ABSTRACT
Several infectious disease outbreaks with high mortality in humans have been attributed to viruses that are thought to have evolved from bat viruses. In this study from Luxembourg, the genetic diversity and epidemiology of paramyxoviruses and coronaviruses shed by the bat species Rhinolophus ferrumequinum and Myotis emarginatus were evaluated. Feces collection (n = 624) was performed longitudinally in a mixed-species colony in 2015 and 2016. In addition, feces (n = 254) were collected cross-sectionally from six Myotis emarginatus colonies in 2016. By use of degenerate primers in a nested format, overall prevalences of 1.1% (10/878) and 4.9% (43/878) were determined for paramyxoviruses and coronaviruses. Sequences of the partial RNA-dependent RNA polymerase and spike glycoprotein genes of coronaviruses, as well as sequences of the partial L gene of paramyxoviruses, were obtained. Novel paramyxovirus and Alphacoronavirus strains were identified in different Myotis emarginatus colonies, and severe acute respiratory syndrome (SARS)-related Betacoronavirus strains were shed by Rhinolophus ferrumequinum. Logistic regression revealed that the level of Alphacoronavirus shedding was highest in July (odds ratio, 2.8; P < 0.01), probably due to periparturient stress. Phylogenetic analyses point to close virus-host coevolution, and the high genetic similarity of the study strains suggests that the Myotis emarginatus colonies in Luxembourg are socially connected. Most interestingly, we show that bats also host Betacoronavirus 1 strains. The high similarity of the spike gene sequences of these viruses with mammalian Betacoronavirus 1 strains may be of concern. Both the SARS-related and Betacoronavirus 1 strains detected in bats in Luxembourg may cross the species barrier after a host adaptation process.
IMPORTANCE Bats are a natural reservoir of a number of zoonotic pathogens. Several severe outbreaks in humans (e.g., a Nipah virus outbreak in Malaysia in 1998, and the almost global spread of severe acute respiratory syndrome in 2003) have been caused by bat-borne viruses that were transmitted to humans mostly after virus adaptation (e.g., in intermediate animal hosts). Despite the indigenousness of bat species that host viruses with suspected zoonotic potential and despite the zoonotic transmission of European bat 1 lyssavirus in Luxembourg, knowledge about the diversity and epidemiology of bat viruses remains limited in this country. Moreover, in contrast to other European countries, bat viruses are currently not included in the national surveillance activities of this land-locked country. We suggest that this gap in disease surveillance should be addressed, since we show here that synanthropic bats host viruses that may be able to cross the species barrier.
KEYWORDS: Chiroptera, Luxembourg, coronavirus, molecular epidemiology, paramyxovirus, phylogenetic analysis, surveillance studies, virology, zoonotic infections
INTRODUCTION
The ability of bats (Chiroptera) to fly long distances and their longevity enable them to spread viruses across time and space. Large colony sizes, close social interactions, and coroosting of different bat species favor intraspecies and interspecies transmission of viruses (1). Moreover, the low pathogenicity of viruses and their persistence in bats are indicative of ancient cospeciation between bats and different virus families (e.g., Paramyxoviridae and Coronaviridae [2–5]). It has been suggested that most human coronaviruses (CoV) evolved from bat counterparts (5–7). For instance, severe acute respiratory syndrome CoV (SARS-CoV) (8) and Middle East respiratory syndrome CoV (MERS-CoV) (9, 10), as well as the paramyxoviruses (PV) Nipah virus and Hendra virus (11, 12), originated in bats and have caused severe outbreaks in humans. While for some viruses, viral adaptation processes in intermediate animal hosts were presumably required before zoonotic transmission (see, e.g., references 9 and 13), direct transmission of Nipah virus between bats and humans has occurred repeatedly in Bangladesh (14). The spike glycoproteins of several bat CoV strains share features with human strains that have been critical for bat-to-human transmission events (15). In particular, the receptor-binding domain of the spike gene determines the host range and tissue tropism of CoV (16–18). Nevertheless, the risk of zoonotic infection with bat viruses is low for humans, since direct contacts with bat excretions are rare (19, 20). In addition, the risk can be monitored by virus surveillance in synanthropic bats (20), such as vespertilionid (e.g., Myotis emarginatus) and rhinolophid (e.g., Rhinolophus ferrumequinum) bats, which have been shown to host a number of viruses with zoonotic potential (5, 21–23).
In Western and Central Europe, M. emarginatus and R. ferrumequinum are endangered (24, 25) due to ongoing habitat fragmentation (26). After hibernating in underground sites, R. ferrumequinum females return to their natal colonies in March, while M. emarginatus females follow only in May (27–29). They form matrilineal maternity colonies in attics and barns (27–29). Around mid-June, each female gives birth to a single pup. Intralineage polygyny is common for R. ferrumequinum (30, 31), and extracolony mating of R. ferrumequinum and M. emarginatus bats occurs during the swarming of the males, between September and October (32, 33).
Despite a growing interest in these animals as hosts of emerging viruses, knowledge about bat viruses in Luxembourg remains limited. In a single study, European bat 1 lyssavirus was isolated and the risk of zoonotic transmission in the country shown (34).
Here we report the shedding of PV and CoV by R. ferrumequinum and M. emarginatus, two sympatric and synanthropic bat species. Virus diversity and prevalence were assessed in six nursing colonies of M. emarginatus in a cross-sectional manner. In addition, we investigated the seasonal patterns of both viruses in a mixed R. ferrumequinum–M. emarginatus colony, in a parallel longitudinal study. Several novel viruses of both families were detected, and we show that bats are also a host for Betacoronavirus 1 strains.
RESULTS
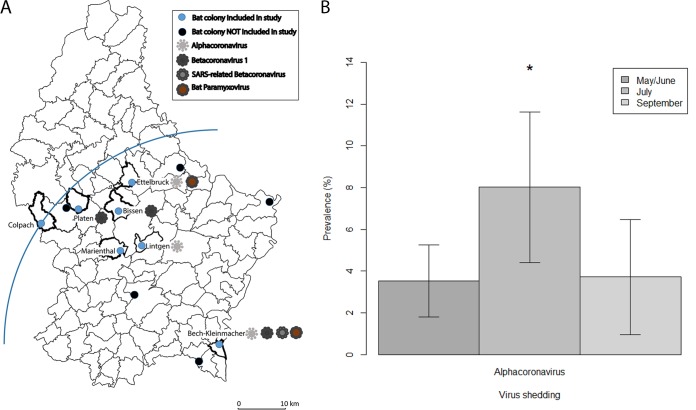
To assess the prevalence and diversity of PV and CoV shedding among bats in Luxembourg, fecal samples from 7 colonies (Fig. 1A) were screened using degenerate primers in a nested format. The overall prevalence of PV was 1.1% (10/878), and that of CoV was 4.9% (43/878); viruses were found in every colony except for those at Colpach and Marienthal (Fig. 1A; Table 1). No PV–CoV coinfections were detected.
FIG 1.
(A) Municipalities with known Myotis emarginatus colonies in Luxembourg and circulation of coronavirus and paramyxovirus strains. The blue quadrant with the mixed colony of Bech-Kleinmacher as the center has a radius of 45 km and includes all colonies investigated. The base map is from the Land Registry Office of the Grand Duchy of Luxembourg. (B) Seasonality of alphacoronavirus shedding in Bech-Kleinmacher. Error bars represent the 95% confidence interval; *, P < 0.05.
TABLE 1.
