Abstract
Background: The mechanism leading to the development of metabolic complications in obese individuals is not fully understood. Thus, the objective of this study was to examine differences in insulin resistance, inflammation, cytokine and adipokine levels, and expression of selected genes across obese individuals with different number of metabolic syndrome (MetS) components.
Methods: Forty obese individuals who underwent bariatric surgery, divided in three groups based on the number of components of MetS, in addition to abdominal obesity (0, 1, and 2–3 additional components), were studied. Levels of inflammatory proteins, insulin resistance, cytokines, adipokines, and gene expression in subcutaneous (SAT) and visceral adipose tissue (VAT) were compared.
Results: There was a significantly higher expression of MYD88 in SAT among those with more components of MetS (P = 0.008). In SAT, but not in VAT, MYD88 expression was significantly correlated with toll-like receptor 4 expression (r = 0.7, P < 0.05). Expression of adipsin in SAT was also associated with the presence of more components of MetS, but with borderline statistical significance (P = 0.05). There were no significant differences in insulin resistance, inflammation, and cytokine and adipokine levels by the number of components of MetS.
Conclusions: Our study suggests that MYD88 expression in SAT of obese subjects could be associated with the development of components of MetS.
Keywords: : metabolic syndrome, obesity, inflammation, myd88
Introduction
The prevalence of obesity has been increasing worldwide over the past 30 years, affecting developed and developing countries. Obesity is associated with a number of metabolic disturbances, including insulin resistance, glucose intolerance, dyslipidemia, and high blood pressure.1 All of these conditions are risk factors for type 2 diabetes and cardiovascular disease. Moreover, it has been shown that obese patients, in particular those with a predominance of abdominal fat, develop a low-grade chronic systemic inflammation,2–5 which undoubtedly plays a critical role in the development of these conditions.
However, a number of epidemiological studies have shown that some obese individuals, despite having excessive body fat, exhibit a healthy metabolic status, comparable to lean people. This condition had been called “healthy obese phenotype” and represents about 30% of the obese population.6–12
The mechanisms that could explain the favorable profile of individuals that are metabolically healthy obese are poorly understood, but a lower degree of inflammation compared to patients with metabolic abnormalities has been proposed.13 In addition, it is possible that changes in the expression in adipose tissue of key genes associated with insulin resistance, lipid metabolism, and inflammation may have a role.
Thus, the objective of this study was to compare obese individuals with different number of metabolic syndrome (MetS) components with respect to levels of cytokines, adipokines, and selected gene expression.
Materials and Methods
Study population
This was a prospective study involving 40 obese men and women, with body mass index (BMI) between 30 and 45 kg/m2, who underwent bariatric surgery (gastric bypass or gastric sleeve resection) at a single hospital (Clinica Las Condes) in the city of Santiago, Chile.
They were divided into three groups according to the presence of components of the MetS before the surgery: (1) only increased waist circumference; (2) 1 additional component; and (3) 2–3 additional components. The following criteria of the MetS were considered: (1) blood pressure ≥130/85 or use of blood pressure lowering medications, (2) triglycerides (TG) ≥150 mg/dL or pharmacological treatment for hypertriglyceridemia, (3) high-density lipoprotein (HDL) cholesterol <40 and <50 mg/dL for men and women, respectively, or pharmacological treatment for dyslipidemia, and (4) fasting glucose ≥100 mg/dL or use of glucose lowering drugs.
Exclusion criteria were as follows: self-reported malignancies, renal and liver diseases, cardiovascular diseases, endocrinopathies (e.g., hypothyroidism, Cushing syndrome polycystic ovary syndrome), chronic or acute inflammatory disease, excessive alcohol consumption (>15 and 7 drinks/week for men and women, respectively), drug abuse, pregnancy, significant body weight change (>5%) in the previous 6 months, and concomitant medication with effects on body weight (corticoids, orlistat, and anorexigens).
