Abstract
AIM
To observe changes in gastric biomarker levels with age and effects of Helicobacter pylori (H. pylori) infection in a healthy population, and explore factors associated with gastric biomarkers.
METHODS
Three hundred and ninety-five subjects were selected and underwent physical examinations, biochemical tests, and measurement of serum pepsinogen (PG) I and II, gastrin-17 (G-17) and H. pylori antibody levels. Analyses were made by Student’s t-test, ANOVA, Pearson’s correlation and multiple linear regressions.
RESULTS
PGII levels were higher in the ≥ 65-years-old age group (P < 0.05) and PGI/PGII were lower in the ≥ 75-years-old age group (P = 0.035) compared to the 35-44-years-old age group. Levels of low-density lipoprotein cholesterol (LDL-C) were higher (P = 0.009) in H. pylori-infected subjects that were male. LDL-C levels were higher in 55-74-years-old age group (P < 0.05) for H. pylori-infected subjects and 45-64-years-old age group (P < 0.05) for non-infected subjects compared to 35-44-years-old age group. Hp-IgG level positively correlated with PGI, PGII and G-17 (P < 0.001, P < 0.001, P = 0.006), and negatively correlated with PGI/PGII (P < 0.001). Creatinine positively correlated with PGI, PGII and G-17 (P < 0.001, P < 0.001, P < 0.001). Fasting blood glucose (FBG) positively correlated with PGI/PGII and G-17 (P < 0.001, P = 0.037). Age positively correlated with PGII and G-17 (P = 0.005, P = 0.026).
CONCLUSION
PGII levels increased while PGI/PGII declined with age in a healthy population. H. pylori infection had an effect on raising LDL-C levels to increase the risk of atherosclerosis in males, especially those of elderly age. Age, H. pylori infection, levels of renal function and FBG were associated with levels of pepsinogens and gastrin.
Keywords: Helicobacter pylori antibody, Pepsinogen, Gastrin, Gastric ageing
Core tip: Our study showed that in an entire healthy population, levels of serum pepsinogen (PG) II increased while PGI/PGII declined with age. We discovered that Helicobacter pylori (H. pylori) infection had an effect on raising levels of low-density lipoprotein cholesterol to increase the risk of atherosclerosis in males, especially those who are elderly. We also found that age, H. pylori infection, serum levels of renal function indicators and fasting blood glucose (FBG) were associated with levels of serum PGs and gastrin; it was assumed that they may influence the secretory function of gastric mucosa and that abnormal serum levels of FBG and renal function might participate in the occurrence and development of gastric diseases.
INTRODUCTION
Ageing of the gastric tract is an early manifestation of overall ageing, and mainly presents as a decline in the secretory function of the gastric mucosa. Histomorphological studies have demonstrated that atrophy of gastric mucosa increases with age[1-3]. In addition, studies have also shown that Helicobacter pylori (H. pylori) infection plays an important role in the progression of gastric mucosa lesions[4]. It has been demonstrated that the prevalence of H. pylori infection increases with age, and H. pylori is closely related with the occurrence and development of peptic ulcers, chronic atrophic gastritis and gastric cancer[5,6].
Serum pepsinogen (PG) levels reflect the number of glands and cells in gastric corpus mucosa. Therefore, they can reflect the secretory function of the gastric mucosa[7-10]. It has been reported that the levels of serum PGs are influenced by age, sex, pathophysiologic status of gastric mucosa and H. pylori infection[11]. Thus, serum PGs are indicators of the functional and morphological status of gastric corpus mucosa, and lower serum levels of PGI or PGI/PGII represent existence and degree of atrophy in gastric corpus mucosa[12].
Serum level of gastrin-17 (G-17) can act as a biomarker that reflects the function and structure of gastric antral mucosa. Combining serum PG and G-17 levels has been shown to provide diagnostic information on gastric mucosa[13-16], and may also reflect the degree of gastric aging. Non-invasive biomarker tests may, therefore, evaluate the secretory function of gastric mucosa and differentiate pathological conditions, such as H. pylori-associated gastritis and atrophic gastritis, from the healthy condition by combining tests for PGs, G-17 and H. pylori-immunoglobulin G (Hp-IgG)[17].
Previous studies have investigated patients with peptic ulcer, chronic atrophic gastritis and gastric cancer. To date, few studies have observed levels of the aforementioned biomarkers and effects of H. pylori infection in a healthy ageing population nor explored the associated factors. In our study, we selected PGs and G-17 as gastric biomarkers and measured their serum levels along with Hp-IgG. The aim of the current study was to observe changes in gastric biomarker levels with age in a healthy Chinese population and effects of H. pylori infection on biochemical tests, as well as to explore associated factors which influence the levels of gastric biomarkers.
MATERIALS AND METHODS
Study subjects
This was a cross-sectional study of a healthy population, defined as having no respiratory, cardiovascular, digestive, neurological, endocrine or urinary system diseases, as well as having absence of neoplastic and chronic infectious diseases and no history of psychiatric disorders. We screened 505 healthy persons out of 1500 volunteers in Shenyang, China between September 2007 and June 2008. The screening included inquiries on medical history, symptoms, smoking, alcohol intake, diet and family history obtained by a questionnaire that was completed by each participant. Physical examinations (i.e., electrocardiogram, chest radiograph, etc.) were carried out along with biochemical tests, including assessments of fasting blood glucose (FBG), blood lipids, liver function, renal function and uric acid levels.
A total of 395 subjects (168 males and 227 females) out of the 505 persons, having a mean age of 59.4 years (range: 37-87 years), were enrolled from November 2010 to May 2011 by the same screening method. Patients with circulatory, respiratory, endocrine, neurological, digestive, urinary diseases and chronic infections or neoplastic diseases, or abnormal physical examinations and test results, as well as those with psychiatric disorders or who were unable to complete instructions and self-evaluations were excluded. Blood samples were obtained and sera were stored (within 2 h of collection) at -75 °C until use for measurement of gastric biomarker levels (within 6 mo).
