Abstract
AIM
To evaluate a novel grading system to predict lymph node metastasis (LNM) in patients with submucosal invasive colorectal carcinoma (SICRC).
METHODS
We analyzed the associations between LNM and various clinicopathological features in 252 patients with SICRC who had undergone radical surgery at the Seoul Saint Mary’s hospital between 2000 and 2015.
RESULTS
LNM was observed in 31 patients (12.3%). The depth and width of the submucosal invasion, lymphatic invasion, tumor budding, and the presence of poorly differentiated clusters (PDCs) were significantly associated with the incidence of LNM. Using multivariate analysis, the receiver operating characteristic curvewas calculated and the area under curve (AUC) was used to compare the ability of the different parameters to identify the risk of LNM. The most powerful clinicopathological parameter for predicting LNM was lymphatic invasion (difference AUC = 0.204), followed by the presence or absence of tumor budding (difference AUC = 0.190), presence of PDCs (difference AUC = 0.172) and tumor budding graded by the Ueno method (difference AUC = 0.128).
CONCLUSION
Our results indicate that the tumor budding and the depth multiplied by the width measurements of submucosal invasion can provide important information for patients with SICRC.
Keywords: Colorectal cancer, Neoplasm invasion, Lymph node, Metastasis
Core tip: The appropriacy of endoscopic resection for patients with submucosal invasive colorectal carcinoma (SICRC) is still questionable. Therefore, highly precise predictors of lymph node metastasis (LNM) are needed to optimize the outcome of treatments for SICRC. We determined the value of a novel grading system based on histopatholological parameters to predict LNM in patients with SICRC. Our results indicate that the presence or absence of tumor budding and the depth multiplied by the width measurements of the submucosal invasion can provide important information regarding the treatment options for patients with SICRC.
INTRODUCTION
Endoscopic resection of intramucosal carcinomas is considered to be a curative therapy since the risk of lymph node metastasis (LNM) is low[1]. However, up to 12% of patients with submucosal invasive colorectal carcinoma (SICRC) have LNM[2,3], and it is unclear whether endoscopic resection is the most appropriate treatment option for these patients. Prior to endoscopic resection, patients with SICRC require careful pathological assessment to determine whether there is a significant risk of LNM which may require additional surgical treatment[4].
Various histopathological parameters such as angiolymphatic invasion, poor tumor differentiation, and the depth and/or width of submucosal invasion are reported to be associated with LNM in patients with SICRC[3-6]. The 2014 guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) for the treatment of colorectal cancer (CRC) by endoscopic resection, suggest that cases with tumor-positive vertical resection margin, submucosal invasion of ≥ 1000 μm, and unfavorable histology such as poorly differentiated adenocarcinoma, signet-ring cell carcinoma, or mucinous carcinoma should be considered for additional surgery with LN dissection[7]. The presence of vascular invasion and/or grade 2/3 budding at the site of the deepest invasion are also included as surgical candidates.
However, not all surgical cases of SICRC present LNM, resulting in overtreatment. Conversely, some patients without surgery eventually present LNM[2,6,8,9]. In order to provide the appropriate treatment to patients with SICRC, and reduce the number of unnecessary additional surgical resections, there needs to be an improvement in discriminating between patients with a high risk of developing LNM and those with a low risk of developing LNM.
In this study, we analyzed the incidence of LNM in relation to several histopathological findings in a large cohort of patients with SICRC, with an aim to help pathologists and clinicians in identifying the best treatment strategy for such patients.
MATERIALS AND METHODS
Patients and clinicopathological data
A total of 252 patients with SICRC who had undergone surgery for systematic lymph node dissection in Seoul Saint Mary’s hospital between 2000 and 2015 were enrolled in this study. Clinicopathological parameters including age, sex, tumor location, size, and LNM status were reviewed retrospectively from the medical records. Approval for this study was acquired from the Institutional Review Board of the College of Medicine at the Catholic University of Korea (KC16RISI0817).
Histopathological analysis
Hematoxylin and eosin stained tumor sections were evaluated for the following: depth and width of the submucosal invasion, tumor budding, poorly differentiated clusters (PDCs), histological grade, lymphatic invasion, venous invasion, perineural invasion, peritumoral inflammation, and desmoplasia. Two pathologists (Lee SH and Yim KI) independently examined each tumor section.
