We have found sex differences in the cerebrovascular response to the Valsalva maneuver and standing. Men have greater cerebral vasoconstriction (or women have greater cerebral vasodilation) during late phase II of the Valsalva maneuver, and the cerebrovascular resistance index increases in men, but not in women, during standing. Furthermore, our findings indicate that both the menstrual cycle and oral contraceptive use can influence cardiovascular function both at rest and during active standing.
Keywords: hemodynamics, end-tidal gases, center of balance, heart rate variability, cardiovagal baroreceptor sensitivity
Abstract
Women experience orthostatic intolerance more than men, and they experience faintness more in the early follicular [i.e., low-hormone (LH)] than luteal [i.e., high-hormone (HH)] phase of the menstrual cycle. Men (n = 13, 25.8 ± 1.8 yr old) and women in the LH (days 2–5; placebo) and HH (days 18–24; high dose) phases of the menstrual cycle with (OC; n = 14, 22.0 ± 0.8 yr old) or without (NOC; n = 12, 21.8 ± 0.5 yr old) oral contraceptive (OC) use underwent the Valsalva maneuver and a supine-sit-stand protocol. Blood pressure, normalized stroke volume [stroke volume index (SVi)], cardiac output index, heart rate, end-tidal CO2, and middle cerebral artery (MCA) blood flow velocity were measured. When subjected to the Valsalva maneuver, all women had a greater increase in diastolic and mean MCA blood flow velocity than men (P ≤ 0.065), with no significant effect of menstrual cycle phase or OC use. When subjected to the supine-sit-stand protocol, men had lower MCA blood flow velocity (P < 0.038) than all women, and SVi was higher in men than in the NOC group in all postures (P < 0.011) and in the OC group in the LH phase of the menstrual cycle during standing (P = 0.010). Only men experienced higher resistance index (P < 0.001) and pulsatility index (P < 0.001) with standing. The OC group had lower end-tidal CO2 (P = 0.002) than the NOC group (P = 0.030) and men (P ≤ 0.067). SVi (P = 0.004) and cardiac output index (P = 0.008) were higher in the OC than NOC group. A tendency toward a lower mean MCA blood flow velocity (P = 0.058) and higher SVi (P = 0.059) and pulsatility index (P = 0.058) was noted in the HH than LH phase. Mean arterial pressure was higher in the OC than NOC group in the LH phase (P = 0.049) and lower in the HH than LH phase (P = 0.014). Our results indicate that cycling estrogens/progestins can influence ventilatory, cardiovascular, and/or cerebrovascular physiology.
NEW & NOTEWORTHY We have found sex differences in the cerebrovascular response to the Valsalva maneuver and standing. Men have greater cerebral vasoconstriction (or women have greater cerebral vasodilation) during late phase II of the Valsalva maneuver, and the cerebrovascular resistance index increases in men, but not in women, during standing. Furthermore, our findings indicate that both the menstrual cycle phase and oral contraceptive use can influence cardiovascular function both at rest and during active standing.
women are known to have greater orthostatic hypotension (i.e., drop in blood pressure upon erect posture) (1, 11), as well as a greater propensity for postural orthostatic tachycardia syndrome (30, 31), than men. Although the exact mechanisms for the greater incidence of orthostatic hypotension in women are unclear, differently regulated cardiovascular responses to physiological stressors between men and women and throughout the menstrual cycle could play a role. Indeed, women have a greater increase in heart rate (HR) and a greater reduction of stroke volume (SV) during standing than age-matched men (17). Furthermore, a greater incidence of lightheadedness has been observed during menses (i.e., the early follicular phase of the menstrual cycle, when estrogen or progesterone levels are very low) than in the late follicular (high-estrogen) and luteal (high-estrogen and high-progesterone) phases in healthy women and in those with vasovagal syncope (35) or postural orthostatic tachycardia syndrome (38). Lower sympathetic nerve activity in the early follicular than luteal phase during orthostatic stress could be a contributing factor (19).
This evidence of a potential role for cycling female sex hormones in presyncope suggests that the role of oral contraceptives (OC) should also be considered. A recent national survey concerning contraceptive use in Canada found that 43.7% of sexually active women between 15 and 50 yr of age who were trying not to conceive were taking OC (6). This high rate of pharmaceutical use highlights the importance of investigating the effect of OC on the physiological responses of women. Data on the effects of OC on cardiovascular and autonomic responses to upright posture are limited. In their investigation of blood pressure, HR, and sympathetic nerve activity in response to lower-body negative pressure in women taking OC (9), Carter et al. found no effect of OC use between the high-hormone (HH, maximal-dose pill) and low-hormone (LH, placebo) phases. However, unlike standing, lower-body negative pressure does not activate skeletal muscle in the lower body, which influences total peripheral resistance (TPR) via vasodilation. SV index (SVi) and cardiac output (Q̇) index (Q̇i) were not reported in their study. The finding of Limberg et al. that increases in forearm vascular conductance due to isoproterenol (i.e., a β-adrenergic receptor agonist), acetylcholine, and nitroprusside were greater in OC users than non-OC users (28) implies that OC users could have a greater reduction of peripheral resistance during standing, thus reducing blood pressure and/or increasing Q̇.
Many investigations using orthostatic stress do not concurrently measure indexes of cerebrovascular resistance (CVR) or end-tidal CO2 (ETco2) (9, 10, 19, 20). Upright posture leads to a reduction of ETco2 (17, 40, 47), which, in turn, contributes to the reduction of brain blood flow. While recent evidence suggests that there is no difference in this reduction of ETco2 between men and women (17), women were only studied from day 8 to day 11 of the menstrual cycle, and all were taking cyclic types of OC (i.e., all were taking the 1st wk of OC pills consisting of estrogen analog and the lowest dose of progesterone). Cerebrovascular reactivity to CO2 has been shown to be greater in women than men (24), implying that, for each decrement in ETco2, women would have a greater drop in brain blood flow/higher CVR and, potentially, an increase in the incidence of presyncope or fainting during upright posture.
The current study has investigated the role of sex difference, menstrual cycle phase, and OC use on cardiovascular, respiratory, and cerebrovascular responses to the Valsalva maneuver or orthostatic stress. These variables have been concurrently measured in men and women previously (17), but not during investigation of the menstrual cycle and OC use. Because of the lower sympathetic activity previously observed in the early follicular than luteal phase during upright posture (19), we hypothesized that women will exhibit a smaller HR response, smaller increase in blood pressure, and smaller increase in brain blood flow velocity during late phase II of the Valsalva maneuver in the early follicular phase/placebo pill week (i.e., LH phase) than during the luteal/maximal-dose week (i.e., HH phase). Furthermore, because of smaller left ventricular dimensions in women (4), greater cerebrovascular reactivity to changes in ETco2 in women (24), and reduced vasodilatory capacity from low estrogen levels in the LH phase of the menstrual cycle (25), we also hypothesized that, in the upright posture, 1) women will exhibit lower SVi, lower Q̇i, and higher CVR than men and 2) women (OC and non-OC users) will have lower SVi, lower Q̇i, and higher CVR in the LH than HH phase. Lastly, because of previous observations of greater vasodilatory capacity in OC users (28), we hypothesized that OC users will have higher SVi, higher Q̇i, and lower CVR than non-OC users.
