Abstract
AIM
To determine the sensitivity and specificity of the 13C-urea breath test (UBT) in patients taking proton pump inhibitors (PPIs), using a new test meal Refex.
METHODS
One hundred and fourteen consecutive patients with dyspepsia, 53 Helicobacter pylori (H. pylori) positive, 49 H. pylori negative, were included in the study. The patients were then given esomeprazole 40 mg for 29 consecutive days, and the 13C-UBT with the new test meal was performed the next morning.
RESULTS
The sensitivity of the 13C-UBT with a cut off 2.5‰ was 92.45% (95%CI: 81.79%-97.91%) by per-protocol (PP) analysis and 78.13% (95%CI: 66.03%-87.49%) by intention-to-treat (ITT) analysis. The specificity of the 13C-UBT test was 96.00% in the ITT population (95%CI: 86.29%-99.51%) and 97.96% in the PP population (95%CI: 89.15%-99.95%).
CONCLUSION
The new test meal based 13C-UBT is highly accurate in patients on PPIs and can be used in those unable to stop their PPI treatment.
Keywords: Urea breath test, New test meal, Proton pump inhibitors, Prospective randomized clinical trial, Helicobacter pylori
Core tip: The urea breath test (UBT) with new test meal Reflex (5.5 g powder mixture of citric, malic and tartaric acid) was tested in one hundred and fourteen consecutive patients with dyspepsia, 53 Helicobacter pylori (H. pylori) positive, 49 H. pylori negative. After being on esomeprazole 40 mg for 29 consecutive days, the 13C-UBT was performed the next morning. The sensitivity of the 13C-UBT (cut off 2.5‰) was 92.45% by per-protocol (PP) analysis and 78.13% by intention-to-treat (ITT) analysis. The specificity of the 13C-UBT test was 96.00% in the ITT population and 97.96% in the PP population.
INTRODUCTION
The urea breath test (UBT) is recommended as the test of choice for determining the success of eradication treatment[1]. In the management of dyspeptic patients in primary care settings, non-invasive Helicobacter pylori (H. pylori) testing is the initial step in the management of dyspeptic patients (i.e., test and treat strategy)[2,3]. The UBT is highly sensitive and specific, except in patients taking proton pump inhibitors (PPIs)[4]. In studies with patients on PPI therapy, the UBT resulted in 10%-40% false negatives[5-7]. Current guidelines recommend stopping these medications for 14 d before the UBT or stool test[2].
PPIs are widely available and are over-the-counter agents in some countries[8]. Clinicians are frequently confronted with making a diagnosis of H. pylori infection in patients who may knowingly or unknowingly be taking a PPI. Patients who self-administer certain medications that can cause dyspepsia (e.g., low dose aspirin to prevent myocardial infarction or nonsteroidal anti-inflammatory drugs) often take PPIs to treat dyspepsia symptoms, and the majority of these patients cannot stop PPI therapy for two weeks without suffering dyspeptic symptoms. Therefore, the UBT might not be properly performed in a substantial number of these patients. If H. pylori is diagnosed late or remains undiagnosed, the risk of stomach cancer is increased[9,10].
The breath tests that are currently available are reliable 12-14 d after discontinuing PPI therapy[4,11]. Acid inhibition with PPIs can reduce the number of H. pylori colonies, especially in the antrum, which may be one possible explanation for a false negative UBT[12]. Some studies have suggested that acidification of the stomach may partially reverse a false negative UBT[11,13]. However, the results have been inconsistent, and the correct procedure for acidifying the stomach has not been established.
Refex is a new acidified test meal for the 13C-UBT that contains a mixture of three organic acids - citric acid, malic acid and tartaric acid - and has been developed to increase the sensitivity of the test in patients taking PPIs.
The aim of this study was to determine the sensitivity, specificity and accuracy of a specially formulated UBT test meal, Refex, in patients taking proton pump inhibitors.
MATERIALS AND METHODS
Study objectives
Primary objective: To determine the sensitivity of the 13C-UBT test using the new test meal for H. pylori in patients with dyspepsia taking PPIs with a one day break in medication.
Secondary objectives: To determine the specificity of the 13C-UBT using the new test meal for H. pylori in patients with dyspepsia taking PPIs with a one day break in medication and to determine the safety and tolerance of the new test meal.