Informed consent was obtained from each participant. This study was reviewed and approved by the Medical Ethics Committees of General Hospital of Chinese People's Liberation Army and China Medical University.
Serological assays
Serum PGI and PGII, G-17 and H. pylori antibody levels were measured with enzyme-linked immunosorbent assay (ELISA)[18] (Biohit Oyj, Laippatie 1, FIN-00880 Helsinki, Finland). All procedures were carried out according to the manufacturer’s instructions.
Study groups
Subjects were divided into five age groups (35-44, 45-54, 55-64, 65-74 and ≥ 75 years). Hp-IgG-positive or -negative groups (Hp-IgG-positive defined as serum Hp-IgG ≥ 35 EIU)[19] were also established.
Statistical analysis
Serum biomarker levels and serum biochemical tests were analyzed in H. pylori-positive and H. pylori-negative patients, separately in male and female subjects, by Student’s t-test. Levels of serum gastric biomarkers among age groups and levels of serum gastric biomarkers and biochemical tests among age groups divided by H. pylori infection status were compared by ANOVA, and multiple comparisons were carried out by the Bonferroni method (homogeneity of variance) or Tamhane method (heterogeneity of variance). Relationships among serum gastric biomarker levels, age and biochemical tests were analyzed by Pearson’s correlation coefficient matrix. Serum gastric biomarkers as dependent variables and other related factors as independent variables were analyzed by multiple linear regression analysis with stepwise method and multiple-colinearity. For all statistical analyses, we used SPSS V.17.0, and a two-sided P value of < 0.05 was considered statistically significant.
RESULTS
Comparison of serum gastric biomarker levels in various age groups
There was no significant difference in serum levels of PGI and G-17 between each age group with increasing age. In contrast, serum levels of PGII increased with age, and were significantly higher in subjects ≥ 65-years-old compared to the 35-44-years-old group (P = 0.024, P = 0.004). The ratio of PGI/PGII decreased with age and was significantly lower in subjects ≥ 75-years-old compared to those in the 35-44-years-old group (P = 0.035) (Table 1 and Figure 1).
Table 1.
Comparison of serum gastric biomarker levels in various age groups
| 35-44 yr, n = 58 | 45-54 yr, n = 84 | 55-64 yr, n = 117 | 65-74 yr, n = 76 | ≥ 75 yr, n = 60 | F | P value | |
| PGI, μg/L | 92.98 ± 5.16 | 98.47 ± 4.15 | 98.65 ± 3.66 | 108.56 ± 8.01 | 117.04 ± 8.30 | 2.326 | 0.056 |
| PGII, μg/L | 9.65 ± 0.73 | 12.26 ± 0.91 | 12.43 ± 0.76 | 14.23 ± 1.171a | 15.33 ± 1.251b | 3.915 | 0.004 |
| PGI/PGII | 10.94 ± 0.44 | 9.89 ± 0.40 | 9.67 ± 0.36 | 9.29 ± 0.61 | 8.71 ± 0.521a | 2.407 | 0.049 |
| G-17, pmol/L | 2.93 ± 0.55 | 5.10 ± 1.29 | 5.75 ± 1.49 | 9.93 ± 3.00 | 10.03 ± 3.18 | 1.950 | 0.101 |
Data are presented as mean ± SD.
Comparison with the 35-44-years-old group,
P < 0.05,
P < 0.01. G-17: Gastrin-17; PGI: Pepsinogen I; PGII: Pepsinogen II.
Figure 1.
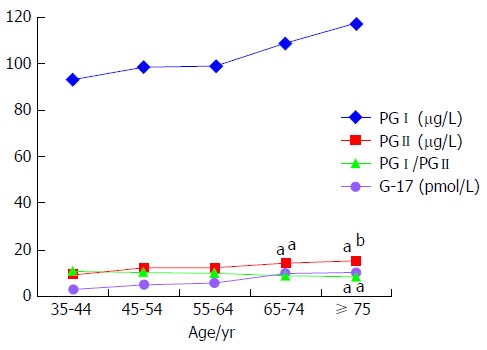
Comparison of serum gastric biomarker levels in various age groups. There was no significant difference in serum levels of PGI and G-17 between each age group with increasing age. In contrast, serum levels of PGII increased with age, and were significantly higher in subjects ≥ 65-years-old compared to 35-44-years-old group. The ratio of PGI/PGII decreased with age, and was significantly lower in subjects ≥ 75-years-old compared to 35-44-years-old group. The “a” denotes comparison with 35-44-years-old age group, aP < 0.05, bP < 0.01. G-17: Gastrin-17; PGI: Pepsinogen I; PGII: Pepsinogen II.
Comparison of serum gastric biomarker levels by H. pylori infection status
Compared to non-infected subjects, serum levels of PGI, PGII and G-17 were significantly higher (P < 0.001, P < 0.001, P = 0.025), while the ratio of PGI/PGII was significantly lower (P < 0.001), in the H. pylori-infected subjects (Figure 2).
Figure 2.
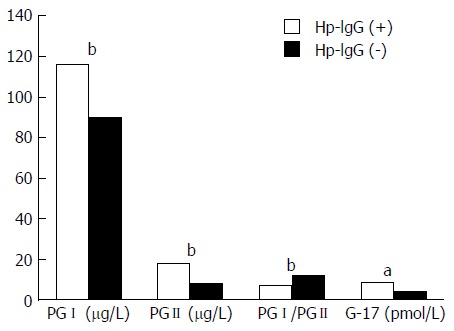
Comparison of serum gastric biomarker levels by Helicobacter pylori infection status. Compared to non-infected subjects, serum levels of PGI, PGII and G-17 were significantly higher, while the ratio of PGI/PGII was significantly lower in Helicobacter pylori-infected subjects. aP < 0.05, bP < 0.01. G-17: Gastrin-17; PGI: Pepsinogen I; PGII: Pepsinogen II.
