Abstract
Objective
To compare the efficacy of bazedoxifene and oral bisphosphonates for the prevention of nonvertebral fractures (NVFs) in women with higher risk of postmenopausal osteoporosis (i.e., the Fracture Risk Assessment Tool [FRAX] score ≥ 20%), based on currently available evidence from randomized controlled trials.
Methods
Randomized controlled trials evaluating the NVF relative risk reduction (RRR) with oral bisphosphonates or bazedoxifene were identified by a systematic literature review and combined by means of a network meta-analysis. A subgroup of patients with a FRAX score of 20% or more in the bazedoxifene phase III osteoporosis study was selected as the population of interest on the basis of the bazedoxifene label. In one analysis (analysis 1), the placebo response of the subgroup with a FRAX score of 20% or more was the benchmark to select comparable bisphosphonate trials. Additional analyses incorporated the aggregate data from the bisphosphonate trials with all the FRAX subgroups (analysis 2) or with the individual patient data from the bazedoxifene trial (analysis 3).
Results
Nine identified bisphosphonate trials (alendronate, ibandronate, risedronate; N = 23,440 patients) with a similar placebo response as observed for the subgroup of high risk patients in the bazedoxifene trial were included in analysis 1. The results of the network meta-analysis of this study set suggest that bazedoxifene is expected to have an RRR of 0.43 (95% credible interval [CrI] −0.19 to 0.72) versus alendronate, 0.58 (95% CrI 0.05–0.81) versus ibandronate, and 0.39 (95% CrI –0.29 to 0.70) versus risedronate. Analyses in which treatment effects with bisphosphonates were projected to a population with a FRAX score of 20% or more with meta-regression approaches (analysis 2 and analysis 3) provide similar findings.
Conclusion
Based on an indirect comparison of randomized trials, bazedoxifene is expected to have at least a comparable RRR of NVF as alendronate, ibandronate, and risedronate in women with higher risk of postmenopausal osteoporosis.
Keywords: meta-analysis, osteoporosis, systematic review, treatment comparisons
Introduction
Osteoporosis, characterized by low bone mineral density and deterioration of bone structure, is primarily present in postmenopausal women and is associated with an increased risk of vertebral, hip, and other nonvertebral fractures [1]. Of the fractures attributed to osteoporosis, approximately 70% occur in nonvertebral locations, including the hip, forearm, and humerus [2,3]. These nonvertebral fractures are associated with substantial mortality and health care costs [4–6].
The most commonly prescribed treatments for osteoporosis are oral bisphosphonates (e.g., alendronate, risedronate, and ibandronate). Bazedoxifene, a novel selective estrogen receptor modulator, is approved in the European Union and in some Asian countries for the treatment of postmenopausal osteoporosis in women at an increased risk of fracture [7,8]. Furthermore, in phase III trials, it has been shown to reduce the risk of nonvertebral fractures in a subgroup of postmenopausal patients with a 10-year fracture risk at or above 20% as assessed by the Fracture Risk Assessment Tool (FRAX). The FRAX tool, developed by the World Health Organization, incorporates clinical risk factors such as age, body mass index, previous fracture, current smoking status, and bone mineral density to estimate the 10-year probability of a hip fracture and the 10-year probability of a major osteoporosis event (i.e., vertebral, hip, forearm, or shoulder fracture) [9].
To aid evidence-based medical decisions, comparisons of all available therapies should be available for all populations of interest. The current evidence base for nonvertebral fracture risk reduction in osteoporotic women, however, consists of many randomized controlled trials (RCTs) that are placebo controlled and do not provide head-to-head direct comparisons. Furthermore, the bisphosphonate RCTs do not present subgroup results according to the FRAX score. In the absence of an RCT comparing all relevant therapies, an indirect treatment comparison by means of a network meta-analysis (NMA) of clinical trial evidence is a valid alternative to provide relevant comparative evidence when there are no systematic differences across comparisons related to the treatment effects.
The objective of this study was to compare the reduction in nonvertebral fracture risk with bazedoxifene relative to oral bisphosphonates for those patients who are at a higher risk of a clinical osteoporotic fracture (i.e., with a FRAX score of at least 20%).
