Abstract
The National Heart, Lung, and Blood Institute (NHLBI) AIDS Program's goal is to provide direction and support for research and training programs in areas of HIV-related heart, lung, blood, and sleep (HLBS) diseases. To better define NHLBI current HIV-related scientific priorities and with the goal of identifying new scientific priorities and gaps in HIV-related HLBS research, a wide group of investigators gathered for a scientific NHLBI HIV Working Group on December 14–15, 2015, in Bethesda, MD. The core objectives of the Working Group included discussions on: (1) HIV-related HLBS comorbidities in the antiretroviral era; (2) HIV cure; (3) HIV prevention; and (4) mechanisms to implement new scientific discoveries in an efficient and timely manner so as to have the most impact on people living with HIV. The 2015 Working Group represented an opportunity for the NHLBI to obtain expert advice on HIV/AIDS scientific priorities and approaches over the next decade.
Keywords: : HIV/AIDS pathogenesis, HIV epidemiology, implementation science
Introduction
Despite advances in the understanding and treatment of HIV that have improved the duration and quality of life of people living with HIV (PLWH), challenges remain. These include the need to better understand the basis of HIV persistence, drug susceptibility, and HIV-related comorbidities that plague infected individuals. According to the United Nations Joint Programme on HIV/AIDS, in 2015, the number of people living worldwide with HIV was about 37 million, of whom ∼2.1 million had acquired their infection during the previous year.
Fortunately, there has been a concomitant increase in the number of PLWH who have access to antiretroviral therapy (ART), reaching 17 million people in 2015. The increased utilization of ART has resulted in decreased HIV/AIDS mortality. AIDS-related deaths have decreased by 43% worldwide; even in the most affected regions, such as eastern and southern Africa, AIDS-related deaths have decreased by 36% since 2010.
Improvement in ART over the past two decades, together with a broader outreach effort to prevent and diagnose infection, has led to increased survival and a change in HIV epidemiology. The HIV-infected population is becoming older and is now facing an increasing number of chronic diseases associated with aging. HIV comorbidities such as cardiovascular, lung, and blood diseases, along with sleep disorders, are more prevalent in PLWH and represent an impending public health problem.1–4 These HIV-associated comorbidities are significantly higher than the current prevalence rates in the non-HIV population. It is estimated that by 2030, 84% of the HIV population will have at least one of these comorbidities, 28% will have more than three comorbidities, and 78% will be diagnosed with cardiovascular disease (CVD).5 The factors that influence the increased risk of comorbidities in PLWH are still unclear, but elements such as long-term use of ART, smoking, sustained HIV-induced immune activation, chronic inflammation, and interaction with other non-ART drugs are likely to play a role in end-organ complications.
Ever since the beginning of the AIDS epidemic, the National Heart, Lung, and Blood Institute (NHLBI) has been at the forefront by supporting research that has spanned fundamental basic science discoveries to clinical investigations. More recently, implementation research has led to a better understanding of HIV in many populations and provided insight on how to best move scientific discoveries from the bench into clinical practice. Given the changing spectrum of HIV disease in the ART era, in December 2015, the NHLBI convened a working group of researchers, academicians, clinicians, and implementation research scientists to discuss scientific gaps that when addressed would lead to better understanding of how to prevent, diagnose, and treat HIV-associated comorbidities.
Furthermore, recognizing that fundamental discoveries need to be translated into public health gains for PLWH, implementation science research priorities that can take the discoveries from the clinic or laboratory to the “real world” were also discussed. What follows is an executive summary of the working group proceedings.
Working Group Overview and Scientific Themes
Four subgroups were created. Three subgroups focused on priorities specific to their area of expertise (heart, lung, blood), and a fourth (implementation science) was charged with developing general scientific approaches that could be used to take scientific discovery from the laboratory to the clinical arena and to improve population health in communities. The core objective of each subgroup was to have discussions on: (1) HIV-related comorbidities; (2) HIV cure; (3) HIV prevention; and (4) how to use evidenced-based interventions to impact and improve the health of PLWH.
To achieve these core objectives, subgroup participants were asked to develop recommendations aligned with the NIH Office of AIDS Research Strategic Plan as follows: (1) highest scientific priorities in HIV-related heart, lung, blood, and sleep (HLBS) and implementation science issues; (2) strategies to enhance dialogue and collaboration among different scientific communities; and (3) approaches to leveraging existing resources (e.g., cohorts, datasets, and so on).
The meeting was structured as two plenary sessions to cover broad basic and clinical research talks followed by breakout sessions for each of the HBLS and implementation science areas, during which specific challenges and future directions for each field were addressed, and in some cases, disease-specific issues were discussed. Subgroup participants were asked to identify the highest priority research gaps within their areas of focus and recommend future research strategies to address those gaps. Subgroups were asked to center discussions on how new epidemiological data could lead to novel hypotheses on the pathogenesis of HIV-associated complications, and how results of pathogenesis studies could inform novel prevention and treatment approaches (Fig. 1). Finally, implementation science researchers were asked to address how to best create a scientific environment that would best address these steps and move new discoveries into clinical practice, utilizing evidence-based interventions in clinical settings and communities.
