Abstract
The role of mast cells in contractile bronchial smooth muscle activity has been evaluated in a model of chronic obstructive pulmonary disease induced in rats that were intermittently exposed to nitrogen dioxide (NO2) for 60 days. Starting from the 31st day, one group of rats inhaled sodium cromoglycate before exposure to NO2 to stabilize mast cell membranes. The second group (control) was not treated. Isometric smooth muscle contraction was analysed in isolated bronchial samples in response to nerve and smooth muscle stimulation. Histological analysis revealed large numbers of mast cells in lung tissue of COPD model rats. The inhibition of mast cell degranulation by sodium cromoglycate prevented the development of nerve-stimulated bronchial smooth muscle hyperactivity in COPD model rats. Histamine or adenosine-induced hyperactivity on nerve stimulation was also inhibited by sodium cromoglycate in bronchial smooth muscle in both control and COPD model rats. This suggests that the mechanism of contractile activity enhancement of bronchial wall smooth muscle cells may be mediated through the activation of resident mast cells transmembrane adenosine receptors resulting in their partial degranulation, with the released histamine acting upon histamine H1-receptors which trigger reflex pathways via intramural ganglion neurons.
Keywords: mast cells, chronic obstructive pulmonary disease, histamine, adenosine, smooth muscle, bronchi
Introduction
Regulating mechanisms of respiratory tract smooth muscle contractile activity determine to a large extent the character and course of pulmonary diseases associated with bronchial obstruction development (1,2,3). Regulatory imbalance in respiratory tract smooth muscle tonus results in increasing airflow limitation, ventilation and lung perfusion imbalance, hypoxemia, and eventually severe respiratory failure. At present the role of mast cells in the development of inflammatory neurogenic mechanisms and bronchial obstruction is being given considerable attention (4). It has been argued that in the pathogenesis of respiratory disorders mast cells play a major role in the initiation of the hypersensitivity reaction owing to their multipotent capacity (5). Whereas the role of these multifunctional cells in the allergic state pathogenesis (e.g., bronchial asthma) is widely recognized, their significance in the bronchial obstruction development in the case of non-allergic airway disease, e.g., chronic obstructive pulmonary disease, has not been properly investigated, and recent findings are highly controversial.
The aim of the study is to evaluate the role of mast cells in the mechanism of contractile activity enhancement in bronchial wall smooth muscle cells using a model of chronic obstructive pulmonary disease (COPD) in rats.
Methods
Experimental animals
Experiments were performed in a total of twenty-nine male juvenile Wistar rats weighing 150–170 g (Laboratory Animal Breeding “Rappolovo” of the Russian Academy of Sciences, St. Petersburg, Russia). All procedures and experiments were carried out in accordance with the internationally accepted guidelines for the care and use of laboratory animals (6). Rats were housed in cages (250 cm2/rat) with free access to drinking water and standard lab rodent chow and maintained at 20–22 °C and 55–60% air temperature and humidity respectively.
COPD model
The experimental model for the formation of COPD was based on exposure of rats to nitrogen dioxide (NO2) (7). Rats (n=22) were placed in a chamber within a fume hood and connected with a NO2-generating laboratory device. A mixture of nitrogen oxides was produced in a chemical reaction between sodium nitrite and sulfuric acid, and then pumped into the exposure chamber which had been provided with an exhaust tube. The colorless nitrogen oxide (NO) reacted with atmospheric oxygen and was converted to the more stable yellow-brown NO2. Nitrogen dioxide concentration in the chamber was equal to 30–40 mg/m3 (15–19 ppm) as determined colorimetrically. Rats were exposed to NO2 in the following intermittent regime: three 30-min exposures/day with 30 min intervals for 60 days. The adequacy of the model was confirmed by the development of symptoms characteristic of COPD, i.e., hyperplasia and hypersecretion of goblet cells, squamous cell metaplasia of the ciliary epithelium, emphysema and focal fibrosis, hyperexpression of CD3 lymphocytes in the bronchial wall and parenchyma, manifold increased production of TNF-α and TGF-β, and high concentrations of circulating pathogenic immune complexes. Persistence of the structural and functional shifts for 6 months following exposure to NO2 indicated a chronic course of the pathological process (7).