Comparison of serum biochemical tests between H. pylori infection statuses by sex
There was no significant difference in serum levels of biochemical tests between H. pylori-infected and non-infected female subjects. In males, levels of low-density lipoprotein cholesterol (LDL-C) were higher (P = 0.009) in H. pylori-infected subjects compared to non-infected subjects (Table 2).
Table 2.
Comparison of serum biochemical tests between Helicobacter pylori infection statuses by sex
|
Male |
Female |
|||||
| Hp-IgG (+), n = 81 | Hp -IgG (-), n = 87 | P value | Hp-IgG (+), n = 104 | Hp-IgG (-), n = 123 | P value | |
| TG, mmol/L | 1.33 ± 0.11 | 1.30 ± 0.17 | 0.875 | 1.24 ± 0.06 | 1.26 ± 0.06 | 0.767 |
| TC, mmol/L | 5.07 ± 0.11 | 4.80 ± 0.09 | 0.052 | 5.16 ± 0.09 | 5.42 ± 0.09 | 0.050 |
| HDL-C, mmol/L | 1.31 ± 0.04 | 1.35 ± 0.03 | 0.381 | 1.52 ± 0.03 | 1.54 ± 0.03 | 0.575 |
| LDL-C, mmol/L | 3.30 ± 0.10 | 2.99 ± 0.07 | 0.009 | 3.26 ± 0.09 | 3.48 ± 0.08 | 0.071 |
| FBG, mmol/L | 5.45 ± 0.09 | 5.26 ± 0.06 | 0.073 | 5.29 ± 0.06 | 5.27 ± 0.09 | 0.857 |
| Cr, μmol/L | 72.58 ± 1.76 | 73.26 ± 1.40 | 0.760 | 60.66 ± 2.59 | 55.34 ± 0.89 | 0.054 |
| Cys-C, mg/L | 0.93 ± 0.02 | 0.91 ± 0.02 | 0.520 | 0.88 ± 0.03 | 0.81 ± 0.02 | 0.059 |
| UA, μmol/L | 339.05 ± 8.19 | 337.48 ± 9.04 | 0.898 | 265.13 ± 6.19 | 273.22 ± 5.30 | 0.319 |
Data are presented as mean ± SD. H. pylori-IgG (+) is defined as H. pylori-IgG ≥ 35 EIU. Cr: Creatinine; Cys-C: Cystatin-C; FBG: Fasting blood glucose; HDL-C: High-density lipoprotein cholesterol; Hp-IgG: Helicobacter pylori-immunoglobulin G; LDL-C: Low-density lipoprotein cholesterol; TC: Total cholesterol; TG: Triglycerides; UA: Uric acid; H. pylori: Helicobacter pylori.
Comparison of serum gastric biomarker levels and biochemical tests in various age groups by H. pylori infection status
There was no significant difference in serum levels of gastric biomarkers between each age group with increasing age in H. pylori-infected subjects. In non-infected subjects, levels of serum PGII increased with age and were significantly higher in subjects ≥ 75-years-old compared to subjects between 35- and 54-years-old (P = 0.007, P = 0.004).
In H. pylori-infected subjects, serum levels of total cholesterol (P = 0.002, P = 0.001) and LDL-C (P = 0.016, P = 0.002) were significantly higher in subjects between 55- and 74-years-old compared to those in the 35-44-years-old age group. In non-infected subjects, serum levels of total cholesterol (P = 0.023, P = 0.035) and LDL-C (P = 0.015, P = 0.006) were significantly higher in subjects between 45- and 64-years-old compared to those in the 35-44-years-old group (Table 3 and Figure 3).
Table 3.
Comparison of serum gastric biomarker levels and biochemical tests in various age groups by Helicobacter pylori infection status
| 35-44 yr | 45-54 yr | 55-64 yr | 65-74 yr | ≥ 75 yr | F | P value | |
| n1 = 21 | n1 = 39 | n1 = 57 | n1 = 36 | n1 = 33 | |||
| n2 = 37 | n2 = 45 | n2 = 60 | n2 = 40 | n2 = 27 | |||
| PGI, μg/L | |||||||
| Hp-IgG (+) | 89.43 ± 7.46 | 100.03 ± 6.45 | 90.25 ± 5.10 | 92.61 ± 12.23 | 94.95 ± 13.47 | 0.824 | 0.920 |
| Hp-IgG (-) | 83.6 ± 6.47 | 81.28 ± 3.89 | 83.96 ± 5.04 | 96.67 ± 10.31 | 105.49 ± 8.08 | 1.730 | 0.143 |
| PGII, μg/L | |||||||
| Hp-IgG (+) | 14.68 ± 1.08 | 18.45 ± 1.34 | 17.47 ± 1.16 | 19.52 ± 1.46 | 19.51 ± 1.91 | 1.393 | 0.260 |