MATERIALS AND METHODS
Participant Description
Young healthy participants with no history of cardiovascular or respiratory disease were recruited for the study [13 men, 12 women not taking OC (NOC group), and 14 women taking OC (OC group); Table 1]. The types of OC include a placebo week, where pills contain no estrogen or progesterone analogs (menses occurs) and then, in the subsequent 3 wk, pills contain both estrogen and progesterone analogs. In noncyclic types of OC, the dose of estrogen/progesterone analogs is the same in every pill; in cyclic types of OC, the dose of estrogen analog is the same in every pill, but the dose of progesterone analog increases with each week. In the OC group, six women were taking Tri-Cyclen [0.18, 0.215, and 0.250 mg of noregestimate and 0.035 mg of ethinyl estradiol (EE) (cyclic)], one was taking Tri-Cyclen Lo [0.18, 0.215, and 0.250 mg of noregestimate and 0.025 mg of EE (cyclic)], five were taking Alesse [100 μg of levonorgestrel and 0.020 mg of EE (noncyclic)], one was taking Marvelon [0.15 mg of desogestrel and 0.03 mg of EE (noncyclic)], and one was taking Novo-Cyprotenone/EE [2 mg of cyproterone acetate and 0.035 mg of EE (noncyclic)]. Women were tested twice during the menstrual cycle at the same time of day. The LH phase was defined as days 2–5 in the NOC group and during the placebo week in the OC group. The HH phase was defined as days 18–24 in the NOC group and the last week of pills containing both EE and progesterone analog in the OC group. These phases were determined by self-report with guidance from the researchers. Participants were not fasted but were asked to refrain from eating fatty food, to abstain from caffeine and alcohol, and to refrain from physical exercise for ≥12 h before assessment. All participants gave written informed consent to participate in the study, which was approved by the Office of Research Ethics at York University.
Table 1.
Anthropometric data
| Women |
||||||
|---|---|---|---|---|---|---|
| NOC (n = 12) |
OC (n = 14) |
|||||
| Men (n = 13) | LH | HH | LH | HH | Significance | |
| Age, yr | 25.8 ± 1.8 | 21.8 ± 0.5 | 22.0 ± 0.7 | *Men vs. OC; Men vs. NOC (P = 0.07) | ||
| Height, cm | 176.7 ± 2.1 | 157.8 ± 1.6 | 164.0 ± 1.7 | *Sex; *OC vs. NOC | ||
| Weight, kg | 83.3 ± 2.9 | 61.0 ± 3.1 | 59.7 ± 3.3 | 58.9 ± 2.0 | 60.4 ± 1.9 | *Sex |
| BMI, kg/m2 | 26.6 ± 0.6 | 24.5 ± 1.0 | 24.1 ± 1.3 | 21.8 ± 0.4 | 22.3 ± 0.4 | *OC vs. NOC; *Men vs. OC |
Values are means ± SE. NOC, non-oral contraceptive users; OC, oral contraceptive users; LH and HH, low- and high-hormone phases of the menstrual cycle; BMI, body mass index.
P < 0.05.
Hemodynamic Measurements
HR was determined using the R-R interval of a standard electrocardiogram. Beat-to-beat blood pressure and Q̇ were determined using a noninvasive blood pressure device (Finometer Pro, Finapres Medical Systems, Amsterdam, The Netherlands). Resting blood pressure was calibrated with a standard manual blood pressure measurement. SV was calculated as Q̇ ÷ HR. SV and Q̇ were normalized to body surface area. TPR index (TPRi) was calculated as mean arterial pressure (MAP) ÷ Q̇i. Cerebral perfusion pressure (CPP) was calculated as MAP – 0.7355 mmHg/cmH2O × distance from the transcranial Doppler probe to the heart during upright posture.
Transcranial Doppler
A 2-MHz ultrasound probe (Multigon Industries, Yonkers, NY) was positioned on the left temple with an adjustable headband and used to measure blood flow velocity of the middle cerebral artery (MCA). The CVR index (CVRi) was calculated as CPP ÷ mean MCA blood flow velocity. Resistance index (RI) was calculated as RI = (MCAsystolic – MCAdiastolic)/MCAsystolic. Pulsatility index (PI) was calculated as PI = (MCAsystolic – MCAdiastolic)/MCAmean.
End-Tidal Gases
Participants were fitted with a nasal cannula to continuously sample end-tidal gases (O2 and CO2) for analysis via infrared spectroscopy (VacuMed, Ventura, CA). Respiratory rate was determined from the rate of CO2 peaks.
HR Variability and Cardiovagal Baroreceptor Sensitivity
HR variability (HRV), an indicator of the autonomic control of HR (39), was determined using 5 min of data in each of the supine, sitting, and upright postures. HRV (time domain and spectral analysis) was analyzed using the HRV module of LabChart Pro 8.0 software (ADInstruments, Colorado Springs, CO).
Cardiovagal baroreceptor sensitivity was determined (lag 0 data) using the sequence method on 5 min of uninterrupted beat-by-beat blood pressure and ECG recordings in the supine, seated, and upright postures (3, 5).
Stress Questionnaires
To control for effects of psychological stress on the physiological responses during repeat visits, female participants were asked to complete the Cohen perceived stress scale questionnaire (13) at each visit. There were no effects of OC (P = 0.26) or menstrual cycle phase (P = 0.65) on the perceived stress scale; scores were 18.6 ± 1.7 for NOC-LH, 17.3 ± 1.4 for NOC-HH, 16.3 ± 1.8 for OC-LH, and 16.7 ± 1.9 for OC-HH (a maximum score is 40). These results indicate that there was no confounding effect of psychological stress between visits in the women.
Wii Balance Board
For the stand portion of the supine-sit-stand test, participants stood on a Wii balance board to obtain their lateral and medial movement. The maximal range of movement in the lateral (i.e., side-to-side) and medial (i.e., front-to-back) directions for the last 30 s of the stand test was determined using LabChart software.
Experimental Protocol
Valsalva.
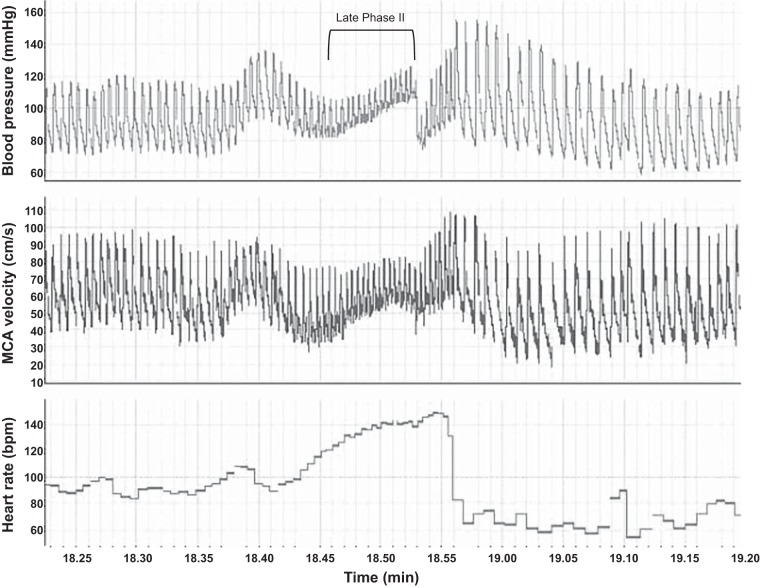
In the supine position, participants exhaled into a tube attached to a pressure gauge and maintained a pressure of 40 mmHg for 15 s (visual feedback of exhalation pressure was provided). The Valsalva HR ratio was calculated as maximal HR during Valsalva ÷ lowest HR within 30 s of the end of exhalation. Late phase II of the Valsalva maneuver is defined as the blood pressure increase in the latter half of exhalation (Fig. 1).
Fig. 1.
Raw blood pressure, middle cerebral artery (MCA) blood flow velocity, and heart rate data collected during the Valsalva maneuver in a female participant in the high-hormone phase of the menstrual cycle.
Supine-sit-stand.
Measurements were obtained for 5 min of supine rest. Participants then moved to the seated position for 5 min. If needed, assistance was provided by a researcher. Lastly, participants stood on a Wii balance board for 10 min. The test was terminated early if systolic pressure fell below 70 mmHg or if the participant experienced dizziness, nausea, or light-headedness. The test was terminated early for two men, and data for the standing position were averaged for the 1 min before termination.
