We compared the vasodilatory capacities of NTG vs. SNP at similar concentration doses and rates into the forearm. Based on the results of the study, it may be feasible to use intra-arterial NTG as a measure of endothelial-independent vasodilator in research studies. However, NTG dosing may need to be higher if used as an endothelial-independent vasodilator due to significant differences in the vasodilatory effects during higher doses of SNP compared with NTG.
Keywords: vasodilators, venous occlusion plethysmography, Doppler ultrasound
Abstract
The vasodilatory mechanism of Nntroglycerin (NTG) is similar to sodium nitroprusside (SNP) in regard to action on guanosine 3′5′-monophosphate (cyclic GMP) via nitric oxide. However, it is unknown whether NTG can achieve the same magnitude of vasodilation in the forearm as SNP. Therefore, the purpose of the study was to evaluate the differences in forearm blood flow (FBF) and forearm vascular conductance (FVC) during escalating infusions of NTG vs. SNP at similar concentration doses and rates. We measured FBF using venous occlusion plethysmography (VOP) and Doppler ultrasound in eight young, healthy participants (mean age = 28 ± 2 yr) during four forearm volume (FAV)-specific doses (0.25, 0.5, 1, and 2 µg·100 ml FAV−1·min−1) of SNP and NTG infused via a brachial artery catheter. There was a significant difference in FVC of SNP vs. NTG only at the higher doses, as measured by VOP (14.9 ± 1.4 and 18.3 ± 1.5 vs. 11.6 ± 1.2 and 12.5 ± 1.2 ml/dl FAV−1·min−1·100 mmHg−1). FVC as measured by Doppler ultrasound unadjusted for FAV was significantly different at the lowest and the higher two doses of SNP compared with NTG (202.1 ± 25.8, 329.4 ± 46.7, and 408 ± 63.5 vs. 142.9 ± 22.4, 217.2 ± 18.8, and 247.5 ± 18.2 ml·min−1·100 mmHg−1). SNP induces significantly higher vasodilatory actions compared with NTG. However, NTG is comparable in eliciting equivalent vasodilator effects to SNP during low concentration doses when measured by VOP. Importantly, for forearm pharmacology studies, NTG can elicit marked endothelium-independent forearm vasodilation.
NEW & NOTEWORTHY We compared the vasodilatory capacities of NTG vs. SNP at similar concentration doses and rates into the forearm. Based on the results of the study, it may be feasible to use intra-arterial NTG as a measure of endothelial-independent vasodilator in research studies. However, NTG dosing may need to be higher if used as an endothelial-independent vasodilator due to significant differences in the vasodilatory effects during higher doses of SNP compared with NTG.
sodium nitroprusside (SNP) is a commonly used vasodilator in medicine and research. SNP is an inorganic hypotensive prodrug that functions primarily as a vasodilator by increasing guanosine 3′5′-monophosphate (cyclic GMP) via release of nitric oxide (NO) (3). This results in relaxation of the vascular smooth muscle, which subsequently causes dilation in both peripheral arteries and veins. Similarly, nitroglycerin (NTG) is a well-established prodrug vasodilator with a similar mechanism to SNP in respect to its action on guanosine 3′5-monophosphate (cyclic GMP) via nitric oxide (1). However, unlike SNP, NTG is an organic nitrate and requires the presence of the enzyme aldehyde dehydrogenase and/or other specific thiol-containing compounds for bioactivation and generation of NO (4, 7).
Vasodilator responses to SNP and NTG are diverse in different vascular beds and may also differ depending on isolated vascular bed infusions vs. whole body systemic infusions (1, 5). Currently, human vascular research typically uses SNP, an expensive drug of choice (price range from July 2015 to December 2016 for 2 ml of SNP was $880.88 to $900), in the isolated human forearm model to assess endothelium-independent vascular function. In this context, it is currently unknown whether SNP and NTG, a cheaper option (price for 10 ml of NTG was $12.48), would have similar dose responses in the isolated forearm vascular bed. To adopt NTG as a potential endothelial-independent drug in this type of human vascular research, a dose-response comparison to SNP is needed. Therefore, the aim of the current study was to examine the forearm blood flow dose responses of NTG and SNP. We hypothesized that NTG would have a similar dose-response relationship between dose and response as SNP in isolated human forearm vasculature.