Inclusion criteria and study protocol
This was an observer-blind, multicentre study (one in Slovenia and two in Germany) in which consecutive dyspeptic H. pylori positive or negative patients were included. The inclusion criteria were as follows: male and female patients of at least 18 year of age; all acid-related disorders requiring long-term PPI treatment, including functional dyspepsia, according to the Rome II classification; and positive or negative standard 13C-UBT at screening. Diagnosis of H. pylori infection was confirmed or excluded by a combination of culture, histology and the rapid urease test (RUT; PyloriTek®, Serim Research Corp., Elkhart, United States) on samples obtained by endoscopy. “True positive patients” were patients with a positive culture or when at least two of the following tests were positive: UBT, histology, or rapid urease test (RUT). “True negative patients” were patients with at least two negative tests and a negative culture. True negative patients were also those with non-evaluable cultures and negative histology and urease test. Patients with negative UBT underwent upper endoscopy only if this was deemed necessary by the investigator for medical reasons. This study was conducted in outpatients.
Two biopsy samples were obtained from the antrum and corpus for histology. One biopsy sample for RUT was taken from the angular fold, and two samples from the antrum were taken for culture.
The biopsies for histology were stained with haematoxylin and eosin and Giemsa stains, and gastritis was scored using the Updated Sydney System. All biopsy samples were analysed at each respective medical centre.
Gastric biopsies for culture were collected and transported in Portagerm pylori (bioMerieiux, France) transport medium. After homogenization in 1 mL PBS, 0.1 mL aliquots were inoculated for gram stain and culture. Two selective and one non-selective media were used. Plates were incubated at 37 °C in a microaerophilic atmosphere for 9 d and inspected for growth every 72 h. An enriched atmosphere was created using Anoxomat (Mart Microbiology). Typical colonies were identified with a typical gram stain and positive urease, catalase and oxidase reactions.
Starting on Day 1, H. pylori positive and negative patients in both study arms took Nexium capsules (40 mg) orally once daily 30 min before breakfast. They were instructed not to take antibiotics, bismuth compounds, H2 receptor antagonists or other acid-suppressive agents during the treatment period. All other concomitant medications were recorded in the case report form with the name of the drug, active ingredient(s), strength of active ingredient(s), indication, single dose, daily dose, dosage interval, route of administration, and the times of initiation and discontinuation.
Patients returned to the hospital/medical practice for breath tests on day 30. Nexium capsules were discontinued after day 29. The patients were requested to return unused PPI medication on day 30. Treatment compliance was assessed by calculating the difference in the number of tablets issued and returned.
The 13C-UBT was performed in H. pylori positive and negative patients using the new test meal Refex on day 30. A delta value ≥ 2.5‰ was set as a positive result. The test started with a breath sample taken at baseline. Thereafter, the patient had to ingest the new test meal Refex dissolved in 200 mL tap water and 75 mg 13C-urea dissolved in 30 mL tap water. The new test meal had to be ingested and was followed by the 13C-urea solution. A second breath sample was taken 30 min after ingestion of the test meal. Breath samples were collected in pre-labelled test-tubes. The breath samples were sent in the original outer packaging to the laboratory in Germany.
We could not compare the new test meal Reflex with the classic meal with 2.0 g of citric acid, because according the UBT protocol the second test should be performed earliest one day later in order to avoid a false positive result. This means second UBT meal can be performed earliest after two days break of PPI treatment instead one day what will implement great bias in the study.
Patients were followed-up for 14 d after discontinuation of PPI treatment. At the end of the study, positive patients were offered eradication therapy according to the current European guidelines. H. pylori negative patients were treated according to national dyspepsia guidelines.
