Abstract
BACKGROUND
In the care of patients with congenital heart disease, percutaneous interventional treatments have supplanted many surgical approaches for simple lesions, such as atrial septal defect. By contrast, complex congenital heart defects continue to require open-heart surgery. In single-ventricle patients, a staged approach is employed, which requires multiple open-heart surgeries and significant attendant morbidity and mortality. A nonsurgical transcatheter alternative would be attractive.
OBJECTIVES
We sought to show the feasibility of catheter-only, closed-chest, large-vessel anastomosis (superior vena cava and pulmonary artery [PA] or bidirectional Glenn operation equivalent) in a patient.
METHODS
In preclinical testing over a decade, we developed the techniques and technology needed for nonsurgical crossing from a donor (superior vena cava) to a recipient (PA) vessel and endovascular stent-based anastomosis of those blood vessels. We undertook this transcatheter approach for an adult with untreated congenital heart disease with severe cyanosis and significant surgical risk. We rehearsed the procedure step by step using contrast-enhanced cardiac computed tomography and a patient-specific 3-dimensional printed heart model.
RESULTS
We described a first-in-human, fully percutaneous superior cavopulmonary anastomosis (bidirectional Glenn operation equivalent). The patient, a 35-year-old woman, was homebound due to dyspnea and worsening cyanosis. She was diagnosed with functional single ventricle and very limited pulmonary blood flow. The heart team believed surgical palliation conferred high operative risk due to the patient’s complete condition. With the percutaneous procedure, the patient suffered little morbidity and remained improved clinically after 6 months.
CONCLUSIONS
This procedure may provide a viable alternative to one of the foundational open-heart surgeries currently performed to treat single-ventricle congenital heart disease.
Keywords: adult congenital heart disease, catheterization, Glenn shunt, image-guided intervention, single ventricle, transcatheter electrosurgery
Graphical abstract
Introduction
Many patients with congenital heart disease are born with 1 main pumping chamber or a functional single ventricle rather than the normal 2-ventricle circulation. Infants born with a single ventricle number about 5 per 10,000 live births (1). Without multiple palliative open-heart surgeries, their prognosis is poor and mortality is high. Occasionally, patients with a single ventricle present in adulthood (1).
In single-ventricle patients, surgery is often required in infancy to establish a temporizing source of pulmonary blood flow (2,3). Thereafter, improved and stable pulmonary blood flow is established by the bidirectional Glenn shunt (superior cavopulmonary anastomosis) surgery, which diverts superior vena cava (SVC) blood flow into the pulmonary arteries (PA) (4,5). Months or years later, a passive pulmonary blood flow circuit is completed with the modified Fontan surgery, which consists of an inferior cavopulmonary anastomosis (6). This allows the single ventricle to exclusively support the systemic circulation.
Multiple open-heart surgeries, each requiring median sternotomy and cardiopulmonary bypass, come at the expense of cumulative morbidity and mortality. The superior cavopulmonary anastomosis has a morbidity rate close to 20% and mortality approximately 5% (7). Some post-surgical patients eventually require heart transplantation (8). Pre-operative atrioventricular valve regurgitation is an independent risk factor for death or heart transplantation (7,8). Post-operative morbidity can affect many organ systems, ranging from chylothorax and diaphragm paralysis to extracorporeal membrane oxygenation and cardiac arrest (7,9). Surgery in adults with single-ventricle congenital heart disease confers additional risk (10).
For more than 2 decades, our group and others have been working to develop minimally invasive, nonsurgical therapy for patients with single-ventricle physiology (11–13). We describe a novel entirely transcatheter nonsurgical closed-chest procedure, which is the anatomic equivalent of the contemporary surgical superior cavopulmonary anastomosis. Our approach used transcatheter electro-wire perforation to cross unoperated or naive intact blood vessel walls and transcatheter anastomosis using stents to connect adjacent large blood vessels.
Methods
Previously, we investigated a percutaneous approach to cavopulmonary anastomosis using modified magnet catheters. Ultimately, we were successful in using trans-septal needle puncture aimed at balloon targets, followed by covered stent placement in animals to create a “classic Glenn” or unidirectional cavopulmonary anastomosis (SVC connected exclusively to the right PA and excluding the left PA) (12). More recently, we accomplished transcatheter bidirectional cavopulmonary anastomosis (SVC blood flow distributed to both the right and left PA) using real-time cardiac magnetic resonance imaging guidance in animals (13).