METHODS
Subjects.
A total of eight young, healthy, recreationally active subjects (5 men and 3 women) completed the study after written, informed consent was acquired. All participants were free of acute and chronic cardiovascular or respiratory disease and not taking any medication or supplements, and all were nonsmoking and nonobese (BMI <30 kg/m2). All participants refrained from exercise, alcohol, and caffeine for ≥24 h and fasted for 12 h before the start of the study. All female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives to control the effects of the reproductive hormones on cardiovascular function. All female participants had a negative pregnancy test the morning of the study day (8). Women with intrauterine devices were excluded. All study procedures were approved by the Institutional Review Board of the Mayo Clinic and performed according to the Declaration of Helsinki.
Experimental protocol.
The study was performed in a single-day experimental session in the Clinical Research and Trials Unit at the Mayo Clinic. The room was maintained at an ambient temperature between 22 and 24°C. Demographics, pregnancy test (for women), and forearm volume were obtained on the day of the testing.
Brachial artery catheterization and blood pressure and heart rate.
A 20-gauge, 5-cm (model RA-04020; Arrow International, Reading, PA) catheter for measurement of arterial pressure and drug infusion was placed into the brachial arterial of the nondominant arm under aseptic conditions after local anesthesia (2% lidocaine) in all of the participants. A three-way port system connector in series and transducer (model PX600F, Edwards Lifescience, Irvine, CA) allowed the simultaneous infusion of SNP or NTG and measurement of beat-by-beat brachial arterial pressure, as described previously in detail (2). Heart rate was recorded via a continuous three-lead electrocardiogram.
Drugs administered.
SNP (Nitropress; Valent, Laval, QC, Canada) and NTG (Sagent Pharmaceutical, Schaumburg, IL) were dissolved in isotonic saline (1 mg/ml) to a concentration of 200 µg/ml. The doses for both SNP and NTG were 0.25, 0.5, 1, and 2 µg/100 ml·FAV−1·min−1. These doses of SNP have been used previously by our group in multiple studies (3, 6, 9). The infusion rate adjustments were made based on each participant’s forearm volume. The same infusion rates were used during saline control infusions. Each dose of SNP and NTG was infused for a period of 3 min. The order of drugs was randomized between participants.
Forearm blood flow and forearm vascular conductance using venous occlusion plethysmography.
A strain gauge was placed around the nondominant forearm 5 cm below the antecubital fold. During recording, a wrist cuff was continuously inflated to suprasystolic pressure to occlude arterial blood flow to the hand while a venous occlusion cuff around the upper arm was inflated to 50 mmHg for 7.5 of every 15 s, providing one blood flow measurement every 15 s. Forearm blood flow (FBF) was determined as the first derivative of the recordings. The average of four flows from the last minute each dose is presented. Mean arterial pressure from the last minute of each dose and corresponding flows is presented. Forearm vascular conductance (FVC) was calculated as (FBF/mean arterial pressure) × 100 and expressed as ml/dl·FAV−1·min−1·100 mmHg−1.
Forearm blood flow using Doppler ultrasound.
Brachial artery mean blood velocity (MBV) and brachial artery diameter were obtained with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7; General Electric, Milwaukee, WI). Brachial artery blood velocity was measured during rest and at all doses of SNP and NTG with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole at rest and during the last 20 s of each drug dose. Mean blood velocity, heart rate (HR), and MAP were determined by averaging the last 60 s of each baseline and drug infusion. Forearm blood flow (FBF) was calculated as FBF = MBV × π × (brachial artery diameter/2)2 × 60 with MBV (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute without normalization for forearm volume. FVC was calculated as (FBF/mean arterial pressure) × 100 and expressed as ml·min−1·100 mmHg−1, so that FVC will be quantitatively similar to the standard units of FBF.
Data analysis.