| Hp-IgG (-) | 6.80 ± 0.58 | 6.89 ± 0.41 | 7.64 ± 0.46 | 9.46 ± 1.42 | 10.23 ± 0.761b2b | 1.115 | 0.011 |
| PGI/PGII | |||||||
| Hp-IgG (+) | 7.92 ± 0.53 | 7.17 ± 0.39 | 7.03 ± 0.38 | 6.65 ± 0.58 | 6.97 ± 0.52 | 0.662 | 0.616 |
| Hp-IgG (-) | 12.66 ± 0.41 | 12.25 ± 0.42 | 12.18 ± 0.39 | 11.68 ± 0.89 | 10.85 ± 0.80 | 1.163 | 0.353 |
| G-17, pmol/L | |||||||
| Hp-IgG (+) | 4.11 ± 0.74 | 9.81 ± 2.58 | 8.18 ± 2.17 | 7.16 ± 0.95 | 13.7 ± 4.79 | 1.258 | 0.285 |
| Hp-IgG (-) | 2.26 ± 0.73 | 1.02 ± 0.26 | 3.44 ± 2.03 | 12.43 ± 5.65 | 5.54 ± 3.90 | 2.254 | 0.066 |
| TC, mmol/L | |||||||
| Hp-IgG (+) | 4.42 ± 0.14 | 4.96 ± 0.14 | 5.30 ± 0.111b | 5.51 ± 0.191b | 5.00 ± 0.15 | 6.604 | < 0.001 |
| Hp-IgG (-) | 4.77 ± 0.10 | 5.41 ± 0.171a | 5.35 ± 0.131a | 5.18 ± 0.16 | 4.98 ± 0.14 | 2.709 | 0.031 |
| LDL-C, mmol/L | |||||||
| Hp-IgG (+) | 2.71 ± 0.14 | 3.10 ± 0.12 | 3.40 ± 0.111a | 3.60 ± 0.171b2a | 3.17 ± 0.14 | 7.291 | < 0.001 |
| Hp-IgG (-) | 2.89 ± 0.09 | 3.47 ± 0.151a | 3.48 ± 0.111b | 3.33 ± 0.13 | 3.08 ± 0.13 | 3.544 | 0.008 |
| FBG, mmol/L | |||||||
| Hp-IgG (+) | 5.25 ± 0.08 | 5.22 ± 0.13 | 5.38 ± 0.12 | 5.46 ± 0.12 | 5.44 ± 0.08 | 0.791 | 0.532 |
| Hp-IgG (-) | 5.01 ± 0.06 | 5.21 ± 0.08 | 5.29 ± 0.08 | 5.34 ± 0.08 | 5.39 ± 0.34 | 1.186 | 0.318 |
| Cr, μmol/L | |||||||
| Hp-IgG (+) | 56.95 ± 2.57 | 60.38 ± 2.35 | 63.79 ± 1.71 | 63.61 ± 3.04 | 81.4 ± 7.241a | 4.974 | 0.001 |
| Hp-IgG (-) | 59.19 ± 2.05 | 62.78 ± 1.95 | 61.20 ± 1.67 | 63.53 ± 2.29 | 73.19 ± 3.401b2a3b | 4.174 | 0.003 |
| Cys-C, mg/dL | |||||||
| Hp-IgG (+) | 0.69 ± 0.02 | 0.78 ± 0.021a | 0.84 ± 0.021b | 0.97 ± 0.031b2b3a | 1.19 ± 0.071b2b3b | 19.952 | < 0.001 |
| Hp-IgG (-) | 0.71 ± 0.02 | 0.78 ± 0.02 | 0.83 ± 0.021b | 0.93 ± 0.031b2b | 1.14 ± 0.041b2b3b4b | 28.435 | < 0.001 |
Data are presented as mean ± SD. Hp-IgG (+) is defined as Hp-IgG ≥ 35 EIU. n1: number in the Hp-IgG (+) group; n2: number in the Hp-IgG(-) group.
35-44-years-old group,
45-54-years-old group,
55-64-years-old group,
65-74-years-old group,
P < 0.05,
P < 0.01. Cr: Creatinine; Cys-C: Cystatin-C; FBG: Fasting blood glucose; G-17: Gastrin-17; Hp-IgG: Helicobacter pylori-immunoglobulin G; LDL-C: Low-density lipoprotein cholesterol; PGI: Pepsinogen I; PGII: Pepsinogen II; TC: Total cholesterol.
Figure 3.
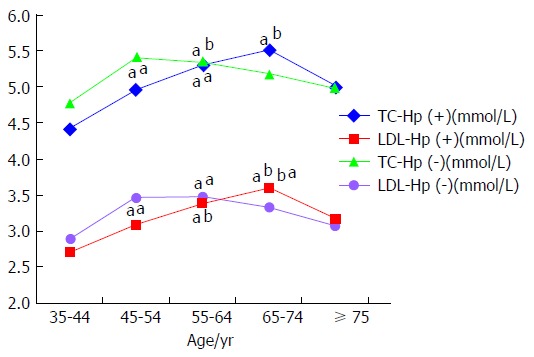
Comparison of serum cholesterol levels in various age groups by Helicobacter pylori infection status. In H. pylori-infected subjects, serum levels of TC and LDL were significantly higher in subjects between 55- and 74-years-old compared to those in the 35-44-years-old age group. In non-infected subjects, serum levels of TC and LDL were significantly higher in subjects between 45- and 64-years-old compared to those in the 35-44-years-old age group. The “a” denotes comparison with the 35-44-years-old age group and the “b” denotes comparison with the 45-54-years-old age group, aP < 0.05, bP < 0.01. H. pylori: Helicobacter pylori; LDL-C: Low-density lipoprotein cholesterol; TC: Total cholesterol.
Correlation analysis among serum gastric biomarker levels, age and biochemical tests
Age positively correlated with serum levels of Hp-IgG, PGI, PGII and G-17 (P = 0.038, P = 0.001, P < 0.001, P = 0.005) and negatively correlated with ratio of PGI/PGII (P = 0.002). Levels of serum Hp-IgG positively correlated with serum levels of PGI, PGII and G-17 (P < 0.001, P < 0.001, P = 0.038) and negatively correlated with ratio of PGI/PGII (P < 0.001).