Data and Statistical Analysis
All signals were collected using PowerLab data acquisition and LabChart software (ADInstruments). During the supine-sit-stand protocol, 1-min averages were obtained at the end of each posture for presentation.
Valsalva maneuver, balance, and MCA blood flow velocity slopes.
Two-way repeated-measures ANOVAs were used to compare responses between OC and NOC groups (OC use and menstrual cycle phase as repeated measures). One-way ANOVAs were used to compare responses between men and each of the OC-LH, OC-HH, NOC-LH, and NOC-HH groups (sex as nonrepeated measure).
Supine-sit-stand protocol.
Three-way repeated-measures ANOVAs were used to compare responses between OC and NOC groups (OC use, menstrual cycle phase, and posture as repeated measures). Two-way repeated-measures ANOVAs were used to compare responses between men and each of the OC-LH, OC-HH, NOC-LH, and NOC HH groups (sex as nonrepeated measure and posture as repeated measure).
Where main or interaction effects were significant, Holm-Sidak post hoc tests were used. All statistical tests were performed with Sigmaplot 13.0 (Systat Software, San Jose, CA). Significance was set at P ≤ 0.05, and trends are noted when P < 0.07. Values are means ± SE.
RESULTS
Cardiovascular Responses to Valsalva
There were no significant effects of OC use, menstrual cycle phase, or sex difference on the increase in diastolic pressure, systolic pressure, and MAP during late phase II of the Valsalva maneuver (Table 2), nor were there any significant effects of OC use, menstrual cycle phase, or sex difference on the Valsalva HR ratio (Table 2).
Table 2.
Blood pressure responses to late phase II of the Valsalva maneuver
| Women |
||||||
|---|---|---|---|---|---|---|
| NOC |
OC |
|||||
| Men | LH | HH | LH | HH | Significance | |
| Change in DBP, mmHg | 19.6 ± 4.0 | 18.4 ± 3.2 | 20.2 ± 4.2 | 20.2 ± 4.0 | 25.0 ± 4.2 | Phase (P = 0.061) |
| Change in SBP, mmHg | 21.9 ± 4.9 | 16.0 ± 4.6 | 21.1 ± 6.5 | 20.7 ± 5.4 | 22.8 ± 4.3 | Phase (P = 0.065) |
| Change in MAP, mmHg | 20.3 ± 4.2 | 17.6 ± 3.5 | 20.5 ± 4.9 | 20.4 ± 4.4 | 24.3 ± 4.0 | Phase (P = 0.051) |
| Valsalva HR ratio | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.1 | |
Values are means ± SE. DBP, diastolic blood pressure; SBP, systolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Cerebrovascular Responses to Valsalva
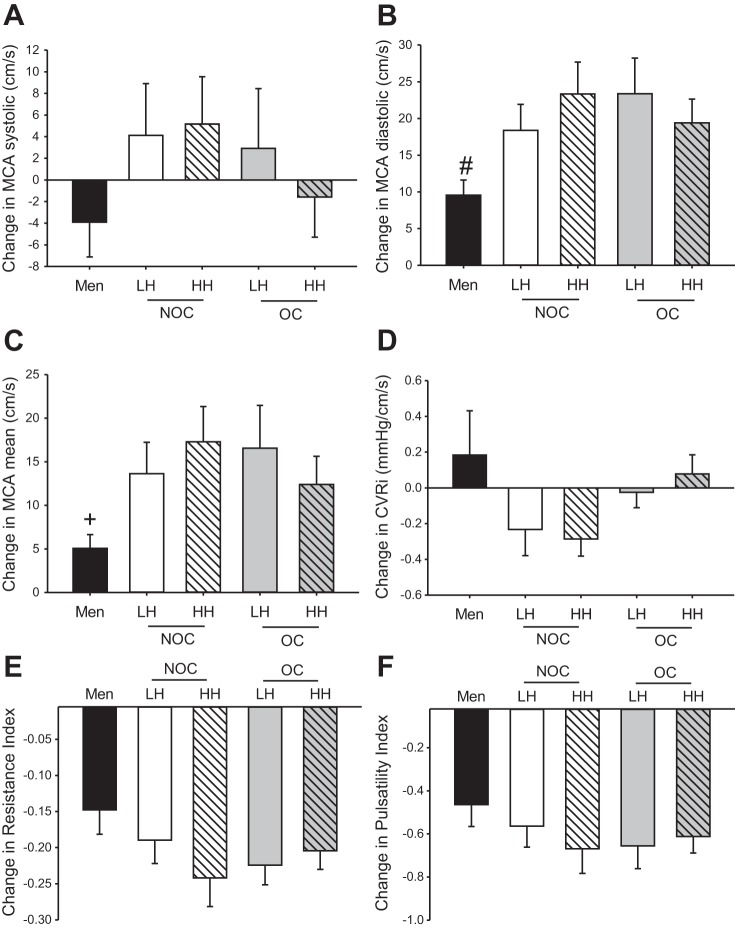
During late phase II of the Valsalva maneuver, men had a significantly smaller increase in diastolic MCA blood flow velocity than OC users (both phases) and the NOC-HH group (Fig. 2B) and a significantly smaller increase in mean MCA blood flow velocity than the NOC-HH group (Fig. 2C). There were no significant sex differences in CVRi (Fig. 2D), RI (Fig. 2E), or PI (Fig. 2F). There were no significant effects of OC use or menstrual cycle phase on cerebrovascular measurements during late phase II of the Valsalva maneuver (Fig. 2).
Fig. 2.
Change in systolic, diastolic, and mean MCA blood flow velocity (A–C), cerebrovascular resistance index (CVRi; D), and resistance and pulsatility indexes (E and F) in response to late phase II Valsalva maneuver. LH, low hormone; HH, high hormone; NOC, non-oral contraceptive (OC) user; OC, OC user. #Significant difference (P < 0.05) between men and OC-LH, OC-HH, and NOC-HH, with a near-significant difference (P < 0.06) between men and NOC-LH. +Significant difference (P < 0.05) between men and NOC-HH, with a near significant difference (P < 0.065) between men and OC-LH, OC-HH, and NOC-LH.
Cardiovascular Responses to Posture Change
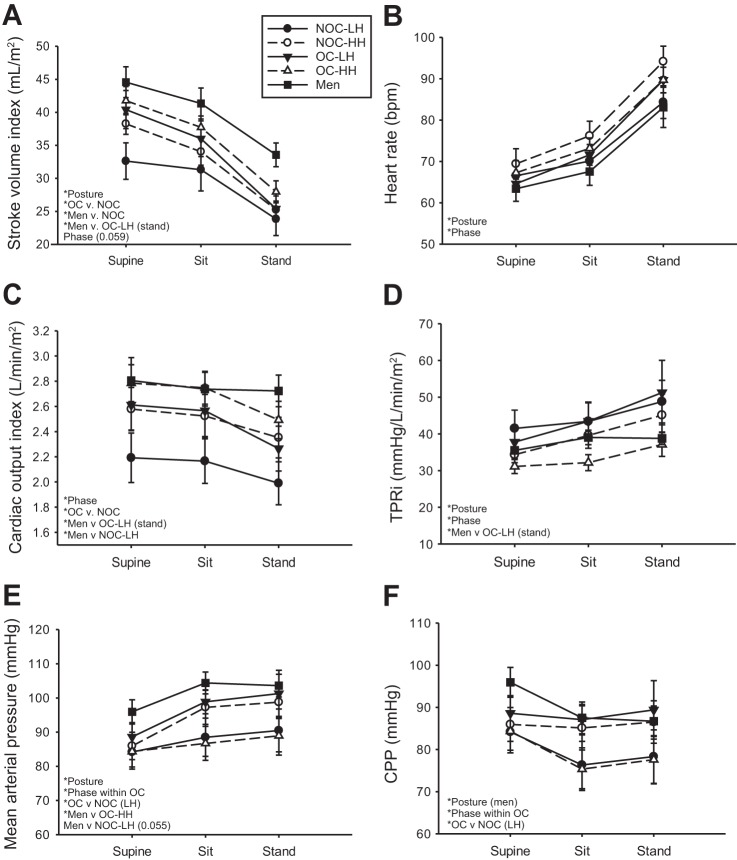
SVi decreased with upright posture in all groups (Fig. 3A). The OC group had significantly higher SVi than the NOC group, and men had significantly higher SVi than the NOC group (both phases) in all postures and the OC-LH group in the standing posture only (Fig. 3A). HR increased with upright posture equally in all groups and was higher in the HH than LH phase of the menstrual cycle (Fig. 3B). Q̇i was significantly higher in the OC than NOC group and significantly higher in the HH than LH phase (Fig. 3C). Men had significantly higher Q̇i than the NOC-LH group in all postures and the OC-LH group during standing (Fig. 3C). TPRi increased with upright posture in all groups (Fig. 3D). TPRi was lower in the HH than LH phase, and during standing, TPRi was lower in men than in the OC-LH group (Fig. 3D). MAP increased with upright posture in all groups (Fig. 3E). In the LH phase, OC users had higher MAP than the NOC group and higher MAP than in the HH phase (Fig. 3E). The OC-HH group also had lower MAP than men (Fig. 3E). CPP decreased significantly with upright posture only in men (Fig. 3F). Similar to the MAP responses, OC users had higher CPP in the LH than HH phase (Fig. 3F).