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Clínica Las Condes and the Johns Hopkins University School of Medicine IRB. All participants gave written informed consent to participate in the study.
Anthropometry
Presurgery body weight and height were measured, and BMI was calculated as weight in kg, divided by height (m2). Blood pressure was measured at the arm in sitting position after 10 min of rest, and the mean of the last two measurements out of three was recorded.
Waist circumference was measured with an inextensible tape in the middle point between the iliac crest and the last rib.
Analyses of blood samples
Fasting blood samples were obtained ∼2 weeks before surgery, and standard methods were used to measure plasma lipids, glucose, and liver enzyme [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl transpeptidase (GGT)] levels.
Lipids [high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG)] were determined by colorimetric methods (Siemens Healthcare Diagnostics, Inc., Nueva York).
Additional fasting blood plasma samples were drawn immediately before the surgery (in the operating room). Serum and plasma samples were kept at −70°C and shipped to The Johns Hopkins School of Medicine, Hopkins Conte Digestive Disease Basic and Translational Core Center, Baltimore, MD, for measurement of insulin (expressed in μIU/mL; Phoenix EK-035-06), leptin (expressed in μIU/mL; Invitrogen KAC2281), adiponectin (expressed as μg/mL; Invitrogen KHP0041), interleukin-6 (IL-6) (expressed as μg/mL; Invitrogen), adipsin (expressed as ng/mL; R&D systems), resistin (expressed as ng/mL; Invitrogen KPH0051), IL-6 (expressed as pg/mL; Invitrogen KHC0069), ghrelin (expressed as ng/mL; Phoenix CEK-031-30) and visfatin (expressed as ng/mL; Phoenix EK-003-80-RIA), and tumor necrosis factor-alpha (TNF-α) (expressed as pg/mL; Invitrogen, KHC3011C).
Insulin resistance was determined by HOMA-IR model (fasting glucose [mg/dL] × fasting insulin [uU/mL]/405). Using IU: (fasting glucose [mmol/L] × fasting insulin [pmol/L]/22.5.
Adipose tissue sample
During the bariatric surgery, 20 grams of adipose visceral omental fat and 3 grams of subcutaneous fat samples were extracted. They were frozen in liquid nitrogen and kept at −70°C until shipped to the Johns Hopkins Medical School, Baltimore, MD.
RNA isolation and quantitation
Serum samples were sent to the University of Maryland, Mid-Atlantic NORC (Nutrition Obesity Research Center), for analyses of gene expression. Adipose tissue was homogenized in 1 mL of TRIzol (Invitrogen, Grand Island, NY), and total RNA was extracted according to the manufacturer's protocol. cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Steady-state mRNA levels were determined by two-step quantitative real-time polymerase chain reaction (qRT-PCR) using the LightCycler 480 (Roche) and normalized to cyclophilin mRNA.
Gene expression of CCAAT Enhancer Binding Protein-α (CEBP-α), Toll-like receptors 4 (TLR4); IL-6, plasminogen activator inhibitor-1 (PAI-1), Myeloid differentiation primary response gene 88 (MYD88), adipsin, peroxisome proliferator activated receptor γ-2 (PPARγ-2), diacylglycerol-acyltransferase-1 (DGAT-1), and adiponectin were determined.
Statistical analyses
For the analyses, we divided the study population into three groups defined by the number of MetS components (0; only having elevated waist circumference; 1; having elevated waist circumference and one additional MetS criteria; 2–3; having elevated waist circumference and two or three more MetS criteria).
Differences in measures of biochemical and metabolic parameters, adipokines, cytokines, and gene expression levels across the groups were tested using chi-square for categorical variables and for continuous variables a nonparametric test for trend across ordered groups, using a modified Wilcoxon rank-sum test (Stata command nptrend).
We further assessed the correlations between metabolic parameters, adipokines, cytokines, and gene expression levels using Pearson's correlation coefficient.
We used Stata 13.1 for all the data analyses.