Exclusion criteria
Patients were not included in the study if they have previously been treated for their H pylori infection, it they have used PPI, H2 receptor antagonists, NSAIDs, antibiotics, antisecretory drugs, bismuth compounds, or sucralfate in the 4 wk prior to enrolment, if they had manifest coagulopathy or any other disorder according to which endoscopy and/or biopsies are contraindicated, if they have participated in a clinical trial with another not approved drug within 30 d before entering the study and in case of pregnancy
Sample size calculations
A total of 114 patients were screened in 3 active study centres. Analyses of the sensitivity and specificity of the modified UBT for H. pylori infection with new test meal were performed for exploratory purposes. Sample size calculations were based on previous experience with the modified UBT for H. pylori infection[14]. This experience showed a sensitivity of at least 90% after 29 d of PPI medication. Although rare cases of false positive breath tests may occur in H. pylori negative patients, if other urea active bacteria than H. pylori such as Proteus mirabilis or Staphylococcus aureus colonize gastric lumen in patients with extensive atrophy or intestinal metaplasia[15]. However, specificity of 90% was still assumed. With a sample size of 43, a two-sided 95%CI for a single proportion using the large sample normal approximation would extend 9 percentage-points from the observed proportion for an expected proportion of 90% (width of the 95%CI of 18%). With a sample size of n = 43 H. pylori positive patients and n = 43 actual H. pylori negative patients, sufficient precision for assessing sensitivity and specificity was expected. The actual sample sizes chosen to be used in the study were slightly larger [H. pylori positive: 63 in intention-to-treat (ITT) population, 53 in the PP population; H. pylori negative: 51 in ITT population, 49 in PP population]. Concerning the primary variable of sensitivity, the asymptotic confidence intervals were quite close to the exact Clopper-Pearson intervals (ITT: asymptotic 95%CI: 64.39%-85.61%, exact 95%CI: 62.60%-84.98%; PP: asymptotic 95%CI: 85.38%-99.56%, exact 95%CI: 81.79%-97.91%). Concerning the secondary variable of specificity, the asymptotic confidence intervals deviate a bit more from the exact Clopper-Pearson intervals (ITT: asymptotic 95%CI: 94.12%-100%, exact 95%CI: 89.35%-99.95%; PP: asymptotic 95%CI: 94.00%-100%, exact 95%CI: 89.15%-99.95%) due to the proximity to 100% of the estimated specificity.
Ethics
The study was carried out in accordance with national laws and regulations, the ICH Guideline E6: Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95), and with the Declaration of Helsinki, revised version, 48th WMA General Assembly, Somerset West, October 1996. Permission of the national regulatory authority was a prerequisite for initiation of the study.
Each patient was supplied with full and adequate verbal and written information on the objectives and procedures of the study as well as potential benefits, discomforts and risks involved prior to inclusion in the study.
Statistical analysis
The sensitivity and specificity of the UBT with the new test meal on day 30 was assessed using relative frequencies and 95%CIs (two-sided). Descriptive statistical methods were applied.
RESULTS
One hundred and fourteen patients were initially included in three centres. Twelve patients were excluded for not fulfilling the inclusion criteria (7 took Nexium 40 mg on day 30, three patients took antibiotics during the study period and two patients did not return on day 30). Altogether, 102 patients were eligible for PP analysis (Figure 1). Demographic data are presented in Table 1. The results of the diagnostic tests are presented in Table 2.
Figure 1.
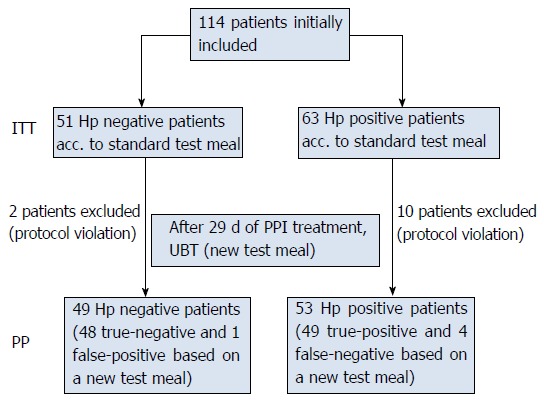
Flow diagram. The patient population and results of Helicobacter pylori diagnosis (based on study criteria and UBT with standard test meal and new test meal after 29 d of PPI treatment). UBT: Urea breath test; ITT: Intention-to-treat; PPI: Proton pump inhibitors; Hp: Helicobacter pylori.
Table 1.
Demographic data n (%)
| Demographic variable | n = 114 |
| Age (yr), mean ± SD | 51.07 ± 14.4 |
| Height (cm), mean ± SD | 168.90 ± 9.3 |
| Weight (kg), mean ± SD | 73.43 ± 14.9 |
| BMI (kg/m²), mean ± SD | 25.66 ± 4.3 |
| Ethnic group | |
| Caucasian | 114 (100.0) |
| Other | 0 (0.0) |
| Gender | |
| Female | 76 (66.7) |
| Male | 38 (33.3) |
Table 2.