FIG. 1.
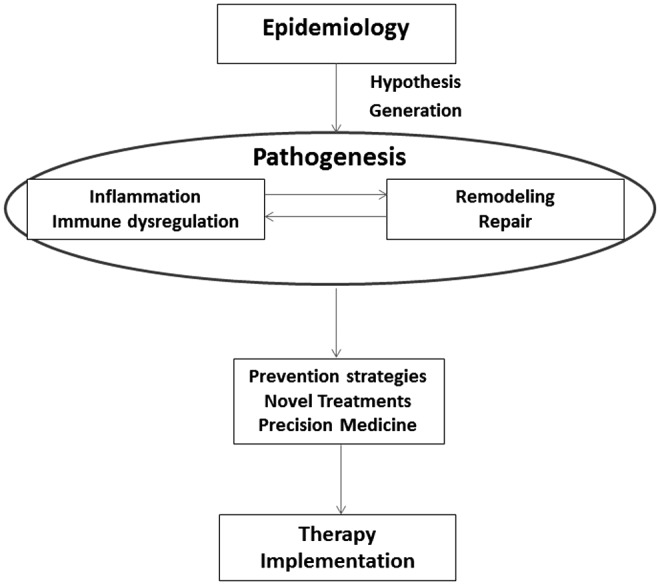
Epidemiological studies lead to novel hypotheses on the pathogenesis of HIV-associated complications, which in turn inform novel prevention and treatment. Ultimately, implementation science is necessary to create a scientific environment to address all these steps and to move new discoveries into clinical practice.
Epidemiology
The development of ART has greatly influenced the morbidity and mortality of HIV infection, and HIV-infected subjects are now living near normal life spans.6 This has generally been attributed to improvements in immunologic function, either by preventing the progressive loss of immunity in HIV infection or by actually promoting immune reconstitution. However, while infectious complications, especially those caused by opportunistic pathogens, have declined, they have been replaced by an increased frequency of pulmonary and vascular complications associated with chronic inflammation (Table 1).7–9 While complications specific to each compartment are listed at the top of the table, it is important to recognize that many epidemiologic risk factors are important contributors to many of the diseases across the spectrum of HIV-associated complications. This includes an increased prevalence of tobacco smoking,10 drug abuse,11 and sedentary life style12 in the HIV-infected population.
Table 1.
Changing Epidemiology of HIV Disease Complications in the Antiretroviral Era
| Heart | Lung | Blood |
|---|---|---|
| Coronary artery disease | COPD | Immune restoration failure |
| Peripheral vascular disease | Pulmonary hypertension | Platelet dysfunction |
| Stroke | Lung cancer | Endothelial activation |
| Heart failure | Lymphoma | Clonal hematopoiesis of indeterminate potential |
| Sudden cardiac death | Pulmonary fibrosis | |
| Immune reconstitution syndrome | ||
| Sleep disorders |
Tobacco smoking, drug abuse, antiretroviral therapy, and other medication use, sedentary life style.
COPD, chronic obstructive pulmonary disease.
Heart disease is increased in HIV infection. Established cohorts of HIV-infected participants from the United States and Europe have demonstrated an increased risk of coronary artery disease and myocardial infarction among patients with treated HIV infection.13 Similarly, heart failure, especially disease associated with a preserved ejection fraction, is increased in the setting of HIV infection and is likely to become an important cause of morbidity in the near future.14 Besides evidence of inflammation, heart failure in HIV infection is associated with myocardial fibrosis and steatosis, the cause of which is unknown. Understanding the differences in cardiac fibrosis and steatosis between HIV-infected and uninfected individuals is a priority. Finally, HIV infection is associated with increased evidence of vascular diseases, including stroke15 and peripheral vascular disease.16
The spectrum of lung disease has greatly changed in the ART era. In the lung, ART has been associated with decreased opportunistic infections,17 specifically decreased mycobacterial infections,18 and decreased incidence of bacterial pneumonia.19 The improved immunologic milieu has led to the development of guidelines on when primary and secondary prophylaxis against opportunistic pathogens can be discontinued.20 However, with the decrease in pulmonary infections in HIV-infected subjects on ART, other pulmonary complications have become more frequently recognized, leading to the currently observed change in the spectrum of pulmonary disease in HIV infection. These complications include pulmonary hypertension,21 emphysema,22,23 a variety of malignancies,9,24 pulmonary fibrosis,25 and the immune reconstitution inflammatory syndrome.26 In addition, a wide variety of sleep disorders have now been described in the HIV-infected population,27 a phenomenon that has been poorly recognized and understudied.