Results
Clinical characteristics of the participants are shown in Table 1: there were 24 women and 16 men, between 24 and 53 years old (mean 36.8 ± 6.8), with a mean BMI of 35.1 ± 3.3 (kg/m2).
Table 1.
Characteristics of the Study Participants, Overall and by Number of Metabolic Abnormalities, in Addition to Increased WC
| Variable | Overall (n = 40) | 0 (n = 11) | 1 (n = 13) | 2–3 (n = 16) | P value for trend |
|---|---|---|---|---|---|
| Age (year) | 36.75 (6.79) | 36.70 (6.85) | 38.75 (8.39) | 35.44 (5.53) | 0.52 |
| BMI (kg/m2) | 35.14 (3.29) | 34.92 (3.47) | 34.67 (3.32) | 35.59 (3.31) | 0.55 |
| Waist circumference (cm) | 108.94 (10.40) | 102.86 (6.26) | 107.91 (13.46) | 112.31 (8.45) | 0.04 |
| SBP (mmHg) | 122.20 (13.32) | 116.00 (6.99) | 118.67 (10.42) | 128.00 (15.61) | 0.01 |
| DBP (mmHg) | 78.38 (8.38) | 76.00 (5.16) | 77.83 (7.74) | 80.06 (10.09) | 0.21 |
| Fasting glucose (mg/dL) | 91.65 (9.39) | 89.30 (7.50) | 92.83 (10.56) | 92.17 (9.78) | 0.50 |
| Total cholesterol (mg/dL) | 197.10 (29.01) | 203.40 (24.28) | 194.42 (31.60) | 195.39 (30.67) | 0.54 |
| HDL-c (mg/dL) | 48.51 (11.37) | 58.40 (11.84) | 48.84 (9.35) | 42.81 (8.57) | <0.001 |
| Median triglycerides (mg/dL) | 125.5 [98.0–180.0] | 96.5 [74.0–125.0] | 111.0 [97.0–134.5] | 176.5 [128.0–275.0] | 0.007 |
| Median AST (U/L) | 27.5 [21.0–46.5] | 27.0 [14.0–46.0] | 27.0 [21.0–47.0] | 29.0 [24.0–38.0] | 0.49 |
| Median ALT (U/L) | 48.5 [20.0–74.0] | 71.0 [68.0–74.0] | 45.0 [25.0–52.0] | 20.0 [14.0–76.0] | 0.55 |
| Median GGT (U/L) | 31.0 [26.0–63.0] | 53.5 [17.0–90.0] | 44.0 [26.0–63.0] | 31.0 [26.0–31.0] | 0.90 |
Data presented as mean (SD), median [p25–p75], or %.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; GGT, gamma glutamyl transpeptidase; HDL-c, high-density lipoprotein cholesterol; SBP, systolic blood pressure; WC, waist circumference.
As expected, there was a significant trend to a higher waist circumference, lower HDL-cholesterol, higher triglyceride plasma levels, and higher systolic blood pressure with the increasing numbers of components of the MetS (Table 1). As shown in Table 2, we did not find any significant association between the levels of insulin resistance markers, cytokines, and adipokines and the number of components of the MetS. Levels of adiponectin tended to be higher with increasing numbers of components of the MetS, but this finding did not reach statistical significance. In addition, ghrelin levels tended to be lower with increasing numbers of the MetS, but the trend was also not statistically significant.
Table 2.
