Abstract
Over the course of the 3 decades, percutaneous coronary intervention (PCI) with stent implantation transformed the practice of cardiology. PCI with stenting is currently the most widely performed procedure for the treatment of symptomatic coronary disease. In large trials, drug-eluting stents (DES) have led to a significant reduction in in-stent restenosis (ISR) rates, one of the major limitations of bare-metal stents. Due to these favorable findings, DES was rapidly and widely adopted enabling more complex coronary interventions. Nevertheless, ISR remains a serious concern as late stent complications. ISR mainly results from aggressive neointimal proliferation and neoatherosclerosis. DES-ISR treatment continues to be challenging complications for interventional cardiologists.
Keywords: Stent, In-stent, Restenosis, Percutaneous coronary intervention
Core tip: Percutaneous coronary intervention with stenting is currently the most widely performed procedure for the treatment of symptomatic coronary disease. In large trials, drug-eluting stents (DES) have led to a significant reduction in in-stent restenosis (ISR) rates, one of the major limitations of bare-metal stents. However, ISR remains a serious concern as late stent complications. ISR mainly results from aggressive neointimal proliferation and neoatherosclerosis. DES-ISR treatment continues to be challenging complications for interventional cardiologists. This review focuses on pathogenesis, diagnosis and treatment options for ISR in the current era of advanced intravascular imaging and intervention.
INTRODUCTION
Percutaneous coronary intervention (PCI) with stenting is currently the most widely performed procedure for the treatment of symptomatic coronary disease[1]. Over the course of the 3 decades, PCI with stent implantation transformed the practice of cardiology. In large trials, drug-eluting stents (DES) have led to a significant reduction in in-stent restenosis (ISR) rates, one of the major limitations of bare-metal stents (BMS)[2]. Due to these favorable findings, DES was rapidly and widely adopted enabling more complex coronary interventions. Nevertheless, ISR remains a serious concern as late stent complications.
DEFINITION
ISR is defined as the gradual re-narrowing of a stented coronary artery lesion due to arterial damage with subsequent neointimal tissue proliferation[3,4]. Angiographically IRS is a binary event defined as recurrent diameter stenosis at the stent segment more than 50% of the vessel diameter as determined by coronary angiography[4]. The angiographic definition remains the main definition since it allows determination of ISR severity and morphological pattern. The clinical definition of ISR requires the presence of greater than 50% diameter in-stent stenosis and one of the following: Clinical symptoms of recurrent angina, objective signs of ischemia (EKG changes), positive coronary hemodynamic assessment with fractional flow reserve (FFR) less than 0.80, intravascular ultrasonography (IVUS) minimum cross-sectional area less than 4 mm2 (6 mm2 for left main), or restenosis with ≥ 70% reduction in lumen diameter even in the absence of clinical symptoms or signs.
CLASSIFICATION
Multiple classification systems have been identified to address the severity of ISR. Mehran system[5] is a morphologic classification of ISR lesions in to four patterns. Pattern I (focal) is ISR (≤ 10 mm in length) lesion within the stent, pattern II (diffuse) is ISR greater than 10 mm within the stent, pattern III (proliferative) is ISR greater than 10 mm extending outside the stent, and pattern IV (occlusion) is totally occluded ISR. This classification system predicts the need for repeat revascularization after intervention (19%, 35%, 50%, and 98%, respectively)[5]. American College of Cardiology/American Heart Association lesion classification has been also validated in patients with ISR[6]. Type A lesions had a probability of success of more than 85% and a low risk of acute occlusion. Type B lesions had a probability of success of between 60% and 85% and a moderate risk of abrupt occlusion. Finally, type C lesions had a probability of success of less than 60% and a high risk of abrupt occlusion following the procedure (Table 1). Lesions B2 and C have been reported to be frequently associated with suboptimal acute results with a higher restenosis rate and poorer long-term clinical outcomes[7].
Table 1.
ACC/AHA lesion-specific classification of the primary target stenosis
| Lesion type | Lesion characteristic according to AHA/ACC classification | |||||
| Type A lesions | Discrete (< 10 mm length) | Concentric | Readily accessible | Non angulated segment < 45° | Smooth contour | Little or no calcification |
| Less than totally occlusive | Not ostial in location | No major branch involvement | Absence of thrombus | |||
| Type B1 lesions | Tubular (10-20 mm length) | Eccentric | Moderate tortuosity of proximal segment | Moderately angulated segment, 45°-90° | Irregular contour | Moderate to heavy calcification |
| Total occlusion < 3-mo-old | Ostial in location | Bifurcation lesions requiring double Guidewires | Some thrombus present | |||
| Type B2 lesions | Two or more “B” characteristics | |||||
| Type C lesions | Diffuse (> 2 cm length) | Total occlusion > 3-mo-old | Excessive tortuosity of proximal segment | Extremely angulated segments, > 90° | Inability to protect major side branches | Degenerated vein grafts with friable lesions |
INCIDENCE
In general, rates of ISR range from 3% to 20% with drug-eluting stents and 16% and 44% with BMS. This occurs mostly between 3 to 20 mo after stent placement[3,8]. The incidence of ISR depends on the definition, stent type, location, patient comorbidities and lesion complexity (i.e., lesion length, vessel size, and bifurcation lesions). The introduction of DES has significantly reduced the occurrence of neointimal proliferation, which is considered the main mechanism for ISR. The decrease in ISR was translated into decreased clinical need for subsequent repeat revascularization[9-11]. A meta-analysis of 38 randomized controlled trials with more than 18000 patients showed significant reduction in TLR with both sirolimus-eluting stent (SES) and paclitaxel-eluting stents (PES) compared with BMS[10]. However, due to the complexity of ISR beyond device and stent design, the rates of ISR in both BMS and DES are still relatively high[12]. Routine angiographic surveillance 6 to 8 mo after stent implantation was done in one study that revealed ISR rates of 30.1%, 14.6%, and 12.2% for BMS, first-generation DES, and second-generation DES, respectively[13].
Bare-metal stents ISR
Despite relatively high restenosis rates, bare-metal stents are still frequently used in clinical practice during PCI[14]. This is related to unaffordable prices of DES and more importantly, lower risk of bleeding due to shorter duration of dual antiplatelet therapy (DAPT) that is required after BMS compared with DES. BMS-ISR causes a significant therapeutic burden in current clinical practice. One pooled analysis reported a one-year TLR and TVR rates after BMS of 12% and 14.1% respectively[15,16]. Clinical restenosis was evident within 6-12 wk after BMS implantation[16]. Beyond 1 year, rate of BMS restenosis is negligible and most stenting events are related to disease progression in vessel segments other than the stented lesion[16].
