Abstract
AIM
To examine the safety and efficacy of mesenchymal stem cell (MSC) therapy for intracerebral haemorrhage with neurological dysfunctions for a year.
METHODS
MSC were ex vivo expanded from 29 mL (17-42 mL) autologous bone marrow. Patients were randomized to have two intravenous injections of autologous MSC or placebos in four weeks apart. Neurological functions and clinical outcomes were monitored before treatment and at 12th, 16th, 24th, 36th and 60th week upon completion of the treatment.
RESULTS
A mean of 4.57 × 107 (range: 1.43 × 107-8.40 × 107) MSC per infusion was administered accounting to 8.54 × 105 (2.65 × 105-1.45 × 106) per kilogram body weight in two occasions. There was neither adverse event at time of administration nor sign of de novo tumour development among patients after monitoring for a year post MSC therapy. Neuro-restoration and clinical improvement in terms of modified Barthel index, functional independence measure and extended Glasgow Outcome Scale were evident among patients having MSC therapy compared to patients receiving placebos.
CONCLUSION
Intravenous administration of autologous bone marrow-derived MSC is safe and has the potential of improving neurological functions in chronic stroke patients with severe disability.
Keywords: Stroke, Intracerebral haemorrhage, Central nervous system, Mesenchymal stem cells, Cell therapy
Core tip: Contemporary treatments are ineffective in restoring lost neurological functions after stroke. Many stroke patients were noted to have lesions close to the sub-ventricular zone. The likely beneficial effects of mesenchymal stem cell (MSC) treatment might correlate with the spatial lesion, not part of the sub-ventricular zone where endogenous neurogenesis persists during adulthood, and indirect chaperon effects of MSC promote endogenous neuro-regeneration. We administered MSC intravenously to patients having severe neurological disability and presenting stable baseline scores one year after the onset of intracerebral haemorrhage to eliminate confounding attributes to the observation of MSC-mediated neurological recovery.
INTRODUCTION
Stroke is a common neurological disorder and is a leading cause of death. More than six million cases of stroke are reported in the world annually[1]. Approximately 50% of the patients died and most of the survivors are left with various degree of neurological dysfunction. There is no effective treatment for restoring the neurological function of the patients to date. Recent studies in animal models of intra-cerebral haemorrhage demonstrated active neurogenesis in the sub-ventricular zone leading to new neurons of phenotypes of their dead counterparts[2,3]. Nevertheless, a majority of newly formed neurons die during the early weeks after stroke, and successful replacement only accounts for a small portion of the mature dead neurons[4]. The feasibility of using a variety of cell types including neural stem cells, embryonic stem cells, umbilical cord blood cells and mesenchymal stem cells (MSCs) to enhance re-innervation has been demonstrated in animal models[5-8]. The breakthrough opens cellular therapy for stroke. In clinical setting a reliable and accessible cell source is requisite. Bone marrow-derived MSCs, which were noted to generate trophic factors, growth stimulants, signalling regulators and cytokines, might help promote neuro-regeneration and neuro-restoration after stroke via neurogenesis, angiogenesis and synaptogenesis[9]. Cellular therapy employing large numbers of ex-vivo expanded viable MSC might be a potential treatment modality to patients after stroke.
An earlier study demonstrated the feasibility and safety of infusion of autologous MSC in patients nine weeks after stroke onset[10]. In the present study we conducted a phase I/II randomized controlled trial of autologous bone marrow-derived MSC therapy in patients one year after onset of stroke with the aim to study the long-term safety and functional efficacy of intravenous administration of MSC.
MATERIALS AND METHODS
Study design
This study is a randomized, controlled, double-blind, phase I/II clinical trial (CREC #2006.425-T) and was approved by the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee of Hong Kong Hospital Authority in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation - Good Clinical Practice. Inclusion criteria are that patient had the onset of stroke for one year ago with stable National Institutes of Health Stroke Scale scores ≥ 7 and Glasgow Outcome Scale score of severe disability and vegetative state at one year after onset of stroke[11,12]. Exclusion criteria are lacunar syndrome, malignant diseases, severe co-morbidity, hepatic/renal dysfunction and unwillingness to participate. Patients who presented stable baseline scores indicating severe neurological disability were recruited to the study. Informed consent was obtained from all subjects - in the case of vegetative state, from their next of kin. Eligible patients were randomly assigned to the treatment group and control group for autologous MSC therapy (Figure 1). The study protocol was developed according to the guidelines of Consolidated Standards of Reporting Trials available on-line at http://www.consort-statement.org/.