Depth of submucosal invasion
The depth of submucosal invasion was measured by three methods. The first method followed the JSCCR guidelines[10]. In brief, when the muscularis mucosa (MM) was clearly visible, the depth of the submucosal invasion was measured from the lowest border of the MM to the deepest invasion front. When the MM was deformed or not easily identified, the depth of the submucosal invasion was measured from the surface of the tumor to the invasion front, for both pedunculated and sessile forms. For pedunculated lesions with tangled MM, the depth of the submucosal invasion was measured as the distance between the boundary line of the head and stalk (level 2 by Haggitt classification) and the deepest point of the tumor. The second method followed the protocol by Kitajima et al[5] The tumor was classified as a pedunculated or sessile type with an identifiable MM or a sessile type without an identifiable MM. For pedunculated lesions, the depth of the submucosal invasion was measured as the distance between Haggitt’s level 2 and the deepest invasion point. For sessile tumor types with an identifiable MM, the depth of invasion was measured from the lowest border of the MM to the deepest invasion front. For sessile tumor types without an identifiable MM, the depth of the submucosal invasion was measured from the surface of the tumor to the invasion front. Lastly, we followed the method by Ueno et al[6]. The depth of the submucosal invasion was simply measured as the distance between the tumor surface and the deepest invasion point.
Width of submucosal invasion
The width of the submucosal invasion was defined as the largest (longest) horizontal measurement of the submucosal invasive area.
Depth multiplied by width
Assuming the shape of the submucosal invasion was an ellipse, the area is π × depth × width. Therefore, we postulated that the depth multiplied by width could represent the area of submucosal invasion. The results of depth multiplied by width were obtained by the three different methods described previously for measuring the depth of invasion.
Lymphatic and vascular invasion
The diagnosis of lymphatic invasion was made based on the presence of at least one tumor cell cluster within vascular space lined by a single layer of endothelial cells with no supporting smooth muscle, elastic lamina and/or red blood cells, whose lumens are sometimes filled with lymphocytes. Similarly, we defined vascular invasion as tumor cell nests in spaces that were lined by endothelium and filled with red blood cells, located in the vicinity of an artery and distant from the main lesion. Tumor cell nests in spaces that were not lined by endothelial cells were considered as stroma-invasive tumor cell nests, that is, retraction artifacts due to tissue shrinkage during fixation. In this study, only tumor cell nests in spaces lined by endothelial cells were counted as lymphovascular invasion.
Additional immunohistochemical staining with Podoplanin (clone D2-40, 1:50, Cell Marque, Hot Springs, AR, the United States of America), to detect lymphatic invasion, and with CD34 (clone QBEnd 10, 1:100, DAKO, Glostrup, Denmark) or CD31 (clone JC70A, 1:200, Dako, Glostrup, Denmark), to detect venous invasion were performed in those sections in which it was difficult to judge the presence or absence of lymphovascular invasion.
Tumor budding
An isolated tumor cell or small clusters of < 5 cancer cells in the invasive front was defined as tumor budding (Figure 1). In the present study, this was assessed by two different methods. The method described by Ueno et al[6] identified a microscopic field with intense budding and counted the number of budding foci using the × 20 objective lens and classified the number of foci by grade (grade 0; 0 focus, grade 1; 1-4 foci, grade 2; 5-9 foci, grade 3; ≥ 10 foci per field). The second method assessed the presence or absence of the tumor budding.
Figure 1.
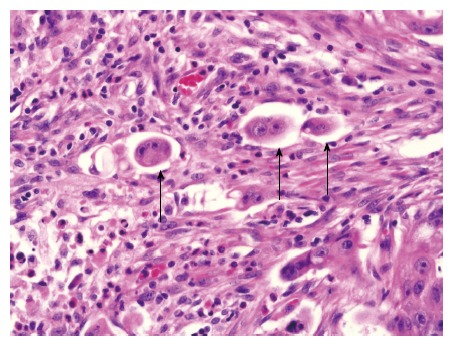
Representative histopathological micrograph of tumor budding (magnification × 400). Hematoxylin and eosin staining of a tumor section showing tumor budding (black arrows) at the invasive front.
PDCs
PDCs were defined as cancer cell clusters of ≥ 5 carcinoma cells that are lacking a glandular formation at the invasive front (Figure 2). However, when evaluating the mucinous adenocarcinoma, cancer cell clusters within a large mucin pool were not classified as PDCs; whereas, cancer cell clusters infiltrating the stroma with minimal extracellular mucin formation were classified as PDCs[11]. In our study, the assessment of PDCs was based on the presence or absence of PDCs.
Figure 2.