Methods
Identification and Selection of Studies
A systematic literature search was performed to identify relevant RCTs published in English from 1990 to November 2011. MEDLINE, Embase, and Cochrane Library Clinical Trials databases were searched according to predefined search strategy with terms relevant to osteoporosis, alendronate, ibandronate, risedronate, bazedoxifene, and RCTs. A hand search of references was also conducted to identify any missing trials. Each identified study was evaluated against the following predefined criteria:
– Population of interest: Postmenopausal women with primary osteoporosis;
– Interventions: Oral bisphosphonates (alendronate 5 and 10 mg, ibandronate 2.5 mg, risedronate 5 mg) and bazedoxifene (20 and 40 mg);
– Comparators: Placebo or one of the regimes described under interventions. Comparisons of different dosages of the same intervention only were excluded because such comparisons have limited ability to strengthen estimates of the comparisons of interest. Comparison of the same interventions with different background treatments were also excluded to limit issues of comparability of patients;
– Outcomes: Incidence of nonvertebral fractures; and
– Study Design: RCTs. Nonexperimental studies (e.g., cohort, case-control, cross-sectional studies) as well as (systematic) literature reviews, commentaries, and other meta-analyses were excluded.
The excluded publications that did not meet the above selection criteria were considered out of scope.
Data Extraction and Quality Assessment
For each identified study that met the selection criteria, data on study design, patient characteristics, interventions, and the number of patients experiencing a nonvertebral fracture during the trial were extracted. For the bazedoxifene trial, subgroup data and individual patient-level data were available and obtained for both placebo and bazedoxifene trial arms.
The quality of each trial was assessed using the National Institute of Health and Care Excellence checklist for RCTs, which consists of seven questions regarding randomization, allocation concealment, similarity of the groups in terms of prognostic factors, blinding, dropouts and imbalances between groups, outcome reporting, and intention to treat methodology [10]. The results of the quality assessment were not explicitly used in the analyses but provide additional information to determine the quality of the evidence base when interpreting the results.
Analyses
Treatment effects of the different studies were synthesized and indirectly compared by means of an NMA [11–14].
The NMAs were performed within a Bayesian framework and involve data, a likelihood distribution, a model with parameters, and prior distributions for these parameters [15]. The model relates the data from the individual studies to basic parameters reflecting the (pooled) relative treatment effect of each intervention compared with an overall reference treatment, namely, placebo. Based on these basic parameters, the relative efficacy between bazedoxifene and each of the competing bisphosphonates was calculated. For nonvertebral fractures, a logistic regression model with a binomial likelihood distribution was used.
The validity of the NMA depends on the comparability or exchangeability of patients across trials. For this analysis, a potential challenge was assessing the differences across patients in terms of baseline fracture risk. For an indirect comparison of bazedoxifene with bisphosphonates in the higher risk population of interest (FRAX score ≥ 20%), it is ideal to have data on treatment effects for these interventions in this population. Although individual patient data (IPD) including FRAX scores were available for the bazedoxifene trial and the relationship between FRAX scores and treatment effects in the subgroup of patients at or above a certain FRAX score threshold could be estimated there, results only with aggregated data (AD) (i.e., the published findings) were available for the bisphosphonate trials, with no information on treatment effects by FRAX subgroup. Because of the lack of FRAX data for the subjects in the bisphosphonate studies, it is difficult to assess comparability of patients across the bisphosphonate and bazedoxifene trials based on the FRAX assessment. To address this issue, the incidence of nonvertebral fracture in the placebo arm was used as an assessment of baseline fracture risk. Given the available evidence, multiple analyses were conducted to obtain treatment effect estimates of bazedoxifene relative to oral bisphosphonates for the population with FRAX scores of 20% or more, namely:
– Comparison based on AD of bisphosphonates and FRAX score of 20% or more subgroup for bazedoxifene: NMA based on AD in which only those bisphosphonate studies were selected that showed a similar (i.e., not statistically significantly different at the 0.05 level) nonvertebral fracture incidence in the placebo arm as did the placebo response of the subgroup with a FRAX score of 20% or more in the bazedoxifene trial.
– Meta-regression based on AD of bisphosphonates and all FRAX subgroups for bazedoxifene: NMA based on AD for all FRAX subgroups of the bazedoxifene trial and all AD for the published bisphosphonate trials. The model includes covariates that capture the effect of placebo response on the treatment effects (i.e., log odds ratio [OR] of nonvertebral fractures) with oral bisphosphonates and bazedoxifene relative to placebo. The relationship between baseline risk and treatment effects was estimated for each treatment separately but assumed exchangeable. The advantage of this analysis over analysis 1 is that all studies and all subgroups were used. The treatment effects for all interventions indirectly compared were centered at a placebo response for nonvertebral fractures corresponding to the subgroup with a FRAX score of 20% or more in the bazedoxifene trial.
