Abstract
Vesicular stomatitis virus (VSV) is a negative-stranded RNA virus that naturally causes disease in livestock including horses, cattle and pigs. The two main identified VSV serotypes are New Jersey (VSNJV) and Indiana (VSIV). VSV is a rapidly replicating, potently immunogenic virus that has been engineered to develop novel oncolytic therapies for cancer treatment. Swine are a natural host for VSV and provide a relevant and well-established model, amenable to biological sampling to monitor virus shedding and neutralizing antibodies. Previous reports have documented the pathogenicity and transmissibility of wild-type isolates and recombinant strains of VSIV and VSNJV using the swine model. Oncolytic VSV engineered to express interferon-beta (IFNβ) and the sodium iodide symporter (NIS), VSV-IFNβ-NIS, has been shown to be a potent new therapeutic agent inducing rapid and durable tumor remission following systemic therapy in preclinical mouse models. VSV-IFNβ-NIS is currently undergoing clinical evaluation for the treatment of advanced cancer in human and canine patients. To support clinical studies and comprehensively assess the risk of transmission to susceptible species, we tested the pathogenicity and transmissibility of oncolytic VSV-IFNβ-NIS using the swine model. Following previously established protocols to evaluate VSV pathogenicity, intradermal inoculation with 107 TCID50 VSV-IFNβ-NIS caused no observable symptoms in pigs. There was no detectable shedding of infectious virus in VSV-IFNβ-NIS in biological excreta of inoculated pigs or exposed naive pigs kept in direct contact throughout the experiment. VSV-IFNβ-NIS inoculated pigs became seropositive for VSV antibodies, while contact pigs displayed no symptoms of VSV infection, and importantly did not seroconvert. These data indicate that oncolytic VSV is both nonpathogenic and not transmissible in pigs, a natural host. These findings support further clinical development of oncolytic VSV-IFNβ-NIS as a safe therapeutic for human and canine cancer.
Keywords: : Oncolytic virus, vesicular stomatitis virus, pig model, biosafety
Introduction
Vesicular stomatitis virus (VSV) is a negative-stranded RNA virus that causes disease in agricultural livestock including horses, cattle, and pigs. Two major serotypes of VSV have been identified, namely, New Jersey (VSNJV) and Indiana (VSIV). While both serotypes have caused epidemics in livestock, VSNJV is considered more pathogenic and has caused recent outbreaks in the United States.1 Pathogenicity and transmissibility of isolated wild-type strains of both VSIV and VSNJV have been previously evaluated in pigs, a natural host for VSV.2–4 These studies indicate that isolated wild-type strains of VSIV and VSNJV cause disease-related clinical symptoms in pigs, including fever, blistering lesions, shedding of infectious virus, and seroconversion in inoculated pigs. It is well documented that wild-type VSV causes clinical infection and is transmissible in the swine model. While wild-type VSNJV is more virulent and transmissible than VSIV, both viruses induce clinical infection in inoculated animals with detectable shedding of infectious virus and clinical disease or seroconversion in contact pigs. These data provide the basis to evaluate and compare the biosafety of recombinant VSV vectors.