Experimental design
After the 30 day exposure to NO2, animals were randomized into two groups with 11 rats in each. Every day during the next 30 days (before exposure to NO2), rats of the first group were treated with an aerosol preparation of cromoglycate sodium (Intal®, Aventis, Great Britain), a mast cell membrane stabilizer. The preparation was supplied through a special mask, which was put on the rat’s muzzle. The nozzle of the aerosol inhaler was inserted into a hole in the mask. After a single spray (1 aerosol dose – 5 mg), the rat was allowed to breath during a 10 sec exposure in which the rat made 20–25 respiratory movements. The rats of the second group (control) did not receive treatment. Seven rats were not exposed to NO2, representing the intact group. At the end of the experiment animals were euthanized with carbon dioxide inhalation.
Measurement smooth muscle force
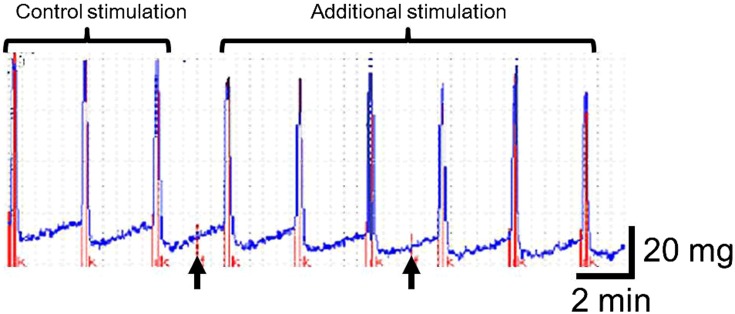
Contractile activity of the bronchial smooth muscle was evaluated in vitro. Three to four longitudinal segments (4–5 mm) with a bifurcation site containing intramural ganglion were isolated from bronchi of the 2nd–4th order in every animal. The bronchial samples were placed into thermostatic flow bath (volume of 2.5 ml) perfused with Krebs−Henseleit solution with the following composition (mM): NaCl 118.0, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaHCO3 11.9, KH2PO4 1.2 and glucose 11.0 (pH 7.4) using a peristaltic pump (Zampl, Poland) with a flow rate of 0.6 ml/min. The solution was aerated with air from a microcompressor MKM-7 (Practik-NC, Russia). The sodium bicarbonate concentration was reduced to 11.9 mM to obtain рН 7.4 (8). The temperature was kept at 37–37.5 °C with the aid of an ultrathermostat U10 (Medingen, GmbH, Germany). One side of the bronchial strips along their longitudinal axis was fastened to bottom of the bath using tungsten needles, the opposite side was combined with an electromechanical transducer (SPA “Introtest”, Russia). The initial force tension of the bronchial preparations was adjusted to 500 mg. Before starting the experiment, bronchial samples were pre-incubated in the solution for 60 min to allow the resting tension to equilibrate. The changes in isometric contraction of the bronchial samples (expressed as tension force in mg) induced by electrical stimulation were evaluated. Bronchial muscle tension was converted into an electrical signal which was fed to an analog-to-digital converter (L-CARD 14-140, Russia) and recorded on a computer (Fig. 1).
Fig. 1.
Myogram. Contraction of a rat isolated bronchial segment in response to field stimulation. Arrows show the commencement of the application of test substances application (in this case histamine and adenosine) to the organ bath.
Preparations of bronchi were stimulated using an electrical stimulator (ES-5, Russia) with the following parameters: 7 Hz, 0.5 ms (stimulation of preganglionic nerves) and 30 Hz, 2.0 ms (stimulation of smooth muscle cells). In all cases the amplitude of impulses was 20 V, the impulse duration 10 sec, with an interval between impulses of 2.5 min. Each bronchial sample underwent four series of electrical stimulation, with 9 stimuli in each series. In the first and second series preganglionic nerve stimulation was performed, while in the third and fourth series smooth muscle was stimulated. Average amplitude of muscle contractions in response to three initial stimuli was considered as a control (baseline) value (expressed in mg). Mean amplitudes of contractions after 4–6, and 7–9 stimulations were referred to as responses to additional long-term stress determining the contractile potential of the airway smooth muscle and were expressed as per cent ratios to contraction amplitude at control stimulation, which was taken as the 100% reference value (9). Bronchial preparations underwent 30-min washings with saline between subsequent experimental series.