Figure 1.
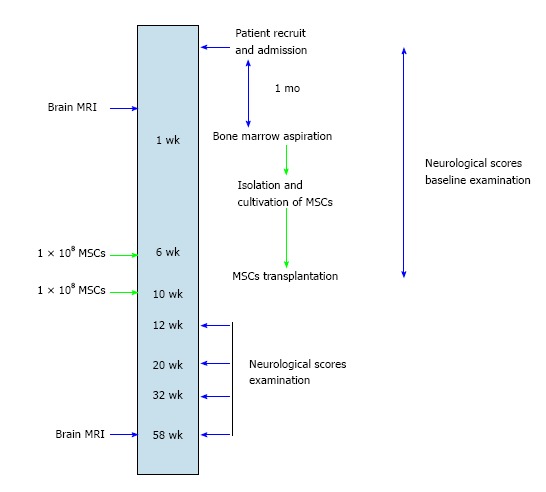
Workup schema for patients recruited to the clinical trial of autologous mesenchymal stem cell therapy. MSC: Mesenchymal stem cell; MRI: Magnetic resonance imaging.
Radio-imaging
Computed tomography (CT) scan was conducted at onset of intracerebral haemorrhage. Haematoma volume in mL was computed by using the formula: ½ × maximal height (cm) × width (cm) × anterior-posterior diameter (cm). Magnetic resonance imaging (MRI, 1.5 Tesla) of the brain were performed on the day before the first injection of either MSC or placebos. A follow-up procedure was conducted on patients at 60th week upon completion of the study (Figure 1).
Ex vivo expansion and infusion of MSC
The procedure of MSC expansion described by Le Blanc et al[13] was adopted with minor modifications. In brief, bone marrow aspirates from the superior iliac crest of patients under local anaesthesia were anti-coagulated in 10 IU/mL preservative-free heparin (DBL, Hospira, Melbourne, Australia). Mononuclear cells were enriched by using density-gradient centrifugation in ficoll-hypaque with specific gravity of 1.077 g/mL (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and cultured in low-glucose Dulbecco modified eagles’ medium (Invitrogen, Life Technologies, Carlsbad, CA, United States) supplemented with 10% foetal bovine serum (Invitrogen). MSC cultures of approximately 80% in confluence were passaged using 0.05% trypsin (Invitrogen) and ex vivo expanded by subcultures in 175 cm2 flasks.
On the day of infusion, MSC cultures were enzymatically segregated and dislodged from culture flasks by trypsin digestion, washed with phosphate-buffered saline, sieved to remove cell aggregates via 40-μm filter and re-suspended in 10 mL 5% normal human albumin (Hong Kong Red Cross Blood Transfusion Service) for intravenous injection in five to ten minutes. Another booster bolus of autologous MSC was prepared and administered to patient four weeks thereafter. A placebo of an equal volume of 5% normal human albumin was administered to patients being allotted to the control group. Cultures and cell processing were conducted under conditions meeting the requirements of good manufacturing practices.
Characterisation of MSC
Immunophenotyping of MSC by flow cytometry was reported elsewhere[14]. Unless stated otherwise, fluorescence-conjugated monoclonal antibodies from Beckman Coulter were used. They were IgG1-FITC, IgG1-PE, HLA-DR-FITC, CD45-FITC, CD3-FITC, CD19-PE, CD16-FITC, CD33-FITC, CD38-FITC, CD34-PE, CD133-PE (Miltenyi Biotec GmbH, Germany), CD29-PE, CD44-FITC, CD73-FITC, CD90-PE, CD105-PE (Serotec, United Kingdom) and CD166-PE were used. At least 10000 events were acquired and signals were analysed by using the Coulter Epic XL MCL flow cytometer (Coulter, Miami, FL, United States).
Procedural details of immunofluorescence staining were described previously[15]. IgM anti-stage-specific embryonic antigen-4 (SSEA-4, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, United States), IgG2b anti-octamer-binding transcription factor-4 (Oct-4; 1:100, Santa Cruz Biotechnology), IgG1 anti-Nestin (1:400; BD Biosciences, San Francisco, CA, United States) were employed.
Cell viability was evaluated by using trypan blue dye exclusion test. Sterility check against microbial contamination was conducted at each MSC passage.