VSV is a rapidly replicating, potently immunogenic virus that is amenable to engineering and has been deployed as a platform to develop prophylactic and therapeutic vaccines to prevent and/or treat infectious disease and oncolytic viruses for the treatment of cancer.5,6 Recombinant attenuated VSV vectors have been shown to replicate selectively in and kill cancer cells and are not pathogenic in humans.7–9 Tumor selectivity is due largely to cancer-specific defects in innate immune response pathways preventing malignant cells from mounting an effective antiviral response rendering them permissive to virus replication and killing.10 VSV is a particularly promising vector for development as an oncolytic therapy due to the lack of preexisting immunity to VSV in the general human population, allowing the development of agents that can potentially be administered systemically for the treatment of disseminated and metastatic cancers.11,12 Oncolytic virus therapies are often attenuated or made tumor selective by experimental evolution or engineering. Oncolytic VSV agents in clinical development for cancer therapy are derived from VSIV, and attenuation is based on their ability to induce expression of type 1 interferons compared with wild-type VSV.8,13 Despite attenuation, clinical translation of recombinant replication competent oncolytic virus is dependent on comprehensive biosafety assessment, including virus pathogenicity and transmissibility, to ensure there is minimal risk of toxicity, transmission to caregivers, or in the case of zoonotic-like VSV, risk of transmission to susceptible species that are in contact with treated patients.14
VSV-IFNβ-NIS is a recombinant oncolytic VSV engineered to express interferon-beta (IFNβ) and the sodium iodide symporter (NIS).15,16 IFNβ expression enhances tumor selectively by activating innate immune responses to promote virus clearance from noncancerous cells, while virus replication and spread continue relatively unhindered in cancer cells. The NIS protein, normally expressed in thyroid tissues, is responsible for accumulation of iodide in cells for hormone production. Viral expression of the NIS gene in infected tumor cells allows serial noninvasive imaging (e.g., single photon emission computed tomography) using NIS-specific radio tracers to track virus biodistribution and replication over time.17 Previous findings demonstrate that VSV-IFNβ-NIS is well tolerated and has potent antitumor activity following intravenous administration in immune competent preclinical rodent tumor models, mediating tumor destruction through a combination of direct virus-induced tumor lysis and stimulation of antitumor immune responses.16 VSV-IFNβ-NIS has also been shown to be nonpathogenic in preclinical toxicity and biodistribution studies with no evidence of neuro- or other toxicities observed following intravenous administration, even in immune-compromised, tumor-bearing mice.15,18 This favorable safety profile is likely associated with virus attenuation due to both incorporation of two transgenes, where maximal VSV-IFNβ-NIS titer is less than that of VSV expressing a single GFP transgene (VSV-GFP), and IFNβ expression that promotes activation of antiviral interferon responses in sensitive cells.16 Preclinical studies in rodents, nonhuman primates, and laboratory dogs indicate the maximum tolerated dose and dose-limiting toxicities and safety and biodistribution of intravenous VSV-IFNβ-NIS.18–20 VSV-IFNβ-NIS is currently in clinical development for the treatment of human and canine cancer. Here, we carried out a biosafety assessment to establish pathogenicity and transmissibility of oncolytic VSV-IFNβ-NIS in pigs to support clinical translation. Human IFNβ has been shown to cross react and induce antiviral activity in bovine and porcine cells.21 This investigation was carried out using well-established protocols that have been previously utilized to characterize isolated wild-type strains of VSIV and VSNJV.2–4 Our findings indicate that oncolytic VSV-IFNβ-NIS is nonpathogenic and nontransmissible in pigs. These data demonstrate the minimal risk of transmission of oncolytic VSV to untreated individuals and to natural animal hosts, supporting the safe use of oncolytic VSV-IFNβ-NIS in both veterinary and human clinical settings.
Results and Discussion
Clinical trial
Recombinant oncolytic VSV-IFNβ-NIS is being tested in clinical trials for the treatment of human and canine cancer. This study provided critical data describing the biosafety, specifically the pathogenicity and transmissibility, of oncolytic VSV-IFNβ-NIS in a susceptible species. These data, in addition to preclinical toxicology, biodistribution, and safety studies18–20 supported submission of an investigational new drug (IND) application to initiate a phase 1 clinical trial evaluating intravenous VSV-IFNβ-NIS therapy in patients with relapsed endometrial cancer and relapsed or refractory hematologic malignancies including multiple myeloma, T-cell lymphoma, and acute myeloid leukemia. These data also supported the initiation of a veterinary clinical study evaluating intravenous VSV-IFNβ-NIS therapy in pet dogs with spontaneous cancer.