To evaluate possible mechanisms of the contribution of mast cells to the contractile activity of bronchial smooth muscle, in the second and fourth series the effect of histamine (main mast cell mediator) and adenosine (mast cell activator) on the bronchial sample contraction amplitude has been studied in the course of nerve and smooth muscle stimulation. Pharmaceutical substances were introduced into the experimental bath: histamine (9 × 10–8 M) 1 min before the 4-th stimulation, adenosine (3.7 × 10–8 M) 1 min before the 7th stimulation. The contractile activity of bronchial samples was evaluated by the contraction amplitude in response to the 4th stimulation for histamine and to the 7th stimulation for adenosine. The interval between histamine and adenosine administration was 7.5 min which was sufficient to wash out previously administered substances from the bath (10).
Histological analysis
To obtain samples for histological studies, rat lungs were expanded by intratracheal infusion of 10% formaldehyde solution. Tissue specimens were embedded in paraffin, microtome slices (5 to 7 μm thick) were stained with toluidine blue to identify mast cells and examined at 120-fold magnification using a binocular microscope MC 300 (S) (Micros, Austria).
Statistical evaluation
Statistical evaluation was performed using Microsoft Excel 7.0 software including calculations of mean values for maximal contraction amplitudes of bronchial smooth muscle with the S.E.M. Comparison of mean values was performed by Student’s t-test, and pairwise comparison methods. A value of P<0.05 was considered to be significant.
Results
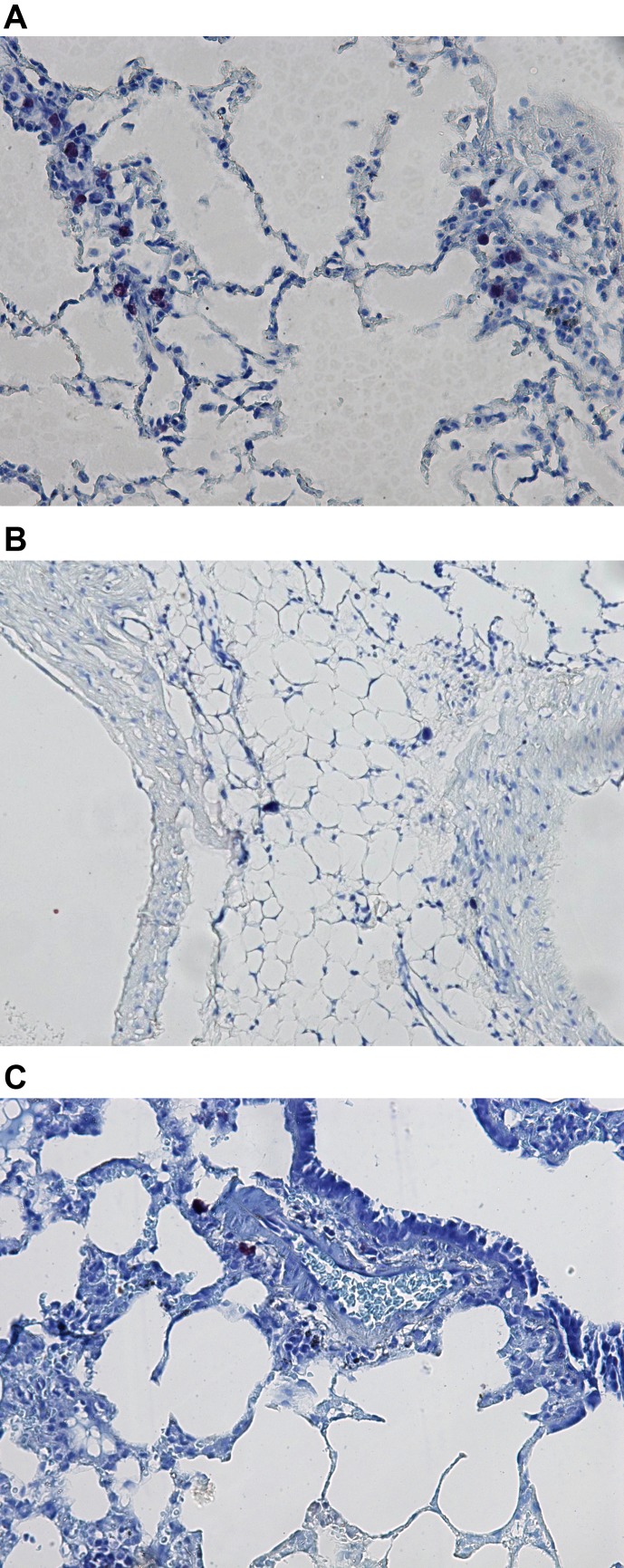
Histological analysis revealed a large number of mast cells in the lung tissue of the control rats for the COPD model (Fig. 2A), in contrast to lung preparations of intact rats (Fig. 2B) and rats treated with cromoglycate sodium (Fig. 2C).