Fig. 3.
Change in stroke volume index (A), heart rate (B), cardiac output index (C), total peripheral resistance index (TPRi; D), mean arterial pressure (E), and cerebral perfusion pressure (CPP; F) in response to sitting and standing. *Significant main or interaction effect. Near-significant differences (P < 0.07) are noted in parentheses.
Cerebrovascular Responses to Posture Change
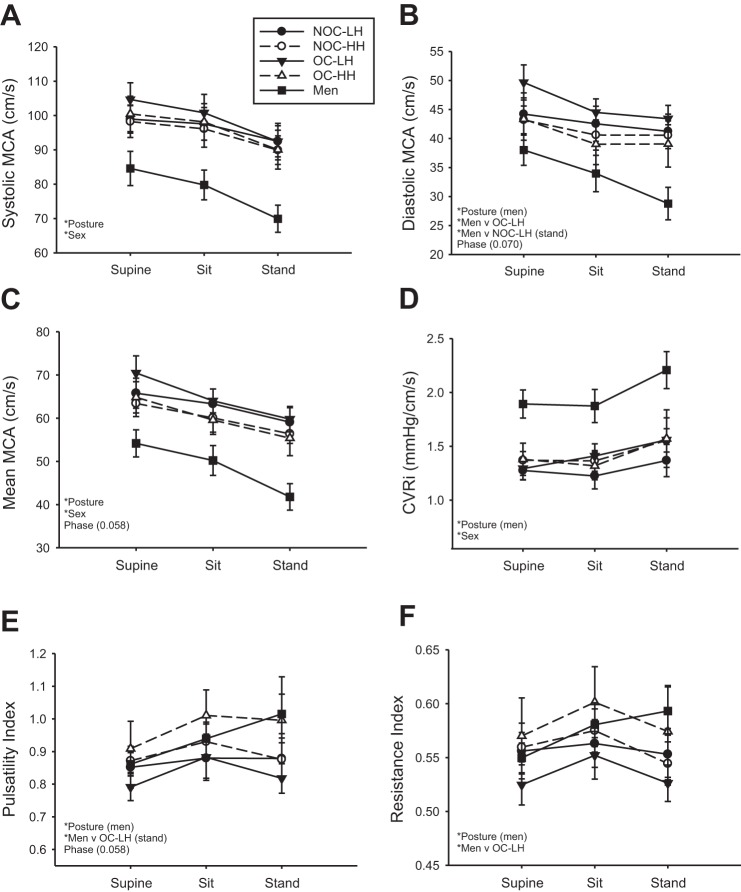
Systolic MCA blood flow velocity decreased with upright posture in all groups and was lower in men than all groups of women (Fig. 4A). Diastolic MCA blood flow velocity decreased significantly with upright posture in men, but not in women (Fig. 4B). Men had lower diastolic MCA blood flow velocity than the OC-LH group in all postures and the NOC-LH group during standing (Fig. 4B). Mean MCA blood flow velocity decreased with upright posture in all groups (Fig. 4C) was lower in men than in all groups of women (Fig. 4C). The CVRi increased with posture in men only and was higher in men than all groups of women (Fig. 4D). PI increased with upright posture in men only and was higher in men than in the OC-LH group during standing (Fig. 4E). RI increased with upright posture in men only and was higher in men than in the OC-LH group (Fig. 4F). There were no significant differences between groups for the relationships between mean MCA blood flow velocity and either ETco2 or CPP during the supine-sit-stand test (Table 3).
Fig. 4.
Change in systolic, diastolic, and mean MCA blood flow velocity (A–C), CVRi (D), and pulsatility and resistance indexes (E and F) in response to sitting and standing. *Significant main or interaction effect. Near-significant differences (P < 0.07) are noted in parentheses.
Table 3.
Slope of MCA blood flow velocity vs. ETco2 and MCA blood flow velocity vs. CPP relationships
| Women |
|||||
|---|---|---|---|---|---|
| NOC |
OC |
||||
| Men | LH | HH | LH | HH | |
| MCAmean vs. ETco2 | 1.64 ± 0.24 | 2.17 ± 0.53 | 2.17 ± 0.60 | 2.54 ± 0.55 | 1.89 ± 0.18 |
| MCAmean vs. CPP | 0.52 ± 0.09 | 0.43 ± 0.19 | 0.85 ± 0.29 | 0.43 ± 0.22 | 0.50 ± 0.19 |
Values are means ± SE. CPP, cerebral perfusion pressure; MCA, middle cerebral artery; ETco2, end-tidal CO2.
Respiratory Responses to Posture Change
ETco2 decreased with upright posture in all groups (Fig. 5A) and was significantly lower in the OC than NOC group (Fig. 5A). End-tidal O2 (ETo2) increased with upright posture in all groups and was significantly higher in the OC than NOC group (Fig. 5B). Respiratory rate decreased with upright posture in all groups of women, but not in men (Fig. 5C).
Fig. 5.