– Meta-regression based on AD of bisphosphonates and IPD for bazedoxifene: NMA based on IPD for the bazedoxifene trial and AD for the published bisphosphonate studies. The advantage of an analysis with IPD is that the relationship between baseline risk and treatment effect with bazedoxifene can be estimated without relying on a linear association while still using all data. Although a model with a linear relationship between baseline risk and treatment effect was tested (results not presented), a model with a quadratic relationship provided a better fit to the IPD and was used for this analysis. The covariate in the model captures the effect of a 10-year baseline fracture risk on the treatment effects (i.e., log OR of nonvertebral fractures) with oral bisphosphonates and bazedoxifene relative to placebo. The FRAX score reflects the 10-year fracture risk available for individuals in the bazedoxifene trial. For the bisphosphonate trials, the placebo response over the follow-up of the trial (e.g., 1–4 years) was transformed to a 10-year (nonvertebral fracture) risk as well. The treatment effects for all interventions indirectly compared were centered at a 10-year fracture risk of 20% or more. The models are presented in Supplemental Materials found at http://dx.doi.org/10.1016/j.jval.2014.01.008.
For each analysis, fixed- and random-effects models were compared regarding the goodness of fit to the data, using the deviance information criterion. The random-effects model resulted in a lower deviance information criterion, and hence was considered appropriate for the synthesis of the available evidence.
To avoid influence of the prior distributions required for Bayesian analyses on the results, noninformative prior distributions were used. Prior distributions of the treatment effects were normal distributions with mean 0 and a variance of 10,000. A uniform distribution with a (noninformative) range of 0 to 2 was used for the prior distribution of heterogeneity needed for the random-effects analyses. WinBugs statistical software was used for the analyses [16].
The results of the NMA are the treatment effects of each treatment versus placebo presented as an OR of a nonvertebral fracture. From the ORs, the risk of a nonvertebral fracture for each treatment can be calculated using the average modeled placebo risk of a fracture for the population of interest and transformed to other effect measures such as the relative risk reduction (RRR) [1–relative risk], which may be a more intuitive measure of effectiveness. Summary statistics are presented for treatment effects, that is, OR and RRR, and expected result (probability of nonvertebral fracture) with corresponding 95% credible intervals (95% CrI) reflecting the range of true underlying effects with 95% probability. The probability that a certain treatment provides greatest RRR out of all those compared was calculated (i.e., probability of being best treatment), as well as the probability that each treatment is better than a certain comparator, which are based on the posterior distribution of the treatment effects.
Results
Study Identification
In Figure 1, the results of the literature search are presented. The database search identified 1462 abstracts. After the duplicates were removed from these databases (n = 488), 974 potentially relevant publications were identified. The abstract screening excluded 882 potentially relevant publications that did not meet selection criteria. The full-text review of the 92 remaining publications excluded 82 studies for reasons including outcomes (n = 48), study design (15), population (10), unable to retrieve full text (4), comparison (2), non-English language (2), and intervention dose (1). In addition, a hand search of references identified a further 2 RCTs to be included in the analyses. Overall, 12 full-text articles corresponding to 11 RCTs were identified in the review. Unpublished subgroup data according to the FRAX score and the IPD for the bazedoxifene trial were also provided [17].
Fig. 1.
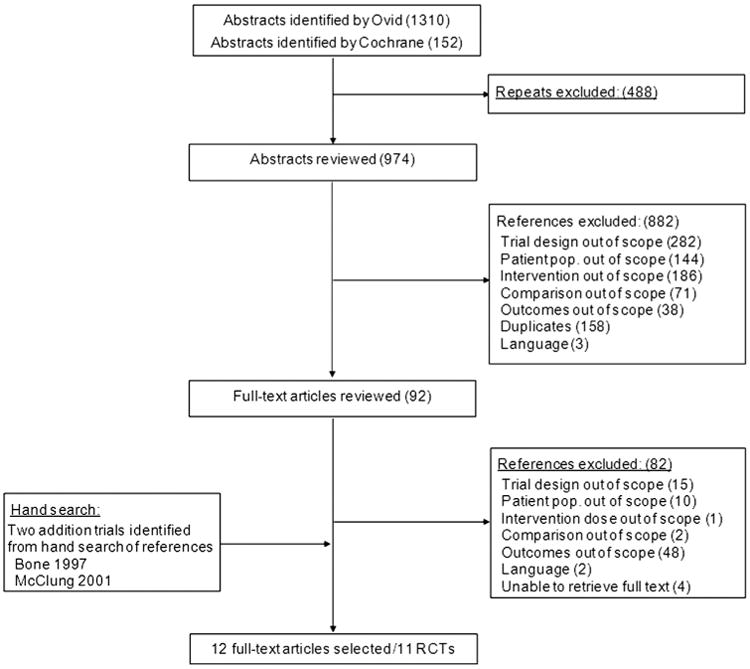
Flow diagram summarizing results of study identification and selection. pop., population; RCTs, randomized controlled trials
Evidence Base
Table 1 provides a summary of the included trials, which were of similar quality. All studies were multicenter, double-blind, placebo-controlled RCTs and, in addition to the study interventions, patients were provided with at least 500 mg of a calcium supplement per day and some patients also received vitamin D. The duration of the trials ranged from 1 year to 4 years, and the patients were predominately from Europe and North America.
