. 1997. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. SEROGEST Cohort Group. J Infect Dis 175:172–175. doi: 10.1093/infdis/175.1.172. [DOI] [PubMed] [Google Scholar]
- 11.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew JF. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med 341:394–402. [DOI] [PubMed] [Google Scholar]
- 12.Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT. 2007. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 196:550–557. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 13.Bashir MF, Elechi HA, Ashir MG, Rabasa AI, Bukbuk DN, Usman AB, Mustapha MG, Alhaji MA. 2016. Neonatal tetanus immunity in Nigeria: the effect of HIV infection on serum levels and transplacental transfer of antibodies. J Trop Med 2016:7439605. doi: 10.1155/2016/7439605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch RM, Shen T, Gnanakaran S, Derdeyn CA. 2009. Appreciating HIV type 1 diversity: subtype differences in Env. AIDS Res Hum Retroviruses 25:237–248. doi: 10.1089/aid.2008.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobin NH, Aldrovandi GM. 2013. Immunology of pediatric HIV infection. Immunol Rev 254:143–169. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickover RE, Garratty EM, Plaeger S, Bryson YJ. 2001. Perinatal transmission of major, minor, and multiple maternal human immunodeficiency virus type 1 variants in utero and intrapartum. J Virol 75:2194–2203. doi: 10.1128/JVI.75.5.2194-2203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawn SD, Butera ST, Folks TM. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev 14:753–777. doi: 10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Vermund SH, Goepfert AR, Andrews WW. 1998. Choriodecidual inflammation: a potentially preventable cause of perinatal HIV-1 transmission? Lancet 352:1927–1930. doi: 10.1016/S0140-6736(98)04453-5. [DOI] [PubMed] [Google Scholar]
- 19.St. Louis ME, Kamenga M, Brown C, Nelson AM, Manzila T, Batter V, Behets F, Kabagabo U, Ryder RW, Oxtoby M, Quinn TC, Heyward WL. 1993. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic, and placental factors. JAMA 269:2853–2859. doi: 10.1001/jama.1993.03500220039023. [DOI] [PubMed] [Google Scholar]
- 20.Wabmire-Mangen F, Gray RH, Mmiro FA, Ndugwa C, Abramowsky C, Wabinga H, Whalen C, Li C, Saah AJ. 1999. Placental membrane inflammation and risks of maternal-to-child transmission of HIV-1 in Uganda. J Acquir Immune Defic Syndr 22:379–385. doi: 10.1097/00042560-199912010-00009. [DOI] [PubMed] [Google Scholar]
- 21.Mandelbrot L, Le Chenadec J, Berrebi A, Bongain A, Bénifla JL, Delfraissy JF, Blanche S, Mayaux MJ. 1998. Perinatal HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA 280:55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 22.Mandelbrot L, Mayaux MJ, Bongain A, Berrebi A, Moudoub-Jeanpetit Y, Bénifla JL, Ciraru-Vigneron N, Le Chenadec J, Blanche S, Delfraissy JF. 1996. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol 175:661–667. [DOI] [PubMed] [Google Scholar]
- 23.Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. 2006. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 168:1210–1226. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell ES, Kwiek JJ, Keys J, Barton K, Mwapasa V, Montefiori DC, Meshnick SR, Swanstrom R. 2011. The genetic bottleneck in vertical transmission of subtype C HIV-1 is not driven by selection of especially neutralization-resistant virus from the maternal viral population. J Virol 85:8253–8262. doi: 10.1128/JVI.00197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Hoffmann F, He J, He X, Kankasa C. 2005. Evolution of subtype C HIV-1 Env in a slowly progressing Zambian infant. Retrovirology 2:67. doi: 10.1186/1742-4690-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, Greene K, Gao H, Daniell X, Sarzotti-Kelsoe M, Gorny MK, Sozza-Pazner SZ, LaBranche CC, Mascola JR, Korber BT, Montefiori DC. 2014. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.