Abstract
Research revealed that the pathogenesis of aortic stenosis (AS) not merely comprises of a mechanical wear and tear process yet that active biological processes, similar to those of coronary artery disease are involved, a promising role for statins in disease-modifying therapy was suggested. However, recently, many prospective studies could not observe decreased progression nor regression of the disease. Here, we review the current knowledge on the pathomechanisms of AS and its similarities and differences with atherosclerosis. Moreover, we discuss whether there is still a place for statins in the treatment of particular AS patient subgroups.
Keywords: Aortic stenosis, Statins, HMG-CoA enzyme inhibitor, Coronary artery stenosis, Aortic valve surgery
Core tip: Aortic stenosis is a age-dependent and growing disease. As there are several similarities with atherosclerotic disease of other regions there are growing research on underlying pathopyhsiology. The treatment benefit of classic atherosclerosis treatment is evaluated in case of aortic valve stenosis.
INTRODUCTION
Aortic stenosis (AS) is the most common heart valve disease in Western countries. It affects 12.4% of the population over the age of 75 years[1] with a male predominance of 80% in symptomatic AS[2]. AS is usually characterized by an asymptomatic latent period of several years or even decades before patients suffer from discomforts. However, once patients with severe AS display symptoms (typically syncope, angina and/or dyspnoea), the prognosis is poor with a 2-year and 5-year survival rate of 50% and 20%, respectively; hence valve replacement surgery is usually mandated[3]. Since the elderly population in Western countries is expected to double by 2050, it is imperative to define early diagnostic and treatment strategies. Even though AS or the fibrous thickening and calcification of the valves is a widespread disease, its underlying molecular mechanisms are still unknown[4]. Traditionally, it was accepted that AS is caused by the progression of calcium deposition continued to aortic valvular leaflets. However, research demonstrating the involvement of active cellular processes over the past 15 years[5]. Genetic polymorphism seems to influence the degree of aortic valve sclerosis[6], with a focus on chronic inflammatory processes. Moreover, it has become clear that the development of AS shows many similarities with atherosclerosis including infiltration and retention of lipoproteins, lipids, T-lymphocyte and other inflammatory cells, as well as osteoblastic activation[7-9]. Accordingly, numerous studies demonstrated a strong associated to coronary artery disease (CAD) and many of its risk factors, including hypercholesterolemia[10,11]. This opened the field to develop effective disease-modifying strategies, which would halt or even regress the disease, thus avoiding the need of surgical or interventional replacement. At first, statins seem to be the most obvious medical treatment choice since its use is well-established for the primary and secondary prevention of CAD[12]. Moreover, statins have been shown to exert pleiotropic effects beyond their cholesterol-lowering effect, such as anti-oxidation, plaques stabilization and reduction of vascular inflammatory processes[13,14]. Numerous retrospective observational studies showed a delay in AS progression when statins were administered concomitantly which encouraged the use of statins for treatment of AS[15-19]. However, 3 recent large-scale prospective randomized clinical trials investigating the effects of intense lipid-lowering therapy with statins showed no effect on neither progression, nor regression of AS. Meanwhile, new insights are emerging, demonstrating distinct differences in (molecular) pathology between CAD and AS. Here, we review what is known today about the pathogenesis of AS and the potential influence of statin therapy in AS patients, with a distinction between degenerative or calcific AS, congenital AS, AS with coronary heart disease as comorbidity, and, treatment before and after aortic valve surgery.