Neurological functional assessments
Patient safety and efficacy of cell therapy were evaluated during the first and second MSC infusion and thereafter at 16th, 24th, 36th and 60th week of the study (Figure 1). The safety of intravenous infusion of either MSC or placebos was assessed in terms of the development of immediate or delayed adverse reactions. Immediate reactions included allergic responses (tachycardia, fever, skin eruption and leucocytosis), local complications (hematoma, local infection at the injection site), vascular obstructions (tachypnea, oliguria, peripheral vascular insufficiency, recurrence of stroke), and systemic complications (systemic infections, increased aspartate aminotransferase, alanine aminotransferase and/or blood urea nitrogen/creatinine levels). Presence of delayed adverse reaction of tumour formation was evaluated by physical examination of skin and oral mucosa and followed up with magnetic resonance imaging (MRI), if necessary. Modified Barthel Index and Functional Independence Measure were monitored by a neurologist being blinded to group allocation and radiological data[16,17]. Scores of Extended Glasgow Outcome Scale were also used to track the progress of disability of patients over time[12]. Stroke scale scores, vascular risk factors, medical history and demographic details were recorded.
Statistical analysis
Means, ranges and standard deviations of continuous variables of years of age, kilogram in body weight, milliliter of hematoma and bone marrow, percentages of cell counts and viability were calculated. Assuming that data were normally distributed, non-parametric Wilcoxon’s rank sum test was used to compare variables derived from the treatment and control groups in the study. Paired t-test with one-sided testing was used to analyse scores of modified Barthel Index, Functional Independence Measure and Extended Glasgow Outcome Scale of patients at time of assessments. Fisher’s exact test was applied to examine the incidence of clinical neurological improvement between the treatment and control groups of patients. Differences between groups were regarded as significant if P ≤ 0.05.
RESULTS
Patient characteristics
We conducted a double-blind, randomized, controlled phase I/II trial to examine the safety and efficacy of autologous MSC therapy in a small cohort of nine patients (four females and five males) with a mean age of 52 years (range: 41-59 years) who had undergone intracerebral haemorrhage (ICH) for a year. CT scan at time of the onset demonstrated cerebral haematoma of 52 mL (12-75 mL) located in the basal ganglion region of the brains of the nine patients in the study cohort. The sizes of the lesion areas were comparable between the treatment and control groups.