Characteristics of the different colonies and of the data set, as well as detection rates of coronaviruses and paramyxoviruses
| Location of colony (UTM coord.a) | Bat species | Population size on 10/06/2016 | Sample collection date (day/mo/yr) | No. of samples (% of population) | No. (%) of bat fecal samples in which nucleic acids of the following virus(es) were detected: |
||||
|---|---|---|---|---|---|---|---|---|---|
| All CoVb,c | alphaCoVb | SARS-related CoVc | betaCoVb | Bat PVb | |||||
| Lintgen (32U 293/5511) | Myotis emarginatus | 65 | 10/06/2016 | 44 (67.7) | 8 (18.2) | 8 (18.2) | 0 (0) | 0 (0) | 0 (0) |
| Ettelbruck (32U 291/5525) | Myotis emarginatus | 220 | 10/06/2016 | 44 (20) | 1 (2.3) | 1 (2.3) | 0 (0) | 0 (0) | 1 (2.3) |
| Marienthal (32U 288/5510) | Myotis emarginatus | 45 | 03/06/2016 | 45 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bissen (32U 288/5519) | Myotis emarginatus | 35 | 03/06/2016 | 33 (94.3) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 0 (0) |
| Colpach (31U 703/5515) | Myotis emarginatus | 70 | 10/06/2016 | 44 (62.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Platen (31U 711/5519) | Myotis emarginatus | 60 | 16/06/2016 | 44 (73.3) | 2 (4.6) | 0 (0) | 0 (0) | 2 (4.6) | 0 (0) |
| Bech-Kleinmacher (32U 308/5489) | Myotis emarginatus and Rhinolophus ferrumequinum | 942 | 09/06/2015 | 100 (10.6) | 1 (1) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| 14/07/2015 | 126 (13.4) | 15 (11.9) | 15 (11.9) | 0 (0) | 0 (0) | 1 (0.8) | |||
| 04/09/2015 | 100 (10.6) | 7 (7) | 6 (6) | 0 (0) | 1 (1) | 2 (2) | |||
| 17/05/2016 | 99 (10.5) | 3 (3) | 1 (1) | 2 (2) | 0 (0) | 1 (1) | |||
| 11/07/2016 | 111 (11.8) | 5 (4.5) | 5 (4.5) | 0 (0) | 0 (0) | 4 (3.6) | |||
| 09/09/2016 | 88 (9.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Total | 1,437 | 878 (61.1) | 43 (4.9) | 36 (4.1) | 2 (0.2) | 5 (0.6) | 10 (1.1) | ||
UTM coord., Universal Transverse Mercator coordinates.
betaCoV, Betacoronavirus 1. Detected in Myotis emarginatus.
Detected in Rhinolophus ferrumequinum.
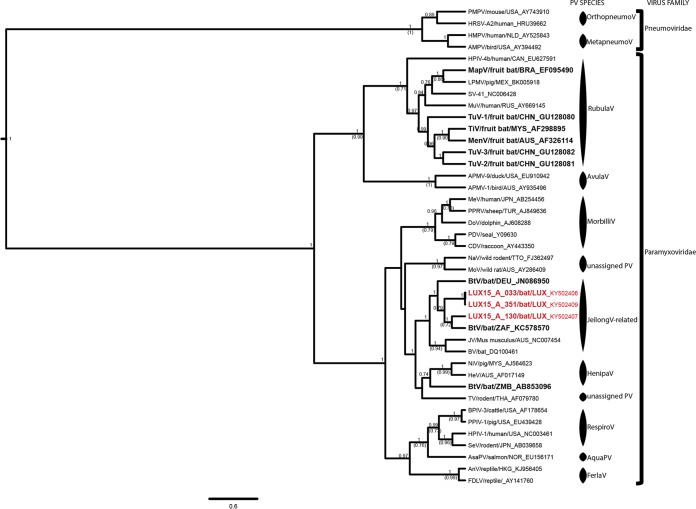
Bat PV were detected only in Ettelbruck and Bech-Kleinmacher (Fig. 1A), and shedding rates never exceeded 0.8 to 3.6% throughout the observation period. Because of the low prevalence rates, statistical analyses of seasonal variation were not possible for PV. Nine of the 10 PV strains detected were nearly identical to each other (represented by LUX15-A-033 and LUX15-A-351 in Fig. 2). BLAST and phylogenetic analyses revealed that our PV strains were most closely related to those of insectivorous bats from China and South Africa, with which they shared <80% nucleotide identity and <92% amino acid identity. Based on the phylogenetic analyses (Fig. 2), all study sequences were grouped into a well-supported cluster, comprising also the unassigned murine J virus (25), Beilong virus (35), and other Jeilong virus-related PV (2, 3).
FIG 2.
Phylogenetic analysis of the partial L genes of Paramyxoviridae. Shown are results of Bayesian analyses of a 410-nt alignment comprising the unique partial L gene sequences of 34 PV strains representing all PV species recognized by the International Committee on Taxonomy of Viruses, as well as novel unassigned but putative PV species. Three of the 10 PV from this study were added to the data set to represent the genetic diversity of PV circulating in Myotis emarginatus populations in Luxembourg. Four Pneumoviridae strains served as the outgroup for the phylogenetic analyses. The study sequences are shown in red, and strains hosted by bats are shown in boldface to reveal the high genetic diversity of bat PV. Only the pp values of well-supported nodes (pp > 0.7) are shown, and if the nodes were also supported by ML inference (bootstrap confidence levels above 0.7), the bootstrap support is shown in parentheses. For each cluster, the PV species, as well as the virus family assignment, are shown. The sequences were named, if the information was available, according to the following nomenclature: abbreviated virus name/host species/three-letter code of the country of origin_GenBank accession number. PMPV, pneumonia virus of mice; HRSV, human respiratory syncytial virus; HMPV, human metapneumovirus; AMPV, avian metapneumovirus; HPIV, human parainfluenza virus; MapV, Mapuera virus; LPMV, porcine rubulavirus; SV, simian virus; MuV, mumps virus; TuV, Tuhoko virus; TiV virus, Tioman virus; MenV, Menangle virus; APMV, avian paramyxovirus; MeV, measles virus; PPRV, peste des petits ruminants virus; DoV, dolphin morbillivirus; PDV, phocine distemper virus; CDV, canine distemper virus; NaV, Nariva virus; MoV, Mossman virus; BtV, bat paramyxovirus; JV, J virus; BV, Beilong virus; NiV, Nipah virus; HeV, Hendra virus; TV, Tupaia paramyxovirus; BPIV, bovine parainfluenza virus; PPIV, swine parainfluenza virus; HPIV, human parainfluenza virus; SeV, Sendai virus; AsaPV, Atlantic salmon paramyxovirus; AnV, anaconda paramyxovirus; FDLV, Fer-de-Lance paramyxovirus.