PATHOMECHANISMS OF DEGENERATIVE OR CALCIFIC AS AND CAD: SIMILARITIES AND DIFFERENCES
Since it became clear that AS is not merely the result of mechanical stress and ageing, but rather an active biological processes involving inflammatory contributing to the disease; pharmacological treatment possibilities were completely opposed to valve replacement surgery inevitable at the time when AS has reached a severe symptomatic status. What has been revealed of the current knowledge of the pathogenesis of AS so far, revealed many similarities with CAD. Hence, it was obvious to turn to statins for the treatment of AS since they have proven efficacy for the primary and secondary prevention of CAD. Nevertheless, it is shown that, besides lipid-lowering, statins display pleiotropic effects such as anti-inflammatory and antioxidant effects as well as plaque-stabilization and improvement of endothelium dysfunction[20] (Figure 1). The potential use of statins was initially encouraged by positive results from numerous retrospective studies. Yet recently, three prospective randomized studies showed neither regression, nor reduction in progression of AS: SEAS (Simvastatin and Ezetimibe in Aortic Stenosis), SALTIRE (Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression) and ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin)[21-23]. All three trials demonstrated that extensive lipid-lowering induced by statins failed to correlate with neither improvement of aortic-jet velocity of the valve, nor with regression of valvular calcification. Such disappointing results were surprising because of the many well recognized similarities between CAD and AS in terms of pathogenesis. Moreover, statin use has become an established principle for CAD. Furthermore, similar risk factors have been identified for both diseases such as age, sex (male gender), dyslipidaemia, hypertension, smoking, diabetes and renal dysfunction[24,25]. Aortic valve stenosis can be characterized an ‘early lesion’, which are similar to those of atherosclerotic plaques in vessels[8] and both CAD and AS are characterized by infiltration and accumulation of (oxidized) low-density lipoproteins (LDL) and T-lymphocytes, thus filling tissue inflammation and the release of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-1α which in turn induce cell proliferation[7,9,26]. Calcification and osteogenesis, mediated by inflammation and processes involving metalloproteinases, have been identified as players in the pathogenesis of AS[27-29]. Finally, neo-angiogenesis contributes to AS development[30,31] and is responsible for reduced concentrations of the anti-angiogenesis protein chondromodulin-1 in damaged aortic valve tissue[32]. The resulting cell apoptosis, extracellular matrix formation and consequent thickening and calcification of the cusps, decreases aortic valve mobility and orifice areas, ultimately leading to an increased pressure gradient[33]. Nevertheless, there are some fundamental differences in the underlying molecular mechanisms with an early inflammation affecting fibroblasts in AS as opposed to late onset of inflammation in smooth muscle cells in CAD. Yet, plaque instability is not the leading clinical problem with AS; however clearly the main cause of symptoms with CAD[34]. It is important to note that those retrospective studies on the effect of statins in AS included patients already exposed to statins prescribed for primary or secondary prevention of CAD. Moreover, study patients had only mild to moderate AS as severe AS was excluded. Based on sub-analyses of the 3
Figure 1.
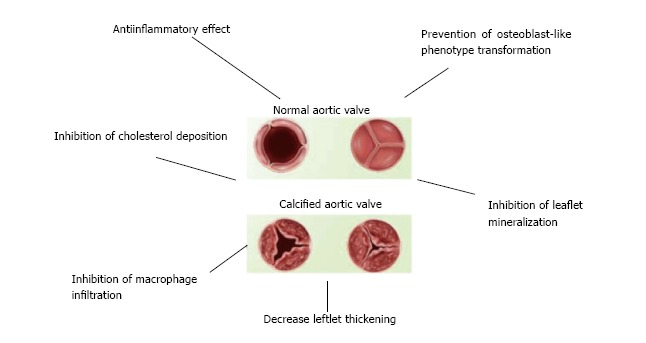
Schematic overview of statin effects on aortic valve calcification.
RCT’s, several research groups support the lipid hypothesis as a common denominator of both diseases, with similarities between atherosclerosis and AS seen in initial stages of AS. Later on, disease progression is mostly determined by plasticity and structure of the leaflets, and by mechanical stress[35,36]. Yet, results of a recent meta-analysis challenged suggested role of statins to prevent the onset of AS in non-symptomatic patients[37]. They pooled high risk patients without known AS from 3 large scale RCT’s who evaluated the effect of high (80 mg) and normal (10 mg) daily doses of atorvastatin and found no significant differences between placebo, low or high atorvastatin with regards to the onset of AS. Furthermore, across the board any subtle correlation between hyperlipidaemia and AS progression[15,17] turned out to be inconsistent[18,19].