Drug-eluting stents ISR
Restenosis rate of DES increased in the recent years due to expanded use to include high-risk patients with complex coronary lesions. The DES-ISR rate has been reported in 3%-20% depending on DES type, the duration of follow-up, and the complexity of the lesions in which the stents were placed[3]. When compared with BMS, DES is associated with lower ISR. At one-year follow up, SES markedly reduced the incidence TLR from 16.6% to 4.1% when compared with BMS[17]. For first-generation DES, j-Cypher registry of 12812 patients who received SES, the TLR rate was 7.3% at 1 year, and 15.9% at 5 years[18]. Ischemia-driven TLR was also the same in patients randomly assigned to SES or PES (13.1% vs 15.1%) in the SIRTAX LATE study[19]. Second generation stents have been associated with lower death and myocardial infarction compared with first-generation DES. However, zotarolimus-eluting stent (ZES) found to be noninferior to PES for TVR at 1 and 5 years[20]. In a pooled analysis of multiple studies comparing everolimus-eluting with ZES, the rates of TVR at up to five years of follow-up were 6.3% and 5.0%, respectively[21].
PREDICTORS OF IN-STENT RESTENOSIS
Patient comorbidities, lesion characteristic, and procedural characteristics are the main predictors of ISR.
Patient characteristics
Patient characteristics and comorbidities that are associated with higher rate of ISR include; metal allergy, local hypersensitivity reactions with immunologic and inflammatory response to the drug or the polymer, age, female gender, diabetes mellitus, chronic kidney disease (including hemodialysis), and multivessel coronary artery disease[3,22,23].
Lesion characteristics
Lesion characteristics associated with ISR include; lesion length, smaller reference artery diameter, ostial lesion, initial plaque burden and residual plaque after implantation. In contrast with BMS, DES tends to have a more focal pattern of ISR, except in diabetics, where the ISR tends to be more confined to the stent edges[24,25]. Focal ISR (Mehran pattern I) has been associated with a lower rate of ISR recurrence than nonfocal (Mehran pattern > I) ISR[25].
Procedural characteristics
Operator and technique dependent characteristics include stent undersizing, incomplete lesion coverage, stent under expansion, and malapposition. Mechanical properties of stents that may lead to recoil because of loss of radial force, stent fractures, and altering increase in shear stress are all associated with higher rates of ISR. For every 10 mm of excess stent length beyond lesion has been independently associated with increased post-procedural percent diameter stenosis by 4% and increased TLR at 9 mo (OR = 1.12, 95%CI: 1.02-1.24)[26-29]. Stent fracture, on the other hand, can trigger focal ISR or thrombosis[30-32] which can result in a reduction in drug delivery at the breakage point of the stent. Stent fracture occurs more frequently in the right coronary artery, overlapping stents, longer stents, SESs (because of the ridged closed cell structure), and excessively tortuous angulated vessels[33]. Malapposition, also known as incomplete stent apposition (ISA), is defined as the absence of contact between stent struts and the vessel wall not overlying a side branch. Malapposition seems to be related to procedural technique due to under-sizing the stent, use of low deployment pressures, and severely calcified lesions, which do not allow for homogenous stent expansion[34]. Oversized stents can also lead to extensive trauma to the vessel wall and increased proliferative reaction[35]. Geographic miss occurs when the stent does not fully cover the injured or diseased segment of the artery (axial miss) or the ratio of balloon to artery size is less than 0.9 or greater than 1.3 (longitudinal miss). Geographic miss is associated with increased risk of TLR and MI at 1 year[36]. DESs decrease neointimal growth. As a result, geographic miss or strut fracture may be larger factors of ISR in DESs compared with BMSs[12].
PATHOGENESIS
The main mechanism of ISR following stent implantation is neointimal tissue proliferation because of arterial wall damage[21,22]. Neointimal tissue proliferation could be focal or distributed uniformly along the length of the stent (Figure 1). ISR, which happens early within days of stent deployment, is due to elastic recoil and relocation of axially transmitted plaque. The causes of late (weeks to months) ISR commonly are reorganization of thrombus, neointima formation and remodeling[37].
Figure 1.
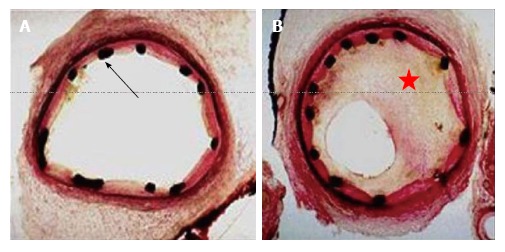
The figure showing cross-section of coronary artery immediately after implantation of a bare metal stent black dots represent the stent struts (red arrow) (A); the figure showing significant in-stent restenosis with neointimal hyperplasia (red star) 6 mo after the implantation of a bare metal stent (B).
Neoatherosclerosis yet is another contributing factor to ISR. The stimulation of neointima formation happens due to injury to the vessel during the PCI and stent deployment. A cascade of events are triggered by the intimal and medial damage, leading to proliferation and migration of vascular smooth muscle cells, extracellular matrix formation which ultimately activates the coagulation-fibrinolysis system[38]. The local inflammation can lead to the development of neoatherosclerosis characterized by accumulation of lipid-laden foamy macrophages within the neointima with or without a necrotic core formation and calcification, which can occur years after stent placement[39]. Neoatherosclerosis is associated with a higher proportion of in-stent atherosclerotic plaque, which could explain unstable symptoms and myocardial infarction presentation of patients with ISR years after PCI. The incidence of neoatherosclerosis was significantly greater in DES compared with BMS (31% vs 16%, P < 0.001)[40]. Younger age, longer implant durations, SES usage, PES usage and underlying unstable plaques, are independent risk factors for neoatherosclerosis[14,40].
CLINICAL PRESENTATION
Due to the gradual and slow progression of ISR compared with stent thrombosis, majority of ISR presents as progressive recurrent angina[40]. The time for symptoms to develop due to DES-ISR is 3 to 12 mo after stent placement[41]. BMS stent on the other hand develop ISR symptoms sooner with reported average period of 6 mo post-PCI[42]. BMS-ISR presented as MI in 3.5%-20% of patients[43]. DES-ISR is similar to that of BMS with approximately 16%-66% of patients presenting with unstable angina and 1%-20% with MI[44,45].
ANATOMIC ASSESSMENT
Routine surveillance
Routine angiographic surveillance is not recommended because it has been shown to increase the rates of oculostenotic revascularization.