Diagnostic investigation for Helicobacter pylori (n = 114)
| Hp diagnostic test | n (%) |
| UBT with standard test meal | |
| Positive | 63 (55.3) |
| Negative | 51 (44.7) |
| Upper endoscopy | |
| Yes | 110 (96.5) |
| No | 4 (3.5) |
| Culture | |
| Positive | 60 (54.5) |
| Negative | 36 (32.7) |
| Not evaluated | 14 (12.7) |
| Histology | |
| Positive | 53 (48.2) |
| Negative | 47 (42.7) |
| Not evaluated | 10 (9.1) |
| Rapid urease test | |
| Positive | 63 (57.3) |
| Negative | 47 (42.7) |
Hp: Helicobacter pylori.
The primary variable in this study was the sensitivity of the 13C-UBT test using the new test meal for H. pylori in patients with dyspepsia taking PPI with a one day break in medication. The sensitivity of the 13C-UBT test was assessed using relative frequency and 95%CI (two-sided).
In our study, the cut-offs were set at 3.0‰, 2.5‰ and 2.0‰. The best sensitivity and specificity were achieved by using cut-off points of 2.5‰ and 2.0‰ combined with a break in PPI intake of one day before performing the UBT (Table 3).
Table 3.
Thirty days of proton pump inhibitors medication, different cut-off points 2‰, 2.5‰, 3‰, sampling time 30 min
| Cut-off | Sensitivity | Specificity | PPV | NPV | Accuracy |
| 2‰ | 92.45% | 97.96% | 98.00% | 92.31% | 95.10% |
| 2.5‰ | 92.45% | 97.96% | 98.00% | 92.31% | 95.10% |
| 3‰ | 86.79% | 97.96% | 97.87% | 87.27% | 92.16% |
The sensitivity of the 13C-UBT test was assessed using relative frequency and 95%CIs (two-sided). The sensitivity of the 13C-UBT was found to be 92.45% (95%CI: 81.79%-97.91%) for the PP population (Table 4).
Table 4.
Sensitivity of the 13C-urea breath test using the new test meal for Helicobacter pylori n (%)
| Population | Result of UBT with new test meal |
Diagnosis of Hp infection |
Sensitivity | 95%CI | |
| Positive | Negative | ||||
| ITT (n = 114) | Positive | 50 (78.1) | 1 (2.0) | 78.13% | 66.03%-87.49% |
| Negative | 13 (20.3) | 48 (96.0) | |||
| Not performed | 1 (1.6) | 1 (2.0) | |||
| PP (n = 102) | Positive | 49 (92.5) | 1 (2.0) | 92.45% | 81.79%-97.91% |
| Negative | 4 (7.5) | 48 (98.0) | |||
UBT: Urea breath test; ITT: Intention-to-treat; PP: Per-protocol; Hp: Helicobacter pylori.
In the PP population, in patients with a positive H. pylori infection, 92.5% also had positive 13C-UBT results, and 7.5% showed (false) negative results. In patients negative for H. pylori infection, 98.0% of the patients had negative 13C-UBT results, and 2.0% showed (false) positive results.
As a secondary variable, the specificity was analysed for the 13C-UBT test using the new test meal for H. pylori in patients with dyspepsia and taking PPI with a one day break in medication. The specificity was found to be 97.96% for the PP population (95%CI: 89.15%-99.95%)(Table 5).
Table 5.
Specificity of the 13C-urea breath test using the new test meal for Helicobacter pylori n (%)
| Population | Result of UBT with new test meal |
Diagnosis of Hp infection |
Specificity | 95%CI | |
| Positive | Negative | ||||
| ITT (n = 114) | Positive | 50 (78.1) | 1 (2.0) | 96.00% | 86.29%-99.51% |
| Negative | 13 (20.3) | 48 (96.0) | |||
| Not performed | 1 (1.6) | 1 (2.0) | |||
| PP (n = 102) | Positive | 49 (92.5) | 1 (2.0) | 97.96% | 89.15%-99.95% |
| Negative | 4 (7.5) | 48 (98.0) | |||
UBT: Urea breath test; ITT: Intention-to-treat; PP: Per-protocol; Hp: Helicobacter pylori.
The analysis of ROC curves considers in principle all cut-offs in order to identify a value with high efficiency. The chosen cut point of 2.5‰ based on the data leads to an excellent sensitivity and specificity. A well the excellent overall performance of the new UBT is substantiated by the ROC curve with a maximum Youden value of 0.90412, corresponding to a measured ∆δ-value of 2.588% (Figure 2).
Figure 2.

Empirical receiver operating characteristic curve by using cut-off values of 2.0‰, 2.5‰ and 3.0‰ (Per-protocol population, n = 102).