Data were collected at 250 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq; DATAq Instruments, Akron, OH; and Powerlab; ADInstruments, Sydney, Australia). Heart rate (HR) and mean arterial pressure (MAP) were analyzed from the electrocardiogram and the continuous brachial artery pressure waveform signals, respectively.
To determine the change in FVC over the dose-response curve of SNP and NTG, we plotted the FVC responses across saline and different doses of SNP and NTG for individual participants.
Statistical analysis.
Mixed-model repeated-measures ANOVA was used to compare the differences between different doses of SNP and NTG. Paired t-tests were used to compare the differences between each of the two drugs at each dose. Independent t-tests were used to compare the FVC slopes of the two drugs. When assessing differences between drugs at each dose level, the comparison at the highest dose level was of primary interest, and the comparisons at lower dose levels were of secondary interest. In all cases, P values of <0.05 were considered statistically significant, and no adjustments were made for multiple comparisons.
RESULTS
All eight participants completed the protocol. The mean age was 28 ± 2 yr, body weight 79 ± 7 kg, height 180 ± 4 cm, and body mass index 24 ± 1 kg/m2.
Systemic hemodynamic variables.
There were no significant systemic differences in MAP (P = 0.924) and HR (P = 0.455) between the SNP and NTG doses.
Effect of SNP and NTG infusion on forearm vascular conductance during venous occlusion plethysmography.
The group data for FVC during SNP infusion are presented in Fig. 1. The four doses of SNP resulted in a 472% increase in FVC at the highest dose (3.2 ± 0.2 vs. 18.3 ± 1.5 ml/dl FAV−1·min−1·100 mmHg−1, P = 0.001). FVC during SNP infusion was significantly higher at each dose compared with the baseline.
Fig. 1.
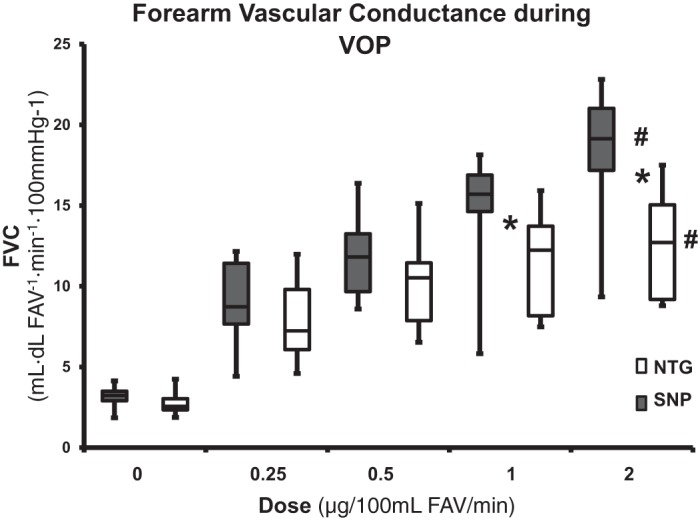
Forearm vascular conductance (FVC) using venous occlusion plethysmography (VOP) during sodium nitroprusside (SNP) and nitroglycerin (NTG) infusion. #P < 0.05, significant interaction between doses; *P < 0.05, significant difference between SNP and NTG. FAV, forearm volume.
The group data for FVC during NTG infusion are presented in Fig. 1. Four linear doses of NTG resulted in a 346% increase in FVC at the highest dose (2.8 ± 0.3 vs. 12.5 ± 1.2 ml/dl FAV−1·min−1·100 mmHg−1, P = 0.001). FVC during NTG infusion was significantly higher at each dose as compared with the baseline.
There was no significant difference in FVC during dose 0.25 (P = 0.22) or 0.5 (P = 0.058) µg·100 ml FAV−1·min−1 SNP compared with NTG (Fig. 1). However, the FVC during 1 and 2 µg·100 ml FAV−1·min−1 SNP infusions was significantly higher as compared with NTG (14.9 ± 1.4 and 18.3 ± 1.5 vs. 11.6 ± 1.2 and 12.5 ± 1.2 ml/dl FAV−1·min−1·100 mmHg−1, P = 0.02 and P = 0.007, respectively).