Table 1.
Study and patient characteristics.
| Author, year | Trial | Active drug | Dosing | Treatment duration (y) | Summary inclusion/exclusion | Calcium/vitamin D | No. of centers/countries | Number randomized | Age (y) (mean) | Years since menopause (mean) | Hologic mean lumbar spine BMD (mean) [T score] | Patients with baseline fractures (%) | Nonvertebral fractures placebo, n/N (%) [annual rate] | Nonvertebral fractures active, n/N (%) [annual rate] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone et al. (1997) [19] | Alendronate Elderly Osteoporosis Study | ALEN | 5 mg/d for 2 y | 2 | Women (age 60–85 y) with low bone mass | 500 mg/NR | 15/United States | 184 | 71 | 24 | 0.72 [NR] | 36 | 16/91 (17.6) [0.09] | 9/93 (9.7) [0.05] |
| Liberman et al. (1995) [20] | Alendronate Phase III Osteoporosis Treatment Study | ALEN | 5 mg/d or 10 mg/d for 3 y; or 20 mg/d for 2 y then 5 mg/d for 1 y | 3 | Postmenopausal (≥5 y) women (age 45–80 y) with low bone mass | 500 mg/NR | NR/Australia, Canada, Europe, Israel, Mexico, New Zealand, South America, United States | 994 | 64 | 16 | 0.71 [NR] | 21 | 38/397 (9.6) [0.03] | 45/597 (7.5) [0.03] |
| Chesnut et al. (2004) [21], Chesnut et al. 2005 [22] | BONE | IBAN | 2.5 mg/d for 3 y | 3 | Postmenopausal (≥5 y) women (age 55–80 y) with low bone mass and one to four vertebral fractures | 500 mg/400 IU | 73/Europe and United States | 1964 | 69 | 21 | NR [−2.8] | 94 | 80/975 (8.2) [0.03] | 89/977 (9.1) [0.03] |
| Black et al. (1996) [23] | FIT I (Vertebral Fractures) | ALEN | 5 mg/d for 2 y then 10 mg/d for 1 y | 3 | Postmenopausal (≥2 y) women (age 55–81 y) with low bone mass and one or more vertebral fracture | 500 mg/250 IU | 11/United States | 2027 | 71 | NR | 0.79 [NR] | 100 | 148/1005 (14.7) [0.05] | 122/1022 (11.9) [0.04] |
| Cummings et al. (1998) [24] | FIT II (Clinical Fractures) | ALEN | 5 mg/d for 2 y then 10 mg/d for 2 y | 4 | Postmenopausal (≥2 y) women (age 55–80 y) with low bone mass and no vertebral fractures | 500 mg/250 IU | 11/United States | 4432 | 68 | NR | 0.84 [NR] | 0 | 294/2218 (13.3) [0.03] | 261/2214 (11.8) [0.03] |
| Pols et al. (1999) [25] | FOSIT | ALEN | 10 mg/d for 1 y | 1 | Postmenopausal (≥3 y) women (age ≤85 y) with low bone bass | 500 mg/NR | 153/Europe, Latin America, Australia, Canada, South Africa, China | 1908 | 63 | 16 | 0.72 [NR] | NR | 37/958 (3.9) [0.04] | 19/950 (2.0) [0.02] |
| McClung et al. (2001) [26] | HIP | RISED | 2.5 mg/d or 5 mg/d for 3 y | 3 | Postmenopausal women (age 70–79 y) with low bone mass or postmenopausal women (age ≥80 y) with low bone mass with at least one nonskeletal risk factor for hip fracture | 1000 mg/up to 500 IU | 183/North America, Europe, New Zealand, Australia | 9331 | 78 | 32 | NR [NR] | 41 | 351/3134 (11.2) [0.04] | 583/6197 (9.4) [0.03] |
| Reginster et al. (2000) [27] | VERT-MN | RISED | 5 mg/d for 3 y | 3 | Postmenopausal (≥5 y) women (age ≤85 y) with two or more vertebral fractures | 1000 mg/up to 500 IU | 80/Europe and Australia | 816 | 71 | 25 | 0.79 [−2.8] | 98 | 51/406 (12.6) [0.04] | 36/406 (8.9) [0.03] |