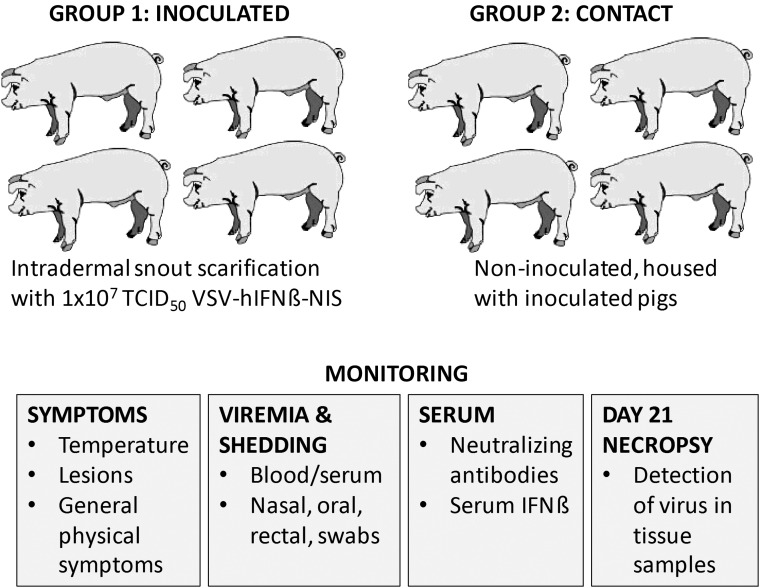
Objectives and study design
Recombinant oncolytic VSV-IFNβ-NIS was previously generated and characterized demonstrating replication and functional transgene expression.16 Figure 1A shows the schematic of the recombinant genome. VSV-IFNβ-NIS anticancer activity was tested in preclinical murine myeloma tumor models, demonstrating that both tumor-selective virus replication and activation of antitumor T-cell responses mediate a therapeutic response against established subcutaneous myeloma tumors in syngeneic immunocompetent mice.15,22 IND-enabling translational steps were carried out including preclinical toxicology and biodistribution studies and manufacture of clinical grade recombinant virus.18,19 Clinical trials evaluating VSV-IFNβ-NIS therapy for the treatment of human cancer are underway. To support clinical development, we wanted to determine the pathogenicity and transmissibility of oncolytic VSV-IFNβ-NIS in a susceptible species. This study was carried out in pigs, a natural host for VSV, similarly to previous investigations that established the pathogenicity of field and laboratory strains of VSIV and VSNJV isolated from naturally occurring infections in livestock (see Supplementary Information for a full description of methods; Supplementary Data are available online at www.liebertpub.com/humc). Figure 1B shows the in vivo study design: four pigs were directly inoculated by intradermal scarification of the snout with 1 × 107 TCID50 of VSV expressing human IFNβ and NIS (VSV-IFNβ-NIS) per animal (inoculated group) and four pigs that remained separated by a fence in the same pen for 24 h postinoculation (contact group), after which the two groups of pigs were housed together in the same pen until the end of the study. Contact animals had direct and continuous exposure to skin, bodily fluids, and excreta from inoculated pigs. Inoculated and contact pigs were housed in BSL-3Ag conditions, and monitored for symptoms of VSV pathogenesis including vesicular lesions and fever by measurement of rectal temperature.
Figure 1.
Evaluation of pathogenicity and transmissibility of recombinant VSV-hIFNβ-NIS in pigs. (A) Schematic of the genome of a recombinant Indiana strain vesicular stomatitis virus (VSV) encoding human interferon-beta (hIFNβ) and the human sodium iodide symporter (hNIS). (B) Study outline indicating treatment groups and monitoring.
Pathogenicity of oncolytic VSV-IFNβ-NIS
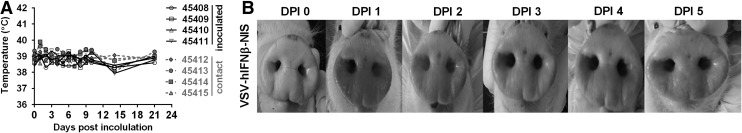
Previous studies demonstrated that pigs inoculated with wild-type VSIV and VSNJV strains caused clinical symptoms of infection, including lesions on the snout and occasional secondary hoof lesions. VSNJV was significantly more pathogenic causing severe lesions on snout, and secondary lesions on the tongue and hooves.2–4 In contrast, there were no observable clinical symptoms in VSV-IFNβ-NIS inoculated pigs. Inoculated pigs did not develop a fever (Fig. 2A), and no lesions were observed at the site of inoculation on the snout or in secondary locations such as hooves (Fig. 2B). These data indicate that oncolytic VSV-IFNβ-NIS is nonpathogenic in pigs, a natural host for VSV.
Figure 2.
Clinical symptoms following VSV-hIFNβ-NIS inoculation. Four pigs were inoculated with 1 × 107 TCID50 VSV-hIFNβ-NIS and housed with four contact pigs. Clinical symptoms were monitored, including (A) measurement of rectal temperature in inoculated (black) and contact (grey) pigs. (B) Pigs were examined for presence of lesions on the snout, mouth, lips and other locations, with representative images indicating absence of lesions from one inoculated pig shown at the indicated days postinoculation (DPI).