Effect of SNP and NTG infusion on resting forearm blood flow and forearm vascular conductance during Doppler ultrasound.
The group data for FVC during SNP infusion are presented in Fig. 2. The four doses of SNP resulted in a 404% increase in FVC at the highest dose (80.9 ± 10.5 vs. 408 ± 63.5 ml·min−1·100 mmHg−1, P = 0.001). FVC during SNP infusion was significantly higher at each dose compared with the baseline.
Fig. 2.
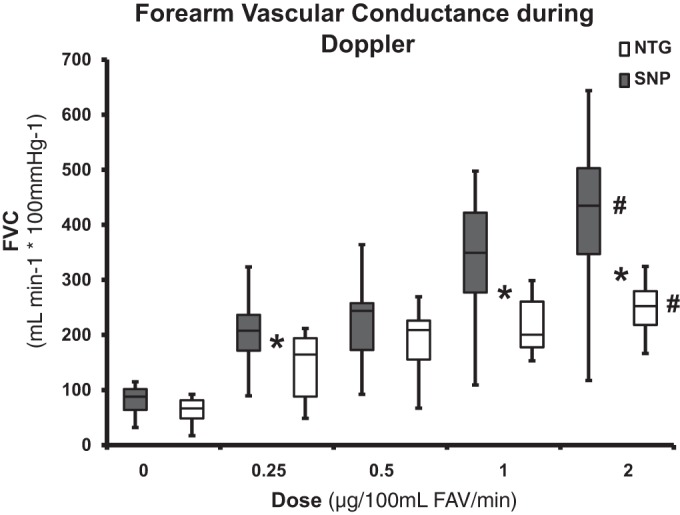
Forearm vascular conductance (FVC) using venous occlusion plethysmography during sodium nitroprusside (SNP) and nitroglycerin (NTG) infusion. #P < 0.05, significant interaction between doses; *P < 0.05, significant difference between SNP and NTG. FAV, forearm volume.
The group data for FVC during NTG infusion are presented in Fig. 2. The four doses of NTG resulted in a 305% increase in FVC at the highest dose (60.9 ± 9.7 vs. 247 ± 18.2 ml·min−1·100 mmHg−1, P = 0.001). FVC during NTG infusion was significantly higher at each dose compared with the baseline.
The FVC during 0.25, 1, and 2 µg·100 ml FAV−1·min−1 SNP infusions was significantly higher compared with NTG (202.1 ± 25.8, 329.4 ± 46.7, and 408 ± 63.5 vs. 142.9 ± 22.4, 217.2 ± 18.8, and 247.5 ± 18.2 ml·min−1·100 mmHg−1; P = 0.001, P = 0.02, and P = 0.02 respectively).
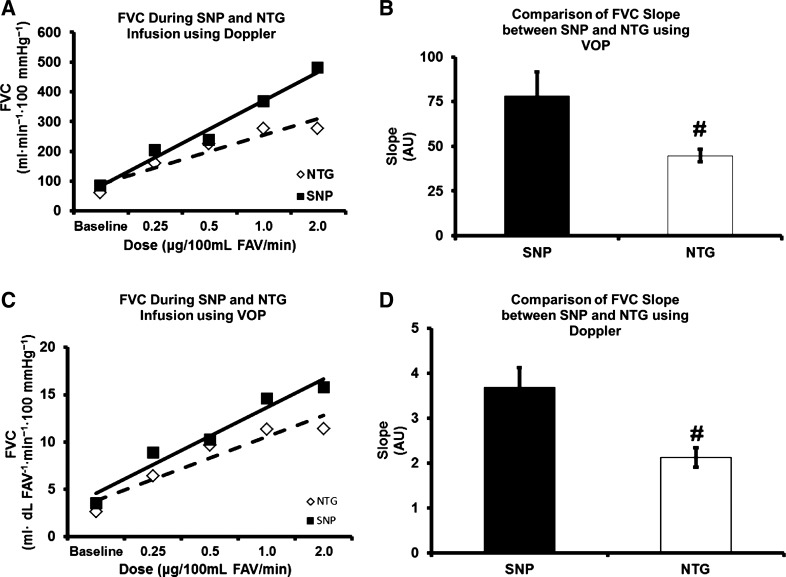
The FVC slope during SNP administration (baseline to maximal dose) was significantly greater compared with FVC slope during NTG administration (Fig. 3, A and B).