Serum Markers of Insulin Resistance, Cytokines, and Adipokines: Overall and by Number of Metabolic Abnormalities
| Variable | Overall (n = 0) | 0 (n = 11) | 1 (n = 13) | 2–3 (n = 16) | P value for trend |
|---|---|---|---|---|---|
| Insulin (μIU/mL) | 8.8 [5.1–12.5] | 9.1 [8.2–12.5] | 6.8 [3.7–11.8] | 9.0 [5.0–12.6] | 0.52 |
| HOMA-IR | 4.3 [3.3–5.5] | 4.2 [3.2–5.4] | 3.8 [2.5–5.3] | 4.6 [4.0–6.0] | 0.62 |
| IL-6 (pg/mL) | 1.0 [0.5–1.5] | 1.1 [0.5–1.4] | 0.6 [0.4–1.1] | 1.4 [0.7–2.2] | 0.70 |
| Resistin (ng/mL) | 6.7 [4.4–9.3] | 6.8 [4.6–12.7] | 5.8 [4.1–8.0] | 7.7 [4.3–9.4] | 0.87 |
| Adipsin (μg/mL) | 3.1 [2.7–3.7] | 2.9 [2.6–3.3] | 2.9 [2.5–3.4] | 3.5 [2.8–3.9] | 0.13 |
| Adiponectin (μg/mL) | 13.5 [10.9–16.6] | 10.9 [7.9–18.5] | 13.7 [11.2–15.1] | 14.0 [11.1–16.9] | 0.36 |
| Ghrelin (pg/mL) | 231.6 [219.9–244.2] | 240.5 [235.8–254.3] | 232.2 [218.8–254.4] | 224.4 [220.1–236.7] | 0.86 |
| Visfatin (ng/mL) | 8.9 [6.7–10.4] | 7.5 [6.6–11.3] | 9.6 [5.8–10.8] | 8.7 [7.6–9.5] | 0.31 |
| Leptin (μg/mL) | 28.4 [15.5–45.7] | 15.5 [12.1–43.8] | 32.1 [17.4–43.6] | 28.4 [17.4–48.6] | 0.28 |
| TNF-alpha (pg/mL) | 11.8 [9.5–13.3] | 9.7 [9.5–12.8] | 12.5 [11.4–13.6] | 11.6 [8.9–13.6] | 0.60 |
Data presented as median [p25–p75].
HOMA-IR, homeostatic model assessment-insulin resistance; IL-6, interleukin-6; TNF-alpha, tumor necrosis factor-alpha.
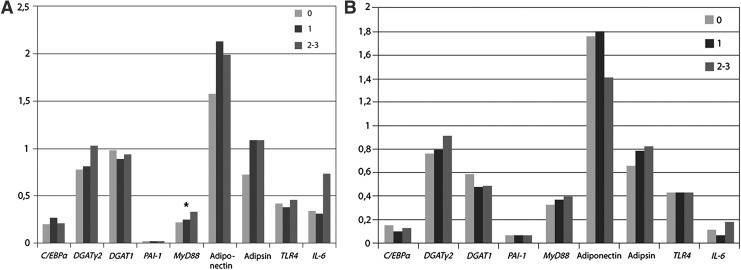
Results of the genetic expression in adipose tissue (subcutaneous and visceral) are shown in Fig. 1A and B.
FIG. 1.
(A) Gene expression by number of metabolic syndrome components in subcutaneous adipose tissue. *P < 0.05. (B) Gene expression by number of metabolic syndrome components in visceral adipose tissue. *P < 0.05. C/EBPα, CAAT/enhancer binding protein α; PAARγ2, peroxisome proliferator activated receptor γ2; DGAT-1, diacylglycerol acyltransferase-1; PAI-1, plasminogen activator inhibitor-1; MYD88, myeloid differentiation primary response gene 88; TLR4, toll-like receptor 4; IL-6, interleukin-6.
There was a significant higher expression of MYD88 in subcutaneous adipose tissue of obese individuals with increasing number of components of the MetS (P < 0.05). Expression of adipsin in subcutaneous fat was also higher with the presence of more components of the MetS, but in the limit of the statistical significance (P = 0.05). There was a trend to a lower expression of adiponectin gene in visceral and subcutaneous adipose tissue in the patients with more components of the MetS, but this difference was not statistically significant.
No significant trends in the expression of the other analyzed genes (PAI-1, TLR4, IL-6, DGAT-1, CEBPA, and PPARγ-2) were detected.