Viremia
Whole blood and sera were collected at indicated time points from both inoculated and contact group pigs following inoculation with VSV-IFNβ-NIS to monitor viremia (Table 1). Samples were analyzed by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) targeting the VSV-N gene, indicating no detectable viral RNA in these samples. No infectious virus or detectable virus genome were observed in any of the samples (not shown).
Table 1.
Collection of biological samples to monitor viremia, virus shedding, and antibody neutralization
| Days Postinoculation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 14 | 21 | |
| Viremia (blood) | Whole blood (qRT-PCR) | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Serum (qRT-PCR) | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Virus shedding | Oral swabs (VI & qRT-PCR) | × | × | × | × | × | × | × | × | × | × | × | × | × |
| Nasal swabs (VI & qRT-PCR) | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Rectal swabs (VI & qRT-PCR) | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Neutralizing antibodies | Serum (antibody neutralization assay) | × | × | × | × | × | × | × | × | × | × | × | × | × |
VI, virus isolation; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction.
Virus shedding
Nasal, oral, and rectal swab samples were collected to monitor virus shedding following VSV-IFNβ-NIS inoculation (Table 1). No infectious virus was recovered from nasal, buccal, or rectal swab samples collected to monitor virus shedding following VSV-IFNβ-NIS inoculation, or from epithelial swabs from the site of inoculation. Quantitative real-time PCR was utilized as previously described23,24 to detect VSV RNA in shedding samples. Results indicated VSV-N RNA was detectable in some early nasal, oral, and rectal swabs (Fig. 3) only in directly inoculated pigs, and no viral RNA was detectable in any samples collected from contact pigs. In summary, there is no recoverable infectious virus being shed from inoculated pigs, and VSV-N RNA was weakly detectable primarily in nasal and oral swabs up to 4 days postinoculation but is not indicative of infectious virus. Both infectious virus and VSV-N RNA are absent in samples from contact pigs. These data indicate that oncolytic VSV-IFNβ-NIS is not shed in nasal, oral, or fecal excreta and is not transmissible in a susceptible species. At the end of the study period (day 21), pigs were euthanized and palatine tonsils and spleen samples were collected and tested to detect infectious virus or VSV-N RNA by qRT-PCR. Postmortem tonsil and spleen tissues were negative for both infectious virus and VSV RNA (N target gene).
Figure 3.
Detection of virus shedding. Biological samples, including nasal, oral, and rectal swabs, were collected at indicated time points after inoculation with VSV-IFNβ-NIS. RNA from biologic samples were analyzed by qRT-PCR to detect VSV nucleocapsid (N) RNA, indicating that low levels of viral RNA were detectable in (A) oral, (B) nasal, and (C) rectal swabs in directly inoculated pigs (Nos. 45408–45411) but not in contact pigs (45412–45415). No infectious virus was detectable in any collected samples.
Neutralizing antibodies
Analysis of serum indicated the presence of neutralizing antibodies against VSV in 3 of 4 pigs directly inoculated with VSV-IFNβ-NIS. No neutralizing antibodies were detected in contact pigs, indicating that oncolytic VSV-IFNβ-NIS is not transmissible to susceptible species in direct physical contact with inoculated animals (Fig. 4). Notably, the antibody levels detected in directly inoculated pigs were markedly lower (∼10-fold lower) than those detected in previous studies in pigs with inoculated with wild-type VSIV or VSNJV.3 Serum was also tested by ELISA indicating no detectable human IFNβ in serum of pigs from inoculated or contact groups (not shown).
Figure 4.
Serum neutralizing antibody titers following VSV-hIFNβ-NIS inoculation. Adaptive immune response was monitored by measuring anti-VSV antibodies in serum collected at indicated time points following inoculation in pigs (45408–45411) or contact pigs (45412–45415) by sero-neutralization test on baby hamster kidney (BHK-21) cells. Anti-VSV antibody titer indicates the maximum serum dilution that protects BHK-21 cells from infection with 500 TCID50 VSV.