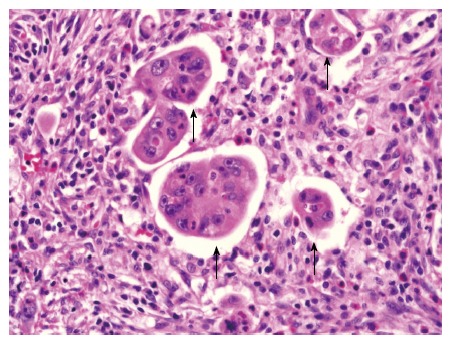
Representative histopathological micrograph of poorly differentiated clusters (magnification × 400). Hematoxylin and eosin stain of tumor section showing cancer cell clusters of ≥ 5 carcinoma cells lacking a glandular formation (poorly differentiated clusters, black arrows).
Statistical analysis
χ2 test, Fisher’s exact test, or Wilcoxon rank sum test were used to analyze the differences between the absence and presence of LNM. When predicting LNM, the sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were evaluated for each factor. Using multivariate logistic regression analysis, ROC was calculated and the AUC was used to compare how effective the parameters were at identifying the risk of LNM; tumor type and desmoplasia were used as compounding factors. Two-sided P values of < 0.05 were considered statistically significant. JMP software (version 9.0.2; SAS Institute, Cary, NV, the United States of America) was used for all statistical calculations.
RESULTS
Patients and clinicopathological data
Of the 252 patients, 130 were male and 122 were female; the mean age was 61.8 years. One hundred and fifty eight patients had tumors in the colon, including 1 in the vermiform appendix, and 94 in the rectum. The mean tumor size was 20.6 mm (range 2.0 mm-65.0 mm). LNM were observed in 31 cases (12.3%). Tumor budding was identified in 133 cases (52.8%) and 86 cases (34.1%) were grade 2 or 3 using the Ueno method [grade 0; 119 cases (47.2%), grade 1; 47 cases (18.7%), grade 2; 46 cases (18.2%), grade 3; 40 cases (15.9%)]. The baseline clinicopathological characteristics are summarized in Table 1.
Table 1.
Univariate analysis of clinicopathological and histological parameters for lymph node metastasis
|
LNM |
P value | ||
| No (n = 221) | Yes (n = 31) | ||
| Age (yr) | 61.81 ± 11.40 | 61.35 ± 10.63 | 0.8334 |
| 63.0 (31.0, 86.0) | 66.0 (38.0, 78.0) | ||
| Sex | |||
| Male | 115 (88.46) | 15 (11.54) | 0.7205 |
| Female | 106 (86.89) | 16 (13.11) | |
| Tumor location | |||
| Ascending colon | 34 (89.48) | 4 (10.52) | 0.6018 |
| Transverse colon | 12 (85.71) | 2 (14.29) | |
| Descending colon | 6 (100.00) | 0 (0.00) | |
| Sigmoid colon | 73 (91.25) | 7 (8.75) | |
| Rectosigmoid colon | 14 (93.33) | 1 (6.67) | |
| Rectum | 78 (82.98) | 16 (17.02) | |
| Cecum | 3 (75.00) | 1 (25.00) | |
| Vermiform appendix | 1 (100.00) | 0 (0.00) | |
| Tumor size (cm) | 2.05 ± 1.67 | 2.07 ± 1.27 | 0.6902 |
| 1.8 (0.2, 6.5) | 2.0 (0.4, 6.5) | ||
| Tumor type | |||
| Pedunculated type | 84 (93.33) | 6 (6.67) | 0.0424 |
| Sessile type | 137 (84.57) | 25 (15.43) | |
| Depth of submucosal invasion | |||
| by JSCCR (μm) | 2473.94 ± 2003.78 | 3777.42 ± 2167.10 | 0.0002 |
| 2200.0 (0.0, 13500.0) | 3500.0 (1050.0, 12000.0) | ||
| < 1000 | 46 (100.00) | 0 (0.00) | 0.0021 |
| ≥ 1000 | 175 (84.95) | 31 (15.05) | |
| by Kitajima (μm) | 1944.3 ± 1885.18 | 3380.65 ± 2390.28 | 0.0002 |
| 1900.0 (0.0, 13500.0) | 3000.0 (500.0, 12000.0) | ||
| < 1000 | 76 (95.00) | 4 (5.00) | 0.0218 |
| ≥ 1000 | 145 (94.30) | 27 (15.70) | |
| by Ueno (μm) | 2671 ± 1898.39 | 3908.06 ± 2147.17 | 0.0002 |
| 2300.0 (50.0, 13500.0) | 3500.0 (1050.0, 12000.0) | ||