Effect of statins on AS in patients with CAD
As much as 40% of patients undergoing aortic valve replacement surgery suffers from atherosclerosis as well. Moreover, AS progresses faster in older patients with CAD[2]. There is evidence of beneficial effects of statins in presence of CAD[38]. Dyslipidaemia is an independent predictor of AS progression and adequate lipid-lowering by statin use in patients with CAD has beneficial effects on valve integrity[39]. Yet, a quantitative relationship between lipid-lowering and changes in AS progression has not been found. It is worth mentioning that most studies investigated patients with intermediate or advanced AS which appears difficult to modify by drugs; however, a potential effect in early AS could still be debated[40]. Another point to address is patient selection bias, especially in the SEAS trial that excluded patients with diabetes or CAD. Even though no correlation between statin use and beneficial effects on AS have been shown, it is highly likely that patients with AS and relevant comorbidities such as atherosclerosis, which is a common clinical scenario, may be positively affected[41,42]. Indeed, statins would mitigate AS progression only when hyperlipidaemia is present[43] but more profound research is needed. Current guidelines suggest offering patients with comorbidities such as CAD, diabetes or hyperlipidaemia statins. Conversely, untreated metabolic syndrome in patients with AS is related to faster stenosis progression and worse prognosis[44]. These observations were more pronounced in younger patients (< 57 years of age). Interestingly, however, statins in younger patients with metabolic syndrome and AS turned out to be disadvantageous with AS progression and lower insulin resistance supporting the notion that lipophilic statins may actually induce diabetes type II[45,46]; this effect is even more pronounced when patients suffer from metabolic syndrome as well[47]. This effect was not seen in older patients, suggesting that the pathophysiological mechanisms of AS progression may be related to the patient’s age[44].
CONGENITAL AORTIC STENOSIS
Congenital defects resulting in AS include partially fused leaflets, thickened leaflets, narrowing of the supravalvular or subvalvular area, and, most frequently, bicuspidity. A biscupid aortic valve is the result of the fusion of two of the three leaflets during the developmental phase and is with an incidence of almost 2% the most common congenital cardiac malformation[48]. The abnormal structure of the bicuspid aortic valve results in excess stress onto leaflets, resulting in valvular thickening, calcification, and increased rigidity and restricted aortic orifice. For that reason, AS is the most frequent complication of bicuspid aortic valve. While randomized prospective trials mainly included older AS patients, the PROCAS (Progression of Stenosis in Adult Patients with Congenital Aortic Stenosis) trial was designed to evaluate the effect of statins on the progression of stenosis in young asymptomatic adults (aged between 18 and 45) with congenital AS. Not surprising, the investigators did not find any benefit from extensive lipid-lowering by 10 mg rosuvastatin, independent of the degree of valve calcification[49]. These findings are in line with those of the bicuspid valve patients subgroup of the ASTRONOMER trial[23].
STATIN THERAPY AND AORTIC VALVE SURGERY
Because of their anti-ischemic and anti-inflammatory effects and protection of the endothelium statins have been suggested to reduce atrial fibrillation after aortic valve surgery, yet with conflicting[50,51]. Some beneficial effects of perioperative statin therapy on mortality, stroke, renal insufficiency, length of ICU and hospital stay were found[52,53] however leading to the conclusion that preoperative statin use is justified in case of coronary artery bypass grafting (CABG). Indeed, advantageous effects on the above-mentioned endpoints have been demonstrated in only CABG patients and not in others[54-56]. Conversely, just to add to the confusion statin were also reported to delay restenosis after balloon aortic valvuloplasty or bioprosthetic valve replacement[57-59], but findings were not[60]. With pre- and postoperative statin therapy in patients undergoing aortic valve replacement an increased long-term survival was found with biological valve replacement but not with mitral valve repair or mechanical valve replacement[61]. Finally, a retrospective study of bicuspid aortic valve patients undergoing surgery showed a significant decrease in ascending aortic dilatation when exposed to statins preoperatively[62]. Clearly, more clinical data are required to justify perioperative use of statin therapy.