Blood complications remain problematic in the treated HIV population. Despite ART, HIV-infected patients remain at increased risk for venous and arterial thromboembolic events. Multiple markers related to inflammation and coagulation are increased in HIV infection, and several are predictive of thrombotic risk and mortality. The mechanisms behind the risk for abnormal coagulation in HIV infection have not been fully elucidated, but may be related to a chronic immune activation and inflammatory state in both untreated and treated HIV infection.28
ART initiation resulted in reductions in levels of CVD-associated biomarkers; although improved, however, markers of endothelial dysfunction and monocyte activation remained elevated. How these persistent abnormalities affect CVD risk in HIV infection remains to be determined.29 In addition, failure to restore immune function still occurs in a significant proportion of HIV-infected subjects on ART.30 Whether this reflects bone marrow, thymus, or peripheral lymphoid organ defects is not clear. Such immune failure is characterized by increased circulating of CD8 T cell counts, but fewer naive CD8 T cells increased plasma indices of inflammation and coagulation, as well as elevated monocyte inflammation and procoagulant activity.
For CD4 lymphopenia, all maturation subsets decreased with increased memory of CD4 T cell turnover. Naive CD4 (and CD8) lymphopenia suggests homeostatic failure. CD8 lymphocytosis tends to be dramatic and persistent. Induction of CD4+ T cell turnover and diminished T cell responsiveness to IL-7 by IL-1β and IL-6 exposure may contribute to the lack of CD4+ T cell reconstitution in treated HIV-infected subjects.31
At the other end of the spectrum is the impact of chronic inflammation on clonal hematopoiesis of indeterminate potential (CHIP) and acquired somatic mutations.32,33 CHIP arises in individuals presumed to be hematologically normal through an age-related acquisition of somatic mutations associated with myeloid malignancy. It is associated with increased mortality from hematologic malignancy, but even more strikingly, from CVD.34 How clonal hematopoiesis influences the response to inflammation or how chronic inflammation can promote emergence and expansion of such clones remains to be investigated.
Finally, the continuing transmission of HIV through blood transfusion in low-resource settings as well as prevention of such transmissions through improved blood safety and availability strategies remain concerns at the global level.35–37
Pathogenesis/Pathophysiology
The chronic diseases now affecting HIV-infected individuals closely mirror those found in an aging uninfected population. It has been known for years that HIV infection is characterized by chronic inflammation.38 This observation has been similarly made in normal aging individuals,39 and in fact HIV infection in the ART era has been suggested as a model of the aging process.40 Whether this represents an accelerated aging process due to higher levels of inflammation in the HIV-infected population or due to a premature “onset” of aging resulting from early inflammation is not clear. The cause of chronic inflammation in HIV-infected subjects is likely multifactorial and includes microbial translocation from a damaged gut mucosa,41 oxidative stress,42 mitochondrial dysfunction,43 and persistence of risk factors frequently seen in the HIV-infected population (smoking and drug injection).44,45
Another important factor leading to chronic inflammation in HIV infection is an altered microbial environment. Patients with advanced HIV infection have altered lung46 and vascular47 microbiomes, the latter likely resulting from a disrupted gut mucosal barrier. Importantly, some of the bacteria found increased in HIV infection are associated with chronic inflammation.48 Potentially even more important is the presence of an altered virome,49,50 since viruses possess the capacity to establish latent infection, resulting in persistent antigenic expression. This results in chronic stimulation of the immune system and accumulation of terminally differentiated lymphocytes (called immune senescence), which paradoxically secrete a proinflammatory cytokine profile.51,52 As a result, tissues containing abundant senescent immune cells are characterized by chronic inflammation.
All three subgroups agreed that chronic inflammation was an important consideration in the pathogenesis of HIV complications, although the manner in which it does so remains unclear. While inflammation can clearly lead to tissue damage, equally important are the concepts of tissue repair and defense mechanisms to try and mitigate the inflammatory challenge. Differences in the host response to inflammation can define whether an individual subject clears the inflammatory challenge, undergoes a fibrotic response, or develops another abnormal response such as fatty infiltration. It is likely that genetic differences influence how one responds to inflammatory challenges, highlighting the need for “mulitiomic” approaches to further our understanding of the pathogenesis underlying HIV-related HLBS complications.
In addition to chronic inflammation, dysregulation of platelet function occurs in HIV-infected patients. Elevated platelet counts (thrombocytosis) are associated with increased risk for cardiovascular sequelae and thrombotic complications.53 Conversely, decreased platelet production and/or enhanced destruction leads to low platelet counts (thrombocytopenia) and an increased risk of bleeding. However, a low platelet count itself is not necessarily associated with diminished proinflammatory and prothrombotic risk, which is largely driven by the platelet's activation status and the many proinflammatory mediators it produces.
Viruses can interact with platelets to induce their activation and/or removal of platelets from the circulation,54 leading them to become proinflammatory and prothrombotic. This activation state leads to the recruitment and infection of other immune cells, such as T lymphocytes and monocytes,55 further stimulating vascular inflammation and an increased risk of CVD. Emerging evidence supports the notion that antiretroviral drugs dysregulate platelets to promote and sustain systemic inflammatory changes that contribute to CVD.56 As smoking also activates platelets,57 it will be important to explore the direct effects of ART and ART in combination with tobacco smoke on platelet function. An understanding of the basic pathways and mechanisms associated with ART-induced changes in platelet function and increased CVD risk is vital, especially in the context of developing and evaluating new antiretroviral agents.