| Harris et al. (1999) [28] | VERT-NA | RISED | 5 mg/d for 3 y | 3 | Postmenopausal (≥5 y) women (age ≤85 y) with two or more vertebral fractures or one vertebral fracture and low lumbar-spine BMD | 1000 mg/up to 500 IU | 110/North America | 1641 | 69 | 24 | 0.83 [−2.4] | 80 | 52/815 (6.4) [0.02] | 33/812 (4.1) [0.01] |
| Fogelman et al. (2000) [29] | – | RISED | 5 mg/d for 2 y | 2 | Postmenopausal (≥1 y) women (age ≤80 y) with low bone mass | 1000 mg/NR | 13/France, United Kingdom, Netherlands, Belgium, Germany | 359 | 64 | 17 | 0.74 [−2.9] | 31 | 13/180 (7.2) [0.04] | 7/177 (4.0) [0.02] |
| Silverman et al. (2008) [17] | – | BAZ | 20 mg/d or 40 mg/d for 3 y | 3 | Postmenopausal (≥2 y) women (age 55–85 y) with low bone mass | Up to 1200 mg/400–800 IU | 206/Asia-Pacific countries, Canada, Europe, Latin America, South Africa, United States | 5643 | 66 | 20 | NR [−2.4] | 56 | 99/1885 (5.3) [0.02] | 174/3758 (4.6) [0.02] |
ALEN, alendronate; BAZ, bazedoxifene; BMD, bone mineral density; BONE, Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe; FIT, Fracture Intervention Trial; FOSIT, Fosomax International Trial; HIP, Hip Intervention Program; IBAN, ibandronate; NR, not reported; RISED, risedronate; VERT, Vertebral Efficacy with Risedronate Therapy; VERT-MN, VERT-Multinational; VERT-NA, VERT-North America.
The enrolled patients were postmenopausal women with low bone mass. Included patients were 45 years or older; the mean average age per study was 69 years and ranged from 63 to 78 years. Note that the mean age for the subgroup with a FRAX score of 20% or more in the bazedoxifene trial is higher than the mean age for the full trial population (73 years vs. 66 years). Other baseline clinical risk factors of interest such as body mass index, bone mineral density, and presence of a baseline vertebral fracture were not consistently reported across the trials.
Results of NMA
Comparison based on AD of bisphosphonates and subgroup with a FRAX score of 20% or more for bazedoxifene
Nine identified bisphosphonate RCTs had a similar (i.e., not statistically significant difference at the 0.05 level) nonvertebral fracture incidence in the placebo arm as did the placebo response of the subgroup with a FRAX score of 20% or more in the bazedoxifene trial and hence were included in this analysis [20–29]. The results of the analysis are provided in Table 2 and suggest that bazedoxifene and risedronate are expected to be more efficacious than placebo in the higher risk population. Alendronate and ibandronate are expected to be comparable with placebo; the point estimates favor alendronate but not ibandronate over placebo in this population. Overall, bazedoxifene shows comparable reductions in nonvertebral fracture risk as do oral bisphosphonates although it has a larger reduction in risk than does ibandronate and more than 90% probability of being better than each of the oral bisphosphonates in the higher risk population. For the higher risk population, there is a 90% probability that bazedoxifene will provide the greatest nonvertebral fracture risk reduction of the five interventions compared. The expected proportion of patients experiencing a nonvertebral fracture for each intervention is presented in Figure 2 to facilitate comparisons.
Table 2.
Results of network meta-analyses.