Intravascular ultrasonography
IVUS is considered a fundamental intracoronary imaging modality to assess ISR. The stent and procedures characteristics can be readily assessed as contributing mechanism of ISR using IVUS[35]. IVUS delineate external elastic lamina behind the stent struts very well, which provides valuable insights on vessel sizing for optimization of stent expansion (Figure 2F and G). IVUS does help detect the presence of neointimal hyperplasia obstructing the stent, stent underexpansion, stent fracture, or edge restenosis. In addition, it can provide insights into optimal vessel sizing for choosing the appropriate stent size (Figure 2K and L). However, IVUS has limited axial resolution (150 μm), which makes neointimal interface hard to define[12].
Figure 2.
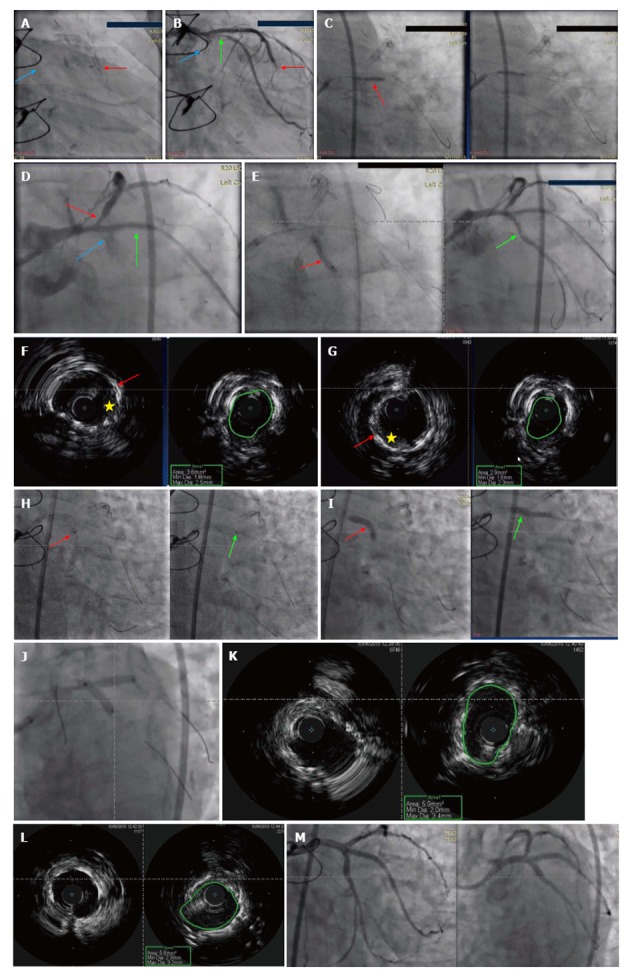
Sixty-seven-year-old man presented with increasing chest pain at rest. He has past medical history significant for coronary artery disease with PCI and coronary artery bypass grafting. He had PCI with (3.0 mm × 12 mm) DES to LCx, (2.5 mm × 16 mm) DES to RI and (2.5 mm × 16 mm) DES to obtuse marginal a year prior to his presentation. The left internal mammary artery to LAD is patent, however, he is known to have occluded SVG to RI and SVG to first diagonal (D1). Given his increasing chest pain, coronary angiogram was done. A: Coronary stents before contrast injection in LAD (red arrow), LCx (blue arrow); B: Coronary angiogram of the same patient showing severe proximal LCx ISR (blue arrow) with no flow, severe proximal RI ISR (green arrow) with slow flow, and mid LAD severe ISR (red arrow); C: Dilation of the RI coronary artery with 2.5 mm × 22 mm NC balloon with 22 atm inflation pressures was done; D: Coronary angiogram showing the proximal RI ISR (green arrow) post balloon dilation. Red arrow shows severe proximal LAD stenosis with poor flow. LCx has completely occluded ISR (blue arrow); E: The left circumflex ISR lesion (red arrow) was wired and with balloon dilation the flow was restored in the LCx (green arrow); F: IVUS imaging of the underexpanded stent in the proximal LCx lesion. Left panel shows stent struts (red arrow) with evidence of neointimal hyperplasia (yellow star). The right panel shows small stent CSA of only 3.6 mm2 which is below the target 5 mm2 in asians and 6 mm2 in non-asians; G: IVUS imaging of the underexpanded stent in the proximal ramus coronary artery. Left panel shows severely under-expanded stent (red arrow) with evidence of neointimal hyperplasia (yellow star). The right panel shows small stent CSA of only 2.9 mm2; H: Excimer Laser Coronary Angioplasty treatment of LCx (left panl - red arow) and ramus artery (right panel - green arrow) using 0.9 mm coronary laser and the heparinized flush technique. Laser catheter was advanced slowly at 0.2-0.5 m/s during laser emission with careful monitoring of heart rate and blood pressure. Vessel injury such as perforation, dissections and acute closure are the main side effects; I: Post laser balloon dilation with (3.5 mm × 20 mm) NC balloon of both LCx (red arrow) and ramus (green arrow) arteries; J: Sequential kissing stenting technique in the proximal LCx and ramus arties with DES 3.5 mm x 18 mm in Ramus and 3.5 mm × 15 mm in LCx; K: IVUS imaging of the stent in the proximal LCx coronary artery that shows good expansion of the stent with great increase in CSA to 5.9 mm2; L: IVUS imaging of the stent in the proximal ramus coronary artery that shows good expansion of the stent with great increase in CSA to 5.6 mm2; M: TIMI III flow was achieved in the LCx and Ramus coronary arteries without any compromise of LAD. PCI: Percutaneous coronary intervention; DES: Drug-eluting stents; LCx: Left circumflex; RI: Ramus intermedius; LAD: Left anterior descending artery; SVG: Saphenous vein graft; ISR: In-stent restenosis; NC: Non-compliant; IVUS: Intravascular ultrasound; CSA: Cross-sectional area.
Optical coherence tomography
Optical coherence tomography (OCT) provides better axial resolution (15 μm), allowing better resolution of the vessel lumen, neointimal tissue, and stent struts distribution. The morphology of ISR can be identified using OCT which could show macrophage infiltration, necrotic core, in-stent calcification and neoatherosclerotic plaque rupture[46,47]. The weakness of the OCT resides in the poor tissue penetration, which cause poor visualization of the residual plaque that is beyond the stent[12].
HEMODYNAMIC ASSESSMENT
Fractional flow reserve
FFR has been validated for clinical decision making in patients with ISR. The clinical outcome of patients with ISR with deferred interventions based on a FFR > 0.75 is excellent[48]. This diagnostic strategy is useful in controversial cases with angiographically moderate or inconclusive ISR.