However, a value that is based on only one study with a limited possible number of cases may lead to slightly higher sensitivity and specificity. Our simulation study for different sample sizes and up to 1000 repeats of the diagnostic test provided an expected average sensitivity of 90%, which is only 2.5% lower than the observed sensitivity of 92.5% in our study. The expected average specificity is 99%, and therefore, higher than the observed value (97.96%). In all simulations, the study shows an expected maximum bias potential of 2.5%.
The accuracy of the method can be derived from 95% confidence limits based on the simulation study. These limits are defined by the 2.5% and 97.5% percentiles of the calculated distributions of the sensitivity and specificity. When reviewing 1000 repeats and a simulated sample size of 400, the limits were 86.8% to 92.5% (sensitivity) and 96.8% to 100% (specificity).
Following adverse events (AEs) are observed: Overall 8 patients (7.0%) experienced AEs, and all AEs were assessed and considered to be related to PPI medication. No serious adverse events, AEs leading to permanent discontinuation of the study medication, or fatal AEs were documented during this study. Mild AEs were reported for 3 patients (2.6%) and moderate AEs for 5 patients (4.4%) related to PPI medication.
DISCUSSION
13C-UBT is the non-invasive method of choice for the detection of H. pylori infection in a test and treat strategy as well as for the assessment of the success of H. pylori eradication[1].
However, the sensitivity of the test decreases to unacceptable levels if patients are on PPI treatment[1,2]. Currently available breath and stool tests are recommended to be performed 14 d after discontinuation of the PPI[16]. This delay results in additional costs and the inconvenience of an additional visit. Dyspeptic symptoms may occur in patients due to acid rebound after the withdrawal of PPI therapy. In this study, we report on the diagnostic performance of a novel 13C-UBT based on a special mixture of acid components in a test meal.
PPIs have a direct antibacterial effect on H. pylori and have been reported to inhibit H. pylori urease activity[17-19]. False negative results, therefore, are a limitation in the use of the standard UBT in patients on PPIs. Up to 40% of individuals taking PPIs had a false negative test result[5,6,19-22]. While 5 d on a PPI had no significant effect on H. pylori and the UBT, one-third of volunteers had a negative UBT after being on PPI therapy for 7 d (omeprazole 20 mg )[12]. The UBT was positive again in all but one patient 4 d after stopping the PPI and in all patients 14 d after stopping the PPI. In all these studies, 2 g of citric acid was used as the test meal for UBT. Patients with a negative 13C-UBT were also negative for H. pylori in antrum biopsies and had reduced H. pylori scores in corpus biopsies[23].
The role of citric acid in the UBT test meal is to acidify the gastric contents and retard gastric emptying[11,12,24-27]. The effect of citric acid on enhancing intragastric urease activity is dose dependent for doses between 1 and 4 g in 200 mL of water[28]. Gastric pH, therefore, plays a major role in H. pylori urease activity.
In a study measuring gastric juice pH in 109 patients on chronic PPI therapy, 74% of the patients presented with gastric hypochlorhydria (pH > 4), and 26% of patients with presented with a pH ≤ 4[28]. False-negative RUT results were prevalent in patients with a pH > 4, whereas with a gastric pH of 2-4 (due to inadequate PPI effect), RUT results were positive[29].
The proton-gated inner membrane urea channel, H. pylori Urel, is essential for the survival of the H. pylori bacteria in the acidic environment of the stomach (pH < 2)[30]. This channel is closed at neutral pH and opens at low pH, allowing urea access to urease. H. pylori urease forms NH3 and CO2, which neutralize incoming protons and thus buffer the periplasmatic space to pH approximately 6, even in gastric juice at a pH < 2.0[7,31-35].
To compensate for the unfavourable gastric pH due to PPI therapy, the concentration of citric acid for UBT has been raised maximally to 4.2 g in 200 mL water[28]. This concentration of citric acid is poorly tolerated and induces symptoms. To avoid inconvenience for patients and to overcome the negative impact of PPIs on H. pylori urease, we used a highly concentrated mixture of organic acids (5.5 g in 200 mL water: tartaric acid, malic acid and citric acid) to reduce patient complaints[14]. Agha et al[13] showed that an enhancement in urease activity can similarly be obtained for citric and malic acid.