| Comparison | Analysis 1* | Analysis 2* | Analysis 3* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| OR (95% CrI) | RRR† (95% CrI) | P(better) | P(best) | OR (95% CrI) | RRR† (95% CrI) | P(better) | P(best) | OR (95% CrI) | RRR† (95% CrI) | P(better) | P(best) | |
| vs placebo | ||||||||||||
| ALEN | 0.81 (0.61– 1.00) | 0.18 (0.00– 0.37) | 97% | 3% | 0.75 (0.59–0.92) | 0.23 (0.07–0.39) | > 99% | 4% | 0.85 (0.47–1.44) | 0.14 (−0.39 to 0.51) | 75% | 6% |
| IBAN | 1.12 (0.70– 1.81) | −0.11 (–0.67 to 0.28) | 29% | 1% | 1.08 (0.46–3.28) | −0.07 (–1.66 to 0.51) | 42% | 4% | 1.17 (0.37–2.50) | −0.16 (−1.20 to 0.61) | 33% | 5% |
| RISED | 0.75 (0.54– 0.93) | 0.23 (0.07– 0.43) | 99% | 7% | 0.76 (0.57–0.96) | 0.22 (0.04–0.40) | > 99% | 4% | 0.69 (0.36–1.09) | 0.29 (−0.08 to 0.62) | 95% | 19% |
| BAZ | 0.44 (0.21– 0.92) | 0.53 (0.08– 0.77) | 98% | 90% | 0.50 (0.36–0.78) | 0.47 (0.20–0.61) | > 99% | 89% | 0.50 (0.24–1.02) | 0.48 (−0.01 to 0.74) | 97% | 70% |
| BAZ vs | ||||||||||||
| ALEN | 0.55 (0.26– 1.21) | 0.43 (−0.19 to 0.72) | 94% | 0.67 (0.45–1.11) | 0.31 (−0.10 to 0.52) | 95% | 0.59 (0.24–1.49) | 0.39 (−0.46 to 0.74) | 88% | |||
| IBAN | 0.40 (0.17– 0.94) | 0.58 (0.05–0.81) | 98% | 0.47 (0.15–1.17) | 0.50 (−0.16 to 0.81) | 95% | 0.43 (0.15–1.56) | 0.55 (−0.52 to 0.83) | 92% | |||
| RISED | 0.59 (0.28– 1.32) | 0.39 (−0.29 to 0.70) | 91% | 0.67 (0.45–1.12) | 0.31 (−0.11 to 0.53) | 95% | 0.73 (0.30–1.84) | 0.26 (−0.78 to 0.68) | 76% | |||
AD, aggregated data; ALEN, alendronate; BAZ, bazedoxifene; FRAX, Fracture Risk Assessment Tool; IBAN, ibandronate; IPD, individual patient data; OR, odds ratio; RISED, risedronate; RRR, relative risk reduction; P(better), probability the intervention is better than comparator.
Analysis 1: Unadjusted comparison based on AD of bisphosphonates and subgroup with a FRAX score of ≥20% for bazedoxifene (N = 24,058); analysis 2: Meta-regression controlling for baseline risk (placebo response) based on AD of bisphosphonates and all FRAX subgroups for bazedoxifene (N = 29,267); analysis 3: Meta-regression controlling for baseline risk (placebo response in bisphosphonate trials and FRAX for bazedoxifme trial) based on AD of bisphosphonates and IPD for bazedoxifene (N = 29,267).
RRR = 1 – relative risk. The relative risk and OR will be similar when the incidence of an event is low, such as with nonvertebral fractures.
Fig. 2.
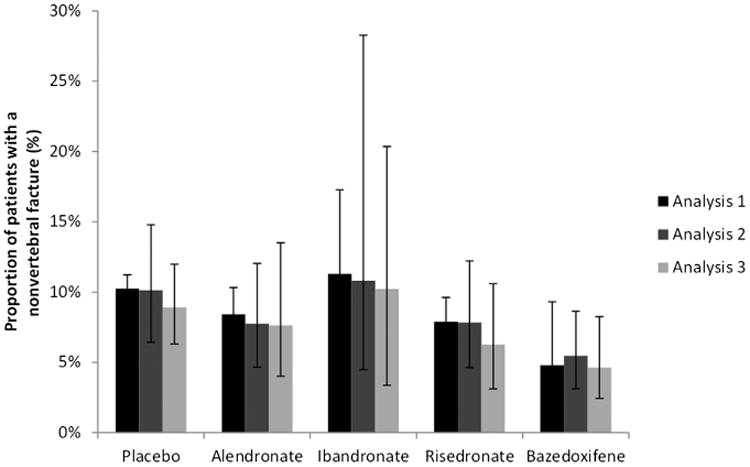
Modeled probability of a nonvertebral fracture (along with 95% credible interval) for each treatment. Analysis 1: Unadjusted Comparison based on AD of bisphosphonates and FRAX > = 20% subgroup for bazedoxifene (N = 24,058); Analysis 2: Meta-regression controlling for baseline risk (placebo response) based on AD of bisphosphonates and all FRAX subgroups for bazedoxifene (N = 29,267); Analysis 3: Meta-regression controlling for baseline risk (placebo response in bisphosphonate trials and FRAX for bazedoxifine trial) based on AD of bisphosphonates and IPD for bazedoxifene (N = 29,267). The treatment effects were centered at a 10-year fracture risk > = 20%. FRAX, Fracture Risk Assessment Tool.