Fig. 2.
A: Lung tissue of rat with induced COPD. Note the large number of mast cells (black “blots”) showing metachromasia in the lung interstitium (toluidine blue staining, × 120). B: Lung tissue of intact rat. Note scattered mast cells (black “blots”) in the lung interstitium (toluidine blue staining, × 120). C: Lung tissue of rat with induced COPD and treated with cromoglycate sodium (Cromo). Note scattered mast cells (black “blots”) in the lung interstitium (toluidin blue staining, × 120).
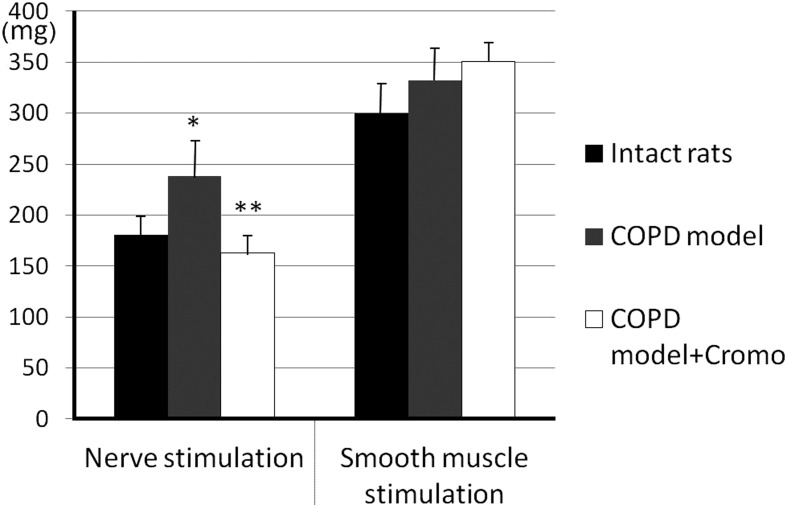
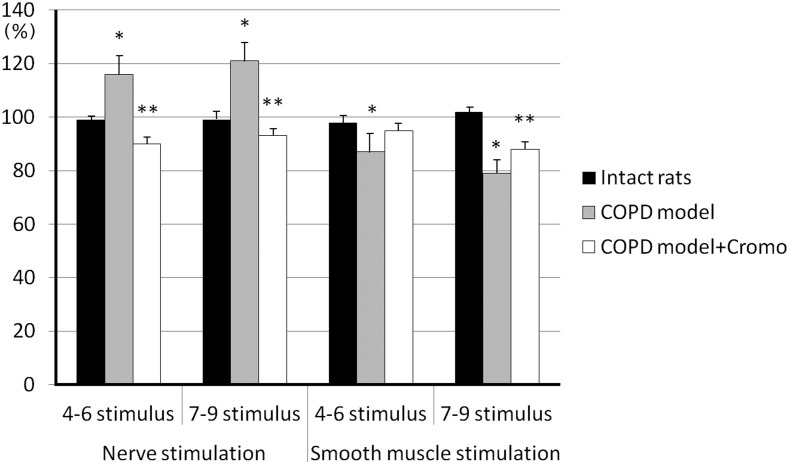
After 60-days exposure to NO2, bronchial smooth muscle contraction amplitude increased in response to control nerve stimulation compared with response of the intact samples: 237.9 ± 24.5 and 181.3 ± 12.3 mg, respectively (P<0.05, n=26) (Fig. 3). Additional nerve stimulations were accompanied by even greater bronchial contractile activity (up to 115.6 ± 3.7% and 121.4 ± 4.8%, P<0.05, n=26) in contrast to the intact samples, the response of which remained unchanged (Fig. 4). Control stimulation of smooth muscle did not cause significant tonus enhancement in COPD samples compared to the intact samples: 331.7 ± 33.9 and 300.4 ± 33.6 mg, respectively (P>0.05, n=26) (Fig. 3). Additional smooth muscle stimulations of COPD samples resulted in response decrease down to 87.2 ± 4.6% and 79.0 ± 2.6% (P<0.05, n=26) (Fig. 4).