| < 1000 | 34 (100.00) | 0 (0.00) | 0.0113 |
| ≥ 1000 | 187 (85.78) | 31 (14.22) | |
| Width of submucosal invasion (μm) | 5831.45 ± 4724.02 | 9261.29 ± 4692.03 | < 0.0001 |
| 5000.0 (50.0, 31000.0) | 8000.0 (1900.0, 21000.0) | ||
| < 4000 μm | 87 (96.67) | 3 (3.33) | 0.0010 |
| ≥ 4000 μm | 134 (82.72) | 28 (17.28) | |
| Depth multiplied by width | |||
| by JSCCR (mm2) | 20.22 ± 35.07 | 38.91 ± 36.50 | < 0.0001 |
| 11.6 (0.0, 303.8) | 26.6 (2.0, 163.8) | ||
| < 6.5 | 84 (98.82) | 1 (1.18) | < 0.0001 |
| ≥ 6.5 | 137 (82.04) | 30 (17.96) | |
| by Kitajima (mm2) | 16.79 ± 34.52 | 36.42 ± 37.97 | < 0.0001 |
| 8.8 (0.0, 303.8) | 23.1 (2.0, 163.8) | ||
| < 6.5 | 100 (95.24) | 5 (4.76) | 0.0017 |
| ≥ 6.5 | 121 (82.31) | 26 (17.69) | |
| by Ueno (mm2) | 20.91 ± 34.82 | 40.12 ± 36.52 | < 0.0001 |
| 12.8 (0.0, 303.8) | 26.6 (2.0, 163.8) | ||
| < 6.5 | 76 (98.70) | 1 (1.30) | 0.0001 |
| ≥ 6.5 | 145 (82.86) | 30 (17.14) | |
| Tumor differentiation | |||
| Well and moderately | 211 (87.92) | 29 (12.08) | 0.6470 |
| Poorly | 10 (83.33) | 2 (16.67) | |
| Lymphatic invasion | |||
| No | 190 (95.96) | 8 (4.04) | < 0.0001 |
| Yes | 31 (57.41) | 23 (42.59) | |
| Venous invasion | |||
| No | 215 (87.76) | 30 (12.24) | 1.0000 |
| Yes | 6 (85.71) | 1 (14.29) | |
| Perineural invasion | |||
| No | 219 (87.60) | 31 (12.40) | 1.0000 |
| Yes | 2 (100) | 0 (0.00) | |
| Inflammation | |||
| No | 100 (87.72) | 14 (12.28) | 1.0000 |
| Yes | 121 (87.68) | 17 (12.32) | |
| Desmoplasia | |||
| No | 106 (92.17) | 9 (7.83) | 0.0550 |
| Yes | 115 (83.94) | 22 (16.06) | |
| Tumor budding | |||
| Ueno | |||
| Grade 0, 1 | 158 (95.18) | 8 (4.82) | < 0.0001 |
| Grade 2, 3 | 63 (73.26) | 23 (26.74) | |
| Present or absent | |||
| No | 119 (100) | 0 (0.00) | < 0.0001 |
| Yes | 102 (76.69) | 31 (23.31) | |
| PDCs | |||
| No | 110 (100.00) | 0 (0.00) | < 0.0001 |
| Yes | 111 (78.17) | 31 (21.83) | |
Data are presented as n (%) and as mean ± SD, median (range). P value of significant difference between present/absent of LMN, by χ2, Fisher's exact, Wilcoxon rank sum test. LNM: Lymph node metastasis; JSCCR: Japanese Society for Cancer of the Colon and Rectum; PDCs: Poorly differentiated clusters.
LNM in relation to histopathological parameters
Table 1 summarizes the correlations between the histopathological parameters and LNM. The depth and width of the submucosal invasion, depth multiplied by width measurement, and the presence of lymphatic invasion, tumor budding, and PDCs were all significantly associated with the incidence of LNM. The depth multiplied by width values were significantly higher in tumors with LNM than in tumors without LNM, irrespective of the method used (P < 0.001). The incidence of LNM was 23.3% in tumors with tumor budding and 0% in cases without tumor budding (P < 0.0001).
Comparison of risk factors for LNM
The sensitivity, specificity, accuracy, PPVs, and NPVs were calculated for the parameters that were significantly associated with LNM using univariate analysis (Table 2). In addition, ROC curves were obtained for each parameter and the AUC was used to compare how well the various risk factors could identify the risk of LNM (Table 3 and Figure 3).
Table 2.