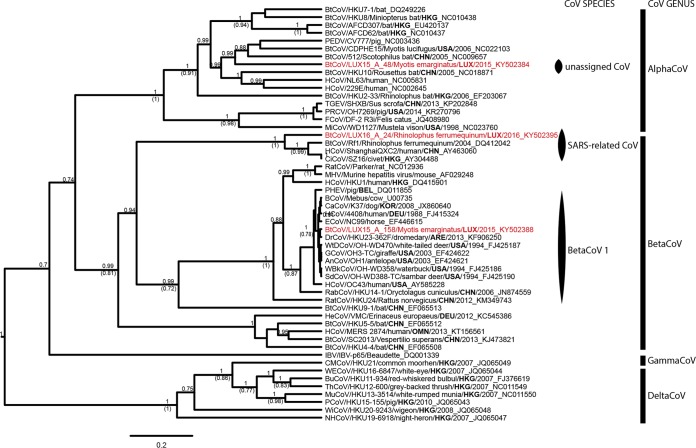
From the CoV strains detected in this study, partial RNA-dependent RNA polymerase (RdRp) gene sequences were obtained. We show that strains of 2 of the 4 currently recognized CoV genera (i.e., Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus) circulate in Luxembourg, and 36 Alphacoronavirus and 7 Betacoronavirus were detected (Fig. 3; Table 1). M. emarginatus bats from three different colonies (i.e., Ettelbruck, Lintgen, and Bech-Kleinmacher) (Fig. 1A; Table 1) shed nearly identical alphacoronaviruses (>99% nucleotide identity between partial RdRp gene sequences), most closely related to CoV circulating among insectivorous bats in China (Fig. 3). In contrast to the PV shedding, Alphacoronavirus shedding was variable in Bech-Kleinmacher; the highest rates were observed in July after parturition (odds ratio [OR], 2.8; P < 0.01) (Fig. 1B). Alphacoronavirus strains from this study (represented by LUX15-A-48 in Fig. 3) formed a distinct cluster, and their RdRp gene sequences shared <86% amino acid identity with other sequences classified as Alphacoronavirus.
FIG 3.
Phylogenetic analysis of the partial RNA-dependent RNA polymerase genes of all CoV genera. Shown are results of Bayesian analyses of an 853-nt alignment comprising unique partial RNA-dependent RNA polymerase gene sequences of 50 CoV strains representing all CoV species recognized by the International Committee on Taxonomy of Viruses (ICTV), as well as novel unassigned but putative CoV species. Three of the 43 CoV from this study were added to the data set to represent the genetic diversity of CoV circulating in Myotis emarginatus and Rhinolophus ferrumequinum populations in Luxembourg. The deltaCoV strains served as the outgroup for the phylogenetic analyses. Only the pp values of well-supported nodes (pp > 0.7) are shown, and if the nodes were also supported by ML inference (bootstrap confidence levels above 0.7), the bootstrap support is shown in parentheses. For each strain, the CoV genus assignment is shown. Assignment to recognized ICTV species is shown only for the study sequences displayed in red. A phylogenetic tree highlighting the CoV species of every strain can be found in the supplemental material (Fig. S3). The code for the country of origin of each strain is shown in boldface to stress the vast geographic spread of CoV. The sequences were named, if the information was available, according to the following nomenclature: abbreviated virus name/virus strain/host species/three-letter code of the country of origin/year of sampling_GenBank accession number. BtCoV, bat coronavirus; PEDV, porcine epidemic diarrhea virus; HCoV, human coronavirus; TGEV, transmissible gastroenteritis virus; PRCV, porcine respiratory coronavirus; FCoV, feline coronavirus; MiCoV, mink coronavirus; CiCoV, civet severe acute respiratory syndrome CoV; RatCoV, rat coronavirus; MHV, murine hepatitis virus; PHEV, porcine hemagglutinating encephalomyelitis virus; BCoV, bovine coronavirus; CaCoV, canine respiratory coronavirus; ECoV, equine coronavirus; DrCoV, dromedary camel coronavirus; WtDCoV, white-tailed deer coronavirus; GCoV, giraffe coronavirus; AnCoV, sable antelope coronavirus; WBkCoV, waterbuck coronavirus; SdCoV, Sambar deer coronavirus; RabCoV, rabbit coronavirus; HeCoV, hedgehog coronavirus; IBV, infectious bronchitis virus; CMCoV, common moorhen coronavirus; WECoV, wigeon coronavirus; BuCoV, bulbul coronavirus; ThCoV, thrush coronavirus; MuCoV, munia coronavirus; PCoV, porcine coronavirus; WiCoV, white-eye coronavirus; NHCoV, night heron coronavirus.
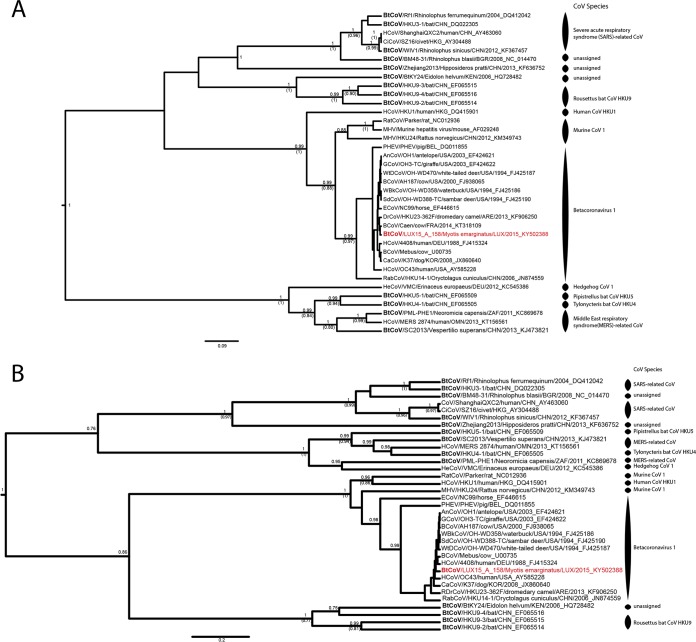
On the phylogenetic tree of the partial RdRp gene, the Betacoronavirus strains from this study clustered within 2 of the 4 recognized lineages (A to D) of Betacoronavirus (https://talk.ictvonline.org): R. ferrumequinum bats shed SARS-related CoV (lineage B, represented by LUX16-A-24 in Fig. 3), and M. emarginatus bats shed Betacoronavirus 1 (lineage A, represented by LUX15-A-158 in Fig. 3). The SARS-related CoV from Bech-Kleinmacher were identical to each other, and BLAST analyses revealed 94% nucleotide identity between partial RdRp gene sequences from this study and SARS-related CoV circulating among rhinolophid bats in Europe (Fig. 1A and 3). Besides, we detected the first bat Betacoronavirus 1 strains (n = 5) in M. emarginatus bats from 3 different colonies in 2015 (Bech-Kleinmacher) and 2016 (Bissen and Platen) (Fig. 1A; Table 1). All strains from Luxembourg were highly similar to each other and to Betacoronavirus 1 strains identified in various mammalian species (>99% nucleotide identity between partial RdRp gene sequences) (Fig. 3 and 4).
FIG 4.
Phylogenetic analysis of the partial RNA-dependent RNA polymerase genes (A) and partial spike glycoprotein genes (B) of betacoronaviruses. Shown are results of Bayesian analyses of a 1,771-nt alignment comprising unique partial RNA-dependent RNA polymerase gene sequences of 38 CoV strains (A) and of a 911-nt alignment comprising unique partial spike glycoprotein gene sequences of 34 CoV strains representing all Betacoronavirus species recognized by the International Committee on Taxonomy of Viruses (ICTV). In addition, 1 of the 5 highly similar Betacoronavirus 1 strains from this study was added to the data set to show the genetic relationship of Betacoronavirus 1 strains circulating in Myotis emarginatus populations in Luxembourg to Betacoronavirus 1 strains of other host species. Only the pp values of well-supported nodes (pp > 0.7) are shown, and if the nodes were also supported by ML inference (bootstrap confidence levels above 0.7), the bootstrap support is shown in parentheses. Assignment to recognized ICTV species is shown for each strain. The study sequence is shown in red, and strains that were detected in bats are displayed in boldface to stress that most Betacoronavirus 1 species comprise CoV strains that were initially detected in bats. The sequences were named, if the information was available, according to the following nomenclature: abbreviated virus name/virus strain/host species/three-letter code of the country of origin/year of sampling_GenBank accession number. BtCoV, bat coronavirus; HCoV, human coronavirus; CiCoV, civet severe acute respiratory syndrome CoV; RatCoV, rat coronavirus; MHV, murine hepatitis virus; PHEV, porcine hemagglutinating encephalomyelitis virus; AnCoV, sable antelope coronavirus; GCoV, giraffe coronavirus; WtDCoV, white-tailed deer coronavirus; BCoV, bovine coronavirus; WBkCoV, waterbuck coronavirus; SdCoV, Sambar deer coronavirus; ECoV, equine coronavirus; DrCoV, dromedary camel coronavirus; CaCoV, canine respiratory coronavirus; RabCoV, rabbit coronavirus; HeCoV, hedgehog coronavirus.