Fig. 3.
Contractile activity of bronchial samples caused by control stimulation of preganglionic nerve and smooth muscle in rats with induced COPD and treated with cromoglycate sodium (Cromo). Y-axis – bronchial contraction, mg; * and ** - significant differences as compared with intact rats and “COPD model”, respectively, P<0.05.
Fig. 4.
Contractile activity of bronchial samples caused by additional stimulation of preganglionic nerve and smooth muscle in rats with induced COPD and treated with cromoglycate sodium (Cromo). Y-axis - changes of bronchial contraction are expressed as per cent ratios to contraction amplitude at control stimulation; * and ** - significant differences as compared with control stimulation and “COPD model”, respectively, P<0.05.
The group of animals receiving cromoglycate sodium demonstrated a decrease in bronchial contraction amplitude in response to control nerve stimulation, in contrast to the COPD group, down to 162.5 ± 9.9 mg (P<0.05, n=26) showing no significant difference from the specimen response in intact rats (P>0.05, n=26) (Fig. 3). Additional stimulation of preganglionic nerves resulted in further contraction amplitude decrease – down to 89.7 ± 2.0% and 93.1 ± 2.5% of the response to control nerve stimulation (P<0.05, n=26) (Fig. 4). Contractile response to control smooth muscle stimulation (351.2 ± 21.4 mg) showed no difference from bronchial samples in control rats, as well as intact rats (P>0.05, n=26) (Fig. 3).
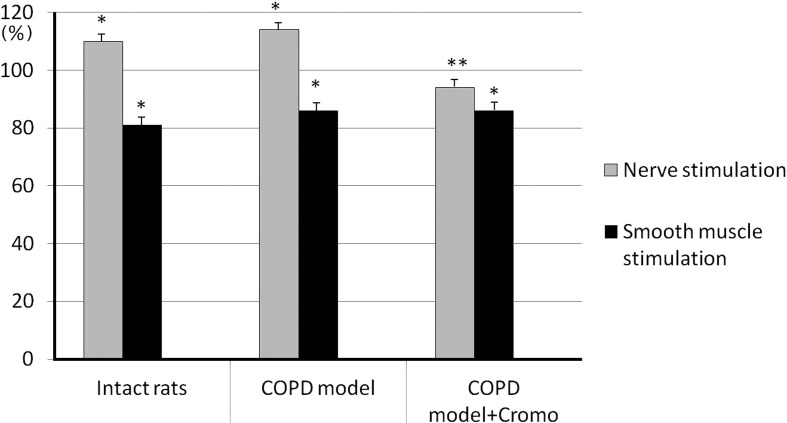
After addition of exogenous histamine into the perfusion medium, nerve stimulation caused bronchial sample contraction amplitude enhancement in both intact and control animals up to 110.2 ± 2.1% and 114.4 ± 2.3%, respectively, (P<0.05, n=26) without affecting bronchial sample contractile responses in rats receiving cromoglycate sodium, 94.0 ± 2.6% (P>0.05, n=26) (Fig. 5). In the case of smooth muscle stimulation, contraction amplitude significantly decreased (P<0.05) in all three groups amounting to 81.4 ± 2.9% of the response to control stimulation in the intact group, 85.9 ± 2.7% in the control group and 85.7 ± 2.3% in the group of animals receiving sodium cromoglycate (Fig. 5).
Fig. 5.
Effect of histamine (9 × 10-8 M) on the contractile activity of bronchial samples caused by additional stimulation of preganglionic nerve and smooth muscle in rats with induced COPD and treated with cromoglycate sodium (Cromo). Y-axis - changes of bronchial contraction are expressed as per cent ratios to contraction amplitude at control stimulation; * and ** - significant differences as compared with control stimulation and “COPD model”, respectively, P<0.05.