We have tested an x-ray fluoroscopy-guided technique to cross between nearby blood vessels (inferior vena cava/abdominal aorta) using an off-the-shelf guidewire electrified by connecting to an electrosurgery pencil or “bovie” in animals (14). To prepare for the procedure, we tested this identical vessel-crossing technique from the SVC into the PA in 6 animals. For the cavopulmonary anastomosis application, we positioned a coaxial crossing system (Figure 1) from the SVC, consisting of a 0.014” Astato XS20 guidewire (Asahi Intecc, Santa Ana, California), 0.035” PiggyBack wire converter (Teleflex Vascular Solutions, Inc. Minneapolis, Minnesota), NAVICROSS microcatheter (Terumo Interventional Systems, Somerset, New Jersey), and JR4 guiding catheter (Cordis, Milpitas, California). We aimed the crossing system toward an Amplatz Gooseneck target snare (Medtronic, Minneapolis, Minneapolis) in the PA. The 0.014” guidewire was electrified by attaching the back end to the electrosurgery pencil (Figure 1) set to pure cutting mode at 50 W prior to advancing the wire across donor and recipient vessel walls. Once across, the guidewire was exchanged for a stent delivery system. Vessel-to-vessel crossing was successful in all. In 3 of those animals, we attempted transcatheter covered stent implantation to achieve cavopulmonary anastomosis. All were successful. Animal procedures were approved by the institutional animal care and use committee, and followed contemporary National Institutes of Health guidelines.
Figure 1. Co-axial Wire-crossing System.
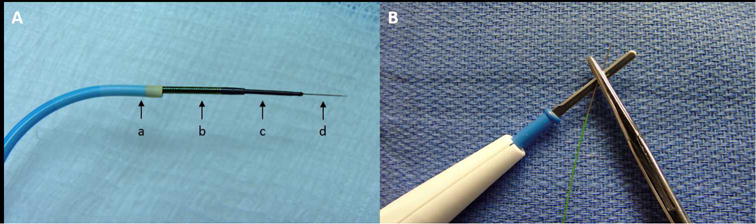
(A) A guiding catheter (a) directed the coaxial wire crossing system consisting of a microcatheter (b), 0.035” wire converter (c), and 0.014” commercial guidewire (d). (B) The guidewire was electrified by connecting the back end to an electrosurgery pencil.
Preliminary Preparation and Procedure
We trained in the electro-wire crossing technique with experienced operators during clinical transcaval access for transcatheter aortic valve replacement (15). We planned the procedure step by step using pre-procedure contrast-enhanced computed tomography (CT) (Figure 2) (16). For vessel-to-vessel crossing, we paid particular attention to an optimal SVC exit point and PA entry point, fluoroscopic visible landmarks and projection angles, and avoiding interposed vital structures. For covered- and bare–metal stent (BMS) anastomosis, target dimensions and distances were planned. We simulated the procedure beforehand on a patient-specific 3-dimensional (3D) printed heart model (Figure 3) based on the contrast-enhanced cardiac CT.
Figure 2. Contrast-enhanced Cardiac CT.
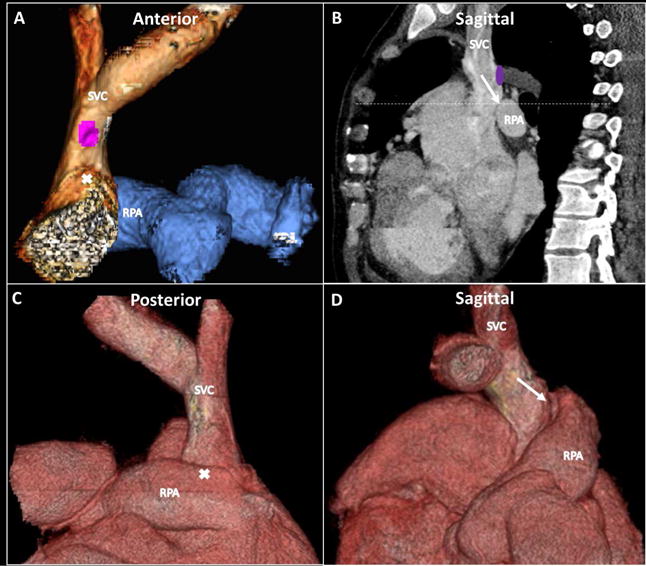
Pre-procedure contrast-enhanced computed tomography (CT) was used for procedure planning (A, C, and D: 3-dimensional (3D) reconstructions; B: sagittal plane) and included fluoroscopic visible landmarks (B, dashed line). CT imaging was used to plan target dimensions (anastomotic balloon expandable covered and anchoring bare-metal stent diameters) and understand distance requirements (covered stent lengths for anastomosis coverage without neighboring vessel occlusion). Purple circle = origin of azygous vein; white arrow = planned point and angle of exit and entry; white X corresponds to white arrow tip on corresponding alternate views. RPA = right pulmonary artery; SVC = superior vena cava.