FUTURE CONSIDERATIONS
Future research in this context needs to focus both, elucidating the molecular pathways involved in the pathogenesis of AS and developing potential pharmacological treatment strategies. In that regard, it is of interest to determine both vascular and valvular aspects and when they occur during the evolution of AS. As such, different treatment approaches might apply for distinct stages of the disease. In addition, defining predisposing genetic deviations may help define preventative and curative approaches to slow disease progression. Additionally, in the quest for new pharmacological agents to treat AS, low-cost accurate animal model are missing; only ageing swine can develop AS[34]. At present, there is no evidence that statins halt the progression or induce the regression of AS. The notion that their administration is harmless and devoid any side effects is untrue as we know adverse effects of statins in asymptomatic AS patients without concomitant diseases may in fact induce new risk factors (diabetes mellitus, aortic valve calcification)[44,63]. In conclusion, the general consensus to date is that treatment with statins is not recommended in patients with valvular aortic stenosis and in absence of standard indications to lipid-lowering treatment.
Footnotes
Conflict-of-interest statement: No conflict of interest for all authors.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: January 7, 2017
First decision: February 17, 2017
Article in press: June 8, 2017
P- Reviewer: Avanzas P, Ueda H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
Ibrahim Akin, Medical Faculty Mannheim, University Heidelberg, 68167 Mannheim, Germany.
Christoph A Nienaber, Royal Brompton Hospital and Harefield Trust, London SW3 6NP, United Kingdom.
References
- 1.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Ramaraj R, Sorrell VL. Degenerative aortic stenosis. BMJ. 2008;336:550–555. doi: 10.1136/bmj.39478.498819.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM. Timing of aortic valve surgery. Heart. 2000;84:211–218. doi: 10.1136/heart.84.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebe V, Brueckmann M, Borggrefe M, Kaden JJ. Statin therapy of calcific aortic stenosis: hype or hope? Eur Heart J. 2006;27:773–778. doi: 10.1093/eurheartj/ehi697. [DOI] [PubMed] [Google Scholar]
- 5.Rossebø AB, Pedersen TR. Hyperlipidaemia and aortic valve disease. Curr Opin Lipidol. 2004;15:447–451. doi: 10.1097/01.mol.0000137229.00020.fe. [DOI] [PubMed] [Google Scholar]
- 6.Ortlepp JR, Schmitz F, Mevissen V, Weiss S, Huster J, Dronskowski R, Langebartels G, Autschbach R, Zerres K, Weber C, et al. The amount of calcium-deficient hexagonal hydroxyapatite in aortic valves is influenced by gender and associated with genetic polymorphisms in patients with severe calcific aortic stenosis. Eur Heart J. 2004;25:514–522. doi: 10.1016/j.ehj.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Rydén L, Nilsson J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 8.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 10.Mautner GC, Roberts WC. Reported frequency of coronary arterial narrowing by angiogram in patients with valvular aortic stenosis. Am J Cardiol. 1992;70:539–540. doi: 10.1016/0002-9149(92)91206-j. [DOI] [PubMed] [Google Scholar]
- 11.Peltier M, Trojette F, Sarano ME, Grigioni F, Slama MA, Tribouilloy CM. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol. 2003;91:97–99. doi: 10.1016/s0002-9149(02)03010-2. [DOI] [PubMed] [Google Scholar]
- 12.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 13.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 14.Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 15.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 16.Shavelle DM, Takasu J, Budoff MJ, Mao S, Zhao XQ, O’Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359:1125–1126. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- 17.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 19.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 20.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 21.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 22.