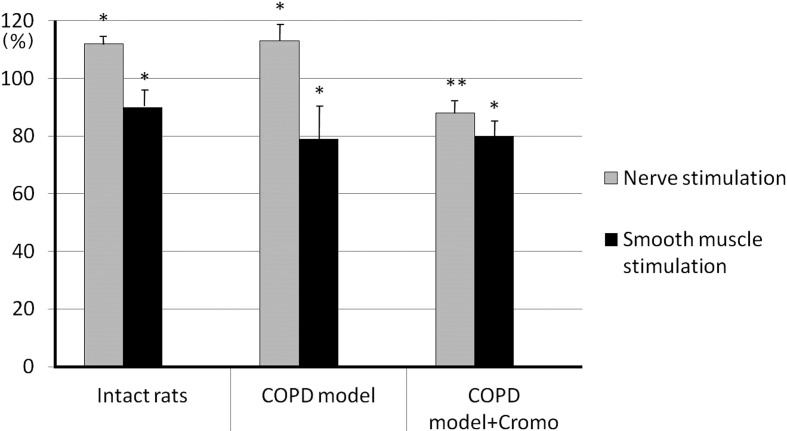
In response to adenosine administration the bronchial samples’ responses, caused by nerve stimulation, intensified both in the intact and control group up to 111.7 ± 1.2% (P<0.05, n=26) and 112.5 ± 3.2% (P<0.05, n=26), respectively. On the contrary, the bronchial samples’ tonus in rats receiving cromoglycate sodium decreased down to 88.5 ± 2.1% (P<0.05, n=26) (Fig. 6). When being stimulated, the smooth muscle demonstrated significant decrease of contractile amplitude (P<0.05, n=26) in all three groups amounting to 89.9 ± 2.8% in the intact group, 79.3 ± 4.0% in the control group and 80.1 ± 3.6% in the group of animals receiving cromoglycate sodium, of the response to control stimulation (Fig. 6).
Fig. 6.
Effect of adenosine (3.7 × 10-8 M) on the contractile activity of bronchial samples caused by additional stimulation of preganglionic nerve and smooth muscle in rats with induced COPD and treated with cromoglycate sodium (Cromo). Y-axis - changes of bronchial contraction are expressed as per cent ratios to contraction amplitude at control stimulation; * and ** - significant differences as compared with control stimulation and “COPD model”, respectively, P<0.05.
Discussion
In recent years there has been increasing evidence that mast cells play a key role in COPD immunopathology (inflammation, remodeling, bronchial obstruction) (11,12,13,14). The mast cell population in pulmonary structures is large and functionally heterogeneous. Airway mast cells may be affected by aggressive pollutants in the inspired air, which results in their activation and release of a great variety of effector molecules, including those accumulated in granules as well as newly synthesized mediators, cytokines and chemokines. Smokers show significant increase in the number of mast cells in bronchoalveolar epithelial lining fluid and in all structural elements of bronchial mucosa, including the smooth muscle layer. Correlation between the increased number of labrocytes and pulmonary function impairment was noted (15,16,17). Gosman et al. (2) have demonstrated an increased number of chymase- and tryptase-positive mast cells in peripheral respiratory tract in COPD patients correlating well with pulmonary function improvement.
In the present experiments, the development of the COPD model, induced by 60-day exposure to NO2 (one of the cigarette smoke components), was accompanied by the development of bronchial wall smooth muscle hyperactivity, which intensified with prolonged nerve stimulation. Bronchial contractile activity decreased with prolonged smooth muscle stimulation. The prevention of mast cell degranulation and biologically active substances release (primarily the main mediator of mast cells histamine) by stabilizing of cell membrane with chromoglycate sodium eliminated these effects, and bronchial specimen responses to the stimulation of both nerves and bronchial smooth muscle approached the responses of intact bronchial preparations.