In summary, all three subgroups (heart, lung, and blood) pointed to chronic inflammation as a major driver of the complications now found in the HIV-infected population. While some compartments may have predominant drivers of inflammation, it is likely that some inflammatory mechanisms are common to more than one compartment (Fig. 2). For example, certain antiretroviral agents and intravenous drug use will affect not only the blood compartment but also the heart and lung as well through effects on platelet dysregulation and alterations the host microbial milieu (i.e., viral infections). Similarly, exposure to oxidative stress in the lung can have similar detrimental effects on the heart. All three compartments are by nature closely intertwined, with effects in one likely to cause downstream effects in the others. This points to a basic need for collaborative studies between scientists from multiple disciplines to move forward.
FIG. 2.
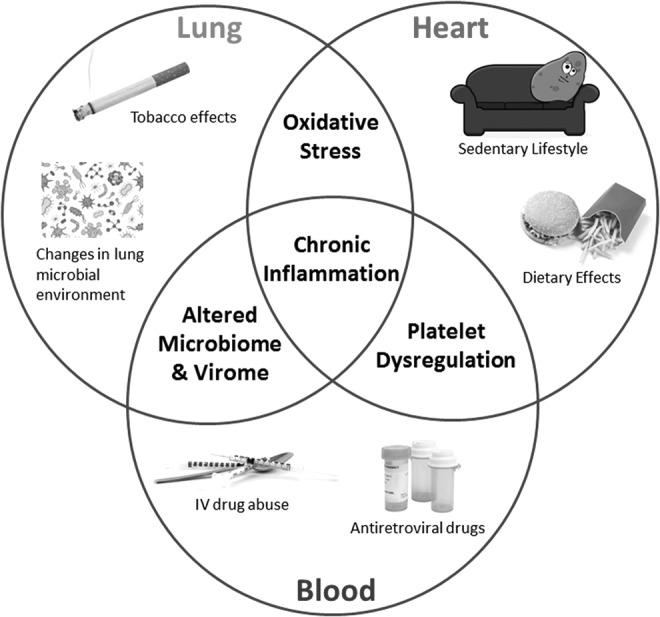
Chronic inflammation is a major driver of heart, lung, and blood complications in the HIV-infected population. While some compartments may have predominant drivers of inflammation, it is likely some inflammatory mechanisms are common to more than one compartment. Thus, platelet dysregulation will have the most effect on the blood and heart. An altered microbiome and virome are important in lung and blood complications. Oxidative stress may preferentially affect the lung and heart. Chronic inflammation is a common unifying end result of perturbations in all three compartments.
Prevention and Treatment
Recognition of the increased prevalence of chronic diseases in HIV infection is just the first step in caring for patients with HIV. New approaches to prevention and treatment are needed (Table 2). All three working groups stressed the need for a better understanding of the cause of chronic disease and inflammation in HIV-infected subjects, including identification of potential biomarkers, to improve risk prediction for specific diseases. Since long-term follow-up is necessary to identify many of the diseases increased in HIV-infected subjects, there is strong rationale for leveraging existing large, well-characterized cohorts of HIV-infected and matched uninfected subjects who can be used to further our understanding of the risks and biomarkers associated with the development and progression of chronic heart, lung, and blood diseases; and thus, identify potential prevention and treatment strategies. For example, new disease assessment tools could be introduced into existing cohorts (i.e., disease questionnaire modifications, pulmonary function tests, and echocardiograms) to better identify and characterize HLBS disorders; and/or enrollment of new participants in ongoing cohorts could allow for the evaluation of HLBS diseases in HIV patients treated with new ART regimens, and treated earlier after diagnosis. Questions needing to be addressed include the effect of ARTs themselves on the chronic disease process, whether the management of chronic disease differs in an HIV-infected population, and the potential role of anti-inflammatory agents in treatment.
Table 2.
Strategies to Address Critical Gaps in Our Understanding of HIV Complications
| Improve risk prediction of disease |
| Identify better biomarkers of disease |
| Leverage existing HIV cohorts, especially those with matched uninfected controls |
| Leverage existing biobanks containing samples from HIV-infected subjects |
| Recruit scientific experts in heart, lung, and blood diseases into the HIV arena |
| Develop animal models of specific diseases |
| Develop the next generation of HIV scientists through targeted research programs |
In addition to patient-focused studies, more translational laboratory investigations are needed to understand the pathogenesis of chronic disease in HIV infection and to guide preventive and treatment strategies. This includes studies on blood and tissue samples obtained from large cohorts as well as the development of new animal models of chronic heart and lung disease. These approaches should encourage new collaborations between existing HIV investigators and investigators with expertise in heart, lung, and blood science who may not be working in the HIV field.