Sequencing of the partial spike gene was attempted for all novel bat CoV strains but was successful only for the Betacoronavirus 1 strains. As with the RdRp gene, all spike gene sequences were highly similar to each other and shared >98% nucleotide identity with the Betacoronavirus 1 strains from other mammalian species (Fig. 4B).
DISCUSSION
Bats are natural reservoirs of numerous viruses with zoonotic potential. Of particular interest are CoV and PV, which share several traits allowing their adaptation to new ecological niches and hosts: high mutation rates, poor RNA proofreading capability, and genetic recombination (36–38). In line with previous studies (see, e.g., references 3, 4, and 23), we found genetically diverse CoV and PV strains in bats that are known to forage in and around human settlements in Luxembourg (Fig. 2 to 4; Table 1). Shedding rates may have been underestimated due to RNA degradation, low viral loads in feces (39), and the reduced sensitivity of degenerate primers. However, the sample collection and processing protocol was optimized to minimize the degradation of viral particles and of RNA, as well as inhibition. We acknowledge that the adenovirus control did not control for inhibition during the reverse transcription step. Although relatively susceptible to PCR inhibition and RNA degradation, fecal samples have been systematically used to investigate virus epidemiology and evolution previously (40–43). Moreover, feces are collected noninvasively and are thus the preferred material for studying viruses circulating among these endangered species (44, 45).
Plowright et al. proposed three scenarios to explain temporal variations in virus shedding in bats: (i) virus reactivation in persistently infected bats, (ii) seasonal epidemic cycles aligning with the physiology of the bats' life cycle, or (iii) transient epidemics due to waning immunity (19, 46). In agreement with a previous study (4), we observed no temporal variation in PV shedding, possibly because of its low prevalence. In contrast, and in line with another study (40), a significant increase in Alphacoronavirus shedding was found in July, possibly due to periparturient stress (40, 47) (Fig. 1B).
The lack of similar reference sequences complicated the genetic and phylogenetic characterization of the virus strains detected. Nevertheless, we identified novel PV and Alphacoronavirus strains that are related to bat viruses from distant regions of the world (Fig. 2 and 3). Also, according to the PV species discrimination criterion published previously (amino acid distance in the L gene, >7 to 7.5% in the L gene) (4), the study sequences may represent putative novel PV strains, but this finding needs confirmation by whole-genome sequencing. Through amino acid sequence analysis of the partial RdRp gene, the topologies of the phylogenetic trees, and BLASTn analyses, the new CoV obtained in this study were found to be sufficiently divergent to represent a novel RdRp-based grouping unit (RGU) (5, 23). We found no evidence of interspecies transmission, although a mixed-species colony was monitored for 2 years (Fig. 1). Taken together, these findings confirm previous studies suggesting an association between Alphacoronavirus and host taxa rather than between geography and viral evolution, and thus close virus-host coevolution (23, 48–50).
On the other hand, the detection of highly similar virus strains in different colonies (Fig. 1A; Table 1) is indicative of a social link between M. emarginatus colonies in Luxembourg. This is of particular interest with respect to ongoing efforts for the conservation of this species. Indeed, short foraging distances (26) and lifelong roost fidelity complicate the preservation of M. emarginatus (51, 52). Since migratory distances of 35 to 126 km between summer and winter roosts have been reported (51, 52), and since all Luxembourgish colonies are within 45 km of each other (Fig. 1A), bats from different colonies may assemble during the autumn swarming of the males (32, 53). Thus, male bats may play a particular role in virus transmission, which warrants further investigation. A better understanding of the dynamics of bat-associated viruses may indirectly benefit these endangered species by providing information about foraging and mating behavior.
In contrast to the pattern of Alphacoronavirus evolution, host switching is a major evolutionary mechanism of Betacoronavirus 1. For instance, SARS-CoV and MERS-CoV circulated in bats before crossing the species barrier to infect an intermediate host, which, in turn, infected humans (8, 13, 54, 55). Bat SARS-CoV even use the same receptor for cell entry as their human counterparts, and they have been detected in rhinolophid bats (8), which also host genetically diverse SARS-related CoV (23, 56–59). Also in our study, R. ferrumequinum from Bech-Kleinmacher shed SARS-related CoV strains (Fig. 3). Although it is unlikely that these CoV represent a direct threat to humans, the potential risk of adaptation to the human host should not be ignored (60–62). The Betacoronavirus 1 species is another exception to the typical host specificity of CoV. This species comprises highly similar viruses of distantly related mammals (6, 63–66), and so far, only a single, short Betacoronavirus 1 sequence has been obtained from a bat (10). Most interestingly, we show here that M. emarginatus bats from different roosts shed Betacoronavirus 1 strains (Fig. 1A) that are highly similar and closely related to Betacoronavirus 1 strains detected in various other animal species (Fig. 4). Most-recent-common-ancestor analyses of Betacoronavirus 1 suggested that the group appeared only recently and has low host specificity (67–69). For example, Betacoronavirus 1 strains detected in exotic ruminants such as giraffes or antelopes are thought to represent spillover viruses of bovine CoV that underwent adaptive mutations (63, 65). Moreover, a possible animal origin of human CoV (HCoV) OC43 has been revealed by molecular clock analysis of the spike gene (68, 69), which provides an indication of host range and tissue tropism. The permissiveness of human cells to certain Betacoronavirus 1 strains further underlines the potential of these strains to be transmitted across species (65, 67). Also in this study, all spike gene sequences were highly similar to Betacoronavirus 1 sequences from other mammalian species, reflecting the genetic stability typical of the lineage (63, 70, 71). To further investigate the role of bats as a reservoir of betacoronavirus 1, studies focusing on the host range of this CoV species are warranted.
In conclusion, we have shown that bats in Luxembourg, Western Europe, are hosts of novel virus strains that may be able to overcome the species barrier. Betacoronavirus 1 strains with spike and RdRp genes genetically highly similar to those of mammalian strains were detected in synanthropic bats. In addition, we identified SARS-related CoV that may infect humans after a viral adaptation process (60–62). As shown before for bat lyssaviruses (34), our study highlights a certain risk for zoonotic transmission of bat viruses in particular, since the foraging and roosting sites of most indigenous bat species overlap with human and animal habitats. To mitigate this risk, it is important to monitor viruses circulating in synanthropic bats and putative intermediate hosts and to identify factors that affect bat populations.
MATERIALS AND METHODS
Samples.