Figure 3. 3D-printed Heart Model.
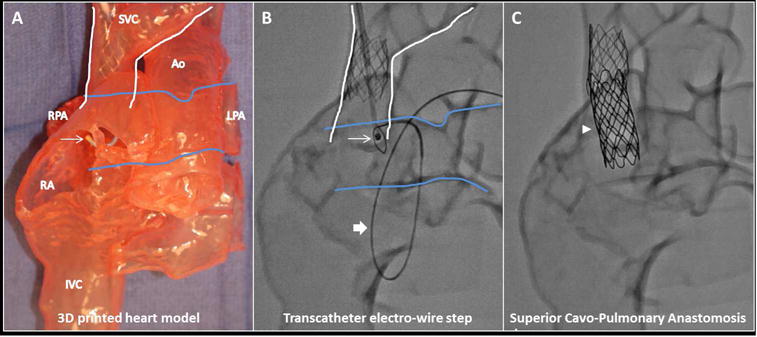
(A) In this 3D-printed heart model based on the contrast-enhanced CT (view from anterior), the SVC is outlined in white and pulmonary artery in blue. The anchoring SVC stent is in place with the guiding catheter (arrow) positioned in the SVC prior to vessel traversal. Subsequent key procedural steps seen with x-ray fluoroscopy of the 3D-printed heart model show (B) the transcatheter electro-wire step with electrified 0.014” guidewire aimed from SVC (arrow) to RPA (loop snare catheter; block arrow); the snare catheter course was retrograde from aorta to single ventricle to right pulmonary artery; and (C) end-to-side anastomotic endograft (arrowhead) in place. Ao = aorta, IVC = inferior vena cava, LPA = left pulmonary artery, RA = right atrium, other abbreviations as in Figure 2.
The procedure has 3 key steps: 1) the walls of 2 nearby blood vessels to be connected (SVC and right PA) were traversed by electrifying a small, insulated commercial 0.014” guidewire as described (14,15); 2) after the wire connected the 2 large blood vessels, we implanted a covered stent (“endograft”) to bridge the 2 vessels; and 3) BMS anchoring was used as an SVC end-to-end anastomosis landing zone and to secure the right PA end-to-side anastomosis. In addition, the azygous vein was occluded with a vascular plug device to complete the flow diversion.
Patient Description
A 35-year-old woman first sought medical attention for resting cyanosis (blood oxygen saturation levels: 60% to 70%) and was homebound due to dyspnea. She was diagnosed with functional single ventricle and very limited pulmonary blood flow (dextrocardia, “criss-cross” atrioventricular connections, L-looped ventricles, hypoplastic tricuspid valve and morphologic right ventricle [moderate], ventricular septal defect [large], and subvalvar and valvar pulmonary stenosis [severe]). There was moderate left-sided atrioventricular valve regurgitation and paroxysmal atrial tachycardia. Her dyspnea precluded exercise stress testing. Diagnostic cardiac catheterization showed mean PA pressure of 13 mm Hg, transpulmonary pressure gradient of 4 mm Hg, and normal pulmonary vascular resistance (1.2 Wood units).