Predictive powers of histopathological factors for lymph node metastasis
| Sensitivity | Specificity | Accuracy | Positive P value | Negative P value | |
| Depth of submucosal invasion | |||||
| by JSCCR | 100.00% | 20.81% | 30.56% | 15.05% | 100.00% |
| by Kitajima | 87.10% | 34.39% | 40.87% | 15.70% | 95.00% |
| by Ueno | 100.00% | 15.38% | 25.79% | 14.22% | 100.00% |
| Width of submucosal invasion | 90.32% | 39.37% | 45.63% | 17.28% | 96.67% |
| Depth multiplied by width | |||||
| by JSCCR | 96.77% | 38.01% | 45.24% | 17.96% | 98.82% |
| by Kitajima | 83.87% | 45.25% | 50.00% | 17.69% | 95.24% |
| by Ueno | 96.77% | 34.39% | 42.06% | 17.14% | 98.70% |
| Lymphatic invasion | 74.19% | 85.97% | 84.52% | 42.59% | 95.96% |
| Tumor budding | |||||
| by Ueno | 74.19% | 71.49% | 71.83% | 26.74% | 95.18% |
| Present or absent | 100.00% | 53.84% | 59.52% | 23.31% | 100.00% |
| PDCs | 100.00% | 49.77% | 55.95% | 21.83% | 100.00% |
JSCCR: Japanese Society for Cancer of the Colon and Rectum; PDCs: Poorly differentiated clusters.
Table 3.
Comparison of histopathological factors for predicting of lymph node metastasis by multivariate analysis
| Model | AUC | SE | 95%CI | Difference AUC | 95%CI | P value |
| Crude (unadjusted) | 0.640 | 0.046 | (0.549, 0.730) | |||
| JSCCR (depth) | 0.666 | 0.041 | (0.585, 0.747) | 0.026 | (0.009, 0.043) | 0.0022 |
| Kitajima (depth) | 0.650 | 0.044 | (0.565, 0.736) | 0.011 | (-0.022, 0.043) | 0.5103 |
| Ueno (depth) | 0.664 | 0.042 | (0.582, 0.746) | 0.024 | (0.009, 0.040) | 0.0024 |
| Width | 0.692 | 0.040 | (0.613, 0.771) | 0.053 | (-0.007, 0.112) | 0.0829 |
| JSCCR (depth × width) | 0.703 | 0.038 | (0.629, 0.776) | 0.063 | (0.024, 0.103) | 0.0017 |
| Kitajima (depth × width) | 0.681 | 0.042 | (0.599, 0.763) | 0.042 | (-0.002, 0.085) | 0.0612 |
| Ueno (depth × width) | 0.699 | 0.038 | (0.624, 0.775) | 0.060 | (0.020, 0.099) | 0.0030 |
| Budding (ueno) | 0.768 | 0.044 | (0.682, 0.853) | 0.128 | (0.043, 0.214) | 0.0033 |
| Lymphatic invasion | 0.843 | 0.041 | (0.763, 0.924) | 0.204 | (0.114, 0.294) | < 0.0001 |
| Budding present itself | 0.830 | 0.0277 | (0.776, 0.884) | 0.190 | (0.106, 0.274) | < 0.0001 |
| PDCs | 0.811 | 0.0296 | (0.753, 0.870) | 0.172 | (0.087, 0.256) | < 0.0001 |
Crude: Tumor type, desmoplasia each model was adjusted to the tumor type and desmoplasia. AUC: Area under curve; JSCCR: Japanese Society for Cancer of the Colon and Rectum; PDCs: Poorly differentiated clusters.
Figure 3.
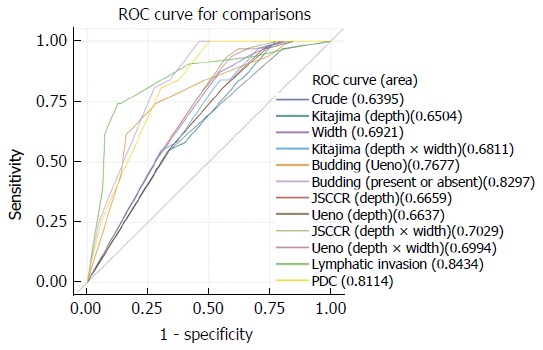
Comparison of receiver operating characteristic curves by multivariate logistic regression tests. Desmoplaisa and tumor type were used as compounding factors.