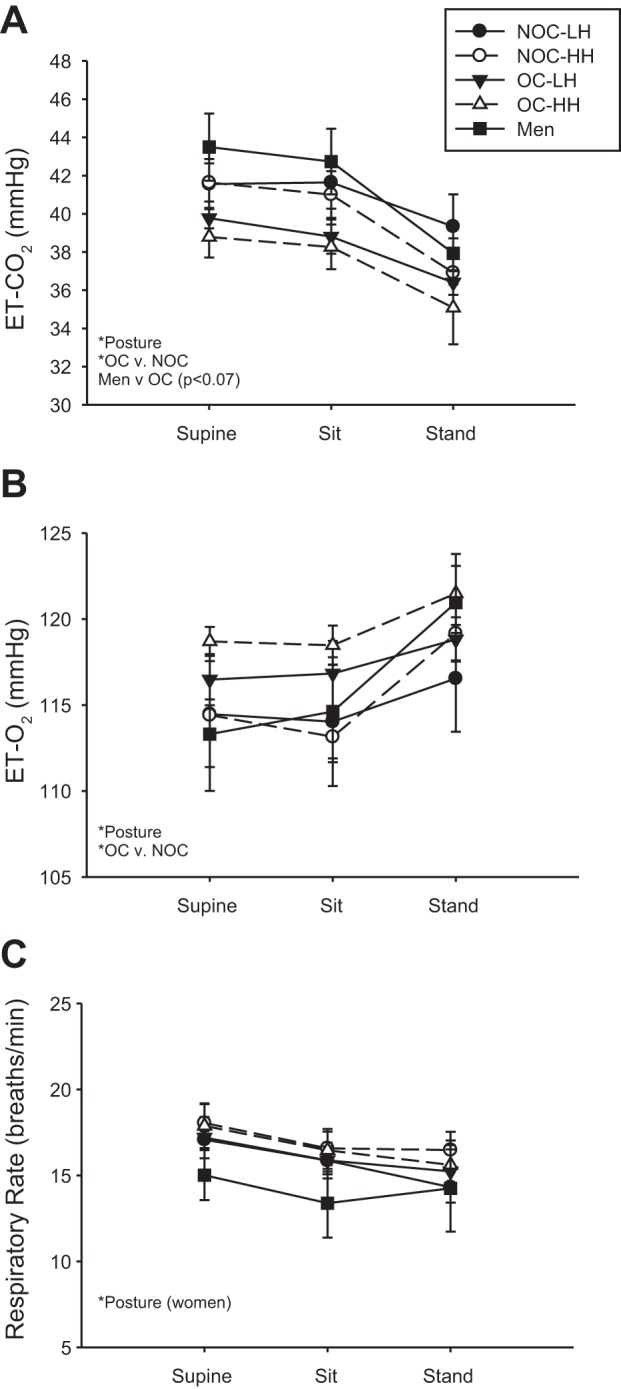
Change in end-tidal CO2 (ETco2; A), end-tidal O2 (ET-O2; B), and respiratory rate (C) in response to sitting and standing. *Significant main or interaction effect. Near-significant differences (P < 0.07) are noted in parentheses.
Autonomic Responses to Posture Change
The time domain of HRV (SDRR), the high-frequency component of the frequency domain of HRV (HF), and cardiovagal baroreceptor sensitivity significantly decreased with upright posture in all groups (Table 4). The low-frequency component of HRV variability (LF) and the LF-to-HF ratio increased with upright posture in all groups (Table 4). SDRR was significantly higher in the OC than NOC group (Table 4).
Table 4.
Heart rate variability and cardiovagal baroreceptor sensitivity
| Women |
||||||
|---|---|---|---|---|---|---|
| NOC |
OC |
|||||
| Men | LH | HH | LH | HH | Significance | |
| SDRR, ms | ||||||
| Supine | 83.9 ± 12.1 | 70.7 ± 12.1 | 76.8 ± 12.2 | 70.9 ± 8.6 | 85.0 ± 8.4 | *Posture; *OC vs. NOC |
| Seated | 91.2 ± 11.3 | 78.6 ± 8.9 | 69.6 ± 7.6 | 90.9 ± 9.1 | 95.2 ± 10.8 | |
| Standing | 56.0 ± 5.1 | 48.2 ± 6.7 | 42.0 ± 3.5 | 48.6 ± 3.6 | 55.4 ± 4.6 | |
| LF, nu | ||||||
| Supine | 41.4 ± 5.1 | 31.1 ± 3.8 | 39.6 ± 5.7 | 35.8 ± 4.8 | 42.3 ± 4.4 | *Posture |
| Seated | 70.8 ± 4.8 | 51.8 ± 8.7 | 57.5 ± 4.7 | 55.8 ± 6.8 | 52.8 ± 5.8 | |
| Standing | 76.2 ± 5.3 | 70.4 ± 5.2 | 75.7 ± 4.5 | 70.6 ± 6.0 | 74.9 ± 4.3 | |
| HF, nu | ||||||
| Supine | 55.7 ± 4.5 | 67.9 ± 3.5 | 58.0 ± 4.7 | 62.2 ± 4.4 | 56.3 ± 4.3 | *Posture; Men vs. NOC-LH (P = 0.056) |
| Seated | 28.6 ± 4.4 | 48.0 ± 8.4 | 42.1 ± 4.7 | 43.9 ± 6.5 | 46.9 ± 5.6 | |
| Standing | 23.1 ± 4.8 | 29.9 ± 5.0 | 24.1 ± 4.3 | 30.2 ± 5.9 | 25.6 ± 4.2 | |
| LF/HF | ||||||
| Supine | 0.9 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.1 | *Posture |
| Seated | 4.4 ± 1.9 | 2.3 ± 1.3 | 2.4 ± 1.2 | 1.9 ± 0.5 | 1.5 ± 0.3 | |
| Standing | 6.6 ± 1.8 | 3.8 ± 1.3 | 5.1 ± 1.3 | 4.1 ± 1.0 | 4.5 ± 1.2 | |
| cBRS slope, ms/mmHg | ||||||
| Supine | 36.0 ± 5.3 | 44.7 ± 10.7 | 33.1 ± 5.4 | 42.4 ± 11.3 | 37.6 ± 4.8 | *Posture |
| Seated | 21.1 ± 3.1 | 23.4 ± 2.9 | 23.2 ± 6.1 | 30.2 ± 8.1 | 21.6 ± 2.3 | |
| Standing | 12.6 ± 3.1 | 12.2 ± 2.4 | 8.0 ± 1.0 | 10.5 ± 1.8 | 12.7 ± 1.9 | |
Values are means ± SE. SDRR, SD between R-R intervals; LF, low frequency; HF, high frequency; cBRS, cardiovagal baroreceptor sensitivity; nu, normalized units.
P < 0.05.
Center of Balance
OC use significantly increased the maximal range of movement of lateral (side-to-side) and medial (front-to-back) center of balance during the last 30 s of the stand test compared with the NOC group (P < 0.05). Men had significantly greater maximal range of movement than only the NOC-HH group in both the lateral and medial directions (P < 0.05): in the lateral direction, 24.5 ± 5.8 kg for men, 14.1 ± 3.0 kg for the NOC-LH group, 13.5 ± 2.5 kg for the NOC-HH group, 24.8 ± 4.3 kg for the OC-LH group, and 36.0 ± 4.5 kg for the OC-HH group; in the medial direction, 12.3 ± 3.4 kg for men, 6.1 ± 1.4 kg for the NOC-LH group, 4.8 ± 0.9 kg for the NOC-HH group, 8.5 ± 1.2 kg for the OC-LH group, and 10.1 ± 1.2 kg for the OC-HH group.
DISCUSSION
Summary
OC use did not significantly affect blood pressure, HR, or cerebrovascular responses to late phase II of the Valsalva maneuver in women. Similarly, menstrual cycle phase did not significantly affect HR or cerebrovascular responses to the Valsalva maneuver; however, there was a strong tendency (P = 0.051) toward a greater increase in MAP during late phase II for women in the HH than women in the LH phase of the menstrual cycle. OC users (LH and HH phases) and non-OC users in the HH phase exhibited a greater increase in diastolic MCA blood flow velocity than men, suggesting a greater increase in brain blood flow in women during late phase II of the Valsalva maneuver. However, a concurrent reduction of CVR indexes in women was not observed.
Throughout the posture change protocol, women in the HH phase of the menstrual cycle had significantly higher HR and Q̇i with lower TPRi than women in the LH phase. OC users had significantly higher SVi and Q̇i with lower ETco2 and higher ETo2 than non-OC users throughout the posture change protocol. In OC users, MAP and, therefore, CPP decreased in the HH phase. These results suggest that the presence of cycling estrogen and progesterone (HH phase) can result in peripheral vasodilation, allowing for greater Q̇, and that the presence of pharmaceutical estrogen and progesterone analogs (active pill use in OC users) can lower blood pressure. The results further suggest that the use of OC in women could potentially be leading to cardiac remodeling with hypoventilation and/or changes in resting metabolism; however, this cannot be proven from this cross-sectional study of OC users. Further studies are needed to determine the mechanism(s) behind higher Q̇ and lower ETco2 in OC users.