Levels of serum PGI positively correlated with serum levels of uric acid, creatinine and cystatin-C (P < 0.001, P < 0.001, P < 0.001). Levels of serum PGII positively correlated with serum levels of creatinine and cystatin-C (P < 0.001, P < 0.001). Levels of serum G-17 positively correlated with serum levels of FBG, creatinine and cystatin-C (P = 0.018, P = 0.011, P = 0.037).
Levels of serum Hp-IgG were strongly associated with serum levels of PGII and PGI/PGII (r = 0.592, P < 0.001; r = -0.587, P < 0.001), and levels of serum PGII were strongly associated with serum levels of PGI and PGI/PGII (r = 0.682, P < 0.001; r = -0.588, P < 0.001)(Table 4).
Table 4.
Correlation matrix among serum gastric biomarker levels, age and biochemical tests
| Age | PGI | PGII | PGI/II | G-17 | Hp-IgG | TG | TC | HDL-C | LDL-C | FBG | UA | Cr | Cys-C | BMI | |
| Age | 1 | 0.161b | 0.215b | -0.155b | 0.140b | 0.104a | -0.009 | 0.108a | 0.010 | 0.136b | 0.145b | 0.148b | 0.265b | 0.548b | -0.016 |
| 0.001 | 0.000 | 0.002 | 0.005 | 0.038 | 0.854 | 0.033 | 0.844 | 0.007 | 0.004 | 0.003 | 0.000 | 0.000 | 0.781 | ||
| PGI | 1.000 | 0.682b | 0.047 | -0.140b | 0.260b | -0.014 | -0.066 | -0.051 | -0.023 | 0.056 | 0.188b | 0.301b | 0.355b | 0.011 | |
| 0.000 | 0.357 | 0.005 | 0.000 | 0.788 | 0.188 | 0.308 | 0.654 | 0.266 | 0.000 | 0.000 | 0.000 | 0.843 | |||
| PGII | 1.000 | -0.588b | 0.149b | 0.592b | -0.029 | -0.062 | -0.075 | -0.019 | -0.005 | 0.077 | 0.209b | 0.278b | 0.006 | ||
| 0.000 | 0.003 | 0.000 | 0.571 | 0.216 | 0.135 | 0.705 | 0.915 | 0.124 | 0.000 | 0.000 | 0.913 | ||||
| PGI/II | 1.000 | -0.384b | -0.587b | 0.035 | 0.032 | 0.040 | 0.027 | 0.074 | 0.080 | -0.019 | -0.060 | 0.003 | |||
| 0.000 | 0.000 | 0.488 | 0.532 | 0.427 | 0.590 | 0.140 | 0.112 | 0.712 | 0.234 | 0.961 | |||||
| G-17 | 1.000 | 0.105a | 0.055 | -0.020 | -0.068 | -0.016 | 0.119a | -0.062 | 0.129a | 0.105a | -0.023 | ||||
| 0.038 | 0.279 | 0.695 | 0.177 | 0.749 | 0.018 | 0.219 | 0.011 | 0.037 | 0.690 | ||||||
| Hp- IgG | 1.000 | 0.000 | -0.025 | -0.062 | -0.004 | 0.057 | 0.009 | 0.058 | 0.095 | 0.006 | |||||
| 0.985 | 0.618 | 0.220 | 0.938 | 0.257 | 0.865 | 0.250 | 0.059 | 0.919 | |||||||
| TG | 1.000 | 0.278b | -0.327b | 0.145b | 0.166b | 0.156b | 0.059 | 0.068 | 0.048 | ||||||
| 0.000 | 0.000 | 0.004 | 0.001 | 0.002 | 0.243 | 0.176 | 0.402 | ||||||||
| TC | 1.000 | 0.295b | 0.896b | 0.123a | 0.023 | -0.032 | -0.039 | 0.111 | |||||||
| 0.000 | 0.000 | 0.015 | 0.647 | 0.530 | 0.442 | 0.053 | |||||||||
| HDL-C | 1.000 | 0.032 | -0.093 | -0.282b | -0.176b | -0.164b | 0.017 | ||||||||
| 0.528 | 0.064 | 0.000 | 0.000 | 0.001 | 0.762 | ||||||||||
| LDL-C | 1.000 | 0.149b | 0.074 | 0.000 | 0.022 | 0.085 | |||||||||
| 0.003 | 0.142 | 10.000 | 0.664 | 0.138 | |||||||||||
| FBG | 1.000 | 0.119a | 0.111a | 0.086 | 0.034 | ||||||||||
| 0.018 | 0.027 | 0.086 | 0.558 | ||||||||||||
| UA | 1.000 | 0.465b | 0.403b | -0.003 | |||||||||||
| 0.000 | 0.000 | 0.961 | |||||||||||||
| Cr | 1.000 | 0.706b | -0.010 | ||||||||||||
| 0.000 | 0.858 | ||||||||||||||
| Cys-C | 1.000 | -0.026 | |||||||||||||
| 0.648 | |||||||||||||||
| BMI | 1.000 |
P < 0.05,
P < 0.01. BMI: Body mass index; Cr: Creatinine; Cys-C: Cystatin-C; FBG: Fasting blood glucose; G-17: Gastrin-17; Hp-IgG: Helicobacter pylori-immunoglobulin G; LDL-C: Low-density lipoprotein cholesterol; PGI: Pepsinogen I; PGII: Pepsinogen II; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; TG: Triglyceride; UA: Uric acid.