TREATMENT
Balloon angioplasty
Balloon angioplasty (BA) is one of the earliest interventions that were used to treat ISR by displacing in-stent tissue from the lumen in axial and longitudinal direction to the outer portion of the vessel wall as well as further expanding the stent[49] (Figure 2). This intervention could be useful in focal ISR. The outcome of BA for focal ISR However, during balloon inflation, slippage or watermelon seeding can occur, leading to edge-related complications. Cutting or scoring balloons can help minimize this, but also have limitations in delivery through stented regions or distal areas[50]. Lateral blades or atherotomes anchor the balloon in the lesion and minimize slippage[51]. Progressive balloon dilations and small/short balloons can also prevent side effects from balloon slippage[52]. One of the limitations of BA is that subacute tissue re-intrusion back to the lumen tends to occur within minutes after the last balloon inflation. This explains the early lumen loss phenomenon detected in BA studies in this setting, a finding also associated with subsequent recurrent restenosis.
Vascular brachytherapy
Brachytherapy inhibits neointimal formation within the stent, but not the stent edges, by delivering radiation to the areas of ISR. Brachytherapy effectively suppressed the proliferative response and significantly reduced clinical and angiographic restenosis rates (Figure 3C). Both beta and gamma radiation sources could achieve major reductions in the angiographic restenosis rates[53]. Gamma emitters had profound tissue penetration, whereas beta emitters had less tissue penetration (Figure 3E). Randomized clinical trials in patients with ISR demonstrated the superiority of brachytherapy compared with conventional BA or atheroablative techniques[53-55]. Adding an extra layer of metal to treat DES or BMS-ISR is not ideal and will continue to place patients at future risk for ISR. Therefore, DEB and vascular brachytherapy are better options compared with DES. Vascular brachytherapy is available in few centers in the United States and is used primarily for recurrence of DES-ISR, but logistic issues and lack of radiation oncology support impede its uses. Therefore, restenting with second-generation DES became the default therapy for DES-ISR.
Figure 3.
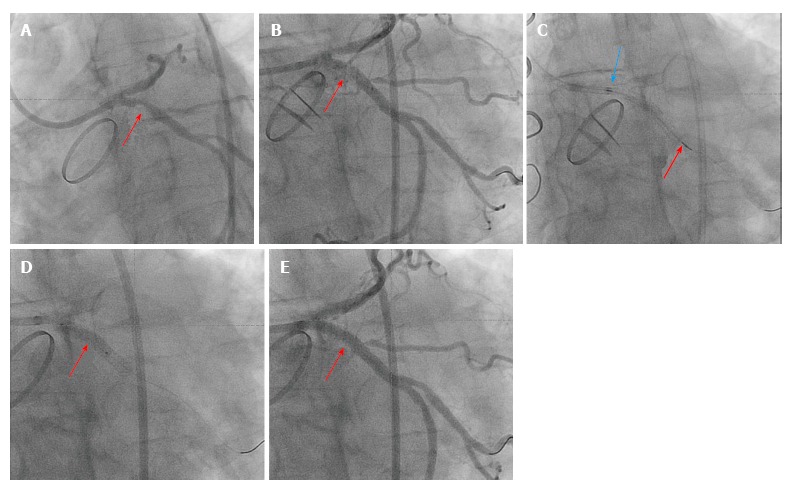
Fifty-five-years-old caucasian male with mantle cell radiation for Hodgkin's lymphoma complicated with radiation heart valve disease with severe aortic valve stenosis status post mehanical aortic valve replacement surgery. Few years later he presented with chest pain and had PCI to the proximal LAD and LCx with DES. However, both few months later he developed ISR and underwent another PCI with DES to the proximal LAD and LCx. Patient was referred for vascular brachytherapy for the treatment of ISR of the LCx due to increased chest pain at rest and recurrent ISR of proximal LCx. A: Coronary angiogram showing 90% focal proximal LCx ISR (red arrow); B: Balloon angioplasty of the proximal LCx lesion (red arrow) to prepare the lesion before brachytherapy delivery; C: Coronary angiogram showing the Novoste Beta-Cath™ System that was used to deliver a source train that contains 12 individual radioactive seeds blue arrow to red arrow). Once properly positioned, 23 Gy from the center of the source center was prescribed. The patient was monitored during the dwell time which required 4 min and 49 s; D: Another balloon angioplasty was done after the radioactive seeds are pulled from LCx; E: Final TIMI-III flow in the LCx. PCI: Percutaneous coronary intervention; LAD: Left anterior descending artery; LCx: Left circumflex; DES: Drug-eluting stents; ISR: In-stent restenosis.
Excimer laser angioplasty
Excimer laser angioplasty (ELA) produces monochromatic light energy, which generates heat and shock waves that disrupt plaque (Figure 2H). Mehran et al[56] compared results of ELA vs rotational atherectomy (RA), both followed by percutaneous transluminal coronary angioplasty (PTCA). 119 patients with 158 ISR lesions were treated with ELA plus PTCA and 130 patients with 161 ISR lesions were treated with RA plus PTCA. Volumetric IVUS analysis showed a greater reduction in intimal hyperplasia volume after RA than after ELA (43 mm3 vs 19 mm3, P < 0.001). However, the 1-year TLR rates were similar: 26% with ELA plus PTCA vs 28% with RA plus PTCA (P = nonsignificant). ELA is not currently a well-accepted treatment for ISR, but the ultimate role of this therapy is still unclear.
Drug-eluting balloon angioplasty
It was proposed that repeat stenting for ISR raises concerns for creating multiple stent layers. Therefore, the use of DEB angioplasty should minimize the metal layer and eventually decrease future ISR. For that purpose, multiple randomized studies have been done to evaluate the efficacy and durability of DEB compared with DES in treating BMS or DES-ISR. Few studies have shown that DEB is non-inferior to DES for BMS and DES-ISR[52-61]. However, none of these studies have been powered for clinical endpoints. DEB is currently not available for use in the United States. In addition, their use has been associated with issues that may limit their use mostly related to the use of paclitaxel and potential of particulates showering to the distal vessel bed, as well as the high profile of the device. Comparison of DEB with DES for treatment of ISR will be discussed in the following section.