MSC were ex vivo expanded from a mean volume of 29 mL (17-42 mL) autologous bone marrow. Patients were randomized to have two intravenous injections of autologous MSC (treatment group of MSC: n = 5 or control group of placebos: n = 4) four weeks apart. The body weight of patients in the treatment group were statistically lighter than those of the control group [treatment vs control; 54.2 kg (42-60 kg) vs 67.2 kg (64-72.7 kg), P = 0.03], however the years of age of both groups were comparable [treatment vs control; 53.4 (48-56) vs 51.5 (41-59); P = 0.64]. There was no difference between the severities of disability in terms of neurologic scores of patients assigned to both groups (data not shown).
MSC autograft
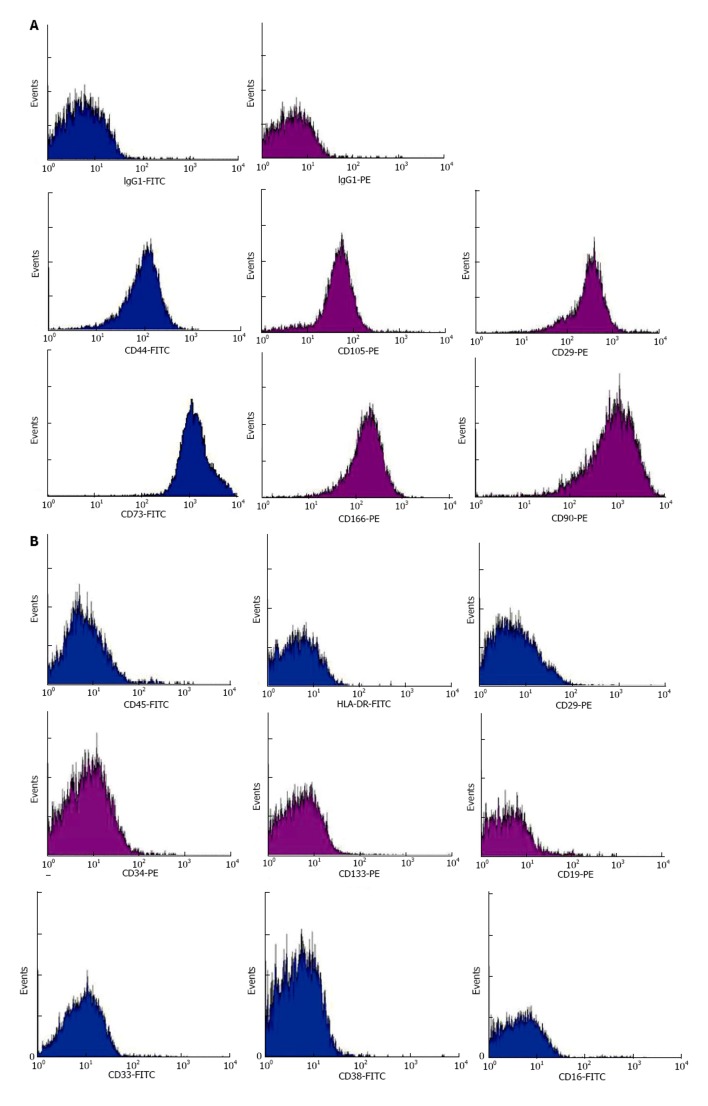
Ex vivo expanded MSC at a mean of 4 passages (1-8) were used for infusion. MSC up to passage-8 displayed longitudinal, bi-polar, spindle-shaped and fibroblast-like morphology. Immunofluorescence staining demonstrated expressions of embryonic stem cell marker SSEA-4, transcription factor Oct-4 and neural stem cell marker Nestin; suggesting the pluripotency and neurogenesis of MSC (Figure 2). Flow cytometry demonstrated that they were immunophenotypically positive for CD29, CD44, CD73, CD90, CD105 and CD166 (Figure 3A), but negative for haematopoietic stem cell markers (CD34 and CD133), myeloid progenitor cell markers (CD33 and CD38), leucocyte markers (HLA-DR and CD45), T-cell marker CD3, NK cell marker CD16 and B-cell marker CD19 (Figure 3B).
Figure 2.
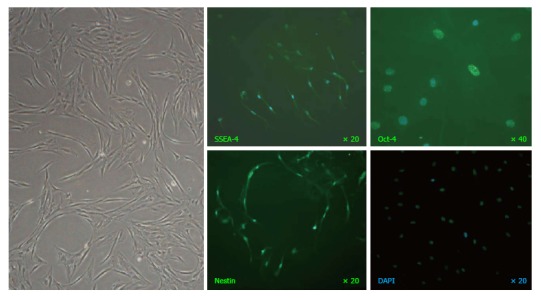
A representative image of mesenchymal stem cells at passage-8 captured under phase-contrast microscopy (left panel) and immunofluorescence staining of stage-specific embryonic antigen SSEA-4, transcription factor Oct-4 and neural stem cell marker Nestin (green fluorescence) with nuclei counterstained by DAPI (blue fluorescence).
Figure 3.
Representative histograms derived from infusates by flow cytometric analyses of mesenchymal stem cell markers CD29, CD44, CD73, CD90, CD105 and CD166 (A) and haemic markers HLA-DR, CD45, CD3, CD19, CD16, CD33, CD38, CD34 and CD133 (B). FITC conjugation in blue and PE conjugation in purple.
MSC infusions
Table 1 shows numbers and doses of MSC in 11 episodes of infusion into five patients (three females and two males). A mean of 4.57 × 107 (1.43 × 107-8.40 × 107) MSC per infusion was administered accounting to 8.54 × 105 (2.65 × 105-1.45 × 106) per kilogram body weight in two occasions except Patient NSCT02 underwent three infusions. Infused cells were immunophenotypically homogenous; HLA-DR-, CD45-, CD3-, CD19-, CD16-, CD33-, CD38-, CD34- and CD133-positive cells were less than 1% on average, whereas CD29-, CD44-, CD73-, CD90-, CD105- and CD166-positive cells were more than 96% (Supplementary Tables 1 and 2). Cell viability was 94.4% (88.5%-99.0%). There was no microbial growth as evident by aerobic and anaerobic cultures of 11 infusates. The control group of four patients (one female and three males) received placebos in an identical manner. No adverse reaction of acute infusion-related toxicity, transient fever, complication in organs or infection was experienced by both groups of patients at time of and a day following MSC administration. There was no sign of tumour development among patients in the study cohort having monitored for a year.
Table 1.