H. pylori urease is a nickel-containing enzyme[36], and preliminary data have suggested that changes in intracellular H. pylori nickel levels may influence urease activity[37]. H. pylori urease and the membrane-bound hydrogenase enzyme are both H. pylori metalloenzymes, which are nickel-dependent. Moreover, the nickel transporter NixA and accessory proteins such as HypA and HypB serve to increase intracellular H. pylori nickel levels and enhance urease activity[37].
Citric acid, tartaric acid and malic acid are organic acids that bind many trace metals, including nickel, and they can increase H. pylori urease activity both by lowering pH as well as by providing nickel to H. pylori.
The new test meal, Refex, has a unique 5.5 g powder mixture of three organic acids: citric, malic and tartaric acid dissolved in 200 mL water (pH 1.8). This highly concentrated organic acid mixture increases the acidity of the stomach for a short period of time and permits an increase in the bacterial urease activity to the point that urease activity becomes detectable in patients on PPIs.
We could not compare the new test meal Reflex with the classic meal with 2.0 g of citric acid, because according the UBT protocol the second test should be performed earliest one day later in order to avoid a false positive result. This means second UBT meal can be performed earliest after two days break of PPI treatment instead one day what will implement great bias in the study.
For optimizing the sensitivity of the UBT on Refex, we adjusted the cut-off point. We analysed the cut-off point of 4‰ for the standard test meal and for the new test meal (Refex), but we also investigated cut-off points of 3.0‰, 2.5‰ and 2.0‰. The best sensitivity and specificity were achieved with cut-off points at 2.5‰ and 2.0‰. With these modifications, we were able to reach a sensitivity of 92.5% (95%CI: 81.79%-97.91%) and specificity of 97.96% (95%CI: 89.15%-99.95%) for the PP population.
The UBT test using the new test meal Refex was well tolerated, with 7.2% of patients reporting dyspeptic effects during test meal intake. No severe side effects were noted.
With good patient compliance (PP population), we were able to demonstrate that the new UBT Refex can be reliable enough to be used in everyday clinical practice in patients who cannot stop their PPI therapy for more than one day.
ACKNOWLEDGMENTS
The authors thank Dr. Jörg Schnitker for his support with the statistical analysis.
COMMENTS
Background
The urea breath test (UBT) is recommended as the test of choice for determining the success of eradication treatment. The UBT is highly sensitive and specific, except in patients taking proton pump inhibitors (PPIs) where e UBT be false negatives in 10%-40% of patients. Current guidelines recommend stopping these medications for 14 d before the UBT or stool test.
Research frontiers
PPIs have a direct antibacterial effect on Helicobacter pylori (H. pylori) and can inhibit H. pylori urease activity. The H. pylori colonisation of the stomach can be also be reduced, especially in antrum. Citric acid test meal is used to acidify the gastric contents and retard gastric emptying. The effect of citric acid on enhancing intragastric urease activity is dose dependent for doses between 1 and 4 g in 200 mL of water. High concentration of citric acid is poorly tolerated and induces symptoms.
Innovations and breakthroughs
A highly concentrated mixture of organic acids (5.5 g in 200 mL water: tartaric acid, malic acid and citric acid; pH 1.8) was used to reduce patient complaints and increase the accuracy of the UBT. This mixture can increase the H. pylori urease activity by the influence of low pH on the Urel chanel, or by providing additional nickel to H. pylori. With the adjustment of the cut-off point to 2.5‰ sensitivity of UBT wit new test meal can be improved.
Applications
The new UBT test meal can be used as the H. pylori diagnostic test in patients on PPI who can not stop their PPI therapy for two weeks or more. Recommendations for the use of UBT after antimicrobial therapy should not be changed.
Peer-review
This work is good and it will help us in further clinical work in the detection of H. pylori positive and negative dyspepsia patients on proton pump inhibitors.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Slovenia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the institutional review boards of AM DC Rogaška, Slovenia, Universitätsklinikum Magdeburg Magdeburg, Germany, Diakonie Klinikum, Siegen, Germany.
Clinical trial registration statement: The study was registered under EudraCT Number: 2008-008010-39.
Informed consent statement: All study participants, or their legal guardian, provided written consent prior to study enrollment.
Conflict-of-interest statement: The authors of this manuscript having no conflicts of interest to disclose.
Data sharing statement: There is no additional data available.
Peer-review started: January 27, 2017
First decision: March 16, 2017
Article in press: June 1, 2017
P- Reviewer: Marusic M, Yamaoka Y S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Bojan Tepeš, AM DC Rogaška, Prvomajska 29 A, 3250 Rogaška Slatina, Slovenia. bojan.tepes@siol.net.