Drug-eluting stents
Balloon angioplasty alone carries a high risk of recurrent stenosis, especially in diffuse and/or severe ISR[44,62,63]. The randomized trials Paclitaxel-eluting Stents vs Brachytherapy for In-stent Restenosis (TAXUS V ISR) and Sirolimus-eluting Stents vs Vascular brachytherapy (SISR) trial showed better outcomes for DES compared with brachytherapy[64,65]. Two major randomized trials compared DES with DEB for patients with ISR. The ISAR-DESIRE 3 trial randomized 402 patients with ISR in DES to paclitaxel-eluting balloon (PEB) vs first generation DES (PES) vs balloon angioplasty[52]. At a median follow-up of 3 years, the risk of TLR was similar with PEB vs PES (HR = 1.46, 95%CI: 0.91-2.33, P = 0.11) and lower with PEB vs balloon angioplasty (HR = 0.51, 95%CI: 0.34-0.74, P < 0.001). The risk of death/MI was lower, but not statistically significant, with PEB vs PES (HR = 0.55, 95%CI: 0.28-1.07, P = 0.08). This finding was driven by a lower risk of death (HR = 0.38, 95%CI: 0.17-0.87, P = 0.02). The risk of death/MI was similar with PEB vs balloon angioplasty (HR = 0.96, 95%CI: 0.46-2.0, P = 0.91). Using the second generation DES, Restenosis Intra-Stent of Drug-Eluting Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS IV) trial, evaluated the comparative efficacy of DEB and EES in patients presenting with DES-ISR[66,67]. A total of 309 patients with DES-ISR were randomly allocated to DEB, or second generation DES (EES) patients in the EES arm had a significantly larger minimal lumen diameter (2.03 ± 0.7 mm vs 1.80 ± 0.6 mm, P < 0.01) net lumen gain (1.28 ± 0.7 mm vs 1.01 ± 0.7 mm, P < 0.01), and lower percent diameter stenosis (23% ± 22% vs 30% ± 22%, P < 0.01) and binary restenosis rate (11% vs 19%, P = 0.06), compared with patients in the DEB arm. At the 1-year clinical follow-up (100% of patients), the main clinical outcome measure (composite of cardiac death, myocardial infarction, and target vessel revascularization) was significantly reduced in the EES arm (10% vs 18%, P = 0.04, HR = 0.58, 95%CI: 0.35-0.98), mainly driven by a lower need for target vessel revascularization (8% vs 16%, P = 0.035).
A meta-analysis looked into treatment of ISR comparing DEB, DES, and BA reported that treatment with DEB had a trend toward better outcomes than with DES[68-72]. The risk of TLR was lower in patients treated with DEB (OR = 0.22, 95%CI: 0.10-0.42) or DES (OR = 0.24, 95%CI: 0.11-0.47) than in those treated with BA. In a comparison of DEB and DES, the risk of TLR (OR = 0.92, 95%CI: 0.43-1.90) was similar. The risk of major adverse cardiac events, which was mainly driven by TLR, was also significantly lower in the DEB and DES groups (OR = 0.28, 95%CI: 0.14-0.53) than in the BA group, but it was similar between the DEB and DES groups (OR = 0.84, 95%CI: 0.45-1.50). For TLR, the probability of being ranked as the best treatment was 59.9% (DEB), 40.1% (DES), and 0.1% (BA).
There is no clear evidence on which type of DES should be used to treat ISR of a DES. Some experts argue that using a different type of DES helps to overcome drug resistance, but no strong data support this practice. A recently published network meta-analysis addressed the question of which strategy is preferred for the treatment of ISR, with the primary outcome defined as the percent diameter stenosis at angiographic follow-up[73]. This analysis suggested that PCI with second-generation DES (EES) was the most effective treatment, whereas percutaneous coronary intervention with DEB was ranked as the second most effective treatment but without significant differences from first-generation DES. Two additional similar design meta-analyses have reported similar findings suggesting second generation DES as treatment of choice for BMS and DES-ISR[74,75]. As a result, the 2009 update of the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guideline update for PCI and the 2005 European Society of Cardiology Task Force recommend DES for ISR whether the initial stent was BMS or DES[76-78].
A recently published pooled analysis of the RIBS V and RIBS IV compared the efficacy of EES in patients with BMS-ISR and DES-ISR[79-82]. The study detected clinical and morphological differences of ISR in BMS vs DES, including for the later more focal ISR pattern and delayed onset of presentation. Nevertheless, the outcome of the patients with DES restenosis was less favorable with regard to the angiographic indices, including lumen diameter post procedure and at follow-up. DES-ISR group treated with EES had both increased mortality and need for target vessel revascularization as compared with BMS-ISR group at one year follow up. The authors conclude that EES provides favorable outcomes in patients with ISR and that the results of EES are less satisfactory in patients with DES-ISR than in those with BMS-ISR.
CONCLUSION
In-stent restenosis remains a prevailing clinical problem. The substrate of ISR includes a pathological spectrum ranging from smooth muscle cell proliferation to neoatherosclerosis. Optimal stent deployment, utilization of imaging-guided implantation by IVUS or OCT, adequate coverage of the lesion, verifying stent expansion and apposition to the vessel wall and minimal use of BMS are considered the main strategies to decrease ISR. Based on the currently available literature, the use of BMS should be minimal in clinical practice and replaced with second generation DES. For patients presenting with first ISR, vascular brachytherapy should be considered in patients with focal ISR, or high bleeding risk or requiring DAPT interruption. 2nd generation DES should be a second line therapy to avoid adding an extra layer of metal to treat DES. For patients presenting with recurrent ISR, second generation DES have better long-term outcomes specially if combined with DEB. DEB should be used as first line therapy for bifurcation restenosis to prevent excess metal at the carina.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 5, 2016
First decision: December 19, 2016
Article in press: July 17, 2017
P- Reviewer: Rauch B, Ueda H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
M Chadi Alraies, Heart and Vascular Institute, Department of Interventional Cardiology, Georgetown University/MedStar Washington Hospital Center, Washington, DC 20010, United States.
Fahed Darmoch, Internal Medicine Department, St Vincent Charity Medical Center/Case Western Reserve University, Cleveland, OH 44115, United States.
Ramyashree Tummala, Internal Medicine Department, St Vincent Charity Medical Center/Case Western Reserve University, Cleveland, OH 44115, United States.
Ron Waksman, Heart and Vascular Institute, Department of Interventional Cardiology, Georgetown University/MedStar Washington Hospital Center, Washington, DC 20010, United States.