Characteristics of intracerebral haemorrhage patients and infuses for autologous bone marrow-derived mesenchymal stem cell therapy
| Patients | Sex/age in years | Location of haemorrhage | CT readout (cm) | Hemorrhage volume (mL) | MSC passage numbers | Viability (%) | MSC (× 107) | MSC/kg (× 105) |
| UPN02 | F/50 | Basal ganglia, left | 4.4 × 5.8 × 5.9 | 75 | 4, 7 | 96.8 | 3.1 | 5.44 |
| 3-5 | 92.1 | 3.2 | 5.61 | |||||
| 3-6 | 92.6 | 3.1 | 5.44 | |||||
| UPN08 | F/48 | Basal ganglia, left | 4.0 × 2.0 × 3.0 | 12 | 3, 4 | 95.8 | 1.43 | 2.65 |
| 3, 4 | 96.5 | 1.43 | 2.65 | |||||
| UPN09 | M/56 | Basal ganglia, right | 6.5 × 5.0 × 4.0 | 65 | 3-8 | 98.5 | 7.6 | 13.1 |
| 2-6 | 96.8 | 4 | 6.84 | |||||
| UPN10 | F/55 | Frontal lobe, right | 5.6 × 4.5 × 5.0 | 63 | 1-6 | 88.5 | 5.5 | 13.1 |
| 1-6 | 98 | 5.6 | 12.4 | |||||
| UPN11 | M/55 | Basal ganglia, left | 2.4 × 5.0 × 3.5 | 21 | 1-6 | 90.5 | 5.4 | 9 |
| 1-7 | 99 | 8.4 | 14.5 | |||||
| UPN05 | F/59 | Basal ganglia, left | 6.0 × 4.0 × 4.0 | 48 | ||||
| UPN06 | M/56 | Basal ganglia, frontoparietal temporal lobe, right | 7.3 × 3.5 × 5.0 | 64 | ||||
| UPN07 | M/41 | Basal ganglia, right | 4.3 × 6.4 × 5.0 | 69 | ||||
| UPN12 | M/50 | Basal ganglia, | NA | 55 | ||||
| putaminal, right |
MSC: Mesenchymal stem cell; CT: Computed tomography; NA: Not available.
Table 2.
Modified Barthel indices of the treatment group (n = 5) having mesenchymal stem cell therapy at the 1st, 12th, 16th, 24th, 36th and 60th week upon completion of the study
| Study duration (wk) |
Modified barthel indices |
P value | ||||
| UPN02 | UPN08 | UPN09 | UPN10 | UPN11 | ||
| 1st | 19 | 66 | 0 | 4 | 69 | |
| 12th | 19 | 73 | 0 | 5 | 70 | 0.12 |
| 16th | 30 | 76 | 0 | 5 | 77 | 0.03 |
| 24th | 32 | 76 | 0 | 5 | 77 | 0.03 |
| 36th | 32 | 78 | 1 | 6 | 77 | 0.02 |
| 60th | 32 | 78 | 1 | - | 77 | 0.03 |
Data derived from different time points were compared to the baseline values established at the first week of the study.
Functional outcomes
Neurological functions and clinical outcomes were monitored before and at 12th, 16th, 24th, 36th and 60th week upon completion of the treatment. In terms of the scores of modified Barthel Index and Functional Independence Measure, the magnitudes of physical and cognitive disability were comparable between the treatment and control groups. Improvements of motor disability and cognitive impairment were observed over the course of a year among patients undergoing MSC therapy (Tables 2 and 3). Similar progresses were not apparent in the control group receiving placebos (Supplementary Tables 3 and 5). Scores of Extended Glasgow Outcome Scale demonstrated a trend of improvement of clinical outcomes of patients at 24th, 36th and 60th week upon completion of the MSC therapy (Table 4). Evident clinical improvement in patients of both groups were comparable (Patients with higher scores of Extended Glasgow Outcome Scale: MSC vs placebos; 3/5 vs 1/4; P = 0.52). There was no re-occurrence of ICH among patients in the study.
Table 3.
Functional independence measure of the treatment group (n = 5) having mesenchymal stem cell therapy at the 1st, 12th, 16th, 24th, 36th and 60th week upon completion of the study
| Study duration (wk) |
Scores of functional independence measure |
P value | ||||
| UPN02 | UPN08 | UPN09 | UPN10 | UPN11 | ||
| 1st | 36 | 72 | 20 | 83 | 70 | |
| 12th | 35 | 73 | 21 | 88 | 74 | 0.07 |
| 16th | 40 | 79 | 21 | 89 | 80 | 0.01 |
| 24th | 41 | 85 | 21 | 89 | 84 | 0.02 |
| 36th | 44 | 87 | 22 | 102 | 84 | 0.01 |
| 60th | 42 | 88 | 22 | - | 85 | 0.03 |
Data derived from different time points were compared to the baseline values established at the first week of the study.
Table 4.