Activation of type 1 Interferon responses by oncolytic VSV-IFNβ-NIS
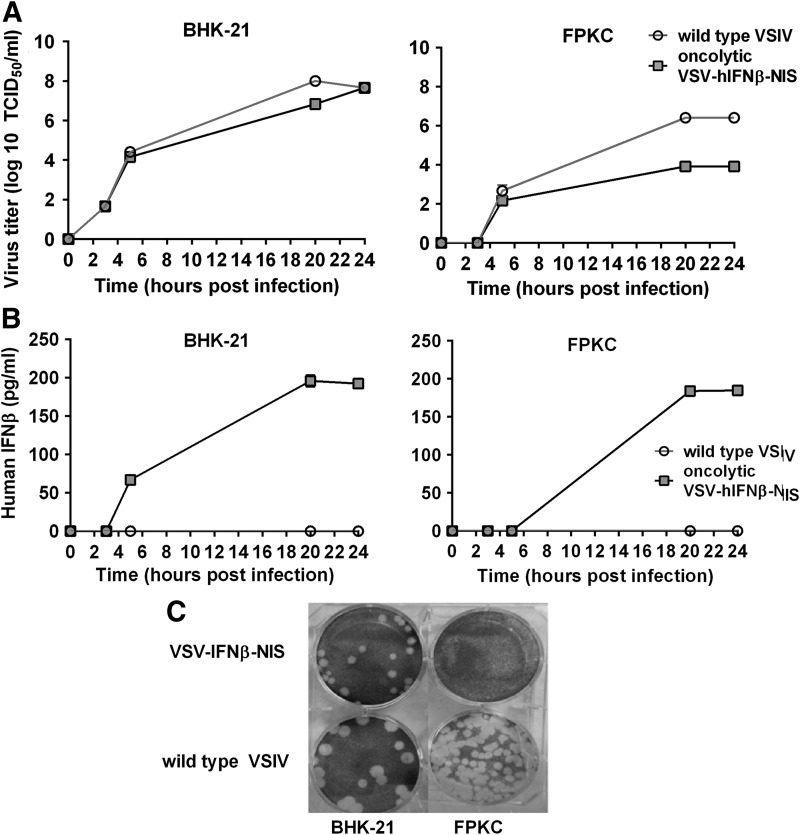
Tumor cells with defective innate immune responses are selectively susceptible to virus replication and oncolysis.10 We hypothesize that activation of innate immune response by virally encoded IFNβ attenuates VSV pathogenicity thereby rendering oncolytic VSV-IFNβ-NIS nonpathogenic and nontransmissible, making it ideally suited for development as an oncolytic agent for the treatment of cancer. Human IFNβ has been previously shown to cross react and induce antiviral responses in bovine and porcine cells.21 To evaluate the correlation between type 1 IFN induction and attenuation in porcine cells, we compared growth kinetics of recombinant VSV-IFNβ-NIS and wild-type VSIV in baby hamster kidney (BHK-21) cells, a cell line that is highly susceptible to VSV infection, or locally derived and cultured primary fetal porcine kidney cells (FPKC)25 (Fig. 5A). These data indicate that VSV-IFNβ-NIS replication is attenuated by approximately 2-log in FPKC, but not attenuated in BHK-21 cells. VSV-IFNβ-NIS replication in both cell lines results in expression and secretion of human IFNβ measurable by 5 h postinfection in BHK-21 cells, and at 20 h postinfection in FPKC (Fig. 5B). Comparison of plaque formation of VSV-IFNβ-NIS to wild-type VSIV in BHK-21 or FPKC indicate that both viruses form comparably large plaques in BHK-21 cells, while oncolytic VSV-IFNβ-NIS plaque formation is notably attenuated in FPKC (Fig. 5C). Finally, gene expression changes following infection with wild-type VSIV or oncolytic VSV-IFNβ-NIS were measured, indicating strong induction of a type 1 IFN response following VSV-IFNβ-NIS infection in FPKC and robust upregulation of antiviral IFN stimulated genes (ISGs). Upregulation of ISGs is highlighted in Table 2. These data suggest induction of Type 1 IFN responses plays a key role in attenuation of VSV pathogenicity following VSV-IFNβ-NIS infection.
Figure 5.
Replication, virus gene expression, and cytotoxicity of oncolytic VSV-IFNβ-NIS versus wild-type VSV Indiana (VSIV). Adapted cell line BHK-21 and primary cell cultures of FPKC were infected with VSV-IFNb-NIS or wild-type VSIV at an MOI of 0.1. Media were replaced at the indicated time points and supernatant collected to determine (A) virus replication by measurement of virus titer in TCID50/mL by titration on BHK-21 cells and (B) human IFNβ expression, which was quantified by ELISA. (C) Plaque morphology on BHK-21 and FPKC by VSV-IFNb-NIS or wild-type VSIV were evaluated by standard plaque assay, where plates were stained with crystal violet after 48 h of incubation.