In 2015 and 2016, fecal samples (n = 624) were collected from a mixed R. ferrumequinum–M. emarginatus nursing colony in Bech-Kleinmacher, using a longitudinal approach. Samples were collected (i) after the resettling of the colony in the summer roost and before the birth of the juveniles (June 2015 [n = 100]; May 2016 [n = 99]), (ii) during lactation (July 2015 [n = 126]; June 2016 [n = 111]), and (iii) before the colony returned to the winter roost (September 2015 [n = 100]; September 2016 [n = 88]). In 2016, in the framework of a cross-sectional study, fecal samples (n = 254) were collected from 6 of the 14 synanthropic M. emarginatus colonies known in Luxembourg (Table 1; Fig. 1). At the beginning of June 2016 and before the birth of the juveniles, the population size of every known M. emarginatus maternity colony in Luxembourg (Table 1; Fig. 1A) was assessed by counting the bats emerging from the roost and/or the bats from a photograph taken in the roost, according to the Guidelines for Surveillance and Monitoring of European Bats (72).
The monitoring and sample collection were approved by the Ministry of Sustainable Development and Infrastructure Luxembourg (reference no. 86503 CG/ne).
Fresh feces were collected on a clean tarpaulin (left for 2 to 12 h underneath the roost) and were individually placed in 2-ml tubes using single-use spatulas. Samples were kept at +4°C during transport to the laboratory, where they were directly processed. The bat species was identified by visual inspection of the feces and of the bat cluster hanging above the collection site. Species identification was confirmed for virus-positive samples by sequencing of mitochondrial DNA (see below).
The study data set is described in Table 1, and the primer sequences can be found in Table 2.
TABLE 2.
Primers used for detection and sequencing of coronaviruses and paramyxoviruses
| Virus | Target protein | Primer sense | Primer sequence (5′–3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| Detection | |||||
| Paramyxoviridae | L protein, subunit of RNA-dependent RNA polymerase | Forward | GAAGGITATTGTCAIAARNTNTGGAC | 660 | 76 |
| Reverse | GCTGAAGTTACIGGITCICCDATRTTNC | ||||
| Forward seminested PCR | GTTGCTTCAATGGTTCARGGNGAYAA | 580 | |||
| Coronaviridae | Replicase polyprotein 1ab | Forward | GGKTGGGAYTAYCCKAARTG | 602 | 75 |
| Reverse | TGYTGTSWRCARAAYTCRTG | ||||
| Forward nested PCR | GGTTGGGACTATCCTAAGTGTGA | 555 | |||
| Reverse nested PCR | CCAACAYTTNGARTCWGCCAT | This study | |||
| Human adenovirus | Hexon gene | Forward | GCCACSGTGGGGTTYCTAAACTT | 130 | This study |
| Reverse | GCCSCAGTGGKCDTACATGCACATC | This study | |||
| Probe | FAM-TGCACCAGACCCGGGCTCAGGTACTCCGA-TAMRA | 74 | |||
| Sequencing | |||||
| Alphacoronavirus | Replicase polyprotein 1ab | Forward | TGTGAAGGCCTTACAGCGTC | 670 | This study |
| Reverse | AGAGCCACAWACAACACACA | ||||
| Replicase polyprotein 1ab | Forward | TGATGCAGCTGTYARAGACTTC | 690 | This study | |
| Reverse | CCAGAAGTCGTACCACCAGG | ||||
| Betacoronavirus 1 | Replicase polyprotein 1ab | Forward | AGACATCGTCCCCATCCATC | 729 | This study |
| Reverse | AGCTACACGTGGTGTTCCTG | ||||
| Replicase polyprotein 1ab | Forward | CATATCATCCCAGCCGCCAT | 584 | This study | |
| Reverse | TGCTGTTTTAGTGTTGCGGC | ||||
| Replicase polyprotein 1ab | Forward | CCGCTTGTTATAGCCGCAAC | 613 | This study | |
| Reverse | AGCGCTACTGAGTTTGCAGA | ||||
| Spike gene | Forward | GTGAGCACTGTTCGGGTCTT | 432 | This study | |
| Reverse | AGCAATGCTGGTTCGGAAGA | ||||
| Spike gene | Forward | ATGGCATTGGGATACAG | 492 | 65 | |
| Reverse | TAATGGAGAGGGCACCGACTT | ||||
| Spike gene | Forward | GGGTTACACCTCTCACTTCT | 767 | 65 | |
| Reverse | GCAGGACAAGTGCCTATACC | ||||
| SARS-related coronavirus | Replicase polyprotein 1ab | Forward | AGTTGAGGTGGTCGACAAGT | 650 | This study |
| Reverse | GCAGTGGTAGCATCTCCTGA | ||||
| Bat species identification | Cytochrome b | Forward | ATGACCAACATTCGMAARTCYCAC | 390 | This study |
| Reverse | TGATGACGGTTGCTCCTCA |
Nucleic acid extraction.
Entire bat droppings (approximately the size of a long grain of rice) were individually resuspended in 1 ml of prechilled virus transport medium (prepared according to the WHO protocol [73]) and were homogenized using stainless steel beads (Qiagen, Venlo, The Netherlands) and a TissueLyser II system (Qiagen).
After centrifugation at 2,200 × g for 20 min, the supernatant was transferred to a new 2-ml tube and was stored at −80°C until further processing. Before nucleic acid extraction, each sample was centrifuged at 2,200 × g for 10 min and was spiked with an extraction control (i.e., human adenovirus C5). Concurrent extraction of DNA and RNA was performed with the QIAamp viral RNA minikit (Qiagen) according to the manufacturer's protocol. To test for inhibition and to confirm successful extraction, each sample was tested using a real-time PCR specific for adenovirus (74).
Virus detection.
All samples were tested for CoV and PV by reverse transcription-PCRs (RT-PCRs) with degenerate primers in a nested format. The PCRs were performed in a final volume of 25 μl. In the first step of the nested PCR, the Qiagen One-Step RT-PCR kit (Qiagen) was used. The CoV PCR master mix contained 2 μl of RNA, 1 μM each primer, 1 mM MgCl2, and 1 mM each deoxynucleoside triphosphate (dNTP), and the PV PCR master mix contained 250 nM each primer, 1.5 mM MgCl2, and 100 μM each dNTP. In the second step of the nested PCRs, the CoV PCR master mix contained 2.5 μl of 1:5-diluted PCR product, 700 nM each primer, 2 mM MgCl2, and 200 μM each dNTP, whereas the PV PCR master mix contained 0.1 μl of undiluted PCR product, 600 nM each primer, 2 mM MgCl2, and 200 μM each dNTP. The adenovirus detection PCR was similar to the CoV PCR, but 2.5 μl of DNA was used and 560 nM probe was added to the mix. In the second step of the nested PCRs, in the adenovirus detection PCR, and in the bat species identification PCR, the Platinum Taq DNA polymerase kit (Life Technologies Europe B.V., Ghent, Belgium) was used. The CoV primers target the RNA-dependent RNA polymerase (RdRp) (modified from reference 75), whereas the PV primers target the L genes (76) of all known strains of the respective viral families. An avian infectious bronchitis virus (an avian CoV) and a measles virus (a human PV) served as positive controls in the CoV and PV PCRs. Details about the primers can be found in Table 2.
Sequencing.