Men had lower systolic and mean MCA blood flow velocity with higher CVRi than all groups of women throughout the posture change protocol. In men only during the posture change protocol, CPP and diastolic MCA blood flow velocity decreased while CVRi, PI, and RI increased, suggesting that, in response to upright posture, only men experienced a reduction of CPP with cerebral vasoconstriction. In women only, during the posture change protocol, respiratory rate decreased, perhaps indicating hypoventilation; however, changes in tidal volume were not measured; therefore, changes in ventilation cannot be concluded from the results of this study. Men had higher SVi than non-OC users (LH and HH phases) and higher Q̇i than non-OC users (LH phase) throughout the posture change protocol. Similarly, men had higher SVi and Q̇i than OC users (LH phase) during standing. These sex differences are likely due to larger left ventricular dimensions in men. Men had lower TPRi (standing), lower diastolic MCA blood flow velocity (all postures), higher PI (standing), and higher RI (all postures) than OC users (LH phase). These results suggest that, during standing, men may have less peripheral vasoconstriction, yet more cerebral vasoconstriction, than women taking OC (LH phase).
Valsalva Maneuver
We hypothesized that women in the LH phase of the menstrual cycle would exhibit a smaller HR response, smaller increase in blood pressure, and smaller increase in brain blood flow velocity during late phase II of the Valsalva maneuver. Although there were no significant differences in HR during late phase II, women in the HH phase (both estrogen and progesterone) tended to have a greater increase in blood pressure than women in the LH phase (neither hormone). Fu et al. found no differences between menstrual cycle phases in the sympathetic response to the Valsalva maneuver (19). Therefore, the tendency for a greater blood pressure response in the HH than LH phase could be due to somewhat improved peripheral neurovascular transduction (i.e., the ability of the vasculature to constrict in response to an increase in sympathetic activity), as observed by Lawrence et al. in the forearm (27). There were no significant differences between sexes or OC users in blood pressure or HR responses to the Valsalva maneuver. This similarity between groups might imply no difference in the autonomic response to an acute fall in blood pressure (i.e., early phase I) between groups; however we did not directly test sympathetic or parasympathetic activity. Despite this similarity between groups, we did observe sex differences in brain blood flow responses to late phase II of the Valsalva maneuver.
Sex differences in the response to increases in ETco2 have been noted by Kastrup et al., who reported a greater cerebrovasodilatory response to CO2 in women (24). Similarly, cerebral autoregulation has been shown to be better in adult women than age-matched men (15, 16, 45). Zhang et al. noted that a 15-s Valsalva maneuver was not expected to change ETco2 significantly (46). Therefore, we suggest that any changes in brain blood flow during the Valsalva maneuver are not due to changes in ETco2. Indeed, after ganglionic blockade, the increase in brain blood flow during late phase II of the Valsalva maneuver is enhanced (46), implying a restrictive effect of sympathetic activity. Fu et al. found no differences between sexes in the sympathetic response to falling diastolic blood pressure during phase II of the Valsalva maneuver (19), yet we observed a greater increase in diastolic MCA blood flow velocity in women. Therefore, we suggest greater neurovascular transduction of cerebral arteries in men, as seen by Hart et al. in the forearm (22). Indeed, during the posture change protocol, we observed increased CVRi, PI, and RI in men only. Investigations into neurovascular transduction (cerebral and peripheral) throughout the menstrual cycle are needed.
Considering the tendency toward a greater increase in blood pressure during the Valsalva maneuver with similar increases in brain blood flow velocity in the HH phase of the menstrual cycle, we investigated CVR indexes during the Valsalva maneuver. There is currently no information about changes in MCA diameter during the Valsalva maneuver; however, there is growing evidence that the MCA constricts during sympathetic activation. For example, using MRI, Verbree et al. found that, at constant ETco2, the MCA vasoconstricts by 2% during 5 min of rhythmic handgrip exercise in a mixed-sex population (43). In the current study, CVR indexes were calculated during the Valsalva maneuver in an attempt to determine cerebrovascular dilation/constriction, yet we did not observe differences between sexes, menstrual cycle phases, or OC use in CVRi, RI, or PI. During the Valsalva maneuver, the RI values were based on single heartbeats at the beginning and end of late phase II and, therefore, may be underpowered. This is a major limitation of this analysis, and we suggest the use of MRI for measurement of MCA diameter and flow during the Valsalva maneuver in future studies. However, when we used 1-min averages during the posture change protocol, we found tendencies toward higher PI and lower mean and diastolic MCA blood flow velocity in women in the HH phase of the menstrual cycle, implying greater vasoconstriction of the cerebral vessels, both at rest and during an orthostatic challenge. Indeed, women in the HH phase have been shown to have greater sympathetic activity at rest and during orthostasis than women in the LH phase (8, 19).
In the current study we did not find evidence of hemodynamic differences in the response to the Valsalva maneuver between sexes or with OC use. We did, however, find a strong tendency for a larger blood pressure response in the HH than LH phase of the menstrual cycle, possibly due to greater peripheral neurovascular transduction. Men had a smaller increase in diastolic MCA blood flow velocity than women during late phase II of the Valsalva maneuver, implying a smaller increase in brain blood flow, which we suggest could be due to enhanced neurovascular transduction in the cerebral vessels of men.
Standing
Cardiorespiratory response.
We hypothesized that, in the upright posture, 1) women would exhibit lower SVi and lower Q̇i than men, 2) women in the LH phase of the menstrual cycle would have lower SVi and lower Q̇i than women in the HH phase, and 3) OC users would have higher SVi and higher Q̇i than non-OC users. These hypotheses are supported by the results. As expected, SVi and Q̇i were higher in men than most groups of women. However, SVi and Q̇i were not higher in men than in OC users in the HH phase due to augmentation from both menstrual cycle phase and OC use.
Estrogen is well known to have vasodilatory effects on peripheral vasculature (23, 26, 32). This vasodilation could contribute to the lower TPRi in the HH phase of the menstrual cycle, in turn, leading to the increase in Q̇i and SVi due to reduced cardiac afterload. Indeed, Minson et al. found greater calf blood flow in the HH than LH phase of OC users (33). Furthermore, the OC users experienced lower MAP in the HH than LH phase throughout the posture change protocol, as seen previously at rest (33). This reduction of MAP could be due to a combination of factors, including 1) greater β-adrenergic-mediated vasodilation in OC users than non-OC users (28) combined with the vasodilatory effects of estradiol in the HH phase, 2) reduced sympathetic and cardiovagal baroreflex sensitivity in the HH phase of OC users (33), 3) impaired postural stability/greater postural sway in OC users, as similarly observed by Mokosakova et al. (34), leading to greater muscle activation and, therefore, vasodilation, or 4) the slight, but significant, hypocapnia and hyperoxia experienced by OC users compared with non-OC users throughout the posture change protocol. This reduction of ETco2 and increase in ETo2 could be due to greater ventilation, leading to attenuation of the central and peripheral chemoreflexes, respectively, which would, in turn, lead to reductions in sympathetic output. Indeed, Usselman et al. found attenuated chemoreflex function in the HH phase of OC users (42). We did not observe changes in respiratory rate due to OC use or menstrual cycle phase; however, we did not measure tidal volume/ventilation in this study.
Cerebrovascular resistance.
We hypothesized that, in the upright posture, 1) women would exhibit higher CVR than men, 2) women in the LH phase of the menstrual cycle would have higher CVR than women in the HH phase, and 3) OC users would have lower CVR than non-OC users. Interestingly, these hypotheses were not supported by the results. CVR is dependent on both CPP and arterial CO2 pressure (measured by ETco2). In the upright posture, we observed lower CPP and higher CVRi in men only, suggesting greater cerebrovascular vasoconstriction in men than women (regardless of menstrual cycle phase or OC use). There were no sex differences in ETco2 responses to standing.