Table 2.
Gene expression changes in fetal porcine kidney cells after infection with oncolytic VSV-IFNβ-NIS or wild-type VSIV
| Type I Interferon | VSV-IFNβ-NIS RQ mRNA | SD | VSVIND 4.1 RQ mRNA | SD |
|---|---|---|---|---|
| Interferon beta 1 (IFNβ1) | 3372.94 | 250.03 | 30.31 | 4.12 |
| Interferon alpha 2 (IFNα2) | 25.95 | 2.36 | 0.63 | 0.27 |
| Interferon alpha 1 (IFNα1) | 3.83 | 1.71 | 1.16 | 0.09 |
| Interferon regulatory factors | VSV-IFNβ-NIS RQ mRNA | SD | VSVIND 4.1 RQ mRNA | SD |
|---|---|---|---|---|
| Interferon Regulatory Factor 7 (IRF7) | 21.53 | 5.05 | 0.56 | 0.17 |
| Interferon Regulatory Factor 9 (IRF9) | 3.68 | 0.26 | 0.55 | 0.10 |
| Interferon Regulatory Factor 1 (IRF1) | 2.62 | 0.20 | 0.48 | 0.06 |
| Interferon Regulatory Factor 6 (IRF6) | 1.70 | 0.19 | 1.00 | 0.29 |
| Interferon Regulatory Factor 3 (IRF3) | 0.62 | 0.34 | 0.78 | 0.20 |
| Trascrpition factors | VSV-IFNβ-NIS RQ mRNA | SD | VSVIND 4.1 RQ mRNA | SD |
|---|---|---|---|---|
| Signal Transducer And Activator Of Transcription 2 (STAT2) | 5.88 | 2.61 | 0.52 | 0.16 |
| Signal Transducer And Activator Of Transcription 1 (STAT1) | 2.81 | 0.39 | 0.78 | 0.24 |
| Antiviral INF -stimulated genes (ISG) | VSV-IFNβ-NIS RQ mRNA | SD | VSVIND 4.1 RQ mRNA | SD |
|---|---|---|---|---|
| 2'-5'-Oligoadenylate Synthetase 1 (OAS1) | 2489.64 | 187.82 | 2.31 | 0.58 |
| Retinoic acid-inducible gene I (RIG-I) | 1913.37 | 173.72 | 0.72 | 0.27 |
| MX Dynamin Like GTPase 2 (MX2) | 1002.02 | 34.78 | 0.98 | 0.19 |
| Interferon Induced Protein With Tetratricopeptide Repeats 1 (IFIT1) | 468.61 | 79.28 | 2.28 | 0.40 |
| Guanylate Binding Protein 1 (GBP1) | 150.81 | 33.68 | 0.46 | 0.01 |
| Interferon-induced protein with tetratricopeptide repeats 2 (IFIT2) | 58.83 | 2.87 | 0.59 | 0.26 |
| Interferon-induced GTP-binding protein (MX1) | 56.05 | 3.71 | 1.16 | 0.45 |
| Bone Marrow Stromal Cell Antigen 2 (BST2) | 12.21 | 2.08 | 0.91 | 0.25 |
| Guanylate Binding Protein 2 (GBP2) | 7.07 | 2.12 | 0.81 | 0.37 |
| Protein kinase R (PKR) | 3.99 | 0.49 | 0.57 | 0.17 |
| Tripartite motif-containing protein 25 (TRIM25) | 1.53 | 0.70 | 0.99 | 0.38 |
| Interferon Stimulated Exonuclease Gene 20 (ISG20) | 0.68 | 0.32 | 0.31 | 0.12 |
Gene expression quantification was assessed by quantitative reverse transcription real-time polymerase chain reaction (qrt-PCR). Values are represented as relative quantities (RQ) of mRNA accumulation (estimated by 2−ΔΔct) with their corresponding standard deviations (SD). RQ values over 3 (shown in boldface) are considered positive.