We found that the JSCCR method for measuring the depth of invasion was the most predictive for LNM (difference AUC = 0.026). In addition, the width of the submucosal invasion was more accurate for predicting LNM than the depth of invasion (difference AUC = 0.053). Furthermore, when using the JSCCR method for the depth of invasion, we found that the depth multiplied by width was even more powerful than the depth of invasion or width of invasion alone (difference AUC = 0.063). Meanwhile, the most powerful clinicopathological parameter for predicting LNM was lymphatic invasion (difference AUC = 0.204), followed by the presence or absence of tumor budding (difference AUC = 0.190), PDCs (difference AUC = 0.172) and tumor budding assessed using the Ueno method (difference AUC = 0.128). Simply classifying tumor budding by the presence or absence was more predictive than the method proposed by Ueno.
DISCUSSION
The presence of LNM is known to be one of the most significant and independent predictor for 5 years cancer specific survival and 5 years disease-free survival in patients with SICRC[12]. It is essential to select the most appropriate treatment options considering the risk of LNM. However, each treatment modality needs to balance a potential cure with the mortality and morbidity risks that accompany such treatment options. Therefore, it is crucial to identify those patients with a potential risk for LNM before proceeding with additional surgical treatments.
To date, various histopathological parameters, including those related to the degree of submucosal invasion (measured as the depth or width of the submucosal invasion) and the status of the MM, have been proposed to predict the risk of LNM in patients with SICRC. The depth of submucosal invasion has long been identified as a predictor of LNM in SICRC, but the level of submucosal invasion associated with LNM and how the depth of invasion should be measured remain undefined. Several measurement systems have been proposed to evaluate the depth of the submucosal invasion in SICRC, although there are still controversies regarding their ability to accurately predict LNM. Haggitt et al[8] revealed that level 4 invasion is an adverse prognostic factor associated with LNM in cases of the pedunculated type of SICRC[8]. Similarly, Kikuchi et al[2] showed that the submucosal invasion level in sessile type tumors is an important risk factor for the development of LNM and local recurrence.However, the Haggitt system is of little use in sessile type tumors without an identifiable stalk, which are always classified as level 4. The Kikuchi system is difficult to apply to specimens obtained by endoscopic resection as they do not usually include the muscularis propria and therefore submucosal invasion levels cannot easily be evaluated. For both the Haggitt and Kickuchi systems to be successfully applied, the tumors should be resected en bloc.
Owing to the variability in the shapes of SICRCs, the usefulness of quantitatively measuring the actual submucosal depth from the MM as an invasion value is being widely accepted[5,7]. In the JSCCR guidelines, a depth of submucosal invasion of 1000 μm or more has been adopted as the criteria for additional intestinal resection[7]. Similarly, in the present study, we found that a submucosal invasion depth of 1000 μm or more was significantly correlated with the incidence of LNM.
In the evaluation of SICRC, there have been difficulties in identifying the depth of the submucosal invasion, because the MM is sometimes poorly defined and disrupted, leading to variability in the measurements. Moreover, the greater the length of the submucosal invasive fronts, the higher the chances of contact with and subsequent invasion into the lymphovascular structures. Thus, Ueno et al[6] addressed the importance of the width of submucosal invasion in predicting LNM. They suggested that a submucosal invasion width of ≥ 4000 μm together with an invasion depth of ≥ 2000 μm could increase the probability of LNM[6]. The chances of the lymphovascular invasion would be higher with a greater area of submucosal invasion. Toh et al[13] introduced the area of tumor involvement within the submucosa as a predictor for LNM, suggesting that the assessment of the submucosal invasion area would provide more valuable information.
Our study revealed that lymph node-positive SICRCs had a significantly greater width of invasion (P = 0.001) and multiplication of depth and width of invasion (P < 0.001) compared with those of lymph node-negative SICRCs. Additionally, the ROC analysis demonstrated that the width of invasion and multiplication of depth and width of invasion had increased sensitivity and specificity compared with the submucosal invasion depth in predicting LNM. Toh et al[13] measured the area of submucosal tumor involvement very accurately using digital pathology, which is impractical and laborious for routine clinical use. On the other hand, in the present study, we assessed the depth and width of the submucosa invasion, and then simply multiplied these two parameters. This can easily be applied in routine practice. Moreover, we have found that the multiplication of depth and width of submucosal invasion, roughly reflects the area of submucosal invasion. It showed to be a good predictor for LNM.