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 23.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J; ASTRONOMER Investigators. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 24.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkilä J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 25.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 26.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kiliç R, Sarikoç A, Piñol R, Hagl S, Lang S, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kiliç R, Sarikoç A, Brueckmann M, Vahl C, Hagl S, Haase KK, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 28.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 29.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol. 2003;34:756–763. doi: 10.1016/s0046-8177(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 31.Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 33.De Vecchis R, Di Biase G, Esposito C, Ciccarelli A, Cioppa C, Giasi A, Ariano C, Cantatrione S. Statin use for nonrheumatic calcific aortic valve stenosis: a review with meta-analysis. J Cardiovasc Med (Hagerstown) 2013;14:559–567. doi: 10.2459/JCM.0b013e3283587267. [DOI] [PubMed] [Google Scholar]
- 34.Hermans H, Herijgers P, Holvoet P, Verbeken E, Meuris B, Flameng W, Herregods MC. Statins for calcific aortic valve stenosis: into oblivion after SALTIRE and SEAS? An extensive review from bench to bedside. Curr Probl Cardiol. 2010;35:284–306. doi: 10.1016/j.cpcardiol.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27:800–808. doi: 10.1016/j.cjca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Holme I, Boman K, Brudi P, Egstrup K, Gohlke-Baerwolf C, Kesäniemi YA, Malbecq W, Rossebø AB, Wachtell K, Willenheimer R, et al. Observed and predicted reduction of ischemic cardiovascular events in the Simvastatin and Ezetimibe in Aortic Stenosis trial. Am J Cardiol. 2010;105:1802–1808. doi: 10.1016/j.amjcard.2010.01.363. [DOI] [PubMed] [Google Scholar]
- 37.Arsenault BJ, Boekholdt SM, Mora S, DeMicco DA, Bao W, Tardif JC, Amarenco P, Pedersen T, Barter P, Waters DD. Impact of high-dose atorvastatin therapy and clinical risk factors on incident aortic valve stenosis in patients with cardiovascular disease (from TNT, IDEAL, and SPARCL) Am J Cardiol. 2014;113:1378–1382. doi: 10.1016/j.amjcard.2014.01.414. [DOI] [PubMed] [Google Scholar]
- 38.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohle K, Mäffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–1932. doi: 10.1161/hc4101.097527. [DOI] [PubMed] [Google Scholar]
- 40.Griffin BP. Statins in aortic stenosis: new data from a prospective clinical trial. J Am Coll Cardiol. 2007;49:562–564. doi: 10.1016/j.jacc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Ge H, Zhang Q, Wang BY, He B. Therapeutic effect of statin on aortic stenosis: a review with meta-analysis. J Clin Pharm Ther. 2010;35:385–393. doi: 10.1111/j.1365-2710.2009.01137.x. [DOI] [PubMed] [Google Scholar]
- 42.Mohler ER 3rd, Wang H, Medenilla E, Scott C. Effect of statin treatment on aortic valve and coronary artery calcification. J Heart Valve Dis. 2007;16:378–386. [PubMed] [Google Scholar]
- 43.Rosenhek R, Baumgartner H. Aortic sclerosis, aortic stenosis and lipid-lowering therapy. Expert Rev Cardiovasc Ther. 2008;6:385–390. doi: 10.1586/14779072.6.3.385. [DOI] [PubMed] [Google Scholar]
- 44.Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, Côté N, Mathieu P, Després JP, Pibarot P; ASTRONOMER Investigators. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. doi: 10.1016/j.jacc.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 45.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64 Suppl 2:43–60. doi: 10.2165/00003495-200464002-00005. [DOI] [PubMed] [Google Scholar]
- 46.Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. [Google Scholar]