Conclusions
Vesicular stomatitis virus causes outbreaks of vesicular disease, that when occurring in cattle or pigs are clinically undistinguishable from foot-and-mouth disease, a devastating foreign animal disease for the United States. Therefore, outbreaks must be reported to State and USDA-APHIS animal health authorities for appropriate diagnostics and rule out of foot-and-mouth disease. Vesicular stomatitis virus outbreaks occur sporadically in the United States, causing acute disease in cattle, horses, and pigs and can cause major economic losses due to quarantines and other animal movement control measures. Pathogenicity and transmissibility of VSV following epithelial snout scarification inoculation in pigs has been previously utilized to characterize wild-type VSV strains including both laboratory and field strains of VSIV and VSNJV.2–4 Here, we used established protocols to evaluate the pathogenicity and transmissibility of oncolytic VSV-IFNβ-NIS following intradermal snout scarification in pigs. The data indicate that oncolytic VSV-IFNβ-NIS is nonpathogenic in pigs with no measurable or observable indications of infection. Monitoring of viremia and virus shedding indicate there is no active infection following VSV-IFNβ-NIS inoculation, with no detectable viremia and no infectious virus detectable in samples tested for virus shedding in pigs from inoculated or contact groups. Samples were also analyzed by qRT-PCR, a highly sensitive method to detect VSV RNA. VSV RNA was detectable in nasal swabs immediately following snout inoculation with VSV-IFNβ-NIS. VSV RNA quantification was below the limit of detection in occasional nasal, oral, and rectal swabs, all within the first 4 days following inoculation. No VSV RNA was detectable in contact pigs. These data indicate the highly sensitive qRT-PCR method may be detecting residual VSV inoculum, and there is no active infection or transmission of infection to contact pigs. The most definitive evidence of the lack of VSV-IFNβ-NIS transmissibility in pigs is the absence of seroconversion in contact pigs. Three out of four inoculated pigs developed a low antibody response against VSV, detectable approximately 6–7 days post inoculation. The maximal antibody titer in VSV-IFNβ-NIS inoculated pigs was ∼10-fold lower than those detected in pigs inoculated with wild-type VSIV.3 None of the contact pigs developed neutralizing antibodies to VSV, indicating that there was no exposure to VSV-IFNβ-NIS even when in direct physical contact with inoculated animals. These data indicate that oncolytic VSV-IFNβ-NIS is nonpathogenic and nontransmissible in pigs, a naturally susceptible host for VSV.
Comparison of in vitro infections of susceptible BHK-21 or FPKC porcine kidney cells to infection by wild-type VSIV or oncolytic VSV-IFNβ-NIS indicates that VSV-IFNβ-NIS is attenuated in porcine cells, with corollary detection of potent activation of Type 1 interferon responses and expression of ISGs. Previous studies indicate human interferon-β can cross react and induce antiviral responses in bovine or porcine cells.21 These data suggest that VSV attenuation is associated with ability of VSV strains to induce activation of type 1 interferon responses. This is likely in addition to attenuation of VSV due to the insertion of the two transgenes. It has previously been shown that the addition of transcriptional units to the genome of nonsegmented negative-strand viruses can have an attenuating effect on virus replication.26 VSV-IFNβ-NIS has lower maximal titer in vitro compared with recombinant VSV expressing a single GFP transgene in an interferon-resistant cell line.16 These data collectively demonstrate the minimal biosafety, and environmental risk related to use of oncolytic VSV-IFNβ-NIS to individuals in contact with patients receiving treatment, and for patients or families in contact with livestock that are natural hosts of VSV.
This study has provided critical data indicating that recombinant oncolytic VSV-IFNβ-NIS is nonpathogenic and nontransmissible in a susceptible species. These findings supported that VSV-IFNβ-NIS can be safely utilized in clinical settings for the treatment of cancer in human and canine cancer patients, both that may come into contact with agricultural livestock that are naturally susceptible to VSV infection. Veterinary clinical trials are underway to test clinical efficacy of intravenous VSV-IFNβ-NIS therapy in pet dogs with advanced cancers. Human clinical trials are underway to evaluate intratumoral and intravenous oncolytic VSV therapy in patients with relapsed or refractory cancer.
Supplementary Material
Acknowledgments
The authors thank Dr Gail W. Wertz for kindly providing the VSIV San Juan Strain. Additionally we thank the Animal Resource Branch at PIADC for assistance and support during animal studies. This study was supported by U.S. Department of Agriculture Agricultural Research Service Project No. 8064-32000-058-00D, under a Specific Cooperative Agreement between ARS and Vyriad Inc.