Fig. 3.
A [venous occlusion plethysmography (VOP)] and B (Doppler ultrasound): representative slopes of one of the participants for forearm vascular conductance (FVC) during sodium nitroprusside (SNP) and nitroglycerin (NTG). C and D: FVC slope during SNP and NTG. #P < 0.05, significant difference between SNP and NTG.
DISCUSSION
In the present study, we found that there was a significant difference in the vasodilatory actions of SNP and NTG only at doses of 1 and 2 µg·100 ml FAV−1·min−1when measured using venous occlusion plethysmography. However, the vasodilatory actions were significantly different between SNP and NTG at doses of 0.25, 1, and 2 µg·100 ml FAV−1 min−1 using Doppler ultrasound on the brachial artery. Additionally, the FVC slope during SNP administration (baseline to maximal dose) was significantly greater compared with FVC slope during NTG administration. Importantly, it is possible to generate robust forearm vasodilator responses with NTG.
Comparison of the two drugs.
To our knowledge, this is the first study to compare the vasodilatory effects of intra-arterial dose responses of SNP and NTG by two different methods in a human pharmacology model. Previously, the direct effects of SNP and NTG have been compared on the coronary circulation and other vascular beds in dogs (1). The study reported that the vasodilatory effects of SNP and NTG were different in different vascular beds. Although the direct vasodilatory effects of SNP and NTG were similar in the coronary collateral circulation, SNP caused a greater vasodilatory effect in the peripheral arterial circulation compared with NTG (1). Our data are consistent with the latter findings where SNP caused greater vasodilatory effects compared with NTG. Gerson et.al. (5) compared the whole body arterial and venous dilatory properties of SNP and NTG in adults undergoing cardiopulmonary bypass. That study reported no difference in the forearm vascular resistance, as measured by forearm plethysmography between the two drugs at any of the three doses; however, SNP caused significantly higher arterial dilation compared with NTG at the two higher doses (1.5 and 2.0 µg·kg−1·min−1) (5). These results are partially consistent with our present study, as we observed greater vasodilatory effects with SNP at the higher doses. However, the study by Gerson et al. (5) had certain differences from the present study that could have led to differences in the forearm vasodilatory effects of the two drugs: 1) their study used a whole body dose, whereas we performed a local infusion; and 2) their study was conducted in participants undergoing cardiopulmonary bypass surgery as opposed to healthy adults in the present study.
In the present study, the FVC slope during SNP was significantly higher than NTG. This suggests that the kinetics involved with increase in flow during SNP could be different compared with NTG. However, there seems to be a greater variability in vasodilatory responses to SNP compared with NTG. This suggests that there is a higher interparticipant variability to dose response of SNP compared with NTG.
In the present study, one of the primary reasons for the difference between SNP and NTG could be because SNP causes both arterial and venous dilation, whereas NTG is thought primarily to cause venodilation. A second potential explanation for the differences in the dilator responses between the two drugs could be that less NO was released by NTG. This could reflect differences in the molecular weight of the drugs and the fact that our dosing scheme was based on µg/100 ml vs. molar calculations. However, the molecular weights of the SNP and NTG are similar (261.92 vs. 227.08 g/mol, respectively). The molecular weights are even closer, especially if we remove the molecular weight of sodium from SNP (215.96 vs. 227.08 g/mol). Finally, SNP has been used extensively as a potent endothelial-independent vasodilator in human research primarily because it is a direct and spontaneous NO donor by interacting with oxyhemoglobin (4). On the contrary, NTG is an organic nitrate and requires the presence of the enzyme aldehyde dehydrogenase and/or other specific thiol-containing compounds for bioactivation and generation of NO (4, 7). This system could have been the rate-limiting step at higher doses. That the slope of the dose-response curve plateaus during NTG infusions further elucidates to our rate-limiting step point.