The mechanisms of mast cell activation in COPD patient airways are poorly understood. The mechanisms of mast cells activation and degranulation mediated by immunoglobulin E are not considered to be critical for COPD, in contrast to asthma (12). The activation of adenosine receptors of A2B subtype in resident pulmonary mast cells membrane stimulated the production of pro-inflammatory cytokines and histamine release from granules, which resulted in smooth muscle contraction (18). In asthma patients adenosine or adenosine-5’-monophosphate provocative test caused short-term bronchoconstriction, which could be prevented by prophylactic administration of the histamine H1-receptor antagonist, chromoglycate sodium, local anesthetics and atropine (19). This wide range of bronchoconstriction blockers means that mast cell mediators and neuronal structures are involved in the adenosine effect (20). Along with its effect on mast cells, adenosine can excite C-fibers of nonadrenergic noncholinergic system with tachykinins secretion (14) as well as smooth muscle adenosine receptors (21), which can result in the constriction effect. In our experiments, adenosine application on bronchial samples of intact rats and control COPD rats caused an increased smooth muscle contraction, and induced nerve stimulation. The bronchial specimens of rats receiving chromoglycate sodium, in contrast, showed significant decrease in their contraction amplitude. Similar results were obtained when histamine was applied to bronchial samples. The study of isolated trachea and bronchi samples of intact rats showed that histamine stimulates histamine H1- receptors, located at postganglionic cholinergic nerves, which is accompanied by smooth muscle contraction enhancement in response to nerve stimulation (10). It is supposed that in the COPD model the enhancement mechanism of bronchial smooth muscle contractile activity could be due to activation of the transmembrane adenosine receptors of resident mast cells, leading to their partial degranulation and histamine release. Histamine H1-receptors excitation, in its turn, is transmitted to the smooth muscle by reflex pathways through intramural ganglion neurons, that initiate smooth muscle contraction enhancement. Since, as previously noted, long-term NO2 effect results in capsaicin-sensitive C-fibers desensitization (22), their role in bronchial smooth muscle tonus enhancement within the present COPD model seem to be insignificant. Mast cell degranulation and histamine release inhibition prevented the development of bronchial smooth muscle hyperactivity in rats treated with membrane stabilizer, chromoglycate sodium.
The lack of a contractile reaction in the bronchial samples in all three animal groups in response to adenosine and histamine application, while the smooth muscle was stimulated, may be due to the fact that the low concentrations of these agents that we used were insufficient to reach sensitivity threshold in smooth muscle receptors. Furthermore, histamine H2-receptors in the smooth muscle are activated under the action of histamine in low concentrations, which stimulates cyclic adenosine monophosphate synthesis in smooth muscle cells leading to muscle relaxation (23).
Thus, the prevention of mast cell degranulation and endogenous histamine release by cellular membrane stabilization with chromoglycate sodium prevents the development of bronchial smooth muscle hyperactivity due to long-term inhalation exposure to NO2 in the experimental COPD model. The study results give us reason to believe that the mechanism of bronchial wall smooth muscle contractile activity enhancement is mediated by transmembrane adenosine receptors activation of resident mast cells resulting in their partial degranulation with histamine release which affects histamine H1-receptors triggering reflex pathways through intramural ganglion neurons. The revealed dilatation effect of chromoglycate sodium on bronchial smooth muscle together with its anti-inflammatory effect allows to consider the clinical perspectives of using this preparation at early stages of COPD development.
Conflict of interests
All authors declare that they have no conflicting interests.
References
- 1.Tunon-de-Lara JM, Berger P, Bégueret H. Mast cells in airway smooth muscle. N Engl J Med. 2002; 347(13): 1040–1. doi: 10.1056/NEJM200209263471318 [DOI] [PubMed] [Google Scholar]