Author Disclosure
Authors S.N., S.J.R., and K.W.P. are co-founders and officers of Vyriad, Inc., a biotech company developing engineered viruses for cancer therapy including the vesicular stomatitis virus oncolytic virus platform described in this study.
References
- 1.USDA Animal and Plant Health Inspection Service, Veterinary Services. 2015 Vesicular Stomatitis Virus (VSV) Situation Report—March 4, 2016. Riverdale, MD: United States Department of Agriculture, Animal and Plant Health Inspection Service, 2016 [Google Scholar]
- 2.Stallknecht DE, Greer JB, Murphy MD, Mead DG, Howerth EW. Effect of strain and serotype of vesicular stomatitis virus on viral shedding, vesicular lesion development, and contact transmission in pigs. Am J Vet Res 2004;65:1233–1239 [DOI] [PubMed] [Google Scholar]
- 3.Martinez I, Barrera JC, Rodriguez LL, Wertz GW. Recombinant vesicular stomatitis (Indiana) virus expressing New Jersey and Indiana glycoproteins induces neutralizing antibodies to each serotype in swine, a natural host. Vaccine 2004;22:4035–4043 [DOI] [PubMed] [Google Scholar]
- 4.Martinez I, Rodriguez LL, Jimenez C, Pauszek SJ, Wertz GW. Vesicular stomatitis virus glycoprotein is a determinant of pathogenesis in swine, a natural host. J Virol 2003;77:8039–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A 1995;92:4477–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 2000;50:135–138 [DOI] [PubMed] [Google Scholar]
- 7.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med 2004;10:210–216 [DOI] [PubMed] [Google Scholar]
- 8.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003;4:263–275 [DOI] [PubMed] [Google Scholar]
- 9.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015;386:857–866 [DOI] [PubMed] [Google Scholar]
- 10.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther 2009;9:1163–1176 [DOI] [PubMed] [Google Scholar]
- 11.Naik S, Nace R, Barber GN, Russell SJ. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-beta. Cancer Gene Ther 2012;19:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel A, Carlson SK, Classic KL, et al. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood 2007;110:2342–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 2003;77:8843–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products—Guidance for Industry. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, 2015 [Google Scholar]
- 15.Bailey K, Kirk A, Naik S, et al. Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS One 2013;8:e73759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik S, Nace R, Federspiel MJ, Barber GN, Peng KW, Russell SJ. Curative one-shot systemic virotherapy in murine myeloma. Leukemia 2012;26:1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingli D, Russell SJ, Morris JC., 3rd In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem 2003;90:1079–1086 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Steele MB, Jenks N, et al. Safety studies in tumor and non-tumor-bearing mice in support of clinical trials using oncolytic VSV-IFNbeta-NIS. Hum Gene Ther Clin Dev 2016;27:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Steele MB, Jenks N, et al. Robust oncolytic virotherapy induces tumor lysis syndrome and associated toxicities in the MPC-11 plasmacytoma model. Mol Ther 2016;24:2109–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBlanc AK, Naik S, Galyon GD, et al. Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Hum Gene Ther Clin Dev 2013;24:174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gresser I, Bandu MT, Brouty-boye D, Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature 1974;251:543–545 [DOI] [PubMed] [Google Scholar]
- 22.Miller A, Suksanpaisan L, Naik S, et al. Reporter gene imaging identifies intratumoral infection voids as a critical barrier to systemic oncolytic virus efficacy. Mol Ther Oncolytics 2014;1:14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson WC, Letchworth GJ, Jimenez C, et al. Field evaluation of a multiplex real-time reverse transcription polymerase chain reaction assay for detection of Vesicular stomatitis virus. J Vet Diagn Invest 2009;21:179–186 [DOI] [PubMed] [Google Scholar]
- 24.Arzt J, Pacheco JM, Rodriguez LL. The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation. Identification of the nasopharynx as the primary site of infection. Vet Pathol 2010;47:1048–1063 [DOI] [PubMed] [Google Scholar]
- 25.Pacheco JM, Mason PW. Evaluation of infectivity and transmission of different Asian foot-and-mouth disease viruses in swine. J Vet Sci 2010;11:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. Nonsegmented negative-strand viruses as vaccine vectors. J Virol 2006;80:10293–10306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.