Tumor budding means individual malignant cells and/or small clusters of undifferentiated malignant cells seen in the tumor stroma, which are located near the invasive front of the tumor[14]. It has been associated with the process of epithelial-mesenchymal transition (EMT), which gives tumor cells a more mesenchymal phenotype with increased migratory capacity and invasiveness[15].Tumor budding is postulated to be akin to EMT, and is now considered a predictor of LNM, lymphovascular invasion, tumor relapse and poor prognosis among CRC patients of all stages[14]. In the same context, it is proposed as an adverse prognostic factor in early CRC by the European Society for Medical Oncology consensus guidelines[16]. Nevertheless, tumor budding has not yet been used in routine clinical practice, because there is no consensus criteria concerning the exact definition, and methodology of assessment. Therefore, standardization of methods for defining and quantifying tumor budding is needed to unequivocally confirm its prognostic value.
The present study defined tumor budding by its presence or absence and demonstrated that tumor budding was the second highest impact factor for predicting LNM in patients with SICRC, after lymphatic invasion. Moreover, in the evaluation of tumor budding, we revealed that our classification is superior to that of Ueno et al[6] suggesting that presence of tumor budding itself can significantly affect patient outcomes.In addition, our defining system is more practical since it does not require any additional grading procedures[17].
Recent studies have emphasized the potential role of PDC as a prognostic marker for LNM in SICRC, and the present study confirmed the significant impact of PDC in identifying the risk of LNM[11,18]. Furthermore, both ROC curves of tumor budding and PDC showed a relatively large AUC, suggesting that these two parameters had high sensitivity and specificity in predicting LNM compared with submucosal invasion depth, width and multiplication of depth and width.
Many researchers have shown that there is a directly proportional relationship between the number of examined lymph nodes and survival, particularly in patients with advanced CRC[19-22]. Similarly, Wang et al[23] revealed that the total number of lymph nodes sampled significantly correlated with the prognosis of SICRC. Therefore, retrieving a sufficient number of nodes is crucial for investigating the relationship between clinicopathological parameters and lymph node status in CRC. However, most previous studies with early CRC have reported upon a relatively small number of lymph nodes: mean number of 9.4 ± 7.8 retrieved lymph nodes in the study by Wang et al[23] 10 in the study by Okabe et al[24] and 14 in the study by Tateishi et al[25]. A median of 18 lymph nodes were retrieved in our study, which is the largest number collected to date.
This study has several limitations. First, it is a retrospective cohort study that has a relatively low statistical power due to the small number of SICRC with LNM. In addition, SICRC case selection in this study might have been biased toward those with high risk for LNM. Therefore, a prospective trial with large scaled cohort is necessary to verify the findings of this study. Despite these limitations, our study revealed relatively simple and novel predictors for LNM in SICRC.
In conclusion, we have revealed that the presence of tumor budding might be a powerful predictor for LNM in patients with SICRC. In addition, we have found that submucosal invasion depth and width were significantly correlated with the incidence of LNM. In particular, the multiplication of depth and width measurements of the submucosal invasion proposed in our study may provide important information regarding treatment options for patients with SICRC.
COMMENTS
Background
Endoscopic resection of intramucosal carcinomas is considered to be a curative therapy since the risk of lymph node metastasis (LNM) is low. However, up to 12% of patients with submucosal invasive colorectal carcinoma (SICRC) have LNM. Thus, it is unclear whether endoscopic resection is appropriate after carcinomatous cells have penetrated the muscularis mucosa. Prior to endoscopic resection, patients with SICRC require careful pathological assessment to determine whether there is a significant risk of LNM which may require additional surgical treatment.
Research frontiers
The depth of submucosal invasion has long been identified as a predictor of LNM in SICRC, but the level of submucosal invasion associated with LNM and how the depth of invasion should be measured remain undefined. Several measurement systems have been proposed to evaluate the depth of the submucosal invasion in SICRC, although there are still controversies regarding their ability to accurately predict LNM. There needs to be an improvement in discriminating between patients with a high risk of developing LNM and those with a low risk of developing LNM.
Innovations and breakthroughs
It has revealed that the width of the submucosal invasion was more accurate for predicting LNM than the depth of invasion and the depth multiplied by width was even more powerful than the depth of invasion or width of invasion alone in the present study. In addition, it has revealed that the presence of tumor budding might be a powerful predictor for LNM in patients with SICRC.
Applications
In the present study, it assessed the depth and width of the submucosa invasion, and then simply multiplied these two parameters. This can easily be applied in routine practice. In the evaluation of tumor budding, it revealed that presence of tumor budding itself can significantly affect patient outcomes. This defining system is more practical since it does not require any additional grading procedures.
Peer-review
This is a well-written manuscript showing predictive factors for LNM of SICRC.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair):0
Grade E (Poor): 0
Conflict-of-interest statement: The authors do not have any conflicts of interest to disclose.