- 2.Gosman MM, Postma DS, Vonk JM, Rutgers B, Lodewijk M, Smith M, Luinge MA, Ten Hacken NH, Timens W. Association of mast cells with lung function in chronic obstructive pulmonary disease. Respir Res. 2008; 9(1): 64. doi: 10.1186/1465-9921-9-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltani A, Ewe YP, Lim ZS, Sohal SS, Reid D, Weston S, Wood-Baker R, Walters EH. Mast cells in COPD airways: relationship to bronchodilator responsiveness and angiogenesis. Eur Respir J. 2012; 39(6): 1361–7. doi: 10.1183/09031936.00084411 [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1. J Smooth Muscle Res. 2008; 44(2): 83–93. doi: 10.1540/jsmr.44.83 [DOI] [PubMed] [Google Scholar]
- 5.Erjefält JS. Mast cells in human airways: the culprit? Eur Respir Rev. 2014; 23(133): 299–307. doi: 10.1183/09059180.00005014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zutphen LFM, Baumans V, Beynen AC, editors Principles of Laboratory Animal Science, rev. ed. Amsterdam: Elsevier; 2001. 428 p. [Google Scholar]
- 7.Lebedeva ES, Kuzubova NA, Danilov LN, Titova ON, Dvorakovskaya IV, Kozlova MY, Preobrazhenskaya TN, Platonova IS. Experimental modelling of chronic obstructive pulmonary disease. Bull Exp Biol Med. 2012; 152(5): 659–63 . doi: 10.1007/s10517-012-1601-3 [DOI] [PubMed] [Google Scholar]
- 8.Blattner R, Classen HG, Dehnert H, Doring HJ. Experiments on isolated smooth muscle preparations. Freiburg: Hugo Sachs Elektronik KG; 1978. 185 p. English edition prepared by Barnden IM, Colson R. 1980. [Google Scholar]
- 9.Kuzubova NA, Lebedeva ES, Fedin AN, Dvorakovskaya IV, Preobrazhenskaya TN, Titova ON. Effect of fenspiride on bronchial smooth muscle of rats with chronic obstructive pulmonary disease. J Smooth Muscle Res. 2013; 49: 46–54. doi: 10.1540/jsmr.49.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedin AN, Kiver EN, Smirnova LE, Kirilina VM, Krivchenko AI. Role of intramural ganglia of airways in histamine action. Russ J Physiol. 2014; 100(9): 1059–67. [PubMed] [Google Scholar]
- 11.Andersson CK, Mori M, Bjermer L, Löfdahl CG, Erjefält JS. Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010; 181(3): 206–17. doi: 10.1164/rccm.200906-0932OC [DOI] [PubMed] [Google Scholar]
- 12.Mortaz E, Folkerts G, Redegeld F. Mast cells and COPD. Pulm Pharmacol Ther. 2011; 24(4): 367–72. doi: 10.1016/j.pupt.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 13.Silva OR, Montes JF, Garcia-Valero J, Olloquequi J. Cellular effectors of the inflammatory response in chronic obstructive pulmonary disease (COPD). Rev Med Chil. 2015; 143(9): 1162–71. doi: 10.4067/S0034-98872015000900009 [DOI] [PubMed] [Google Scholar]
- 14.Cruse G, Bradding P. Mast cells in airway diseases and interstitial lung disease. Eur J Pharmacol. 2016; 778: 125–38. doi: 10.1016/j.ejphar.2015.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekberg-Jansson A, Amin K, Bake B, Rosengren A, Tylén U, Venge P, Löfdahl CG. Bronchial mucosal mast cells in asymptomatic smokers relation to structure, lung function and emphysema. Respir Med. 2005; 99(1): 75–83. doi: 10.1016/j.rmed.2004.05.013 [DOI] [PubMed] [Google Scholar]
- 16.Mortaz E, Redegeld FA, Sarir H, Karimi K, Raats D, Nijkamp FP, Folkerts G. Cigarette smoke stimulates the production of chemokines in mast cells. J Leukoc Biol. 2008; 83(3): 575–80. doi: 10.1189/jlb.0907625 [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, Reid DW, Zhang D, Ward C, Wood-Baker R, Walters EH. Assessment of airway inflammation using sputum, BAL, and endobronchial biopsies in current and ex-smokers with established COPD. Int J Chron Obstruct Pulmon Dis. 2010; 5: 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudich N, Ravid K, Sagi-Eisenberg R. Mast cell adenosine receptors function: a focus on the a3 adenosine receptor and inflammation. Front Immunol. 2012; 3: 134. doi: 10.3389/fimmu.2012.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006; 533(1-3): 77–88. doi: 10.1016/j.ejphar.2005.12.056 [DOI] [PubMed] [Google Scholar]
- 20.Blazhevich LE, Kirilina VM, Fedin AN, Krivchenko AI. Effect of adenosine on the trachea and bronchi smooth muscle contraction in rats. Russ J Physiol. 2016; 102(1): 41–9. [Google Scholar]
- 21.Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol Ther. 2009; 123(1): 105–16. doi: 10.1016/j.pharmthera.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedin AN, Kuzubova NA, Danilov LN, Lebedeva ES, Postnikova TY, Krivchenko AI. Bronchodilator effect of prednisolone in rats inhaled with nitrogen dioxide. Russ J Physiol. 2010; 96(3): 293–300. [PubMed] [Google Scholar]
- 23.Fedin AN, Kryukova EN, Nekrasova EA. Interaction of histamine and glucocorticoids with neural structures of the respiratory tract. Neurosci Behav Physiol. 2003; 33(3): 289–94. doi: 10.1023/A:1022111717241 [DOI] [PubMed] [Google Scholar]