PCR-positive samples were identified by agarose gel electrophoresis. Where multiple bands were present, amplicons of the appropriate size were excised from the gel and were purified with the QIAquick gel extraction kit (Qiagen). PCR products giving a single band in the gel electrophoresis were directly purified using the JetQuick extraction kit (GenoMed, Löhne, Germany). Sequencing was performed using the BigDye Terminator kit (Applied Biosystems, Foster City, CA), run on an ABI 3130 sequencer (Applied Biosystems). Partial L gene sequences of PV were obtained using the detection primers. Partial sequencing of CoV was attempted using specific primers targeting the conserved RdRp gene, as well as the spike glycoprotein gene. To reliably identify the bat species of all virus-positive samples, partial cytochrome b sequences were obtained. The bat species identification PCR was performed using the Platinum Taq DNA polymerase kit (Life Technologies Europe B.V.) in a final volume of 25 μl containing 5 μl of DNA, 700 nM each primer, 4 mM MgCl2, and 400 μM each dNTP. New primer sets were designed and evaluated with Geneious software (version 7.1.7; Biomatters Limited, Auckland, New Zealand) and Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/). Details about the sequencing primers can be found in Table 2.
Sequence and phylogenetic analyses.
Sequence assembly and processing were performed in Geneious, version 7.1.9 (77). A BLASTn search against the sequences in GenBank (https://www.ncbi.nlm.nih.gov/GenBank/) was performed with the default parameters. Phylogenetic trees based on nucleotide sequences of the partial RdRp and spike genes for CoV, and of the partial L gene for PV, were constructed. In order to increase the phylogenetic resolution and because of the high genetic similarity of the virus strains obtained, only the longest sequences of high quality were selected among the novel sequences and were aligned with representative GenBank sequences using the ClustalW algorithm (78), as implemented in Geneious. Phylogenetic trees based on all study sequences of good quality are shown in the supplemental material (Fig. S1 and S3). Poorly aligned positions in the alignments were eliminated using Gblocks (79) as implemented in Seaview, version 4 (80). Maximum likelihood (ML) and Bayesian inference of evolution were estimated in PhyML (81, 82) and BEAST (83, 84), respectively. The best substitution model identified by jModelTest (85) according to the Bayesian information criterion (BIC) and/or Akaike information criterion (AIC) values was used. A bootstrap test including 1,000 replicates was performed for each ML tree. For the Bayesian Markov chain Monte Carlo (MCMC) approach, the parametric model “Constant Size” was used as the prior, and the analyses were performed with a lognormal relaxed clock. The MCMC run was at least 2 × 107 steps long, with sampling every 103 steps. Convergence was assessed on the basis of the effective sampling size using Tracer, version 1.6 (86). The results of the Bayesian phylogenetic inference were summarized in a maximum clade credibility tree using the Tree Annotator program after a 10% burn-in. Tree topology was tested by posterior probability (pp), and only the pp values of well-supported nodes (pp > 0.7) are shown in the figures. Since the topologies of the trees based on Bayesian and ML inference largely overlapped, only the maximum clade credibility trees are shown. However, for the nodes also supported by ML inference (bootstrap confidence levels above 0.7), the bootstrap support is shown in parentheses in the figures. The scale bar for each tree indicates the average number of nucleotide substitutions per site (Fig. 2 to 4).
Statistical analyses.
Statistical analyses were performed in R software (version 3.1.0.; R Foundation for Statistical Computing, Vienna, Austria [https://www.r-project.org/]) (24). Logistic regression was performed to predict the binary outcome (i.e., the presence or absence of detectable Alphacoronavirus shedding by M. emarginatus) based on the categorical predictor “season” with the levels “May/June,” “July,” and “September” and using a logistic function.
Accession number(s).
The viral and mitochondrial sequences obtained in this study have been submitted to GenBank under accession numbers KY502383 to KY502414, as well as KY707827 and MF048874 to MF048903.
Supplementary Material
ACKNOWLEDGMENTS
We thank the many people who contributed significantly to the success of this study by providing logistical support or by supervising or performing the sample collection. In this context, we mention in particular the investigators of a research project on the genetics of M. emarginatus cofinanced by the Ministry of Sustainable Development and Infrastructure (MDDI, Environment Department) and the Natural History Museum of Luxembourg: Simone Schneider and Mara Lang of the Biological Station (SICONA, Luxembourg), as well as Alain Frantz of the Centre de Recherche Scientifique (Musée National d'Histoire Naturelle, Luxembourg). We are also grateful to the owners of the buildings containing the bat roosting sites for approving this study, as well to Martyna Marynowska and Claire Dording for performing part of the laboratory analyses.
This study was funded by the Ministry of Foreign and European Affairs, Luxembourg (project “Microbiology for development IV”), which was not involved in study design, data collection and interpretation, or the decision to submit the work for publication. We declare that we have no conflict of interest relevant to the study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01326-17.
REFERENCES
- 1.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan L, Li M, Li L, Monagin C, Chmura AA, Schneider BS, Epstein JH, Mei X, Shi Z, Daszak P, Chen J. 2014. Evidence for retrovirus and paramyxovirus infection of multiple bat species in China. Viruses 6:2138–2154. doi: 10.3390/v6052138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurth A, Kohl C, Brinkmann A, Ebinger A, Harper JA, Wang LF, Muhldorfer K, Wibbelt G. 2012. Novel paramyxoviruses in free-ranging European bats. PLoS One 7:e38688. doi: 10.1371/journal.pone.0038688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drexler JF, Corman VM, Muller MA, Maganga GD, Vallo P, Binger T, Gloza-Rausch F, Cottontail VM, Rasche A, Yordanov S, Seebens A, Knornschild M, Oppong S, Adu Sarkodie Y, Pongombo C, Lukashev AN, Schmidt-Chanasit J, Stocker A, Carneiro AJ, Erbar S, Maisner A, Fronhoffs F, Buettner R, Kalko EK, Kruppa T, Franke CR, Kallies R, Yandoko ER, Herrler G, Reusken C, Hassanin A, Kruger DH, Matthee S, Ulrich RG, Leroy EM, Drosten C. 2012. Bats host major mammalian paramyxoviruses. Nat Commun 3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drexler JF, Corman VM, Drosten C. 2014. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res 101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. J Virol 86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B, Ge X, Wang LF, Shi Z. 2015. Bat origin of human coronaviruses. Virol J 12:221. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge X-Y, Li J-L, Yang X-L, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang Y-J, Luo C-M, Tan B, Wang N, Zhu Y, Crameri G, Zhang S-Y, Wang L-F, Daszak P, Shi Z-L. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memish ZA, Cotten M, Meyer B, Watson SJ, Alsahafi AJ, Al Rabeeah AA, Corman VM, Sieberg A, Makhdoom HQ, Assiri A, Al Masri M, Aldabbagh S, Bosch BJ, Beer M, Muller MA, Kellam P, Drosten C. 2014. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis 20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, Alhakeem R, Durosinloun A, Al Asmari M, Islam A, Kapoor A, Briese T, Daszak P, Al Rabeeah AA, Lipkin WI. 2013. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis 19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enserink M. 2000. Emerging diseases. Malaysian researchers trace Nipah virus outbreak to bats. Science 289:518–519. [DOI] [PubMed] [Google Scholar]