Institutional review board statement: This study was approved by the Institutional Review Board of the Catholic University of Korea, Seoul St. Mary’s Hospital, College of Medicine (KC16RISI0817).
Informed consent statement: Written informed consent was obtained by all patients.
Data sharing statement: No additional data are available.
Peer-review started: May 26, 2017
First decision: June 23, 2017
Article in press: August 8, 2017
P- Reviewer: Naito Y, Neninger E, Yamagata M S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
Contributor Information
Kwangil Yim, Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea.
Daeyoun David Won, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea.
In Kyu Lee, Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea.
Seong-Taek Oh, Department of Surgery, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea.
Eun Sun Jung, Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea.
Sung Hak Lee, Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, South Korea. hakjjang@catholic.ac.kr.
References
- 1.Nivatvongs S. Surgical management of early colorectal cancer. World J Surg. 2000;24:1052–1055. doi: 10.1007/s002680010148. [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286–1295. doi: 10.1007/BF02049154. [DOI] [PubMed] [Google Scholar]
- 3.Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS, et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007;60:912–915. doi: 10.1136/jcp.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, Noda N, Kurisu Y, Shibayama Y. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004;17:503–511. doi: 10.1038/modpathol.3800030. [DOI] [PubMed] [Google Scholar]
- 5.Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 6.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328–336. doi: 10.1016/0016-5085(85)90333-6. [DOI] [PubMed] [Google Scholar]
- 9.Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, Yoshida S, Arihiro K, Chayama K. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057–1062. doi: 10.1111/j.1440-1746.2011.07041.x. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, Hase K, Hashiguchi Y, Shimazaki H, Yoshii S, Kudo SE, Tanaka M, Akagi Y, Suto T, Nagata S, et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol. 2014;49:1314–1323. doi: 10.1007/s00535-013-0881-3. [DOI] [PubMed] [Google Scholar]
- 12.Chok KS, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surg. 2007;31:1485–1490. doi: 10.1007/s00268-007-9089-0. [DOI] [PubMed] [Google Scholar]
- 13.Toh EW, Brown P, Morris E, Botterill I, Quirke P. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum. 2015;58:393–400. doi: 10.1097/DCR.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 14.Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer. 2012;106:1713–1717. doi: 10.1038/bjc.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 17.Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T, et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28:872–879. doi: 10.1038/modpathol.2015.36. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, Katsurada Y, Nakamura T, Mochizuki H, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36:193–201. doi: 10.1097/PAS.0b013e318235edee. [DOI] [PubMed] [Google Scholar]
- 19.Deodhar KK, Budukh A, Ramadwar M, Bal MM, Shrikhande SV. Are we achieving the benchmark of retrieving 12 lymph nodes in colorectal carcinoma specimens? Experience from a tertiary referral center in India and review of literature. Indian J Pathol Microbiol. 2012;55:38–42. doi: 10.4103/0377-4929.94853. [DOI] [PubMed] [Google Scholar]
- 20.Lagoudianakis E, Pappas A, Koronakis N, Tsekouras D, Dallianoudis J, Kontogianni P, Papanikolaou D, Chrysikos J, Karavitis G, Markogiannakis H, et al. Lymph node harvesting in colorectal carcinoma specimens. Tumori. 2011;97:74–78. doi: 10.1177/030089161109700114. [DOI] [PubMed] [Google Scholar]
- 21.Dekker JW, Peeters KC, Putter H, Vahrmeijer AL, van de Velde CJ. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM classification. Eur J Surg Oncol. 2010;36:1180–1186. doi: 10.1016/j.ejso.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Stocchi L, Fazio VW, Lavery I, Hammel J. Individual surgeon, pathologist, and other factors affecting lymph node harvest in stage II colon carcinoma. is a minimum of 12 examined lymph nodes sufficient? Ann Surg Oncol. 2011;18:405–412. doi: 10.1245/s10434-010-1308-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, Chang SC, Lin JK. Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum. 2005;48:1182–1192. doi: 10.1007/s10350-004-0935-y. [DOI] [PubMed] [Google Scholar]
- 24.Okabe S, Shia J, Nash G, Wong WD, Guillem JG, Weiser MR, Temple L, Sugihara K, Paty PB. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg. 2004;8:1032–1039; discussion 1039-1040. doi: 10.1016/j.gassur.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23:1068–1072. doi: 10.1038/modpathol.2010.88. [DOI] [PubMed] [Google Scholar]