Given the global focus of the HIV epidemic, there is strong support to ensure that heart, lung, and blood research in HIV infection takes into consideration unique factors, which may influence these conditions in low and middle income settings, where access to healthcare (including antiretroviral agents) and social/environmental influences are considerably different. For example, factors that contribute to inflammation in the setting of HIV infection may differ in settings where endemic infectious diseases are more prevalent, highlighting the opportunity to examine the contributions of inflammation to chronic disease.
Finally, focusing on a cure for HIV remains a very high priority and will involve collaborations across disciplines to address such critical issues as viral reservoirs (which tissues? which cells?) and novel immunotherapy-based approaches to target these reservoirs.58–60 Furthermore, newer hematopoietic stem cell approaches, using genetically modified cells resistant to HIV infection, are being identified.61–63 In the field of cell and gene therapy for HIV cure, there are currently ongoing studies to optimize gene editing of T cells and hematopoietic stem/progenitor cells (HSPCs) to better understand the allogeneic effect in HIV eradication or cure and to harness the immune systems using chimeric antigen receptor T cell technology or biospecific antibodies to target HIV-infected cells. In addition, modulation of the immune system with immune checkpoint inhibitors (e.g., PD1/PD-L1 inhibitors) and combinations with latency reversing agents (such as TLR7) are also being actively pursued.64–66
In the blood transfusion field, screening of blood donors can reveal unique HIV-infected subjects. For example, individual nucleic acid test screening of blood donations in South Africa provided a unique opportunity for recruiting a cohort of donors identified during Fiebig stages I and II of their HIV infection and referring them for ART (https://reds-iii.rti.org/ResearchStudies/Phase2Studies.aspx#MATHS). The successful execution of a proof of concept study in South Africa as part of the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) will inform future efforts in early identification of HIV-infected individuals and treatment of such infections for maximal prevention of further transmission. The findings of the study will also evaluate the impact of very early ART on HIV reservoir formation and prevention of HIV comorbidities. ART initiated within 6 months of infection resulted in lower CD4+ and CD8+ lymphocyte activation, as well as lower HIV proviral DNA and lower cell-associated HIV RNA.67 Emerging results from studies conducted in Thailand suggest that ART treatment initiated during Fiebig stage I leads to minimal HIV reservoir establishment, limiting the seeding and persistence of HIV in all subsets of CD4 T memory cells.68,69
Implementation Science Research
The implementation science subgroup focused on utilizing implementation science research to address the health needs of PLWH and was tasked with providing expert advice that would inform and guide NHLBI's implementation science-focused initiatives in the area of HIV/AIDS research. The subgroup identified opportunities in the implementation science arena, which could guide NHLBI as it develops a robust implementation science research program to address comorbidities for PLWH both globally and domestically. Unlike the other subgroups, the implementation science experts identified four interrelated themes that were grouped according to Bronfenbrenner's ecological framework model.
Patient-related themes address the types of research that are acceptable to patients. Concepts within this theme include how the scientific community obtains more data to better treat patients, how patient care should be managed, and what are patient perceptions about managing HIV and other heart, lung, and blood comorbidities.
Provider-related themes represent the knowledge the provider needs to better treat PLWH. This involves not only identifying knowledge gaps for implementing care but also focusing on the infrastructure needed to facilitate research necessary to fill these gaps.
Health systems-related themes include optimizing the health systems to better address patient needs. This includes identifying how health systems should be strengthened both domestically and globally to address the needs of PLWH.
Finally, a crosscutting opportunities theme describes how the NHLBI can facilitate training of new scientists and draw established investigators into the HIV field to develop relevant implementation science research expertise to address health issues for PLWH.
After identifying the constituent framework necessary for interventions, the implementation science research subgroup identified four major opportunities to use evidence-based interactions to impact the health of PLWH. These four major opportunities paralleled the themes aforementioned and included research opportunities encompassing (1) the patient, (2) the provider, (3) the health system, and (4) crosscutting opportunities. Each area is briefly highlighted below:
1. Research Opportunities Encompassing the Patient
Implementation science must encompass patients to achieve true public health impact. This provides significant opportunities to address the needs of PLWH using multilevel interventions that are contextualized in the ecosystem of the person. PLWH have significant challenges in regard to access behavior. Furthermore, PLWH have exposure to significant factors that can negatively impact prolonged outcomes. These include persistent exposure to tobacco and frequent comorbid conditions (e.g., hypertension, kidney disease, endocrine, hepatic, and psychiatric issues). The utilization of implementation research to determine how to best adopt, adapt, scaleup, and sustain routine use of those effective interventions will be essential to prolong the lives of PLWH.