Meta-regression based on AD of bisphosphonates and all FRAX subgroups for bazedoxifene
Data from each of the eight FRAX subgroups in the bazedoxifene trial were combined with all the bisphosphonate AD with covariates to capture the impact of placebo response on the treatment effects (i.e., log OR of nonvertebral fractures) with each intervention relative to placebo. The meta-regression model with placebo response as a covariate indicates that alendronate, risedronate, and bazedoxifene are more efficacious than placebo while ibandronate is comparable to placebo in the higher risk population (Table 2). Overall, bazedoxifene is comparable to the oral bisphosphonates although the point estimates favor bazedoxifene; bazedoxifene has at least a 95% probability of being better than each of the oral bisphosphonates in this population.
Meta-regression based on AD of bisphosphonates and IPD for bazedoxifene
The results of the meta-regression adjusting for placebo response in the bisphosphonate trials and the FRAX score in the bazedoxifene trial also suggest that bazedoxifene is comparable to the oral bisphosphonates in the higher risk population. Although the point estimates favor bazedoxifene, there is considerable uncertainty associated with the estimates. Bazedoxifene has a 70% probability of being the best intervention compared.
Discussion
This study compared bazedoxifene with oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women who are at a higher risk of fracture. To provide comparative evidence on nonvertebral fractures for bazedoxifene versus bisphosphonates for this population, an NMA was performed. When considering only those bisphosphonate trials that reported a placebo nonvertebral fracture response similar to the placebo response of the subgroup with a FRAX score of 20% or more from the bazedoxifene trial, bazedoxifene has at least comparable RRR of non-vertebral fractures as the oral bisphosphonates. This finding was confirmed by analyses in which treatment effects with bisphosphonates were projected to a population with a FRAX score of 20% or more with a meta-regression approach based on all data from all trials in the NMA.
The currently available RCTs did not provide treatment effect estimates of bazedoxifene versus bisphosphonates for the prevention of nonvertebral fractures. Although a meta-analysis has been performed to assess the effect of several osteoporosis treatments including bisphosphonates on the risk of nonvertebral fractures, the study did not include bazedoxifene [17]. Furthermore, the meta-analysis did not provide comparative estimates for the subgroup of women at a higher risk of fracture.
Randomization of patients holds only within an RCT but not across RCTs, and consequently there may be systematic differences in study and/or patient characteristics across the RCTs that are modifiers of the treatment effects, which may bias the results. In all the analyses presented here, the similarity across comparisons was based on the placebo risk of nonvertebral fractures, which reflects the prognostic effect of all study and patient characteristics on the occurrence of nonvertebral fractures. By doing so, all variables that are both prognostic factors and treatment effect modifiers are implicitly captured. The only variables that we did not adjust for by using the placebo risk are variables that are treatment effect modifiers but not prognostic factors of the nonvertebral fracture risk. We consider this, however, to be a minor source of possible bias in the indirect comparison.
The first analysis in this study incorporated the evidence from bisphosphonate trials with a placebo response similar to that of the subgroup with a FRAX score of 20% or more in the bazedoxifene trial. To evaluate the sensitivity of the findings, a second analysis was conducted in which a linear relationship between baseline risk and treatment effects was estimated on the basis of data from all identified trials and all subgroups from the bazedoxifene trial. In a third analysis, IPD was used to model the relationship between baseline severity (as measured with the FRAX score) and treatment effects with bazedoxifene. Given that subjects with a FRAX score of 20% or more account for approximately 13% of the trial population, the effect of this population on the slope of the linear regression across the range of FRAX scores in an IPD analysis was not as great as with the subgroup data and as such, the treatment effect predicted for the population with a FRAX score of 20% or more underestimated the observed treatment effect (results not presented). Given the power of the IPD, however, a nonlinear relationship was modeled with an additional parameter that provided a better fit to the data. These results are presented in analysis 3 of this study and are similar to the results from the first two analyses.
The findings from this study are relevant to the management of patient care for those patients with a higher risk of non-vertebral fractures. By incorporating all relevant RCT evidence identified from a systematic literature review, this study allows for the comparison of all active interventions despite the lack of head-to-head RCT evidence. Furthermore, the methods used allow for treatment comparisons pertaining to specific populations of interest that currently have not been studied in RCTs (e. g., subgroup with a FRAX score of ≥20%). Although results pertaining to the overall population have important implications, clinicians are also faced with individual patients with differing baseline risks and hence results for subgroups may also aid decisions.
Furthermore, the methods used in this study allow for the probabilistic interpretation of results (e.g., the probability of an intervention being better than another and the probability of being the best intervention). With five interventions being compared, there is a 20% chance of any one intervention being the best in the absence of evidence. The results from the analysis, however, suggest that there is at least a 70% chance of bazedoxifene being the best intervention for the population with a FRAX score of 20% or more. In other words, there is a 70% probability that bazedoxifene provides the greatest reduction in the risk of a nonvertebral fracture for a postmenopausal woman with osteoporosis and a FRAX score of 20% or more.