- 12.Halpin K, Young PL, Field HE, Mackenzie JS. 2000. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 13.Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 14.Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, Homaira N, Rota PA, Rollin PE, Comer JA, Kenah E, Ksiazek TG, Rahman M. 2009. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis 15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu G, Wang Q, Gao GF. 2015. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol 23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. 2005. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A 102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, Middleton D, Reid PA, McFarlane RA, Martin G, Tabor GM, Skerratt LF, Anderson DL, Crameri G, Quammen D, Jordan D, Freeman P, Wang LF, Epstein JH, Marsh GA, Kung NY, McCallum H. 2015. Ecological dynamics of emerging bat virus spillover. Proc Biol Sci 282:20142124. doi: 10.1098/rspb.2014.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl C, Kurth A. 2014. European bats as carriers of viruses with zoonotic potential. Viruses 6:3110–3128. doi: 10.3390/v6083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schatz J, Ohlendorf B, Busse P, Pelz G, Dolch D, Teubner J, Encarnacao JA, Muhle RU, Fischer M, Hoffmann B, Kwasnitschka L, Balkema-Buschmann A, Mettenleiter TC, Muller T, Freuling CM. 2014. Twenty years of active bat rabies surveillance in Germany: a detailed analysis and future perspectives. Epidemiol Infect 142:1155–1166. doi: 10.1017/S0950268813002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picard-Meyer E, Robardet E, Arthur L, Larcher G, Harbusch C, Servat A, Cliquet F. 2014. Bat rabies in France: a 24-year retrospective epidemiological study. PLoS One 9:e98622. doi: 10.1371/journal.pone.0098622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drexler JF, Gloza-Rausch F, Glende J, Corman VM, Muth D, Goettsche M, Seebens A, Niedrig M, Pfefferle S, Yordanov S, Zhelyazkov L, Hermanns U, Vallo P, Lukashev A, Muller MA, Deng H, Herrler G, Drosten C. 2010. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol 84:11336–11349. doi: 10.1128/JVI.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Development Core Team. 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 25.Jack PJ, Boyle DB, Eaton BT, Wang LF. 2005. The complete genome sequence of J virus reveals a unique genome structure in the family Paramyxoviridae. J Virol 79:10690–10700. doi: 10.1128/JVI.79.16.10690-10700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietz M, Pir JB, Hillen J. 2013. Does the survival of greater horseshoe bats and Geoffroy's bats in Western Europe depend on traditional cultural landscapes? Biodivers Conserv 22:3007–3025. doi: 10.1007/s10531-013-0567-4. [DOI] [Google Scholar]
- 27.Topal G. 2001. Myotis emarginatus (Geoffroy, 1806)—Wimperfledermaus. In Niethammer J, Krapp F (ed), Handbuch der Säugetiere Europas, vol 4, p 369–404. Aula-Verlag, Wiesbaden, Germany. [Google Scholar]
- 28.Dietz C, Nill D, von Helversen O. 2016. Handbuch der Fledermäuse: Europa und Nordwestafrika, 2nd ed Kosmos Verlag, Stuttgart, Germany. [Google Scholar]
- 29.Schwaab F, Knochel A, Jouan D. 2009. Connaître et protéger les chauves-souris de Lorraine. CPEPESC Lorraine, Strasbourg, France. [Google Scholar]
- 30.Rossiter SJ, Ransome RD, Faulkes CG, Le Comber SC, Jones G. 2005. Mate fidelity and intra-lineage polygyny in greater horseshoe bats. Nature 437:408–411. doi: 10.1038/nature03965. [DOI] [PubMed] [Google Scholar]
- 31.Flanders J, Jones G, Benda P, Dietz C, Zhang S, Li G, Sharifi M, Rossiter SJ. 2009. Phylogeography of the greater horseshoe bat, Rhinolophus ferrumequinum: contrasting results from mitochondrial and microsatellite data. Mol Ecol 18:306–318. doi: 10.1111/j.1365-294X.2008.04021.x. [DOI] [PubMed] [Google Scholar]
- 32.Burns LE, Frasier TR, Broders HG. 2014. Genetic connectivity among swarming sites in the wide ranging and recently declining little brown bat (Myotis lucifugus). Ecol Evol 4:4130–4149. doi: 10.1002/ece3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossiter SJ, Jones G, Ransome RD, Barratt EM. 2000. Parentage, reproductive success and breeding behaviour in the greater horseshoe bat (Rhinolophus ferrumequinum). Proc Biol Sci 267:545–551. doi: 10.1098/rspb.2000.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Servat A, Herr J, Picard-Meyer E, Schley L, Harbusch C, Michaux C, Pir J, Robardet E, Engel E, Cliquet F. 2015. First isolation of a rabid bat infected with European bat lyssavirus in Luxembourg. Zoonoses Public Health 62:7–10. doi: 10.1111/zph.12095. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Yu M, Zhang H, Magoffin DE, Jack PJ, Hyatt A, Wang HY, Wang LF. 2006. Beilong virus, a novel paramyxovirus with the largest genome of non-segmented negative-stranded RNA viruses. Virology 346:219–228. doi: 10.1016/j.virol.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Woo PC, Lau SK, Huang Y, Yuen KY. 2009. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med 234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 37.Moya A, Holmes EC, Gonzalez-Candelas F. 2004. The population genetics and evolutionary epidemiology of RNA viruses. Nat Rev Microbiol 2:279–288. doi: 10.1038/nrmicro863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitchen A, Shackelton LA, Holmes EC. 2011. Family level phylogenies reveal modes of macroevolution in RNA viruses. Proc Natl Acad Sci U S A 108:238–243. doi: 10.1073/pnas.1011090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edson D, Field H, McMichael L, Vidgen M, Goldspink L, Broos A, Melville D, Kristoffersen J, de Jong C, McLaughlin A, Davis R, Kung N, Jordan D, Kirkland P, Smith C. 2015. Routes of Hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS One 10:e0140670. doi: 10.1371/journal.pone.0140670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Seebens A, Muller MA, Drosten C. 2011. Amplification of emerging viruses in a bat colony. Emerg Infect Dis 17:449–456. doi: 10.3201/eid1703.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goffard A, Demanche C, Arthur L, Pincon C, Michaux J, Dubuisson J. 2015. Alphacoronaviruses detected in French bats are phylogeographically linked to coronaviruses of European bats. Viruses 7:6279–6290. doi: 10.3390/v7122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrardy C, Tao Y, Kuzmin IV, Niezgoda M, Agwanda B, Breiman RF, Anderson LJ, Rupprecht CE, Tong S. 2014. Molecular detection of adenoviruses, rhabdoviruses, and paramyxoviruses in bats from Kenya. Am J Trop Med Hyg 91:258–266. doi: 10.4269/ajtmh.13-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YN, Phuong VN, Chen HC, Chou CH, Cheng HC, Wu CH. 2016. Detection of the severe acute respiratory syndrome-related coronavirus and alphacoronavirus in the bat population of Taiwan. Zoonoses Public Health 63:608–615. doi: 10.1111/zph.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piraccini R, Hutson AM, Spitzenberger F, Aulagnier S, Nagy Z. 2016. Myotis emarginatus. The IUCN Red List of Threatened Species 2016:e.T14129A22051191. doi: 10.2305/IUCN.UK.2016-2.RLTS.T14129A22051191.en. [DOI] [Google Scholar]
- 45.Piraccini R, Aulagnier S, Hutson AM, Spitzenberger F, Juste J, Karataş A, Palmeirim J, Paunović M. 2016. Rhinolophus ferrumequinum. The IUCN Red List of Threatened Species 2016:e.T19517A21973253. doi: 10.2305/IUCN.UK.2016-2.RLTS.T19517A21973253.en. [DOI] [Google Scholar]