Very few studies have investigated sex differences in CVR during orthostatic stress. Edgell et al. previously investigated CVR in the upright posture in men and in women taking cyclic OC and in the “late follicular phase” of the menstrual cycle (i.e., 1st wk of hormone pill) (17). Throughout the posture change protocol, they found lower CVR indexes (CVRi, RI, and PI) in women than men, whereas in the current study, only the CVRi was lower in women. This likely stems from an increase in MAP in women after 10 min of standing in the current study (from 84 ± 5 to 89 ± 6 mmHg in the OC-HH group) compared with the finding of Edgell et al. that MAP did not change in women after 5 min of standing (from 84 ± 2 to 82 ± 3 mmHg). These differences could be due to the duration of standing or different amounts of estradiol/progesterone analog (i.e., 1st wk of pill vs. last week of pill), highlighting the importance of controlling for OC use and menstrual cycle phase.
There were no significant effects of menstrual cycle phase or OC use on CVR indexes during the posture change protocol. However, there were trends for lower diastolic (P = 0.07) and mean MCA blood flow velocity (P = 0.06) with higher PI (P = 0.06) in women in the HH than LH phase, implying reduced brain blood flow due to cerebral vasoconstriction. Brackley et al. similarly observed higher PI and RI of the MCA in the luteal than midfollicular phase of the menstrual cycle (7). To our knowledge, this is the first study to investigate the effect of OC use on CVR indexes.
The current study has provided evidence indicating that both menstrual cycle phase and OC use can affect cardiorespiratory responses to standing. We have found that cycling female sex hormones are responsible for peripheral vasodilation, leading to reduced cardiac afterload and, therefore, higher SVi and Q̇i. Furthermore, we have found that the use of pharmaceutical female sex hormones lowers MAP. While there was no statistically significant evidence for an effect of menstrual cycle phase or OC use on cerebrovascular responses during the standing trial, there was a trend for greater cerebrovascular constriction in the HH than LH phase. Lastly, we have provided significant evidence of greater cerebrovascular constriction in men than women (regardless of menstrual cycle phase) during standing.
Limitations
We did not measure ventilation or resting fitness levels in our participants; nor did we investigate differences between groups in lower-body and core strength, muscle fatigability, or mitochondrial activity, all of which could lead to differences in postural sway and metabolite buildup, in turn leading to changes in vasodilation and TPR. OC users in the HH phase of the menstrual cycle experienced lower blood pressure throughout the posture change protocol. We suggested that this could be due to greater peripheral vasodilation, reduced sympathetic reflex function (not directly tested in this study), or postural sway. The increase in postural sway in OC users could be due to 1) increased ventilation, 2) greater height, or 3) greater fatigue in core and/or leg skeletal muscles, perhaps due to changes in mitochondrial activity or a reduction of strength. Further studies are needed to elucidate these possibilities.
Transcranial Doppler measurements of blood flow velocity as an index of brain blood flow are limited because of the unknown diameter of the vessel. In 2000, using a 1.5-T MRI scanner, Serrador et al. found no change in MCA diameter during 10 min of orthostatic stress (41). However, more recently, using a 3-T MRI scanner, Coverdale et al. found that hypocapnia (−13 mmHg) resulted in MCA vasoconstriction (14), yet using a 7-T MRI scanner, Verbree et al. found that milder hypocapnia (still greater than that seen during orthostatic stress; −7.5mmHg) elicited no significant change in MCA diameter (44). All the aforementioned investigations were carried out in a mixed-sex population, and there is no information on differences according to sex, menstrual cycle phase, or OC use.
The current study was a cross-sectional investigation of OC use. In future investigations, women should be studied before and during OC use to strengthen our conclusions. Furthermore, we did not have plasma concentrations of estrogen and progesterone (or analogs) during this study. Therefore, the veracity of data from the HH phase of the menstrual cycle of non-OC users could be in doubt if participants did not ovulate in the month of testing.
Participants were not tested in the fasted state. While women were tested at the same time of day to control for changes in circadian rhythm, we did not control for diet beyond our request that the subjects avoid fatty foods, caffeine, and alcohol. Diet can acutely affect the cardiovascular system. For example, in young healthy participants, consumption of ω-3 fatty acids can improve postprandial deteriorations in endothelial function and arterial stiffness (12, 36), and hypohydration can reduce endothelial function and lower plasma volume (2). Indeed, any food consumption can alter hemodynamics via changes in splanchnic blood flow (21) and sympathetic activity (18).
Conclusions
Compared with the LH phase of the menstrual cycle, there were strong tendencies for women in the HH phase to exhibit a greater increase in MAP during the Valsalva maneuver, potentially indicating greater peripheral neurovascular transduction. Men exhibited a smaller increase in brain blood flow velocity than women toward the end of the Valsalva maneuver, suggesting that 1) men had greater cerebral vasoconstriction or 2) women had greater cerebral vasodilation. However, the RI values used in the current study (calculated using single heartbeats of data) did not support these suggestions. Future studies using MRI to measure both total cerebral blood flow and changes in MCA diameter throughout the Valsalva maneuver are needed for clarification. Similarly, only men experienced an increase in CVR indexes during the posture change protocol, indicating greater cerebral vasoconstriction. There were no statistically significant effects of menstrual cycle phase or OC use on cerebral responses to the Valsalva maneuver or standing; however, women in the HH phase showed strong tendencies toward greater cerebral vasoconstriction than women in the LH phase throughout the posture change protocol.
Women in the HH phase of the menstrual cycle had significantly higher HR and Q̇i with lower TPRi than women in the LH phase, suggesting an acute vasodilatory effect of female sex hormones (endogenous or pharmaceutical). OC users had significantly higher SVi and Q̇i than non-OC users with no effect on TPR or HR. Lastly, OC users exhibited reduced MAP in the HH phase compared with the LH phase, which could be from ventilatory changes, greater postural sway, enhanced vasodilatory capacity, or reduced sympathetic reflex control. Interestingly, these hemodynamic differences due to menstrual cycle phase or OC use are primarily due to baseline differences that persist throughout the posture change protocol.
Very few physiological research studies control for sex difference, menstrual cycle phase, and/or OC use, yet the results of the current study highlight their importance. Furthermore, many clinicians use the cardiovascular responses to the Valsalva maneuver and orthostatic stress as diagnostic tools for conditions such as orthostatic hypotension, postural orthostatic tachycardia syndrome, vasovagal syncope, and dysautonomia (29, 37), yet they do not control for menstrual cycle phase or OC use. These results suggest that controlling for (or at least recording) menstrual cycle phase and/or OC use could improve clinical diagnoses. Future investigations will expand into clinical populations with the goal of improving diagnosis in women.
GRANTS
Funding was provided by the Faculty of Health at York University and a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A., M.N., and H.E. conceived and designed research; S.A., M.N., S.S., S.K., and C.H. performed experiments; S.A., M.N., S.S., S.K., C.H., and H.E. analyzed data; S.A., M.N., and H.E. interpreted results of experiments; S.A., M.N., S.S., S.K., C.H., and H.E. edited and revised manuscript; S.A., M.N., S.S., S.K., C.H., and H.E. approved final version of manuscript; H.E. prepared figures; H.E. drafted manuscript.