Scores of extended Glasgow Outcome Scale of the treatment group (n = 5) having mesenchymal stem cell therapy at the 1st, 12th, 16th, 24th, 36th and 60th week upon completion of the study
| Study duration (wk) |
Scores of extended glasgow outcome scale |
P value | ||||
| UPN02 | UPN08 | UPN09 | UPN10 | UPN11 | ||
| 1st | 3 | 4 | 3 | 3 | 4 | |
| 12th | 3 | 4 | 3 | 3 | 4 | 1 |
| 16th | 3 | 4 | 3 | 3 | 4 | 1 |
| 24th | 3 | 4 | 3 | 3 | 5 | 0.19 |
| 36th | 3 | 5 | 3 | 3 | 5 | 0.09 |
| 60th | 4 | 6 | 3 | - | 5 | 0.05 |
Data derived from different time points were compared to the baseline values established at the first week of the study.
Radio-imaging
Comparing MRI brain before MSC injection and at completion of the study, there was no interval change in morphology.
DISCUSSION
In the study we demonstrated the safety, feasibility and improvement of neurological outcomes of intravenous administration of autologous bone marrow-derived MSC in a small cohort of nine chronic stroke patients one year after intracerebral haemorrhage through a randomized controlled double-blinded phase I/II clinical trial.
Despite advances in neurosurgery and contemporary medical regimes, survivors of intracerebral haemorrhage often suffer long-term to permanent severe disabilities in terms of cognitive impairment and motor dysfunction. Neurological restoration remains poor. There is an imperative to develop therapeutic modalities to promote neurological recovery. Stem cell-based therapy has drawn a lot of attention recently and the therapeutic efficacies of various cell types were studied[18]. Some cell types are deemed difficult for a wide application. Human neural stem cells may be the prototype, however they are not easily harvestable for transplantation unless collected from aborted foetuses or during necropsy[19,20]. Embryonic stem cells are capable to give rise to all cell lineages, but the application to brain therapy is hindered by the risk of teratoma development and not to mention the ethical controversy[21]. Therapeutic potentials of human stem and progenitor cells from other sources; including bone marrow mononuclear cells, umbilical cord blood CD34+ cells, dental pulp stem cells, adipose-derived stem cells and bone marrow-derived MSC, have also been widely investigated[22-26]. Previous studies indicated that only a limited number of extraneous cells had eventually implanted and integrated into neural networks of recipients. The numbers of successfully engrafted cells were far less than those lost and died to facilitate neuro-restoration. Nonetheless, the implanted cells elicited the neurological recovery via indirect chaperon mechanisms of paracrine signalling of cytokines, chemokines, growth factors, trophic factors, signalling regulators and immuno-modulators, which ultimately stimulated endogenous neurogenesis, angiogenesis and synaptogenesis. It is essential to investigate MSC transplantation as a cell therapy for stroke.
Shortly after the first report on the clinical trial of MSC therapy for stroke[10], many issues arose to be resolved before MSC therapy can be safely and effectively administered to stroke patients. A plethora of clinical trials of MSC therapy for stroke, including the present study, were conducted in small cohorts of patients rendering the statistical power of safety and efficacy less valid[10,27-30]. In parallel with studies in large patient cohorts, clinical trials in small cohorts of patients would be more easily manageable and feasible to provide data for meta-analysis. The study serves the goal.
Autologous serum and platelet lysate were used to replace foetal calf serum in the supplement of basal culture media for MSC propagation in fear of the likelihood of zoonosis[28,31]. Likewise, animal serum-free chemically modified culture media are feasible alternatives to override the likely hurdle[27]. There were concerns of loss of stemness, change of functions, senescence and transformation of prolonged cultures of human MSC, nonetheless little report on the clinical trial of human MSC at high passages is available. Honmou et al[28] reported no side effect on the administration of autologous MSC at passages ≤ 3 in stroke patients during one year of follow-up. More pronounced neurogenesis was observed in a rat stroke model receiving human MSC at earlier passage 2 than counterparts having human MSC at later passage 6[32]. Bernardo and co-workers reported that long-term in vitro cultures of human bone marrow-derived MSC up to passage 25 are not susceptible to malignant transformation[33]. In the study we investigated MSC at passages up to 8. There was neither morphological changes, phenotypic alterations nor growth senescence. No infusion-related toxicity and complications were experienced by patients at time and upon completion of MSC infusion. Data of the study suggest that MSC up to passage 8 are applicable to clinical use without a compromise of safety over an observation period of a year.