References
- 1.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 2.Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, Menichelli M, Sabaté M, Suttorp MJ, Baumgart D, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:1030–1039. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 3.Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56:1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Kuntz RE, Baim DS. Defining coronary restenosis. Newer clinical and angiographic paradigms. Circulation. 1993;88:1310–1323. doi: 10.1161/01.cir.88.3.1310. [DOI] [PubMed] [Google Scholar]
- 5.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 6.Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990;82:1193–1202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- 7.Alfonso F, Cequier A, Angel J, Martí V, Zueco J, Bethencourt A, Mantilla R, López-Minguez JR, Gómez-Recio M, Morís C, Perez-Vizcayno MJ, Fernández C, Macaya C, Seabra-Gomes R; Restenosis Intra-stent Balloon angioplasty versus elective Stenting (RIBS) Investigators. Value of the American College of Cardiology/American Heart Association angiographic classification of coronary lesion morphology in patients with in-stent restenosis. Insights from the Restenosis Intra-stent Balloon angioplasty versus elective Stenting (RIBS) randomized trial. Am Heart J. 2006;151:681.e1–681.e9. doi: 10.1016/j.ahj.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv. 2011;4:195–205. doi: 10.1161/CIRCINTERVENTIONS.110.959882. [DOI] [PubMed] [Google Scholar]
- 9.Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 10.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 11.Byrne RA, Sarafoff N, Kastrati A, Schömig A. Drug-eluting stents in percutaneous coronary intervention: a benefit-risk assessment. Drug Saf. 2009;32:749–770. doi: 10.2165/11316500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 13.Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100:153–159. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- 14.García Del Blanco B, Rumoroso Cuevas JR, Hernández Hernández F, Trillo Nouche R. Spanish cardiac catheterization and coronary intervention registry. 22nd official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2012) Rev Esp Cardiol (Engl Ed) 2013;66:894–904. doi: 10.1016/j.rec.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, Cohen DJ, Kuntz RE. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–2089. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, Bakhai A, Cohen DJ, Kuntz RE, Ho KK. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–1230. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 17.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, Shiode N, Namura M, Sone T, Oshima S, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–591. doi: 10.1161/CIRCULATIONAHA.111.046599. [DOI] [PubMed] [Google Scholar]
- 19.Räber L, Wohlwend L, Wigger M, Togni M, Wandel S, Wenaweser P, Cook S, Moschovitis A, Vogel R, Kalesan B, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel-Eluting Stents for Coronary Revascularization LATE trial. Circulation. 2011;123:2819–2828, 6 p following 2828. doi: 10.1161/CIRCULATIONAHA.110.004762. [DOI] [PubMed] [Google Scholar]
- 20.Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O’Shaughnessy C, Overlie PA, McLaurin BT, Solomon SL, Douglas JS Jr, Ball MW, Caputo RP, Jain A, Tolleson TR, Reen BM 3rd, Kirtane AJ, Fitzgerald PJ, Thompson K, Kandzari DE; ENDEAVOR IV Investigators. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010;55:543–554. doi: 10.1016/j.jacc.2009.08.067. [DOI] [PubMed] [Google Scholar]
- 21.Piccolo R, Stefanini GG, Franzone A, Spitzer E, Blöchlinger S, Heg D, Jüni P, Windecker S. Safety and efficacy of resolute zotarolimus-eluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv. 2015;8:e002223. doi: 10.1161/CIRCINTERVENTIONS.114.002223. [DOI] [PubMed] [Google Scholar]
- 22.Kastrati A, Schömig A, Elezi S, Schühlen H, Dirschinger J, Hadamitzky M, Wehinger A, Hausleiter J, Walter H, Neumann FJ. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;30:1428–1436. doi: 10.1016/s0735-1097(97)00334-3. [DOI] [PubMed] [Google Scholar]
- 23.Ivens K, Gradaus F, Heering P, Schoebel FC, Klein M, Schulte HD, Strauer BE, Grabensee B. Myocardial revascularization in patients with end-stage renal disease: comparison of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. Int Urol Nephrol. 2001;32:717–723. doi: 10.1023/a:1015067611958. [DOI] [PubMed] [Google Scholar]
- 24.Rathore S, Kinoshita Y, Terashima M, Katoh O, Matsuo H, Tanaka N, Kimura M, Tsuchikane E, Nasu K, Ehara M, et al. A comparison of clinical presentations, angiographic patterns and outcomes of in-stent restenosis between bare metal stents and drug eluting stents. EuroIntervention. 2010;5:841–846. doi: 10.4244/eijv5i7a141. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi Y, De Gregorio J, Kobayashi N, Akiyama T, Reimers B, Finci L, Di Mario C, Colombo A. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol. 1999;34:651–659. doi: 10.1016/s0735-1097(99)00303-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SL, Loussararian A, De Gregorio J, Di Mario C, Albiero R, Colombo A. Predictors of diffuse and aggressive intra-stent restenosis. J Am Coll Cardiol. 2001;37:1019–1025. doi: 10.1016/s0735-1097(01)01107-x. [DOI] [PubMed] [Google Scholar]
- 28.Serruys PW, Kay IP, Disco C, Deshpande NV, de Feyter PJ. Periprocedural quantitative coronary angiography after Palmaz-Schatz stent implantation predicts the restenosis rate at six months: results of a meta-analysis of the BElgian NEtherlands Stent study (BENESTENT) I, BENESTENT II Pilot, BENESTENT II and MUSIC trials. Multicenter Ultrasound Stent In Coronaries. J Am Coll Cardiol. 1999;34:1067–1074. doi: 10.1016/s0735-1097(99)00308-3. [DOI] [PubMed] [Google Scholar]
- 29.Mauri L, O’Malley AJ, Cutlip DE, Ho KK, Popma JJ, Chauhan MS, Baim DS, Cohen DJ, Kuntz RE. Effects of stent length and lesion length on coronary restenosis. Am J Cardiol. 2004;93:1340–1346, A5. doi: 10.1016/j.amjcard.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, Onuma Y, Higashikuni Y, Otsuki S, Yagishita A, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2007;69:380–386. doi: 10.1002/ccd.20950. [DOI] [PubMed] [Google Scholar]
- 31.Umeda H, Gochi T, Iwase M, Izawa H, Shimizu T, Ishiki R, Inagaki H, Toyama J, Yokota M, Murohara T. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;133:321–326. doi: 10.1016/j.ijcard.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: clinical characteristics and implications. Catheter Cardiovasc Interv. 2007;69:387–394. doi: 10.1002/ccd.20942. [DOI] [PubMed] [Google Scholar]