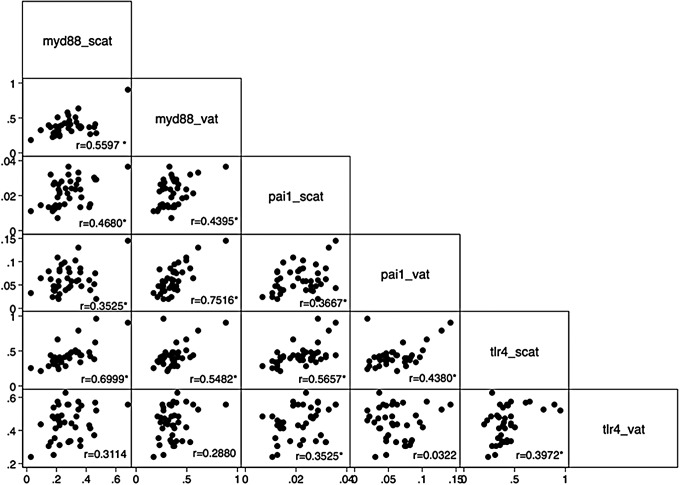
Correlations of the gene expression of MYD88 with other genes are shown in Fig. 2. There was a significant correlation between the visceral and subcutaneous gene expression of MYD88 (r = 0.56, P < 0.05). In addition, we detected a significant correlation of the subcutaneous expression of MYD88 with the subcutaneous expression of TLR4 (r = 0.7, P < 0.05) and a significant correlation between the visceral expression of MYD88 with the subcutaneous expression of TLR4 (r = 0.55). Moreover, a significant correlation between the visceral expression of MYD88 with the expression of PAI-1 in subcutaneous and visceral fat was detected (r = 0.44 and r = 0.75, respectively) and a significant correlation between the subcutaneous expression of MYD88 with the expression of PAI-1 in subcutaneous and visceral fat (r = 0.45 and r = 0.35, respectively).
FIG. 2.
Pairwise correlation between MYD88, PAI, and TLR4 expression in subcutaneous and visceral adipose tissue. *P value <0.05. scat, subcutaneous adipose tissue; vat, visceral adipose tissue.
Discussion
In our study in 40 obese individuals undergoing bariatric surgery, we detected a higher genetic expression of MYD88 in the subcutaneous adipose tissue of those with more metabolic complications. However, although there were differences regarding the extent of clinical manifestations (e.g., waist circumference, dyslipidemia, high blood pressure), we did not find significant differences in plasma levels of cytokines or adipokines across groups defined by the number of MetS components, except for a nonsignificant trend for lower plasma adiponectin levels. It is accordant with the protective role of this adipokine in the development of cardio metabolic complications.
The reasons for the lack of association between the number of MetS components and common cytokines and adipokines are not clear. One possible explanation is that all the participants were obese with an elevated waist circumference (≥88 cm for women and 102 cm for men), a clinical marker of visceral abdominal fat. It is well known that cytokines/adipokines, are mainly produced by this type of adipose tissue,13,14 therefore it could have limited our ability to detect significant differences in these parameters. Another possible explanation could be the use of metformin that may attenuate the inflammatory condition. In our study, three patients with 0–1 metabolic abnormalities and six patients with 2 or more metabolic abnormalities were receiving metformin because of glucose intolerance (one patient) and insulin resistance (eight patients). However, we did the analysis excluding those patients, and the results were similar (data not shown). Moreover, 1 week before the surgery, they were advised to discontinue the drug.
Notwithstanding the limitations of the current study, at least one other study found similar results. It was a cross-sectional analysis, including metabolically healthy and abnormal obese patients and 225 lean subjects as reference. The cardiometabolic and inflammatory profiles were similarly increased in both groups of obese individuals compared to lean controls. In addition, expression of genes involved in inflammation and tissue remodeling in visceral fat and liver showed a similar pattern. These findings indicate a comparably adverse cardiometabolic profile in metabolically healthy and metabolically abnormal obese patients.15 The authors concluded that the concept of “healthy obesity” should be applied with caution and that it is necessary to identify better markers of the metabolic state in obese individuals.