Comparison of methods.
It is interesting to note that there was a difference between the methods of measuring the vasodilatory effects between SNP and NTG. A potential reason for this difference is that venous occlusion plethysmography measures both venous and arterial changes, as it measured at the level of the forearm. Hence, the changes in forearm are contributed from both sides of the vasculature. However, during the Doppler ultrasound measurement, only the brachial artery is imaged and measured. Therefore, given the predominance of SNP in vasodilating the arterial side compared NTG, we could assume that there was a difference in the vasodilatory effects even at the lowest dose with this method. Another reason for the potential differences between the methods could be attributed to the inclusion of hand circulation during Doppler ultrasound compared during venous occlusion plethysmography, where the wrist cuff is inflated to suprasystolic pressure.
Limitations.
There are two main limitations to the current study. First, these data include only young, healthy adults, and we have no current data comparing these drugs on older adults or individuals with cardiovascular disease. Second, due to the lack of repeat testing, the intraparticipant variability in FVC for NTG compared with SNP is unknown.
Significance and conclusions.
Based on the results of the study, it may be feasible to use intra-arterial NTG, ∼70 times cheaper at current prices in the US, as a measure of endothelial-independent vasodilation in research studies, especially given the low intraparticipant variability during NTG infusions. Use of NTG could be preferable for studies where a plateau for the dose-response curve is needed. However, care must be noted, as there are significant differences not only in the vasodilatory effects but also the kinetics involved with increase in flow during higher doses of SNP compared with NTG. Future studies need to focus on the equivalent dose of NTG to match the SNP responses.
GRANTS
This research was supported by National Institutes of Health Research Grants HL-119337 and CTSA UL1-TR000135, the National Institute of General Medical Sciences under award no. T32-GM-008685, and Mayo Clinic BIRCWH Scholarship K12 HD065987. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.R., A.R.E., W.T.N., and M.J.J. conceived and designed research; S.M.R. and G.A.D. performed experiments; S.M.R. and G.A.D. analyzed data; S.M.R., A.R.E., W.T.N., and M.J.J. interpreted results of experiments; S.M.R. and G.A.D. prepared figures; S.M.R. drafted manuscript; S.M.R., A.R.E., G.A.D., W.T.N., and M.J.J. edited and revised manuscript; S.M.R., A.R.E., G.A.D., W.T.N., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Shelly Roberts, Sarah Wolhart, and Pam Engrav for assistance.
REFERENCES
- 1.Capurro NL, Kent KM, Epstein SE. Comparison of nitroglycerin-, nitroprusside-, and phentolamine-induced changes in coronary collateral function in dogs. J Clin Invest 60: 295–301, 1977. doi: 10.1172/JCI108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenach JH, Schroeder DR, Pike TL, Johnson CP, Schrage WG, Snyder EM, Johnson BD, Garovic VD, Turner ST, Joyner MJ. Dietary sodium restriction and beta2-adrenergic receptor polymorphism modulate cardiovascular function in humans. J Physiol 574: 955–965, 2006. doi: 10.1113/jphysiol.2006.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friederich JA, Butterworth JF 4th. Sodium nitroprusside: twenty years and counting. Anesth Analg 81: 152–162, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Gerson JI, Allen FB, Seltzer JL, Parker FB Jr, Markowitz AH. Arterial and venous dilation by nitroprusside and nitroglycerin—is there a difference? Anesth Analg 61: 256–260, 1982. doi: 10.1213/00000539-198203000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hart EC, Wallin BG, Barnes JN, Joyner MJ, Charkoudian N. Sympathetic nerve activity and peripheral vasodilator capacity in young and older men. Am J Physiol Heart Circ Physiol 306: H904–H909, 2014. doi: 10.1152/ajpheart.00181.2013. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, Cheriyan J, Monteith S, Brown MJ, Wilkinson IB. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol 25: 1891–1895, 2005. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- 8.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. doi: 10.1161/01.CIR.101.8.862. [DOI] [PubMed] [Google Scholar]
- 9.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol (1985) 109: 768–777, 2010. doi: 10.1152/japplphysiol.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]