- 46.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, Restif O. 2016. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir-host populations. PLoS Negl Trop Dis 10:e0004796. doi: 10.1371/journal.pntd.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turmelle AS, Allen LC, Jackson FR, Kunz TH, Rupprecht CE, McCracken GF. 2010. Ecology of rabies virus exposure in colonies of Brazilian free-tailed bats (Tadarida brasiliensis) at natural and man-made roosts in Texas. Vector Borne Zoonotic Dis (Larchmont, NY) 10:165–175. doi: 10.1089/vbz.2008.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer K, Zeus V, Kwasnitschka L, Kerth G, Haase M, Groschup MH, Balkema-Buschmann A. 2016. Insectivorous bats carry host specific astroviruses and coronaviruses across different regions in Germany. Infect Genet Evol 37:108–116. doi: 10.1016/j.meegid.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vidgen ME, de Jong C, Rose K, Hall J, Field HE, Smith CS. 2015. Novel paramyxoviruses in Australian flying-fox populations support host-virus co-evolution. J Gen Virol 96:1619–1625. doi: 10.1099/vir.0.000099. [DOI] [PubMed] [Google Scholar]
- 50.Mortlock M, Kuzmin IV, Weyer J, Gilbert AT, Agwanda B, Rupprecht CE, Nel LH, Kearney T, Malekani JM, Markotter W. 2015. Novel paramyxoviruses in bats from sub-Saharan Africa, 2007–2012. Emerg Infect Dis 21:1840–1843. doi: 10.3201/eid2110.140368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arthur L, Lemaire M. 2009. Les chauves-souris de France, Belgique, Luxembourg et Suisse. Musée National d'Histoire Naturelle, Paris, France. [Google Scholar]
- 52.Dietz C, Kiefer A. 2016. Bats of Britain and Europe, 1st ed Bloomsbury Natural History, London, United Kingdom. [Google Scholar]
- 53.van Schaik J, Janssen R, Bosch T, Haarsma AJ, Dekker JJ, Kranstauber B. 2015. Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PLoS One 10:e0130850. doi: 10.1371/journal.pone.0130850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. 2014. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reusken CB, Ababneh M, Raj VS, Meyer B, Eljarah A, Abutarbush S, Godeke GJ, Bestebroer TM, Zutt I, Muller MA, Bosch BJ, Rottier PJ, Osterhaus AD, Drosten C, Haagmans BL, Koopmans MP. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill 18:20662. doi: 10.2807/1560-7917.ES2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- 56.Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 57.Yuan J, Hon CC, Li Y, Wang D, Xu G, Zhang H, Zhou P, Poon LL, Lam TT, Leung FC, Shi Z. 2010. Intraspecies diversity of SARS-like coronaviruses in Rhinolophus sinicus and its implications for the origin of SARS coronaviruses in humans. J Gen Virol 91:1058–1062. doi: 10.1099/vir.0.016378-0. [DOI] [PubMed] [Google Scholar]
- 58.Balboni A, Palladini A, Bogliani G, Battilani M. 2011. Detection of a virus related to betacoronaviruses in Italian greater horseshoe bats. Epidemiol Infect 139:216–219. doi: 10.1017/S0950268810001147. [DOI] [PubMed] [Google Scholar]
- 59.Ren W, Li W, Yu M, Hao P, Zhang Y, Zhou P, Zhang S, Zhao G, Zhong Y, Wang S, Wang LF, Shi Z. 2006. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J Gen Virol 87:3355–3359. doi: 10.1099/vir.0.82220-0. [DOI] [PubMed] [Google Scholar]
- 60.Menachery VD, Yount BL Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS. 2015. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menachery VD, Yount BL Jr, Sims AC, Debbink K, Agnihothram SS, Gralinski LE, Graham RL, Scobey T, Plante JA, Royal SR, Swanstrom J, Sheahan TP, Pickles RJ, Corti D, Randell SH, Lanzavecchia A, Marasco WA, Baric RS. 2016. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A 113:3048–3053. doi: 10.1073/pnas.1517719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hon CC, Lam TY, Shi ZL, Drummond AJ, Yip CW, Zeng F, Lam PY, Leung FC. 2008. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J Virol 82:1819–1826. doi: 10.1128/JVI.01926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alekseev KP, Vlasova AN, Jung K, Hasoksuz M, Zhang X, Halpin R, Wang S, Ghedin E, Spiro D, Saif LJ. 2008. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J Virol 82:12422–12431. doi: 10.1128/JVI.01586-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J, Guy JS, Snijder EJ, Denniston DA, Timoney PJ, Balasuriya UB. 2007. Genomic characterization of equine coronavirus. Virology 369:92–104. doi: 10.1016/j.virol.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasoksuz M, Alekseev K, Vlasova A, Zhang X, Spiro D, Halpin R, Wang S, Ghedin E, Saif LJ. 2007. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J Virol 81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W, Lin XD, Guo WP, Zhou RH, Wang MR, Wang CQ, Ge S, Mei SH, Li MH, Shi M, Holmes EC, Zhang YZ. 2015. Discovery, diversity and evolution of novel coronaviruses sampled from rodents in China. Virology 474:19–27. doi: 10.1016/j.virol.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau SK, Woo PC, Yip CC, Fan RY, Huang Y, Wang M, Guo R, Lam CS, Tsang AK, Lai KK, Chan KH, Che XY, Zheng BJ, Yuen KY. 2012. Isolation and characterization of a novel Betacoronavirus subgroup A coronavirus, rabbit coronavirus HKU14, from domestic rabbits. J Virol 86:5481–5496. doi: 10.1128/JVI.06927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vijgen L, Keyaerts E, Lemey P, Maes P, Van Reeth K, Nauwynck H, Pensaert M, Van Ranst M. 2006. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J Virol 80:7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vijgen L, Keyaerts E, Moes E, Thoelen I, Wollants E, Lemey P, Vandamme AM, Van Ranst M. 2005. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol 79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kin N, Miszczak F, Diancourt L, Caro V, Moutou F, Vabret A, Ar Gouilh M. 2016. Comparative molecular epidemiology of two closely related coronaviruses, bovine coronavirus (BCoV) and human coronavirus OC43 (HCoV-OC43), reveals a different evolutionary pattern. Infect Genet Evol 40:186–191. doi: 10.1016/j.meegid.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bidokhti MR, Traven M, Krishna NK, Munir M, Belak S, Alenius S, Cortey M. 2013. Evolutionary dynamics of bovine coronaviruses: natural selection pattern of the spike gene implies adaptive evolution of the strains. J Gen Virol 94:2036–2049. doi: 10.1099/vir.0.054940-0. [DOI] [PubMed] [Google Scholar]
- 72.Battersby J. 2010. Guidelines for surveillance and monitoring of European bats. UNEP/EUROBATS, Bonn, Germany. [Google Scholar]
- 73.WHO. October 2006. Collecting, preserving and shipping specimens for the diagnosis of avian influenza A(H5N1) virus infection. Guide for field operations. WHO/CDS/EPR/ARO/2006.1. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/surveillance/WHO_CDS_EPR_ARO_2006_1/en/. [Google Scholar]
- 74.Heim A, Ebnet C, Harste G, Pring-Akerblom P. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol 70:228–239. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 75.Chu DK, Leung CY, Gilbert M, Joyner PH, Ng EM, Tse TM, Guan Y, Peiris JS, Poon LL. 2011. Avian coronavirus in wild aquatic birds. J Virol 85:12815–12820. doi: 10.1128/JVI.05838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tong S, Chern SW, Li Y, Pallansch MA, Anderson LJ. 2008. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J Clin Microbiol 46:2652–2658. doi: 10.1128/JCM.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics (Oxford, Engl) 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 80.Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 81.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 82.Guindon S, Lethiec F, Duroux P, Gascuel O. 2005. PHYML Online—a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33:W557–W559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouckaert R, Heled J, Kuhnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol 10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rambaut A, Suchard M, Xie D, Drummond A. 2014. Tracer v1.6. http://tree.bio.ed.ac.uk/software/tracer/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.