After evaluation and deliberation, our multidisciplinary heart team (primary cardiology, interventional cardiology, cardiothoracic surgery, cardiac critical care, cardiac electrophysiology, adult congenital heart disease, radiology, and cardiac anesthesia) (17) concluded that a strategy to augment pulmonary blood flow with superior cavopulmonary anastomosis would improve this patient’s oxygen saturation and relieve her symptoms. The team believed surgical palliation conferred high operative risk because of the combination of: atrioventricular valve regurgitation (7,8); longstanding pre-operative cyanosis; pre-operative arrhythmia; New York Heart Association (NYHA) class IV (18); potential for post-operative bleeding requiring re-exploration (19); dilated systemic ventricle (20); potential for post-operative acute kidney injury (21); and aorto-pulmonary collateral burden. Pre-procedure hemoglobin was 19.3 g/dl, hematocrit was 61.9%, blood urea nitrogen was 12 mg/dl, and creatinine was 0.6 mg/dl. Echocardiogram showed qualitatively normal left ventricular systolic function. The multidisciplinary heart team concluded that a percutaneous approach with provisional surgical bail-out was an appropriate management strategy. Detailed informed consent was obtained from the patient, given the novel nature of the procedure and the “off-label” use of medical devices. Per institutional protocol, we obtained consent to publish directly from the patient.
Results
The procedure was performed under general anesthesia with right internal jugular (16-F) and bilateral femoral arterial (4- and 5-F) and venous (7- and 12-F) vascular access. Image guidance was conventional x-ray fluoroscopy (Central Illustration), as well as 3D rotational angiography and overlaid pre-procedure contrast-enhanced cardiac CT. Transcatheter electro-wire vessel-to-vessel crossing, proximal end-to-end SVC anastomosis, and a distal end-to-side pulmonary anastomosis (preserving blood flow to both branch PAs) were successful. The vessel-to-vessel crossing procedure was performed as described in the methods. Anastomotic endografts (CP8Z45 and CP8Z34, NuMED, Hopkinton, New York) were balloon dilated to 20 mm. The SVC landing zone Intrastent LD Max 26 mm BMS (Medtronic) was also balloon dilated to 20 mm. Right PA end-to-side anastomosis was secured with an Intrastent LD Max 36 mm BMS (Medtronic) balloon dilated to 16 mm. We selected open-cell design anchor stents to allow future access through side struts if indicated. The azygous vein was occluded with an Aplatzer Vascular Plug II 10 mm (St. Jude Medical, Plymouth, Minnesota), but also covered by the endograft. There were no residual pressure gradients and no angiographic stenosis or leak across the cavopulmonary anastomosis.
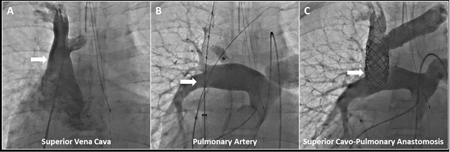
Central Illustration. Transcatheter Superior Cavopulmonary Anastomosis: Contrast X-ray Angiography.
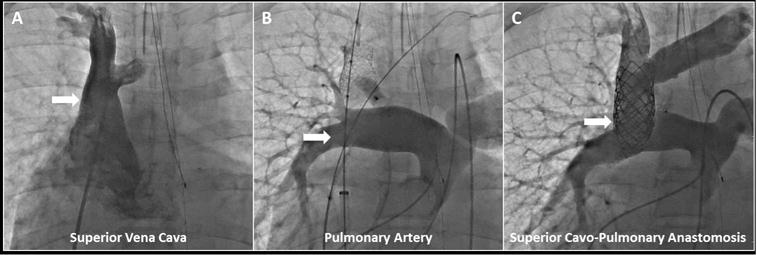
(A) Pre-intervention superior vena cava angiogram was performed with the angiographic catheter introduced from femoral venous access, while (B) pre-intervention pulmonary artery angiogram was performed with the angiographic catheter introduced from femoral arterial access (retrograde to single ventricle through stenotic pulmonary valve). Superior vena cava anchor stent (and post-deployment balloon, sheath, wire), left innominate marking wire, and pacing catheter introduced from femoral venous access are shown. Angiographic catheter (introduced from right internal jugular) and deployed azygous vascular plug are also shown. (C) Post-transcatheter superior cavopulmonary anastomosis is seen via simultaneous angiography performed with previously described angiographic catheters introduced from femoral arterial and right internal jugular access.
Guidewire crossing induced brief intraprocedure runs of supraventricular tachycardia. No other procedural complications were encountered, including adjacent tissue (aorta) injury, hemo-or pneumothorax, hemo- or pneumopericardium, vessel dissection, thrombosis, or sustained dysrhythmia. Chest or mediastinal tube drainage was not indicated.