Peter Malfertheiner, Universitätsklinikum Magdeburg A. ö. R Klinik für Gastroenterologie, Hepatologie und Infektiologie, 39120 Magdeburg, Germany.
Joachim Labenz, Department of Internal Medicine and Gastroenterology, Diakonie Klinikum, Jung-Stilling Hospital, 57074 Siegen, Germany.
Sitke Aygen, Institut für Biomedizinische Analytik und NMR-Imaging GmbH (INFAI), Gottfried-Hagen-Str. 6062, 51105 Köln, Germany.
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 3.American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 1998;114:579–581. doi: 10.1016/s0016-5085(98)70541-4. [DOI] [PubMed] [Google Scholar]
- 4.Gatta L, Vakil N, Ricci C, Osborn JF, Tampieri A, Perna F, Miglioli M, Vaira D. Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol. 2004;99:823–829. doi: 10.1111/j.1572-0241.2004.30162.x. [DOI] [PubMed] [Google Scholar]
- 5.Levine A, Shevah O, Shabat-Sehayek V, Aeed H, Boaz M, Moss SF, Niv Y, Avni Y, Shirin H. Masking of 13C urea breath test by proton pump inhibitors is dependent on type of medication: comparison between omeprazole, pantoprazole, lansoprazole and esomeprazole. Aliment Pharmacol Ther. 2004;20:117–122. doi: 10.1111/j.1365-2036.2004.02021.x. [DOI] [PubMed] [Google Scholar]
- 6.Oztürk E, Yeşilova Z, Ilgan S, Ozgüven M, Dağalp K. Performance of acidified 14C-urea capsule breath test during pantoprazole and ranitidine treatment. J Gastroenterol Hepatol. 2009;24:1248–1251. doi: 10.1111/j.1440-1746.2009.05845.x. [DOI] [PubMed] [Google Scholar]
- 7.Shirin H, Frenkel D, Shevah O, Levine A, Bruck R, Moss SF, Niv Y, Avni Y. Effect of proton pump inhibitors on the continuous real time (13)C-urea breath test. Am J Gastroenterol. 2003;98:46–50. doi: 10.1111/j.1572-0241.2003.07187.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Vakil N. Review article: Helicobacter pylori and reflux disease. Aliment Pharmacol Ther. 2003;17:297–305. doi: 10.1046/j.1365-2036.2003.01428.x. [DOI] [PubMed] [Google Scholar]
- 9.Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013;114:491–497. doi: 10.1002/jcb.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chey WD, Chathadi KV, Montague J, Ahmed F, Murthy U. Intragastric acidification reduces the occurrence of false-negative urea breath test results in patients taking a proton pump inhibitor. Am J Gastroenterol. 2001;96:1028–1032. doi: 10.1111/j.1572-0241.2001.03687.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Opekun AR, Hammoud F, Yamaoka Y, Reddy R, Osato MS, El-Zimaity HM. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 2003;98:1005–1009. doi: 10.1111/j.1572-0241.2003.07426.x. [DOI] [PubMed] [Google Scholar]
- 13.Agha A, Opekun AR, Abudayyeh S, Graham DY. Effect of different organic acids (citric, malic and ascorbic) on intragastric urease activity. Aliment Pharmacol Ther. 2005;21:1145–1148. doi: 10.1111/j.1365-2036.2005.02440.x. [DOI] [PubMed] [Google Scholar]
- 14.Aygen S, inventor A method for the diagnosis of Helicobacter pylori infection and diagnostic kit for performing the method. European Patent EP1685851B1 13.05.2009
- 15.Osaki T, Mabe K, Hanawa T, Kamiya S. Urease-positive bacteria in the stomach induce a false-positive reaction in a urea breath test for diagnosis of Helicobacter pylori infection. J Med Microbiol. 2008;57:814–819. doi: 10.1099/jmm.0.47768-0. [DOI] [PubMed] [Google Scholar]
- 16.Shiotani A, Graham DY. Helicobacter pylori: New Thoughts and Practices. Gastroenterol Clin North Am. 2015;44:15–16. doi: 10.1016/j.gtc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Nagata K, Takagi E, Tsuda M, Nakazawa T, Satoh H, Nakao M, Okamura H, Tamura T. Inhibitory action of lansoprazole and its analogs against Helicobacter pylori: inhibition of growth is not related to inhibition of urease. Antimicrob Agents Chemother. 1995;39:567–570. doi: 10.1128/aac.39.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirshahi F, Fowler G, Patel A, Shaw G. Omeprazole may exert both a bacteriostatic and a bacteriocidal effect on the growth of Helicobacter pylori (NCTC 11637) in vitro by inhibiting bacterial urease activity. J Clin Pathol. 1998;51:220–224. doi: 10.1136/jcp.51.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoschus B, Domínguez-Muñoz JE, Kalhori N, Sauerbruch T, Malfertheiner P. Effect of omeprazole on Helicobacter pylori urease activity in vivo. Eur J Gastroenterol Hepatol. 1996;8:811–813. [PubMed] [Google Scholar]