Several limitations of our study should be mentioned. First, the analyses are based on aggregated published data for the oral bisphosphonates because access to IPD was not available; subgroup data based on FRAX and IPD were available only for the bazedoxifene trial. Consequently, a nonlinear relationship between baseline risk and treatment effects could be modeled only for bazedoxifene while a linear relationship was assumed for the bisphosphonates given the limited data. Second, the current study is limited to nonvertebral fractures. Vertebral fractures are also associated with high morbidity and health care costs, and additional analysis on vertebral fractures should be warranted in the future to have a complete model of all osteoporotic fractures. Furthermore, analyses of adverse events should be considered given concerns over the safety of treatments for osteoporosis. In addition, non-English studies were excluded and the potential for language bias should be considered. Finally, only published studies were included from the literature review and no attempt was made to contact investigators regarding the presence of negative results. Hence, there is potential for publication bias.
In conclusion, based on the currently available RCT evidence, bazedoxifene showed at least a comparable risk reduction in nonvertebral fractures as did alendronate, ibandronate, and risedronate in postmenopausal women with osteoporosis with an elevated FRAX score. Our study suggests that bazedoxifene is likely to be at least as effective as oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis who are at a higher risk of fracture [18].
Supplementary Material
Acknowledgments
Source of financial support: This study was sponsored by Pfizer, Inc. A.G.E. and J.P.J. are former employees of Mapi, who were paid consultants to Pfizer in connection with the development of this manuscript.
Footnotes
Supplemental Materials: Supplemental materials accompanying this article can be found in the online version as a hyperlink at http://dx.doi.org/10.1016/j.jval.2014.01.008 or, if a hard copy of article, at www.valueinhealthjournal.com/issues (select volume, issue, and article).
References
- 1.Deal CL. Osteoporosis: prevention, diagnosis, and management. Am J Med. 1997;102(Suppl):35S–9S. doi: 10.1016/s0002-9343(97)00415-4. [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int. 2005;16:229–38. doi: 10.1007/s00198-004-1811-2. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson M, Jones ML, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:100–60. doi: 10.3310/hta9220. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson M, Davis S, Lloyd-Jones M, et al. The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women. Health Technol Assess. 2007;11:100–34. doi: 10.3310/hta11040. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Assessment report for Conbriza. Procedure No.EMEA/H/C/913. [Accessed October 19, 2011];2009 Available from: http://www.emea.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000913/human_med_000723.jsp.
- 8.Kawate H, Takayanagi R. Efficacy and safety of bazedoxifene for postmenopausal osteoporosis. Clin Interv Aging. 2011;6:151–6. doi: 10.2147/CIA.S15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FRAX. WHO Fracture Assessment Tool. [Accessed October 19, 2011]; Available from: http://www.shef.ac.uk/FRAX/
- 10.National Institute for Health and Care Excellence. The guidelines manual 2009. [Accessed October 19, 2011]; Available from: http://www.nice.org.uk/aboutnice/howwework/developingniceclinicalguidelines/clinicalguidelinedevelopmentmethods/GuidelinesManual2009.jsp?domedia=1&mid=60B7B3CD-BBC2-E1BE-A6D53FD68D8845AD.
- 11.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14:429–37. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14:417–28. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 15.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelhalter D, Thomas A, Best N, et al. WinBUGS User Manual: Version 1.4. Cambridge: MRC Biostatistics Unit; 2003. [Google Scholar]
- 17.Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923–34. doi: 10.1359/jbmr.080710. [DOI] [PubMed] [Google Scholar]
- 18.Boonen S, Laan RF, Barton IP, et al. Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int. 2005;16:1291–8. doi: 10.1007/s00198-005-1945-x. [DOI] [PubMed] [Google Scholar]
- 19.Bone HG, Downs RW, Jr, Tucci JR, et al. Dose-response relationships for alendronate treatment in osteoporotic elderly women. Alendronate Elderly Osteoporosis Study Centers. J Clin Endocrinol Metab. 1997;82:265–74. doi: 10.1210/jcem.82.1.3682. [DOI] [PubMed] [Google Scholar]
- 20.Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 21.Chesnut CH, III, Skag A, Christiansen C, et al. Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 22.Chesnut CH, Ettinger MP, Miller PD, et al. Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONE. Curr Med Res Opin. 2005;21:391–401. doi: 10.1185/030079905X30752. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 24.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 25.Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 26.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 27.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 28.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 29.Fogelman I, Ribot C, Smith R, et al. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab. 2000;85:1895–900. doi: 10.1210/jcem.85.5.6603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