- 33.Lindsey JB, Marso SP. Incomplete stent apposition: should we appose or oppose? JACC Cardiovasc Interv. 2010;3:495–497. doi: 10.1016/j.jcin.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Mintz GS. What to do about late incomplete stent apposition? Circulation. 2007;115:2379–2381. doi: 10.1161/CIRCULATIONAHA.107.697136. [DOI] [PubMed] [Google Scholar]
- 35.Fujii K, Mintz GS, Kobayashi Y, Carlier SG, Takebayashi H, Yasuda T, Moussa I, Dangas G, Mehran R, Lansky AJ, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085–1088. doi: 10.1161/01.CIR.0000121327.67756.19. [DOI] [PubMed] [Google Scholar]
- 36.Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial) Am J Cardiol. 2008;101:1704–1711. doi: 10.1016/j.amjcard.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 37.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89:218–224. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9:53–62. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 39.Tsigkas GG, Karantalis V, Hahalis G, Alexopoulos D. Stent restenosis, pathophysiology and treatment options: a 2010 update. Hellenic J Cardiol. 2011;52:149–157. [PubMed] [Google Scholar]
- 40.Alfonso F, Pérez-Vizcayno MJ, Cruz A, García J, Jimenez-Quevedo P, Escaned J, Hernandez R. Treatment of patients with in-stent restenosis. EuroIntervention. 2009;5 Suppl D:D70–D78. [PubMed] [Google Scholar]
- 41.Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol. 2008;20:401–403. [PubMed] [Google Scholar]
- 42.Nayak AK, Kawamura A, Nesto RW, Davis G, Jarbeau J, Pyne CT, Gossman DE, Piemonte TC, Riskalla N, Chauhan MS. Myocardial infarction as a presentation of clinical in-stent restenosis. Circ J. 2006;70:1026–1029. doi: 10.1253/circj.70.1026. [DOI] [PubMed] [Google Scholar]
- 43.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Bossi I, Klersy C, Black AJ, Cortina R, Choussat R, Cassagneau B, Jordan C, Laborde JC, Laurent JP, Bernies M, et al. In-stent restenosis: long-term outcome and predictors of subsequent target lesion revascularization after repeat balloon angioplasty. J Am Coll Cardiol. 2000;35:1569–1576. doi: 10.1016/s0735-1097(00)00584-2. [DOI] [PubMed] [Google Scholar]
- 45.Mishkel GJ, Moore AL, Markwell S, Shelton MC, Shelton ME. Long-term outcomes after management of restenosis or thrombosis of drug-eluting stents. J Am Coll Cardiol. 2007;49:181–184. doi: 10.1016/j.jacc.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Uchida T, Popma J, Stone GW, Ellis SG, Turco MA, Ormiston JA, Muramatsu T, Nakamura M, Nanto S, Yokoi H, et al. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of “oculostenotic” reintervention in patients with intermediate lesions. JACC Cardiovasc Interv. 2010;3:403–411. doi: 10.1016/j.jcin.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Nakano M, Vorpahl M, Otsuka F, Taniwaki M, Yazdani SK, Finn AV, Ladich ER, Kolodgie FD, Virmani R. Ex vivo assessment of vascular response to coronary stents by optical frequency domain imaging. JACC Cardiovasc Imaging. 2012;5:71–82. doi: 10.1016/j.jcmg.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Nam CW, Rha SW, Koo BK, Doh JH, Chung WY, Yoon MH, Tahk SJ, Lee BK, Lee JB, Yoo KD, et al. Usefulness of coronary pressure measurement for functional evaluation of drug-eluting stent restenosis. Am J Cardiol. 2011;107:1783–1786. doi: 10.1016/j.amjcard.2011.02.328. [DOI] [PubMed] [Google Scholar]
- 49.Macander PJ, Roubin GS, Agrawal SK, Cannon AD, Dean LS, Baxley WA. Balloon angioplasty for treatment of in-stent restenosis: feasibility, safety, and efficacy. Cathet Cardiovasc Diagn. 1994;32:125–131. doi: 10.1002/ccd.1810320206. [DOI] [PubMed] [Google Scholar]
- 50.Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schömig A; ISAR-DESIRE Study Investigators. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293:165–171. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- 51.Alfonso F, Pérez-Vizcayno MJ, Hernandez R, Bethencourt A, Martí V, López-Mínguez JR, Angel J, Mantilla R, Morís C, Cequier A, et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the Restenosis Intrastent: Balloon Angioplasty Versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J Am Coll Cardiol. 2006;47:2152–2160. doi: 10.1016/j.jacc.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 52.Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, Schulz S, Fusaro M, Ott I, Ibrahim T, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381:461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 53.Leon MB, Teirstein PS, Moses JW, Tripuraneni P, Lansky AJ, Jani S, Wong SC, Fish D, Ellis S, Holmes DR, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344:250–256. doi: 10.1056/NEJM200101253440402. [DOI] [PubMed] [Google Scholar]
- 54.Waksman R, White RL, Chan RC, Bass BG, Geirlach L, Mintz GS, Satler LF, Mehran R, Serruys PW, Lansky AJ, et al. Intracoronary gamma-radiation therapy after angioplasty inhibits recurrence in patients with in-stent restenosis. Circulation. 2000;101:2165–2171. doi: 10.1161/01.cir.101.18.2165. [DOI] [PubMed] [Google Scholar]
- 55.Teirstein PS, Massullo V, Jani S, Popma JJ, Mintz GS, Russo RJ, Schatz RA, Guarneri EM, Steuterman S, Morris NB, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med. 1997;336:1697–1703. doi: 10.1056/NEJM199706123362402. [DOI] [PubMed] [Google Scholar]
- 56.Mehran R, Dangas G, Mintz GS, Waksman R, Abizaid A, Satler LF, Pichard AD, Kent KM, Lansky AJ, Stone GW, et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty versus rotational atherectomy: comparative mechanisms and results. Circulation. 2000;101:2484–2489. doi: 10.1161/01.cir.101.21.2484. [DOI] [PubMed] [Google Scholar]
- 57.Dahm JB, Kuon E. High-energy eccentric excimer laser angioplasty for debulking diffuse iIn-stent restenosis leads to better acute- and 6-month follow-up results. J Invasive Cardiol. 2000;12:335–342. [PubMed] [Google Scholar]
- 58.Litvack F, Eigler N, Margolis J, Rothbaum D, Bresnahan JF, Holmes D, Untereker W, Leon M, Kent K, Pichard A. Percutaneous excimer laser coronary angioplasty: results in the first consecutive 3,000 patients. The ELCA Investigators. J Am Coll Cardiol. 1994;23:323–329. doi: 10.1016/0735-1097(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 59.Stone GW, de Marchena E, Dageforde D, Foschi A, Muhlestein JB, McIvor M, Rizik D, Vanderlaan R, McDonnell J. Prospective, randomized, multicenter comparison of laser-facilitated balloon angioplasty versus stand-alone balloon angioplasty in patients with obstructive coronary artery disease. The Laser Angioplasty versus Angioplasty (LAVA) Trial Investigators. J Am Coll Cardiol. 1997;30:1714–1721. doi: 10.1016/s0735-1097(97)00387-2. [DOI] [PubMed] [Google Scholar]