Analysis of factors associated with serum levels of gastric biomarkers
With serum PGI as a dependent variable, serum levels of creatinine, Hp-IgG and FBG positively correlated with levels of serum PGI (P < 0.001, P < 0.001, P = 0.037), while serum levels of G-17 negatively correlated with levels of serum PGI (P < 0.001). With serum PGII as a dependent variable, serum levels of creatinine, Hp-IgG and age positively correlated with levels of serum PGII (P = 0.006, P < 0.001, P = 0.007). With PGI/PGII as a dependent variable, serum levels of FBG positively correlated with PGI/PGII (P < 0.001), while serum levels of Hp-IgG, G-17 and age negatively correlated with PGI/PGII (P < 0.001, P < 0.001, P = 0.024). With serum G-17 as a dependent variable, age and serum levels of creatinine, Hp-IgG and FBG positively correlated with levels of serum G-17 (P = 0.032, P < 0.001, P = 0.037, P = 0.045), while serum levels of PGI and uric acid negatively correlated with levels of serum G-17 (P < 0.001, P = 0.009)(Table 5).
Table 5.
Factors associated with serum levels of gastric biomarkers
| Dependent variable | Associated factors |
Non-standard coefficient |
Standard coefficient | P value | |
| B | SE | β | |||
| PGI | Constant | 50.347 | 200.845 | 0.798 | |
| Cr | 0.712 | 0.138 | 0.273 | 0.000 | |
| Hp-IgG | 0.334 | 0.066 | 0.263 | 0.000 | |
| G-17 | -0.647 | 0.138 | -0.247 | 0.000 | |
| FBG | 70.859 | 30.744 | 0.110 | 0.037 | |
| PGII | Constant | -10.657 | 20.078 | 0.426 | |
| Hp-IgG | 0.120 | 0.010 | 0.556 | 0.000 | |
| Cr | 0.058 | 0.021 | 0.131 | 0.006 | |
| Age | 0.089 | 0.033 | 0.129 | 0.007 | |
| PGI /PGII | Constant | 90.251 | 10.461 | 0.000 | |
| Hp-IgG | -0.054 | 0.004 | -0.520 | 0.000 | |
| G-17 | -0.075 | 0.009 | -0.349 | 0.000 | |
| FBG | 10.037 | 0.255 | 0.177 | 0.000 | |
| Age | -0.033 | 0.015 | -0.101 | 0.024 | |
| G-17 | Constant | -140.817 | 80.992 | 0.100 | |
| Age | 0.192 | 0.089 | 0.1240 | 0.032 | |
| PGI | -0.103 | 0.022 | -0.269 | 0.000 | |
| Cr | 0.228 | 0.063 | 0.228 | 0.000 | |
| Hp-IgG | 0.058 | 0.027 | 0.119 | 0.037 | |
| UA | -0.042 | 0.016 | -0.160 | 0.009 | |
| FBG | 30.054 | 10.520 | 0.112 | 0.045 | |
Cr: Creatinine; FBG: Fasting blood glucose; G-17: Gastrin-17; Hp-IgG: Helicobacter pylori-immunoglobulin G; PGI: Pepsinogen I; PGII: Pepsinogen II; UA: Uric acid.
DISCUSSION
A European gastric biomarkers test[17] has been developed to measure serum PG and G-17 levels, and Hp-IgG antibodies by ELISA technique. Compared to endoscopic biopsy findings, the test classified the subjects into groups with “healthy” or “diseased” gastric mucosa with 94% accuracy, 95% sensitivity and 93% specificity. Compared to endoscopic histological findings, the accuracy of the biomarkers test in diagnosing atrophic gastritis was 87%, with a sensitivity of 40% and a specificity of 94%. Combined testing of Hp-IgG, PG and G-17 levels is of great clinical significance for general assessment of gastric mucosa secretion.
It has been previously shown that levels of serum PGI decreased with age. Levels of serum PGII increased with age, but declined in participants aged over 60. Ratio of PGI/PGII decreased with age, but it increased after age 60[20]. It has also been observed that levels of PGI and PGII increased with age. In a healthy population, levels of PGI and PGII varied amongst age groups, and the average PG level was highest in the senile group[21].
Our study showed that in the entire healthy study population, levels of serum PGII increased with age, while the ratio of PGI/PGII decreased with age. The correlation between age and PGII is stronger and more significant than that of PGI; possibly, the distribution of PGII-secreting cells is more extensive, and this could be one of the reasons to explain this finding. Since the ratio of PGI/PGII reflects the degree of atrophy in gastric mucosa, the current study indicated that atrophy of gastric mucosa occurred and developed with increasing age in a non-invasive serological method.
It has been suggested that serum levels of PGI and PGII significantly correlated with age in H. pylori-positive subjects. Increased PGI and PGII levels associated with age in a healthy population were caused by increased rates of H. pylori infection. Levels of PGI and PGII were dependent on the presence of H. pylori infection[22]. It was suggested that serum levels of G-17, PGI and PGII increased in subjects with H. pylori infection, especially PGII, while the ratio of PGI/PGII decreased.
Hypergastrinemia and hyperpepsinogenemia may be secondary to H. pylori infection[23,24]. The results of the current study on the effects of H. pylori infection on serum gastric biomarker levels were consistent with those of previous studies, and it was suggested that H. pylori infection had a closer correlation with PGII than with PGI and may influence the levels of PGII more.
It has been shown that H. pylori infection was independently associated with elevated LDL-C levels and contributed to the atherosclerotic process[25]. The current study showed the difference on levels of serum LDL-C between H. pylori-infected and non-infected male subjects, which suggested an effect of H. pylori infection on raising levels of LDL-C in males. Meanwhile, the highest level of LDL-C was found in the middle-aged group (45-64 years) in non-infected subjects, while in H. pylori-infected subjects it was found in the elderly group (55-74 years). Increased LDL-C level is a risk factor for the development of atherosclerosis, and the current study indicated that Hp infection may increase the risks of atherosclerosis in males, especially those of elderly age.