- 47.Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, Kastelein JJ, Colhoun H, Barter P. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–1545. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 48.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- 49.van der Linde D, Yap SC, van Dijk AP, Budts W, Pieper PG, van der Burgh PH, Mulder BJ, Witsenburg M, Cuypers JA, Lindemans J, et al. Effects of rosuvastatin on progression of stenosis in adult patients with congenital aortic stenosis (PROCAS Trial) Am J Cardiol. 2011;108:265–271. doi: 10.1016/j.amjcard.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Folkeringa RJ, Tieleman RG, Maessen JG, Prins MH, Nieuwlaat R, Crijns HJ. Statins Do Not Reduce Atrial Fibrillation After Cardiac Valvular Surgery: A Single Centre Observational Study. Neth Heart J. 2011;19:17–23. doi: 10.1007/s12471-010-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kourliouros A, De Souza A, Roberts N, Marciniak A, Tsiouris A, Valencia O, Camm J, Jahangiri M. Dose-related effect of statins on atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2008;85:1515–1520. doi: 10.1016/j.athoracsur.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 52.Paraskevas KI. Applications of statins in cardiothoracic surgery: more than just lipid-lowering. Eur J Cardiothorac Surg. 2008;33:377–390. doi: 10.1016/j.ejcts.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Virani SS, Nambi V, Polsani VR, Lee VV, Elayda M, Kohsaka S, Pan W, Reul RM, Wilson JM, Petersen LA, et al. Preoperative statin therapy decreases risk of postoperative renal insufficiency. Cardiovasc Ther. 2010;28:80–86. doi: 10.1111/j.1755-5922.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 54.Kuhn EW, Liakopoulos OJ, Stange S, Deppe AC, Slottosch I, Scherner M, Choi YH, Wahlers T. Meta-analysis of patients taking statins before revascularization and aortic valve surgery. Ann Thorac Surg. 2013;96:1508–1516. doi: 10.1016/j.athoracsur.2013.04.096. [DOI] [PubMed] [Google Scholar]
- 55.Liakopoulos OJ, Kuhn EW, Slottosch I, Wassmer G, Wahlers T. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev. 2012;(4):CD008493. doi: 10.1002/14651858.CD008493.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Virani SS, Nambi V, Lee VV, Elayda M, Reul RM, Wilson JM, Ballantyne CM. Does preoperative statin therapy improve outcomes in patients undergoing isolated cardiac valve surgery? Am J Cardiol. 2008;102:1235–1239. doi: 10.1016/j.amjcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 57.Roncalli J, Carrié D, Galinier M. Calcific aortic stenosis and statin therapy: a new insight. Int J Cardiol. 2009;131:275–276; author reply 276-277. doi: 10.1016/j.ijcard.2007.06.125. [DOI] [PubMed] [Google Scholar]
- 58.Antonini-Canterin F, Zuppiroli A, Popescu BA, Granata G, Cervesato E, Piazza R, Pavan D, Nicolosi GL. Effect of statins on the progression of bioprosthetic aortic valve degeneration. Am J Cardiol. 2003;92:1479–1482. doi: 10.1016/j.amjcard.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 59.Colli A, Gherli T, Mestres CA, Pomar JL. Degeneration of native and tissue prosthetic valve in aortic position: do statins play an effective role in prevention? Int J Cardiol. 2007;116:144–152. doi: 10.1016/j.ijcard.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 60.Kulik A, Masters RG, Bédard P, Hendry PJ, Lam BK, Rubens FD, Mesana TG, Ruel M. Postoperative lipid-lowering therapy and bioprosthesis structural valve deterioration: justification for a randomised trial? Eur J Cardiothorac Surg. 2010;37:139–144. doi: 10.1016/j.ejcts.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 61.Pullan M, Chalmers J, Mediratta N, Shaw M, McShane J, Poullis M. Statins and long-term survival after isolated valve surgery: the importance of valve type, position and procedure. Eur J Cardiothorac Surg. 2014;45:419–424; discussion 424-425. doi: 10.1093/ejcts/ezt399. [DOI] [PubMed] [Google Scholar]
- 62.Taylor AP, Yadlapati A, Andrei AC, Li Z, Clennon C, McCarthy PM, Thomas JD, Malaisrie SC, Stone NJ, Bonow RO, et al. Statin Use and Aneurysm Risk in Patients With Bicuspid Aortic Valve Disease. Clin Cardiol. 2016;39:41–47. doi: 10.1002/clc.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dichtl W, Alber HF, Feuchtner GM, Hintringer F, Reinthaler M, Bartel T, Süssenbacher A, Grander W, Ulmer H, Pachinger O, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg) Am J Cardiol. 2008;102:743–748. doi: 10.1016/j.amjcard.2008.04.060. [DOI] [PubMed] [Google Scholar]