2. Research Opportunities Encompassing the Provider
Although implementation science has been extensively utilized in global health within the field of HIV research, the focus has been on patients. Provider-focused implementation research has occurred, however, there are significant gaps related to the tools required by physicians to gain competencies to address the health issues of PLWH. This is an area of need and which late-stage implementation research could help mitigate. In the global setting, task shifting might represent ways, in which holistic treatments could occur for PLWH. Other issues implementation science could address include polypharmacy and mechanisms to retain high-risk populations in care.
3. Research Opportunities—Health System
Implementation science research can be used to address the deficiencies in health systems. For example, implementation science can inform how to provide effective, impactful care to PLWH in various settings (e.g., rural, semirural, urban, and periurban), both domestically and internationally. However, optimization of health systems to address the health issues of PLWH is context specific and will require implementation research to address structural barriers in a context-specific manner. While this knowledge could be transferrable, the disparities in resources and access to care in low-resource settings compared with high-resource areas are potentially significant barriers to addressing the needs of specific health systems.
Opportunities also exist to utilize implementation science research to strengthen and develop robust systems that can address noncommunicable diseases in HIV-infected populations. Qualitative research in health systems is also an area of need in addition to health services research and payment modeling. Both aspects contribute to health system strengthening by identifying the best schemes that can be utilized for PLWH in various settings.
4. Research Opportunities—Crosscutting Opportunities
Increasing the number of individuals trained in implementation science via NIH support of career awards (e.g., K awards) or institutional training grants (e.g., T32 awards) would increase the overall pool of investigators and thus positively increase the number of individuals conducting implementation science to address the needs of PLWH. Furthermore, HLBS scientists could consider extending their research to encompass HIV patients. For example, investigators could consider recruiting HIV-infected subjects into existing non-HIV-infected cohorts and making available valuable banked clinical samples from both HIV-infected and non-HIV-infected subjects who have undergone extensive clinical investigation.
Conclusion
ART has greatly improved the outlook of PLWH infection, with a significant reduction in infectious complications and an improved long-term survival. This decrease in HIV-related mortality has been facilitated by novel basic science discoveries, high-quality clinical trials, and implementation science. Nevertheless, PLWH continue to suffer from many complications out of proportion compared with their uninfected counterparts, which in the western world consists primarily of diseases associated with chronic inflammation and aging. To address this disparity, NHLBI convened a scientific working group to identify research gaps in the epidemiology, pathogenesis, and management of HIV-associated HLBS comorbidities.
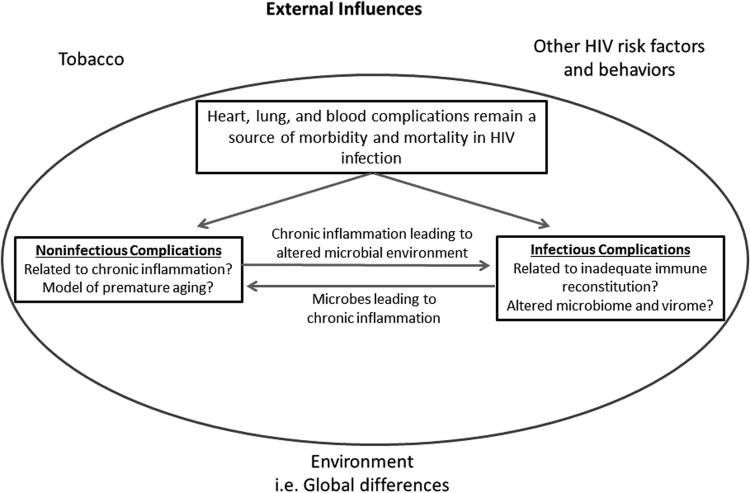
While the focus of the working groups was on chronic inflammation and its resulting comorbidities, it should be emphasized that infectious complications remain critically important for two reasons. First, despite improvements in healthcare delivery, over half the HIV-infected subjects in the world are not on ART and thus remain susceptible to infections that used to be so prevalent in the pre-ART era. Second, and perhaps more importantly, while HIV-infected patients on ART have a reduced incidence of pathologic infections, it is likely that many still have a perturbed microbial milieu that is directly or indirectly contributing to their chronic inflammatory state (Fig. 3).
FIG. 3.
While chronic inflammation contributes substantially to HIV comorbidities, perturbations in the microbial environment remain critically important due to (1) lack of access of many HIV-infected populations to effective antiretroviral therapy, which can still lead to infectious complications, and (2) perturbation in the microbial milieu that directly or indirectly contributes to chronic inflammation. Chronic inflammation and abnormalities in the microbial milieu are further influenced by external factors such as tobacco abuse, other HIV risk factors, and geographic variation.
Finally, the importance of translating new discoveries to the clinical practice and community healthcare delivery cannot be overstated. It is this collaboration along the research continuum from basic to translation science to the final stages of delivery of said intervention that truly promises to reduce HLBS mortality. Engaging this continuum by obtaining input from experts in the fields that were represented at the 2015 NHLBI AIDS Working Group meeting will be a necessary component of NHLBI's strategy to address the health needs of PLWH.