- 60.Reifart N, Vandormael M, Krajcar M, Göhring S, Preusler W, Schwarz F, Störger H, Hofmann M, Klöpper J, Müller S, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) Study. Circulation. 1997;96:91–98. doi: 10.1161/01.cir.96.1.91. [DOI] [PubMed] [Google Scholar]
- 61.Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11:926–934. doi: 10.4244/EIJY14M08_12. [DOI] [PubMed] [Google Scholar]
- 62.Bauters C, Banos JL, Van Belle E, Mc Fadden EP, Lablanche JM, Bertrand ME. Six-month angiographic outcome after successful repeat percutaneous intervention for in-stent restenosis. Circulation. 1998;97:318–321. doi: 10.1161/01.cir.97.4.318. [DOI] [PubMed] [Google Scholar]
- 63.Eltchaninoff H, Koning R, Tron C, Gupta V, Cribier A. Balloon angioplasty for the treatment of coronary in-stent restenosis: immediate results and 6-month angiographic recurrent restenosis rate. J Am Coll Cardiol. 1998;32:980–984. doi: 10.1016/s0735-1097(98)00333-7. [DOI] [PubMed] [Google Scholar]
- 64.Stone GW, Ellis SG, O’Shaughnessy CD, Martin SL, Satler L, McGarry T, Turco MA, Kereiakes DJ, Kelley L, Popma JJ, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295:1253–1263. doi: 10.1001/jama.295.11.1253. [DOI] [PubMed] [Google Scholar]
- 65.Holmes DR Jr, Teirstein P, Satler L, Sketch M, O’Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, Cohen SA; SISR Investigators. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295:1264–1273. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 66.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García Del Blanco B, Seidelberger B, Iñiguez A, Gómez-Recio M, Masotti M, Velázquez MT, Sanchís J, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent) J Am Coll Cardiol. 2014;63:1378–1386. doi: 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, Benedicto A, Masotti M, Zueco J, Iñiguez A, Velázquez M, Moreno R, Mainar V, Domínguez A, Pomar F, Melgares R, Rivero F, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C; RIBS IV Study Investigators (under auspices of Interventional Cardiology Working Group of Spanish Society of Cardiology) A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 68.Lee JM, Park J, Kang J, Jeon KH, Jung JH, Lee SE, Han JK, Kim HL, Yang HM, Park KW, et al. Comparison among drug-eluting balloon, drug-eluting stent, and plain balloon angioplasty for the treatment of in-stent restenosis: a network meta-analysis of 11 randomized, controlled trials. JACC Cardiovasc Interv. 2015;8:382–394. doi: 10.1016/j.jcin.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Garg S, Smith K, Torguson R, Okabe T, Slottow TL, Steinberg DH, Roy P, Xue Z, Gevorkian N, Satler LF, et al. Treatment of drug-eluting stent restenosis with the same versus different drug-eluting stent. Catheter Cardiovasc Interv. 2007;70:9–14. doi: 10.1002/ccd.21106. [DOI] [PubMed] [Google Scholar]
- 70.Alfonso F, Pérez-Vizcayno MJ, Dutary J, Zueco J, Cequier A, García-Touchard A, Martí V, Lozano I, Angel J, Hernández JM, López-Mínguez JR, Melgares R, Moreno R, Seidelberger B, Fernández C, Hernandez R; RIBS-III Study Investigators (under the auspices of the Working Group on Interventional Cardiology of the Spanish Society of Cardiology) Implantation of a drug-eluting stent with a different drug (switch strategy) in patients with drug-eluting stent restenosis. Results from a prospective multicenter study (RIBS III [Restenosis Intra-Stent: Balloon Angioplasty Versus Drug-Eluting Stent]) JACC Cardiovasc Interv. 2012;5:728–737. doi: 10.1016/j.jcin.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Alfonso F, Augé JM, Zueco J, Bethencourt A, López-Mínguez JR, Hernández JM, Bullones JA, Calvo I, Esplugas E, Pérez-Vizcayno MJ, et al. Long-term results (three to five years) of the Restenosis Intrastent: Balloon angioplasty versus elective Stenting (RIBS) randomized study. J Am Coll Cardiol. 2005;46:756–760. doi: 10.1016/j.jacc.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 72.Mehran R, Dangas G, Abizaid A, Lansky AJ, Mintz GS, Pichard AD, Satler LF, Kent KM, Waksman R, Stone GW, et al. Treatment of focal in-stent restenosis with balloon angioplasty alone versus stenting: Short- and long-term results. Am Heart J. 2001;141:610–614. doi: 10.1067/mhj.2001.113998. [DOI] [PubMed] [Google Scholar]
- 73.Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655–664. doi: 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 74.Sethi A, Malhotra G, Singh S, Singh PP, Khosla S. Efficacy of various percutaneous interventions for in-stent restenosis: comprehensive network meta-analysis of randomized controlled trials. Circ Cardiovasc Interv. 2015;8:e002778. doi: 10.1161/CIRCINTERVENTIONS.115.002778. [DOI] [PubMed] [Google Scholar]
- 75.Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ. 2015;351:h5392. doi: 10.1136/bmj.h5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, Morrison DA, O’Neill WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) J Am Coll Cardiol. 2006;47:e1–121. doi: 10.1016/j.jacc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, Jørgensen E, Marco J, Nordrehaug JE, Ruzyllo W, et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–847. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 78.Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Alfonso F, Pérez-Vizcayno MJ, García Del Blanco B, García-Touchard A, López-Mínguez JR, Masotti M, Zueco J, Melgares R, Mainar V, Moreno R, et al. Everolimus-Eluting Stents in Patients With Bare-Metal and Drug-Eluting In-Stent Restenosis: Results From a Patient-Level Pooled Analysis of the RIBS IV and V Trials. Circ Cardiovasc Interv. 2016;9:e003479. doi: 10.1161/CIRCINTERVENTIONS.115.003479. [DOI] [PubMed] [Google Scholar]
- 80.Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation. 2011;123:779–797. doi: 10.1161/CIRCULATIONAHA.110.971606. [DOI] [PubMed] [Google Scholar]
- 81.Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med. 2015;373:1905–1915. doi: 10.1056/NEJMoa1509038. [DOI] [PubMed] [Google Scholar]
- 82.Waksman R, Steinvil A. In-Stent Restenosis? The Raiders of the Magic Remedy. Circ Cardiovasc Interv. 2016;9:e004150. doi: 10.1161/CIRCINTERVENTIONS.116.004150. [DOI] [PubMed] [Google Scholar]