All together, these results add to the controversy related to the real existence of a benign obesity. A recent meta-analysis of eight studies evaluating all-cause mortality and cardiovascular events in obese people did not find differences between obese people with or without metabolic abnormalities.16
Nevertheless, we found that the genetic expression of MYD88 in subcutaneous adipose tissue was different according to the number of MetS components. We detected a significant trend to a higher genetic expression of MYD88 in subcutaneous fat of obese individuals with larger number of metabolic complications. Myd88 mediates inflammatory signaling pathways, in response to activation of TLR.17,18 TLR are transmembrane receptors that play a potential role in pathogen recognition and immune response by activating various inflammatory signaling pathways, including Myd88, which then leads to an activation of NF-κB signaling activity.18
Recent studies provided evidence of increased expression of TLR in murine adipose tissue in response to obesity induced by a high fat diet, causing activation of Myd88 signaling cascades.19 Similarly, Ahmad et al. also found a significantly increased expression of TLR2, TLR4, and MYD88 in both peripheral blood mononuclear cells and subcutaneous adipose tissue of obese and overweight subjects compared to lean controls.20
In agreement with these reports, we detected a significant correlation between the gene expression of TLR4 in subcutaneous fat with the subcutaneous and visceral expression of MYD88. In addition, we detected a significant correlation between the subcutaneous and visceral expression of MYD88 with the expression of PAI-1 in both adipose tissues. We can speculate that there is a positive feedback between the expression of TLR4, MYD88, and PAI-1 in the adipose tissue of obese individuals that develop metabolic complications. It is well known that PAI-1 is an acute response protein that mediates inflammatory responses, and obese people with MetS exhibit higher plasma levels of PAI-1.21
Adipsin (complement factor D) is a key enzyme involved in the activation of alternative pathway of complement activation and is primarily secreted from adipocytes and monocytes/macrophages in human subjects.22 Previous studies have shown a trend of increased adipsin production in subjects with higher BMI and waist circumference23 and others detected a significant correlation between HOMA index and adipsin.24 Similarly, it has been shown that postmenopausal women with MetS displayed higher levels of adipsin compared to those without MetS.25 However, a recent study in animal models of obesity reported that adipsin improves the insulin secretion by the pancreas and lowers plasma levels of adipsin in patients with severe diabetes. The authors hypothesized that the increased production of adipsin in obesity could be necessary to compensate the higher production of insulin.26
We did not find any significant differences in the expression of other analyzed genes or any significant differences in gene expression levels comparing visceral versus subcutaneous adipose tissue.
There are some limitations in our study. First, we did not have a control lean group that would have allowed us to better identify differences in the adipokine/cytokine levels and gene expression compared to obese people. Second, all patients had an abnormal waist circumference (≥88 and 102 cm in women and men, respectively), and it is well known that abdominal fat is the main source of inflammatory mediators and adipokines, thus limiting the presence of some significant difference. Third, the use of metformin in some patients could decrease the inflammatory process associated to obesity and therefore influence the results in the total group (although the analysis excluding them was similar).
In conclusion, the current study provides evidence for the existence of a higher expression of MYD88 in the subcutaneous adipose tissue of obese individuals who are carriers of more metabolic alterations. The clinical relevance of our result regarding gene expression of MYD88 and adipsin in subcutaneous fat and to clarify the role of Myd88 in the development of MetS needs further studies.
Nevertheless, an unexpected result was the lack of significant differences in plasma levels of cytokines and adipokines according to the increasing number of metabolic complications. Our results add to data contradicting the real existence of a “healthy obesity,” suggesting that metabolic abnormalities associated with obesity are merely a question of evolution of the disease.