In the end, the conference allowed for a robust exchange of ideas on how best to enhance the health of PLWH, utilizing the most current epidemiologic data to inform future scientific directions to better define the pathogenesis and management of HIV-associated comorbidities. With the help of implementation science, the NHLBI seeks to rapidly move basic fundamental discoveries to clinical research and subsequently translate those findings into sustainable programs that improve the lives of PLWH.
Notes
The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Acknowledgments
The authors acknowledge the participants in the NHLBI AIDS Working Group: Refining Current Scientific Priorities & Identifying New Scientific Gaps in HLBS Research (https://www.nhlbi.nih.gov/health/resources/nhlbi-aids-working-group-refining-current-scientific-priorities-identifying-new-scientific-gaps-hiv) for their contribution to the discussions.
Funding
The authors prepared this report as part of the objective of the NHLBI AIDS Working Group, and no funding was required.
Authors’ Contributions
All authors participated in the creation of this report. All authors read and approved the final article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Almodovar S: The complexity of HIV persistence and pathogenesis in the lung under antiretroviral therapy: Challenges beyond AIDS. Viral Immunol 2014;27:186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byun E, Gay CL, Lee KA: Sleep, fatigue, and problems with cognitive function in adults living with HIV. J Assoc Nurses AIDS Care 2016;27:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So-Armah K, Freiberg MS: Cardiovascular disease risk in an aging HIV population: Not just a question of biology. Curr Opin HIV AIDS 2014;9:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura K: Current status of HIV/AIDS in the ART era. J Infect Chemother 2017;23:12–16 [DOI] [PubMed] [Google Scholar]
- 5.Smit M, Brinkman K, Geerlings S, et al. : Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis 2015;15:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F: Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010;24:1527–1535 [DOI] [PubMed] [Google Scholar]
- 7.Palella FJ, Jr., Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34 [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr., Phair JP: Cardiovascular disease in HIV infection. Curr Opin HIV AIDS 2011;6:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubb JR, Moorman AC, Baker RK, Masur H: The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS 2006;20:1095–1107 [DOI] [PubMed] [Google Scholar]
- 10.Stall RD, Greenwood GL, Acree M, Paul J, Coates TJ: Cigarette smoking among gay and bisexual men. Am J Public Health 1999;89:1875–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiller MW, Broz D, Wejnert C, et al. : HIV infection and HIV-associated behaviors among persons who inject drugs—20 cities, United States, 2012. MMWR Morb Mortal Wkly Rep 2015;64:270–275 [PMC free article] [PubMed] [Google Scholar]
- 12.Schuelter-Trevisol F, Wolff FH, Alencastro PR, et al. : Physical activity: Do patients infected with HIV practice? How much? A systematic review. Curr HIV Res 2012;10:487–497 [DOI] [PubMed] [Google Scholar]
- 13.Triant VA: HIV infection and coronary heart disease: An intersection of epidemics. J Infect Dis 2012;205 Suppl 3:S355–S361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butt AA, Chang CC, Kuller L, et al. : Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011;171:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA: Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Periard D, Cavassini M, Taffe P, et al. : High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis 2008;46:761–767 [DOI] [PubMed] [Google Scholar]
- 17.Torres RA, Barr M: Impact of combination therapy for HIV infection on inpatient census. N Engl J Med 1997;336:1531–1532 [DOI] [PubMed] [Google Scholar]
- 18.Kirk O, Gatell JM, Mocroft A, et al. : Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med 2000;162:865–872 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan JH, Moore RD, Keruly JC, Chaisson RE: Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med 2000;162:64–67 [DOI] [PubMed] [Google Scholar]
- 20.Kovacs JA, Masur H: Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med 2000;342:1416–1429 [DOI] [PubMed] [Google Scholar]
- 21.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA: HIV-Related pulmonary hypertension: Analytic review of 131 cases. Chest 2000;118:1133–1141 [DOI] [PubMed] [Google Scholar]
- 22.Diaz PT, King MA, Pacht ER, et al. : Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372 [DOI] [PubMed] [Google Scholar]
- 23.Crothers K, Griffith TA, McGinnis KA, et al. : The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med 2005;20:1142–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hessol NA, Martinez-Maza O, Levine AM, et al. : Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS 2015;29:1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crothers K, Huang L, Goulet JL, et al. : HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. : Immune reconstitution inflammatory syndrome: Emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227 [DOI] [PubMed] [Google Scholar]
- 27.Taibi DM: Sleep disturbances in persons living with HIV. J Assoc Nurses AIDS Care 2013;24:S72–S85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funderburg NT, Lederman MM: Coagulation and morbidity in treated HIV infection. Thromb Res 2014;133 Suppl 1:S21–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Halloran JA, Dunne E, Gurwith M, et al. : The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med 2015;16:608–619 [DOI] [PubMed] [Google Scholar]