Before the intervention, the systemic arterial saturation with 100% inspired oxygen was 73%. Afterwards, the systemic arterial saturation without supplemental inspired oxygen was 82%. Mean central and branch PA pressure increased from 13 to 14 mm Hg. As expected, SVC pressure increased from 8 mm Hg (pre-intervention) to 14 mm Hg (post-intervention). Vessel dimensions were similar before and after intervention (SVC: 20 mm; right PA: 16 mm).
The patient was extubated within hours and able to walk without dyspnea on the first post-procedure day, but kept in the hospital out of caution for 5 days before being discharged home. Follow-up chest radiography, cardiac CT (Figure 4), and contrast echocardiography (upper extremity bubble study) demonstrated the intended bypass was without endovascular leak or evident complications. Oxygen saturation (breathing room air) was as expected after superior cavopulmonary anastomosis at discharge (81%), after 10 days (87%), 1 month (82%), 2 months (89%), and 4 months (85%). She has been maintained on aspirin and clopidogrel. The patient reported significant improvement in her ability to walk multiple blocks dyspnea-free and carry out activities of daily living (NYHA class I). With improved physical conditioning, she has been able to exercise for 45 min daily without cardiopulmonary symptoms.
Figure 4. Video: Post-intervention Transcatheter Superior Cavopulmonary Anastomosis.
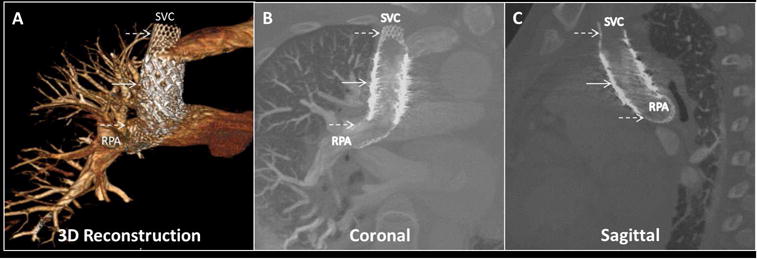
The successful transcatheter superior cavopulmonary anastomosis is seen (A) via a 3D-reconstructed image. Solid arrow = anastomotic covered stent endograft bridging SVC and RPA; dashed arrows = bare-metal locking anchor stents. Traditional oblique (B) coronal and (C) sagittal planes are shown using contrast-enhanced cardiac CT. Abbreviations as in Figure 2.
Discussion
We report successful creation of a large-vessel anastomosis (20 mm) and superior cavopulmonary shunt in an unoperated patient. There was no prior surgical preparation (22). This was a percutaneous transcatheter alternative to a fundamental contemporary congenital heart surgery. In this patient, the percutaneous approach avoided the expected high morbidity of open-heart surgery and cardiopulmonary bypass.
Our group and others have explored numerous related preclinical approaches to endovascular bypass over the past decade (12,13,23). The vessel-to-vessel crossing technique was first described by our group (14). In preparation for the described clinical case, we tested this vessel-to-vessel crossing maneuver at the intended anatomic site and transcatheter cavopulmonary anastomosis in animals and in vitro using patient-specific 3D printed heart models (Figures 1, 2, and 3). The procedure was guided by standard biplane x-ray contrast angiography (Central Illustration, Online Video), 3D rotational angiography, and prior cardiac CT images fused with live x-ray fluoroscopy. Pre-procedure planning and augmented imaging guidance facilitated 3 enabling steps. The trajectory for transcatheter electro-wire traversal across neighboring vessel tissue planes was defined. The anastomotic endograft was positioned to provide coverage for SVC cardiac bypass, ensure bidirectional blood flow to both branch PAs, and avoid catastrophic miss. The sites in the SVC and in the right PA were defined for the bare-metal anchoring stents. The caudal aspect of the right PA was not instrumented and remains available for a possible future transcatheter or surgical inferior cavopulmonary anastomosis.
This procedure reflected a technical evolution in crossing and connecting blood vessels, beginning with the pioneering vascular anastomosis that garnered the 1912 Nobel Prize in Physiology or Medicine. In animals, our group and others have used needle (12) and radiofrequency perforation (23) for unidirectional “classic” Glenn shunts (SVC solely to the right PA) and more recently the contemporary, and more technically demanding, bidirectional (both PAs) version (13). In post-operative Fontan patient case reports, small pulmonary artery-to-atria fenestrations (24) and medium-sized PA reconnections (25) have been created. In unoperated patients, sharp wire puncture was used to create a restrictive 7 mm transcatheter Potts shunt (small descending thoracic aorta to left PA communication) surrogate in a small number of adults with end-stage PA hypertension (26). We employed our new transcatheter electro-wire crossing technique currently being used to enable precise crossing from inferior vena cava to aorta for temporary access prior to tract device closure (14,15).