- 20.Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36:12–16. doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravo LE, Realpe JL, Campo C, Mera R, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroenterol. 1999;94:2380–2383. doi: 10.1111/j.1572-0241.1999.01361.x. [DOI] [PubMed] [Google Scholar]
- 22.Chey WD, Spybrook M, Carpenter S, Nostrant TT, Elta GH, Scheiman JM. Prolonged effect of omeprazole on the 14C-urea breath test. Am J Gastroenterol. 1996;91:89–92. [PubMed] [Google Scholar]
- 23.Malfertheiner P. Diagnostic methods for H. pylori infection: Choices, opportunities and pitfalls. United European Gastroenterol J. 2015;3:429–431. doi: 10.1177/2050640615600968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt JN, KNOX MT. The regulation of gastric emptying of meals containing citric acid and salts of citric acid. J Physiol. 1962;163:34–45. doi: 10.1113/jphysiol.1962.sp006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiotani A, Saeed A, Yamaoka Y, Osato MS, Klein PD, Graham DY. Citric acid-enhanced Helicobacter pylori urease activity in vivo is unrelated to gastric emptying. Aliment Pharmacol Ther. 2001;15:1763–1767. doi: 10.1046/j.1365-2036.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JN, Knox MT. The slowing of gastric emptying by four strong acids and three weak acids. J Physiol. 1972;222:187–208. doi: 10.1113/jphysiol.1972.sp009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt JN, Knox MT. The slowing of gastric emptying by nine acids. J Physiol. 1969;201:161–179. doi: 10.1113/jphysiol.1969.sp008749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham DY, Runke D, Anderson SY, Malaty HM, Klein PD. Citric acid as the test meal for the 13C-urea breath test. Am J Gastroenterol. 1999;94:1214–1217. doi: 10.1111/j.1572-0241.1999.01069.x. [DOI] [PubMed] [Google Scholar]
- 29.Cayado-Lopez R, Bornschein J, Zeki S, Udarbe M, Di Pietro M. PTU-178 clinical utility of endofaster® In patients on chronic PPI therapy undergoing upper GI endoscopy. Gut. 2014;63:A117. [Google Scholar]
- 30.Strugatsky D, McNulty R, Munson K, Chen CK, Soltis SM, Sachs G, Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2013;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485. doi: 10.1126/science.287.5452.482. [DOI] [PubMed] [Google Scholar]
- 32.Pantoflickova D, Scott DR, Sachs G, Dorta G, Blum AL. 13C urea breath test (UBT) in the diagnosis of Helicobacter pylori: why does it work better with acid test meals? Gut. 2003;52:933–937. doi: 10.1136/gut.52.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott DR, Marcus EA, Wen Y, Singh S, Feng J, Sachs G. Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori. J Bacteriol. 2010;192:94–103. doi: 10.1128/JB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachs G, Kraut JA, Wen Y, Feng J, Scott DR. Urea transport in bacteria: acid acclimation by gastric Helicobacter spp. J Membr Biol. 2006;212:71–82. doi: 10.1007/s00232-006-0867-7. [DOI] [PubMed] [Google Scholar]
- 35.Evans DJ Jr, Evans DG, Kirkpatrick SS, Graham DY. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991;10:15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- 36.Benoit S, Maier RJ. Dependence of Helicobacter pylori urease activity on the nickel-sequestering ability of the UreE accessory protein. J Bacteriol. 2003;185:4787–4795. doi: 10.1128/JB.185.16.4787-4795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musiani F, Ciurli S. Evolution of Macromolecular Docking Techniques: The Case Study of Nickel and Iron Metabolism in Pathogenic Bacteria. Molecules. 2015;20:14265–14292. doi: 10.3390/molecules200814265. [DOI] [PMC free article] [PubMed] [Google Scholar]