- 30.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD: Incomplete immune recovery in HIV infection: Mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012;2012:670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shive CL, Mudd JC, Funderburg NT, et al. : Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: Implications for immune failure in HIV disease. J Infect Dis 2014;210:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steensma DP, Bejar R, Jaiswal S, et al. : Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Crumpacker C: Hematopoietic stem and immune cells in chronic HIV infection. Stem Cells Int 2015;2015:148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaiswal S, Fontanillas P, Flannick J, et al. : Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloch EM, Vermeulen M, Murphy E: Blood transfusion safety in Africa: A literature review of infectious disease and organizational challenges. Transfus Med Rev 2012;26:164–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takei T, Amin NA, Schmid G, Dhingra-Kumar N, Rugg D: Progress in global blood safety for HIV. J Acquir Immune Defic Syndr 2009;52 Suppl 2:S127–S131 [DOI] [PubMed] [Google Scholar]
- 37.Lund TC, Hume H, Allain JP, McCullough J, Dzik W: The blood supply in Sub-Saharan Africa: Needs, challenges, and solutions. Transfus Apheresis Sci 2013;49:416–421 [DOI] [PubMed] [Google Scholar]
- 38.Appay V, Sauce D: Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol 2008;214:231–241 [DOI] [PubMed] [Google Scholar]
- 39.Jenny NS. Inflammation in aging: Cause, effect, or both? Discov Med 2012;13:451–460 [PubMed] [Google Scholar]
- 40.Aberg JA: Aging, inflammation, and HIV infection. Top Antivir Med 2012;20:101–105 [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 42.Sharma B: Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res 2014;12:13–21 [DOI] [PubMed] [Google Scholar]
- 43.Polo R, Martinez S, Madrigal P, Gonzalez-Munoz M: Factors associated with mitochondrial dysfunction in circulating peripheral blood lymphocytes from HIV-infected people. J Acquir Immune Defic Syndr 2003;34:32–36 [DOI] [PubMed] [Google Scholar]
- 44.Francis H: Substance abuse and HIV infection. Top HIV Med 2003;11:20–24 [PubMed] [Google Scholar]
- 45.Reynolds NR: Cigarette smoking and HIV: More evidence for action. AIDS Educ Prev 2009;21:106–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twigg HL, 3rd, Knox KS, Zhou J, et al. : Effect of advanced HIV infection on the respiratory microbiome. Am J Respir Crit Care Med 2016;194:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merlini E, Bai F, Bellistri GM, Tincati C, d'Arminio Monforte A, Marchetti G: Evidence for polymicrobic flora translocating in peripheral blood of HIV-infected patients with poor immune response to antiretroviral therapy. PLoS One 2011;6:e18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal LN, Alekseyenko AV, Clemente JC, et al. : Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013;1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young JC, Chehoud C, Bittinger K, et al. : Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant 2015;15:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Twigg HL, 3rd, Weinstock GM, Knox KS: Lung microbiome in human immunodeficiency virus infection. Transl Res 2017;179:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davalos AR, Coppe JP, Campisi J, Desprez PY: Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 2010;29:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppe JP, Patil CK, Rodier F, et al. : Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:2853–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuentes QE, Fuentes QF, Andres V, Pello OM, Font de Mora J, Palomo GI: Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets 2013;24:255–262 [DOI] [PubMed] [Google Scholar]
- 54.Assinger A: Platelets and infection—An emerging role of platelets in viral infection. Front Immunol 2014;5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh MV, Davidson DC, Jackson JW, et al. : Characterization of platelet-monocyte complexes in HIV-1-infected individuals: Possible role in HIV-associated neuroinflammation. J Immunol 2014;192:4674–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falcinelli E, Francisci D, Belfiori B, et al. : In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients. Thromb Haemost 2013;110:349–357 [DOI] [PubMed] [Google Scholar]
- 57.Barua RS, Ambrose JA: Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol 2013;33:1460–1467 [DOI] [PubMed] [Google Scholar]
- 58.International ASSWGoHIVC, Deeks SG, Autran B, et al. : Towards an HIV cure: A global scientific strategy. Nat Rev Immunol 2012;12:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam S, Bollard C: T-cell therapies for HIV. Immunotherapy 2013;5:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leibman RS, Riley JL: Engineering T cells to functionally cure HIV-1 infection. Mol Ther 2015;23:1149–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tebas P, Stein D, Tang WW, et al. : Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 2014;370:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson CW, Haworth KG, Burke BP, et al. : Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS. Mol Ther Methods Clin Dev 2016;3:16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Younan PM, Polacino P, Kowalski JP, et al. : Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood 2013;122:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elliott JH, Wightman F, Solomon A, et al. : Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014;10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rasmussen TA, Tolstrup M, Brinkmann CR, et al. : Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV 2014;1:e13–e21 [DOI] [PubMed] [Google Scholar]
- 66.Sogaard OS, Graversen ME, Leth S, et al. : The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 2015;11:e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain V, Hartogensis W, Bacchetti P, et al. : Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013;208:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ananworanich J, Schuetz A, Vandergeeten C, et al. : Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012;7:e33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuetz A, Deleage C, Sereti I, et al. : Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014;10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]