It has been reported that renal function status may influence levels of serum PG and gastrin. Levels of serum PG and gastrin were found to be increased in patients with renal function insufficiency. This may have been due to reduced renal clearance of PG and gastrin[26,27]. There have been few studies investigating the relationship between renal function and serum PG and gastrin in a healthy population. The current study showed that age and serum levels of Hp-IgG, creatinine and FBG were the main factors associated with levels of serum PG and G-17. Since different levels of PG and G-17 represent different pathophysiological status of gastric mucosa, it was assumed that age, H. pylori infection, and serum levels of FBG and markers of renal function may influence the secretory function of gastric mucosa, and that abnormal serum levels of FBG and renal function might participate in the occurrence and development of gastric diseases.
In summary, the current study observed changes in gastric biomarker levels with age and effects of H. pylori infection in a healthy Chinese population, and explored factors associated with gastric biomarkers. Our data provide a theoretical basis for the recognition of gastric aging and its related diseases, which is of important clinical significance. However, there are some limitations in the study. Firstly, the sample size was relatively small and may, therefore, not represent the whole healthy population. Secondly, we found the effects of H. pylori infection and the correlation between gastric biomarkers and other associated factors, but the mechanisms are not clear. More studies are needed to illustrate the mechanisms in the future.
COMMENTS
Background
Combined testing of Helicobacter pylori (H. pylori)-immunoglobulin G (Hp-IgG), pepsinogen (PG) and G-17 levels is of great clinical significance for general assessment of gastric mucosa secretion, and may also reflect the degree of gastric aging. Previous studies have investigated patients with peptic ulcer, chronic atrophic gastritis and gastric cancer. To date, few studies have observed levels of the gastric biomarkers and effects of H. pylori infection in a healthy ageing population nor explored the associated factors.
Research frontiers
Non-invasive biomarker tests may evaluate the secretory function of gastric mucosa, and distinguish pathological conditions, such as atrophic gastritis, from the healthy condition by combining tests for PGs, G-17 and Hp-IgG. Lower serum levels of PGI or PGI/PGII represent existence and degree of atrophy in gastric corpus mucosa. Studies have indicated that serum levels of PGs and G-17 are related to H. pylori infection and age, and could be significantly influenced by H. pylori infection. Furthermore, it has been shown that H. pylori infection was independently associated with elevated low-density lipoprotein cholesterol (LDL-C) levels and contributed to the atherosclerotic process.
Innovations and breakthroughs
The current study observed changes in gastric biomarker levels with age and effects of H. pylori infection in a healthy Chinese population, and explored factors associated with gastric biomarkers. This study showed that in the entire healthy study population, levels of serum PGII increased while PGI/PGII declined with age, which indicated that atrophy of gastric mucosa occurred and developed with increasing age, observed via a non-invasive serological method. Meanwhile, we discovered that H. pylori infection had an effect on raising levels of LDL-C to increase the risk of atherosclerosis in males, especially those of elderly age. Moreover, it is suggested that age, H. pylori infection, serum levels of renal function and fasting blood glucose (FBG) were associated with levels of serum PGs and gastrin.
Applications
In this study, the authors’ discovered that H. pylori infection had an effect on raising levels of LDL-C to increase the risk of atherosclerosis in males, especially those who were elderly, which indicated that H. pylori infection should be afforded a more important status and given active treatment in elderly males to prevent atherosclerotic diseases. This study suggested that age, H. pylori infection, serum levels of renal function and FBG were associated with levels of serum PGs and gastrin. It was assumed that serum levels of renal function and FBG may influence the secretory function of gastric mucosa, and abnormal serum levels of FBG and renal function might participate in the occurrence and development of gastric diseases.
Terminology
H. pylori: A curved Gram-negative bacillus which is found in gastric mucosa; H. pylori is closely related with multiple gastric diseases, such as peptic ulcers, chronic atrophic gastritis and gastric cancer. PG: A precursor of pepsin which is mainly secreted by cells in the gastric corpus and can be divided into two groups, PGI and PGII; serum PG levels reflect the number of glands and cells, as well as the secretory function in gastric corpus mucosa. G-17: A hormone which is mainly secreted by G cells in gastric antrum and plays multiple physiological roles; serum level of G-17 reflects the number of cells and the secretory function in gastric antral mucosa.
Peer-review
The authors have carried out a detailed study of biomarkers and H. pylori infection in a large cohort of patients. The manuscript is detailed, the study well carried out and the data is comprehensive and complex.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Medical Ethics Committees of the General Hospital of Chinese People’s Liberation Army and China Medical University.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
Data sharing statement: No additional data are available.
Peer-review started: April 17, 2017
First decision: May 16, 2017
Article in press: July 12, 2017
P- Reviewer: Bramhall S, Lombardo L, Yamaoka Y S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
Contributor Information
Jin-Hua Shan, Department of Gerontology and Geriatrics, the First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Xiao-Juan Bai, Department of Gerontology and Geriatrics, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China. baixj@sj-hospital.org.
Lu-Lu Han, Department of Gerontology and Geriatrics, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China.
Yuan Yuan, Department of Tumor Research, the First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Xue-Feng Sun, Department of Kidney, General Hospital of Chinese People’s Liberation Army, Beijing 100853, China.