REFERENCES
- 1.Ali YS, Daamen N, Jacob G, Jordan J, Shannon JR, Biaggioni I, Robertson D. Orthostatic intolerance: a disorder of young women. Obstet Gynecol Surv 55: 251–259, 2000. doi: 10.1097/00006254-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Arnaoutis G, Kavouras SA, Stratakis N, Likka M, Mitrakou A, Papamichael C, Sidossis LS, Stamatelopoulos K. The effect of hypohydration on endothelial function in young healthy adults. Eur J Nutr 56: 1211–1217, 2017. doi: 10.1007/s00394-016-1170-8. [DOI] [PubMed] [Google Scholar]
- 3.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol Heart Circ Physiol 254: H377–H383, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Best SA, Okada Y, Galbreath MM, Jarvis SS, Bivens TB, Adams-Huet B, Fu Q. Age and sex differences in muscle sympathetic nerve activity in relation to haemodynamics, blood volume and left ventricular size. Exp Physiol 99: 839–848, 2014. doi: 10.1113/expphysiol.2013.077248. [DOI] [PubMed] [Google Scholar]
- 5.Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol Heart Circ Physiol 268: H1682–H1687, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Black A, Yang Q, Wu Wen S, Lalonde AB, Guibert E, Fisher W. Contraceptive use among Canadian women of reproductive age: results of a national survey. J Obstet Gynaecol Can 31: 627–640, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Brackley KJ, Ramsay MM, Broughton Pipkin F, Rubin PC. The effect of the menstrual cycle on human cerebral blood flow: studies using Doppler ultrasound. Ultrasound Obstet Gynecol 14: 52–57, 1999. doi: 10.1046/j.1469-0705.1999.14010052.x. [DOI] [PubMed] [Google Scholar]
- 8.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter JR, Klein JC, Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J Physiol Regul Integr Comp Physiol 298: R9–R14, 2010. doi: 10.1152/ajpregu.00554.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YC, Vyas A, Hymen E, Perlmuter LC. Gender differences in orthostatic hypotension. Am J Med Sci 342: 221–225, 2011. doi: 10.1097/MAJ.0b013e318208752b. [DOI] [PubMed] [Google Scholar]
- 12.Chong MF, Lockyer S, Saunders CJ, Lovegrove JA. Long chain n-3 PUFA-rich meal reduced postprandial measures of arterial stiffness. Clin Nutr 29: 678–681, 2010. doi: 10.1016/j.clnu.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 24: 385–396, 1983. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 14.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 15.Deegan BM, Sorond FA, Galica A, Lipsitz LA, O’Laighin G, Serrador JM. Elderly women regulate brain blood flow better than men do. Stroke 42: 1988–1993, 2011. doi: 10.1161/STROKEAHA.110.605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deegan BM, Sorond FA, Lipsitz LA, Olaighin G, Serrador JM. Gender related differences in cerebral autoregulation in older healthy subjects. Conf Proc IEEE Eng Med Biol Soc 2009: 2859–2862, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgell H, Robertson AD, Hughson RL. Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J Appl Physiol (1985) 112: 1482–1493, 2012. doi: 10.1152/japplphysiol.01204.2011. [DOI] [PubMed] [Google Scholar]
- 18.Fagius J, Berne C. Increase in muscle nerve sympathetic activity in humans after food intake. Clin Sci (Lond) 86: 159–167, 1994. doi: 10.1042/cs0860159. [DOI] [PubMed] [Google Scholar]
- 19.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110: 2931–2937, 2004. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- 21.Gatt M, MacFie J, Anderson AD, Howell G, Reddy BS, Suppiah A, Renwick I, Mitchell CJ. Changes in superior mesenteric artery blood flow after oral, enteral, and parenteral feeding in humans. Crit Care Med 37: 171–176, 2009. doi: 10.1097/CCM.0b013e318192fb44. [DOI] [PubMed] [Google Scholar]
- 22.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurtado R, Celani M, Geber S. Effect of short-term estrogen therapy on endothelial function: a double-blinded, randomized, controlled trial. Climacteric 19: 448–451, 2016. doi: 10.1080/13697137.2016.1201809. [DOI] [PubMed] [Google Scholar]
- 24.Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke 28: 2353–2356, 1997. doi: 10.1161/01.STR.28.12.2353. [DOI] [PubMed] [Google Scholar]
- 25.Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Okumura K, Yasue H. Menstrual cyclic variation of endothelium-dependent vasodilation of the brachial artery: possible role of estrogen and nitric oxide. Proc Assoc Am Physicians 108: 473–480, 1996. [PubMed] [Google Scholar]
- 26.Krejza J, Siemkowicz J, Sawicka M, Szylak A, Kochanowicz J, Mariak Z, Lewko J, Spektor V, Babikian V, Bert R. Oscillations of cerebrovascular resistance throughout the menstrual cycle in healthy women. Ultrasound Obstet Gynecol 22: 627–632, 2003. doi: 10.1002/uog.907. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JE, Klein JC, Carter JR. Menstrual cycle elicits divergent forearm vascular responses to vestibular activation in humans. Auton Neurosci 154: 89–93, 2010. doi: 10.1016/j.autneu.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limberg JK, Peltonen GL, Johansson RE, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Walker BJ, Schrage WG. Greater β-adrenergic receptor mediated vasodilation in women using oral contraceptives. Front Physiol 7: 215, 2016. doi: 10.3389/fphys.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low PA, Tomalia VA, Park KJ. Autonomic function tests: some clinical applications. J Clin Neurol 9: 1–8, 2013. doi: 10.3988/jcn.2013.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald C, Koshi S, Busner L, Kavi L, Newton JL. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open 4: e004127, 2014. doi: 10.1136/bmjopen-2013-004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 15: 67–75, 2007. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 32.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473–1476, 2000. doi: 10.1161/01.CIR.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 34.Mokosáková M, Krsková L, Hlavacka F, Zeman M. Diurnal changes of the postural control in young women without and with hormonal contraceptive treatment. Neuro Endocrinol Lett 35: 230–235, 2014. [PubMed] [Google Scholar]
- 35.Muppa P, Sheldon RS, McRae M, Keller NR, Ritchie D, Krahn AD, Morillo CA, Kus T, Talajic M, Raj SR. Gynecological and menstrual disorders in women with vasovagal syncope. Clin Auton Res 23: 117–122, 2013. doi: 10.1007/s10286-013-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newens KJ, Thompson AK, Jackson KG, Wright J, Williams CM. DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. Am J Clin Nutr 94: 742–748, 2011. doi: 10.3945/ajcn.110.009233. [DOI] [PubMed] [Google Scholar]
- 37.Novak P. Quantitative autonomic testing. J Vis Exp 53: 2502, 2011. doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 118: 242–246, 2012. doi: 10.1016/j.ijgo.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, . et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol Heart Circ Physiol 248: H151–H153, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006. doi: 10.1152/ajpregu.00446.2005. [DOI] [PubMed] [Google Scholar]
- 41.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 42.Usselman CW, Luchyshyn TA, Gimon TI, Nielson CA, Van Uum SH, Shoemaker JK. Hormone phase dependency of neural responses to chemoreflex-driven sympathoexcitation in young women using hormonal contraceptives. J Appl Physiol (1985) 115: 1415–1422, 2013. doi: 10.1152/japplphysiol.00681.2013. [DOI] [PubMed] [Google Scholar]
- 43.Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab (January 1, 2016). doi: 10.1177/0271678X16679419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Krishnamurthy S, Evans J, Bhakta D, Justice L, Bruce E, Patwardhan A. Transfer function analysis of gender-related differences in cerebral autoregulation. Biomed Sci Instrum 41: 48–53, 2005. [PubMed] [Google Scholar]
- 46.Zhang R, Crandall CG, Levine BD. Cerebral hemodynamics during the Valsalva maneuver: insights from ganglionic blockade. Stroke 35: 843–847, 2004. doi: 10.1161/01.STR.0000120309.84666.AE. [DOI] [PubMed] [Google Scholar]
- 47.Zuj KA, Arbeille P, Shoemaker JK, Hughson RL. Cerebral critical closing pressure and CO2 responses during the progression toward syncope. J Appl Physiol (1985) 114: 801–807, 2013. doi: 10.1152/japplphysiol.01181.2012. [DOI] [PubMed] [Google Scholar]