Acknowledgments
This study was supported by the Mid-Atlantic Nutrition Obesity Research Center (NORC) and Research Grant of Clinica Las Condes.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med 2000;160:898–904 [DOI] [PubMed] [Google Scholar]
- 2.Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab 2013;27:163–177 [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–2180 [DOI] [PubMed] [Google Scholar]
- 4.Zeyda M, Farmer D, Todoric J, et al. . Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes 2007;31:1420–1428 [DOI] [PubMed] [Google Scholar]
- 5.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010;314:1–16 [DOI] [PubMed] [Google Scholar]
- 6.Stefan N, Kantartzis K, Machann J, et al. . Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 7.Klöting N, Fasshauer M, Dietrich A, et al. . Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Salinas CA, Garcia EG, Robles L, et al. . High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab 2008;93:4075–4079 [DOI] [PubMed] [Google Scholar]
- 9.Samocha-Bonet D, Chisholm DJ, Tonks K, et al. . Insulin-sensitive obesity in humans—A ‘favorable fat’ phenotype? Trends Endocrinol Metab 2012;23:116–124 [DOI] [PubMed] [Google Scholar]
- 10.Stefan N, Häring H-U, Hu FB, et al. . Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152–162 [DOI] [PubMed] [Google Scholar]
- 11.Primeau V, Coderre L, Karelis AD, et al. . Characterizing the profile of obese patients who are metabolically healthy. Int J Obes 2011;35:971–981 [DOI] [PubMed] [Google Scholar]
- 12.Velho S, Paccaud F, Waeber G, et al. . Metabolically healthy obesity: Different prevalences using different criteria. Eur J Clin Nutr 2010;64:1043–1051 [DOI] [PubMed] [Google Scholar]
- 13.Fain JN, Madan AK, Hiler ML, et al. . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adypocites from visceral and subcutaneous abdominal adipose tissue of obese humans. Endocrinology 2004;145:2273–2282 [DOI] [PubMed] [Google Scholar]
- 14.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998;83:847–850 [DOI] [PubMed] [Google Scholar]
- 15.Goméz-Ambrosi J, Catalán V, Rodriguez A, et al. . Increased cardiometabolic risk and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014;37:2813–2821 [DOI] [PubMed] [Google Scholar]
- 16.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy and obesity benign conditions? A systematic review and meta-analysis of the effect of body mass index and metabolic status phenotypes on all-cause mortality and cardiovascular events. Ann Intern Med 2013;159:758–769 [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol 2004;4:499–511 [DOI] [PubMed] [Google Scholar]
- 18.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J Clin Endocrinol Metab 2014;99:39–48 [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Choi Y, Choi YH, et al. . Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J Nutr Biochem 2012;23:113–122 [DOI] [PubMed] [Google Scholar]
- 20.Ahmad R, Al-Mass A, Atizado V, et al. . Elevated expression of the toll-like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J Inflamm 2012;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaraj J, Siegel D, Jialal I. Inflammation and metabolic syndrome. In: Byrne CD, Wild SH. (eds). The Metabolic Syndrome, 2nd ed, Oxford, UK: Wiley-Blackwell; 2011: 210–228 [Google Scholar]
- 22.White RT, Damm D, Hancock N, et al. . Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 1992;267:9210–9213 [PubMed] [Google Scholar]
- 23.Abu-Farha M, Behbehani K, Elkum N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc Diabetol 2014;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derosa G, Fogari E, D'Angelo A, et al. . Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation 2013;36:914–920 [DOI] [PubMed] [Google Scholar]
- 25.Chedraui P, Pérez-López FR, Escobar GS, et al. ; Research Group for the Omega Women's Health Project. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas 2014;79:86–90 [DOI] [PubMed] [Google Scholar]
- 26.Lo JC, Ljubicic S, Leibiger B, et al. . Adipsin is an adipokine that improves β cell function in diabetes. Cell 2014;158:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]