It is intuitive that MSC should rest precisely in the locality of interest in the brain in order to achieve the optimal therapeutic effects. Data of clinical trials of MSC therapy demonstrate that intravenous administration is a feasible approach[10,27,28,30]. However, the homing of MSC into the brain was shown to be limited and many cells were trapped into the peripheral organs especially the lungs. Alternate means of intra-arterial delivery and intracranial injection using stereotactic device were also reported to be safe and feasible in human[29,34,35]. Nonetheless, intra-arterial administration was found not superior to intravenous delivery of bone marrow mononuclear cells in a rat stroke model[36]. Both modes of cell delivery achieve comparable structural and functional outcomes in stroke animals after stem cell therapy despite the low homing efficiency.
The optimal dose of MSC applied to human is largely unknown. The empirical cell numbers of 0.5 - 5 × 108 in human are extrapolated from the effective dose of 0.1 - 3 × 106 cells per rat in rat stroke model[10,28,37]. Cells of 5 × 107 were administered twice in the first report on the clinical trial of MSC therapy for stroke and better outcome in Barthel index was noted one year post-treatment[10]. Bhasin et al[27] transplanted a mean of 5-6 × 107 MSC and reported neural plasticity. Honmou et al[28] administered intravenously 0.6-1.6 × 108 cells per patient and observed reduction of lesion size by > 20% after one week. In the study a mean of 4.6 × 107 MSC was administered twice and improvements of motor disability and cognitive impairment were noted.
At present, available reports on clinical trials of MSC therapy suggest neuro-restoration, increase of neural plasticity and reduction of lesion volume[10,27-30,38]. Many stroke patients were noted to have lesions close to the sub-ventricular zone[39]. The likely beneficial effects of MSC treatment might correlate with the spatial lesion not part of the sub-ventricular zone where endogenous neurogenesis persists during adulthood and indirect chaperon effects of MSC promote endogenous neuro-regeneration[40]. In the study we administered MSC to patients having severe neurological disability and presenting stable baseline scores one year after the onset of intracerebral haemorrhage to eliminate confounding attributes to the observation of MSC-mediated neurological recovery.
Data of the study suggest that intravenous administration of autologous bone marrow-derived MSC is safe and facilitate the recovery of neurological functions in patients with severe disability long after the onset of intracerebral haemorrhage. Clinical neurological improvement of patients having MSC therapy was evident compared to patients receiving placebos. MSC therapy is effective independently of other treatment courses. The work may help define criteria for future phase III studies.
ACKNOWLEDGMENTS
The GMP facility of Stem Cell Laboratory located at Li Ka Shing Institute of Health Sciences, Faculty of Medicine, the Chinese University of Hong Kong was utilised to ex vivo expand bone marrow-derived mesenchymal stem cells. We are thankful to the patients participating in the study. We are also grateful to the occupational therapists and physiotherapists of Prince of Wales Hospital in conducting the functional assessments.
COMMENTS
Background
This study was initiated from the randomised Phase II from the Korean Neurologist, suggesting efficacy. Collaborating with the authors Bone Marrow Transplant Unit, the authors were able to be suing autologous bone marrow mesenchymal stem cells (MSCs) of sufficient number for two interval infusion.
Research frontiers
To generate efficacy data post-stroke is important. At present there has been no effective treatment for improving neurological deficits after any stroke illnesses.
Innovations and breakthroughs
There were more data on ischaemic stroke. For brain haemorrhage, clinical data were even more scarce. This study showed a trend towards improvement for intracerebral haemorrhage: This if confirmed in a bigger future study will be a breakthrough.
Applications
This manuscript provided pilot data on autologous bone marrow MSCs intravenous infusion in two treatment episodes with a 4-wk interval, showing a trend towards benefits.
Terminology
These pilot data provide substances for a phase III randomised controlled trial, when formal funding is required.
Peer-review
This manuscript is worth publishing, reporting the result of the phase I/II clinical trial of autologous BM-MSC transplantation therapy in a small cohort of patients with chronic brain haemorrhage.
Footnotes
Institutional review board statement: The randomized, controlled, double-blind, phase I/II clinical trial was approved by the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee of Hong Kong Hospital Authority in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation - Good Clinical Practice.
Clinical trial registration statement: The clinical trial CREC #2006.425-T was registered at http://crec.cuhk.edu.hk.
Informed consent statement: Written informed consent was obtained from all subjects - in the case of vegetative state, from their next of kin.
Conflict-of-interest statement: All authors declared that no conflict of interest exists.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 15, 2017
First decision: March 8, 2017
Article in press: July 17, 2017
P- Reviewer: Jun Y, Saeki K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Contributor Information
Kam Sze Tsang, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China; Department of Anatomical and Cellular Pathology, the Chinese University of Hong Kong, Hong Kong, China.