References
- 1.Kang JM, Kim N, Kim JH, Oh E, Lee BY, Lee BH, Shin CM, Park JH, Lee MK, Nam RH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147–G1153. doi: 10.1152/ajpgi.00218.2010. [DOI] [PubMed] [Google Scholar]
- 2.Park KS. [Aging and digestive diseases: at the view of the functional change of gastrointestinal tract] Korean J Gastroenterol. 2011;58:3–8. doi: 10.4166/kjg.2011.58.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Salles N. Basic mechanisms of the aging gastrointestinal tract. Dig Dis. 2007;25:112–117. doi: 10.1159/000099474. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, Zendehdel N, Rakhshani N, Stolte M, Mirzaei M, Saliminejhad M, Saeidi S, Shahidi M. Precancerous conditions after H. pylori eradication: a randomized double blind study in first degree relatives of gastric cancer patients. Arch Iran Med. 2012;15:664–669. [PubMed] [Google Scholar]
- 6.Chen TS, Lee YC, Li FY, Chang FY. Smoking and hyperpepsinogenemia are associated with increased risk for duodenal ulcer in Helicobacter pylori-infected patients. J Clin Gastroenterol. 2005;39:699–703. doi: 10.1097/01.mcg.0000173854.55172.ee. [DOI] [PubMed] [Google Scholar]
- 7.Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pyloriinfection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332–338. doi: 10.1007/s005350300060. [DOI] [PubMed] [Google Scholar]
- 8.Derakhshan MH, El-Omar E, Oien K, Gillen D, Fyfe V, Crabtree JE, McColl KE. Gastric histology, serological markers and age as predictors of gastric acid secretion in patients infected with Helicobacter pylori. J Clin Pathol. 2006;59:1293–1299. doi: 10.1136/jcp.2005.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523–530. doi: 10.1016/j.dld.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Sun LP, Gong YH, Wang L, Gong W, Yuan Y. Follow-up study on a high risk population of gastric cancer in north China by serum pepsinogen assay. J Dig Dis. 2008;9:20–26. doi: 10.1111/j.1443-9573.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun LP, Gong YH, Wang L, Yuan Y. Serum pepsinogen levels and their influencing factors: a population-based study in 6990 Chinese from North China. World J Gastroenterol. 2007;13:6562–6567. doi: 10.3748/wjg.v13.i48.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, Shimosegawa T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853–859. doi: 10.3748/wjg.15.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broutet N, Plebani M, Sakarovitch C, Sipponen P, Mégraud F; Eurohepygast Study Group. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi R, Abe Y, Iijima K, Koike T, Ara N, Uno K, Asanuma K, Asano N, Imatani A, Shimosegawa T. Low serum levels of pepsinogen and gastrin 17 are predictive of extensive gastric atrophy with high-risk of early gastric cancer. Tohoku J Exp Med. 2011;223:35–44. doi: 10.1620/tjem.223.35. [DOI] [PubMed] [Google Scholar]
- 15.Cao Q, Ran ZH, Xiao SD. Screening of atrophic gastritis and gastric cancer by serum pepsinogen, gastrin-17 and Helicobacter pylori immunoglobulin G antibodies. J Dig Dis. 2007;8:15–22. doi: 10.1111/j.1443-9573.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 16.Haj-Sheykholeslami A, Rakhshani N, Amirzargar A, Rafiee R, Shahidi SM, Nikbin B, Khosravi F, Massarrat S. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol. 2008;6:174–179. doi: 10.1016/j.cgh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ, Engstrand L, Stolte M, Vieth M, Walker M, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–1455. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 18.González CA, Megraud F, Buissonniere A, Lujan Barroso L, Agudo A, Duell EJ, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, Krogh V, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23:1320–1324. doi: 10.1093/annonc/mdr384. [DOI] [PubMed] [Google Scholar]
- 19.He CY, Sun LP, Gong YH, Xu Q, Dong NN, Yuan Y. Serum pepsinogen II: a neglected but useful biomarker to differentiate between diseased and normal stomachs. J Gastroenterol Hepatol. 2011;26:1039–1046. doi: 10.1111/j.1440-1746.2011.06692.x. [DOI] [PubMed] [Google Scholar]
- 20.Rollan A, Ferreccio C, Gederlini A, Serrano C, Torres J, Harris P. Non-invasive diagnosis of gastric mucosal atrophy in an asymptomatic population with high prevalence of gastric cancer. World J Gastroenterol. 2006;12:7172–7178. doi: 10.3748/wjg.v12.i44.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miki K, Fujishiro M, Kodashima S, Yahagi N. Long-term results of gastric cancer screening using the serum pepsinogen test method among an asymptomatic middle-aged Japanese population. Dig Endosc. 2009;21:78–81. doi: 10.1111/j.1443-1661.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HY, Kim N, Kang JM, Park YS, Lee DH, Kim YR, Kim JS, Jung HC, Song IS. Clinical meaning of pepsinogen test and Helicobacter pylori serology in the health check-up population in Korea. Eur J Gastroenterol Hepatol. 2009;21:606–612. doi: 10.1097/MEG.0b013e3283086757. [DOI] [PubMed] [Google Scholar]
- 23.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 24.Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, Zendehdel N, Aliasgari A, Rakhshani N, Stolte M, Shahidi SM. Pepsinogen II can be a potential surrogate marker of morphological changes in corpus before and after H. pylori eradication. Biomed Res Int. 2014;2014:481607. doi: 10.1155/2014/481607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Y, Wei W, Jingwei L, Nannan D, Yuan Y. Helicobacter pylori Infection Status Correlates with Serum Parameter Levels Responding to Multi-organ Functions. Dig Dis Sci. 2015;60:1748–1754. doi: 10.1007/s10620-015-3522-2. [DOI] [PubMed] [Google Scholar]
- 26.Furgała A, Błaut-Kadzielska U, Stojakowska M, Dobrek Ł, Mazur M, Machowska A, Thor PJ. Gastric dysfunction in dialysed patients with chronic renal failure. Folia Med Cracov. 2012;52:39–55. [PubMed] [Google Scholar]
- 27.Gong Y, Wang W, Li Y, Yuan Y. Serum Indicators Reflecting Gastric Function May Also Correlate with Other Extragastric Diseases. Gastroenterol Res Pract. 2015;2015:867495. doi: 10.1155/2015/867495. [DOI] [PMC free article] [PubMed] [Google Scholar]