Chi Ping Stephanie Ng, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
Xian Lun Zhu, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
George Kwok Chu Wong, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
Gang Lu, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
Anil Tejbhan Ahuja, Department of Imaging and Interventional Radiology, the Chinese University of Hong Kong, Hong Kong, China.
Ka Sing Lawrence Wong, Department of Medicine and Therapeutics, the Chinese University of Hong Kong, Hong Kong, China.
Ho Keung Ng, Department of Anatomical and Cellular Pathology, the Chinese University of Hong Kong, Hong Kong, China.
Wai Sang Poon, Department of Surgery, the Chinese University of Hong Kong, Hong Kong, China.
References
- 1.WHO. Global status report on noncommunicable diseases 2014. Available from: http://www.who.int/nmh/publications/ncd-status-report-2014/en/
- 2.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 3.Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 4.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 5.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizaki T, Inaji M, Kouike H, Shimazaki T, Sawamoto K, Ando K, Date I, Kobayashi K, Suhara T, Uchiyama Y, et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci Lett. 2004;363:33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 9.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 10.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 11.NIH. National Institute of Neurological Disorders and Stroke. Stroke Scale. Available from: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf.
- 12.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Zhang XA, Wang H, Wang X, Meng CL, Chan CY, Yew DT, Tsang KS, Li K, Tsai SN, et al. Comparative proteomic analysis of mesenchymal stem cells derived from human bone marrow, umbilical cord, and placenta: implication in the migration. Proteomics. 2009;9:20–30. doi: 10.1002/pmic.200701195. [DOI] [PubMed] [Google Scholar]
- 15.Fong SP, Tsang KS, Chan AB, Lu G, Poon WS, Li K, Baum LW, Ng HK. Trophism of neural progenitor cells to embryonic stem cells: neural induction and transplantation in a mouse ischemic stroke model. J Neurosci Res. 2007;85:1851–1862. doi: 10.1002/jnr.21319. [DOI] [PubMed] [Google Scholar]
- 16.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 17.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 18.Doeppner TR, Hermann DM. Stem cell-based treatments against stroke: observations from human proof-of-concept studies and considerations regarding clinical applicability. Front Cell Neurosci. 2014;8:357. doi: 10.3389/fncel.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-García Verdugo J, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 21.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 22.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS Jr, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, Otani K, Yamahara K, Ihara M, Harada-Shiba M, et al. Effects of intravenous administration of umbilical cord blood CD34(+) cells in a mouse model of neonatal stroke. Neuroscience. 2014;263:148–158. doi: 10.1016/j.neuroscience.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J, et al. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med. 2012;1:177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang KL, Lee JT, Pang CY, Lee TY, Chen SP, Liew HK, Chen SY, Chen TY, Lin PY. Human adipose-derived stem cells for the treatment of intracerebral hemorrhage in rats via femoral intravenous injection. Cell Mol Biol Lett. 2012;17:376–392. doi: 10.2478/s11658-012-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 2010;1310:8–16. doi: 10.1016/j.brainres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22:2291–2298. doi: 10.3727/096368912X658818. [DOI] [PubMed] [Google Scholar]
- 30.Díez-Tejedor E, Gutiérrez-Fernández M, Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML, Gimeno BF. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23:2694–2700. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 2015;32:199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- 33.Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 34.Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I, Garcia-Solis D, Cayuela A, Montaner J, Boada C, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 35.Suárez-Monteagudo C, Hernández-Ramírez P, Alvarez-González L, García-Maeso I, de la Cuétara-Bernal K, Castillo-Díaz L, Bringas-Vega ML, Martínez-Aching G, Morales-Chacón LM, Báez-Martín MM, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Migliati E, Parsha K, Schaar K, Xi X, Aronowski J, Savitz SI. Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke. Stroke. 2013;44:3463–3472. doi: 10.1161/STROKEAHA.111.000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 39.Delavaran H, Sjunnesson H, Arvidsson A, Lindvall O, Norrving B, van Westen D, Kokaia Z, Lindgren A. Proximity of brain infarcts to regions of endogenous neurogenesis and involvement of striatum in ischaemic stroke. Eur J Neurol. 2013;20:473–479. doi: 10.1111/j.1468-1331.2012.03877.x. [DOI] [PubMed] [Google Scholar]
- 40.Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol. 2014;24:558–563. doi: 10.1016/j.tcb.2014.07.003. [DOI] [PubMed] [Google Scholar]