Diverting upper body systemic venous return to the PAs is a foundational surgery used to manage patients with functional single ventricles. This creates a passive pulmonary circulation. We have reported initial success with the first nonsurgical alternative. Employing a new percutaneous, closed chest, transcatheter technique, we created a large-vessel, end-to-side anastomosis as a replacement for surgical superior cavopulmonary anastomosis (bidirectional Glenn operation). Recovery was rapid; oxygen saturation and dyspnea improvement was immediate. The patient was clinically suitable for discharge the day following the procedure, but was kept in hospital out of an abundance of caution.
Study Limitations
Our technique is new; this is the first-in-human report of a single patient. Current surgical anastomotic techniques have been built on decades of experience. Surgical vascular anastomoses are secured using sutures that create little obstruction to blood flow. Our approach replaced suture fixation with intravascular metallic open-cell stent anchors that crossed neighboring vessels. Both achieve hemostasis by tight apposition of graft material to the rents in the donor and recipient blood vessel. Crossing and connecting blood vessels adds risk of catastrophic miss (26) or unintended endograft vessel exclusion (i.e., left PA) to known risks of interventional cardiac catheterization. We attempted to mitigate this risk with preclinical testing, CT procedure planning, 3D-printed anatomic model simulation, and multimodality image guidance. This early experience was in an adult patient with large PAs using off-the-shelf devices. Our initial impression was that our unpalliated cyanotic adult patient with single-ventricle congenital heart disease and low pulmonary vascular resistance was a rare entity. However, similar patients have surfaced at our institution (including some patients with Ebstein’s Anomaly of the tricuspid valve) who may benefit from this transcatheter strategy. In general, adult patients in whom a one-and-a-half ventricle repair is indicated could be considered. Percutaneous therapy for typical cavopulmonary anastomosis in small children could be achieved through smaller purpose-built devices, tailored to overcome the limitations we described of off-the-shelf devices. We are developing such a device (13).
Conclusions
Large-vessel anastomosis, a mainstay of open-heart surgery, has a long history but carries significant concomitant morbidity and mortality. We reported the first-in-human closed chest, large-vessel anastomosis. This was performed in unoperated anatomy. It was a percutaneous replacement for open-chest surgical therapy. Future advances in imaging guidance (i.e., cardiac magnetic resonance) and purpose-built devices will likely simplify these procedures.
Supplementary Material
CONDENSED ABSTRACT.
We reported a first-in-human, fully percutaneous, closed-chest, large-vessel anastomosis joining the superior vena cava and pulmonary artery (superior cavopulmonary anastomosis or bidirectional Glenn operation equivalent). The patient suffered little morbidity and remained improved 6 months later. Large-vessel anastomosis is a mainstay of open-heart surgery; it has a long history but significant concomitant morbidity and mortality. This approach may offer a viable alternative to one of the foundational open-heart surgeries currently performed to treat single ventricle congenital heart disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Over the past 2 decades, several groups of investigators have pursued transcatheter methods designed to replace surgical palliation for patients with single-ventricle congenital heart disease. Except for our animal studies, previous efforts have focused on performing Fontan completion (stage 3) after surgical preparation, rather than transcatheter creation of a bidirectional Glenn anastomosis (stage 2). We reported the first-in-human nonsurgical, closed-chest, transcatheter large-vessel anastomosis; this was the anatomic equivalent of the contemporary surgical superior cavopulmonary anastomosis (bidirectional Glenn operation).
TRANSLATIONAL OUTLOOK
Ongoing imaging, procedural technique, and device evolution will establish viable nonsurgical, minimally invasive therapeutic options for patients who now require multiple open-heart surgeries with its attendant morbidity and mortality.
Acknowledgments
We would like to thank John Lamberti, Jeffrey Frazer, Michael Worthen, Justin Yeh, Harjot Bassi, Tommy Do, Raghav Murthy, and Adam Greenbaum for clinical support and consultation.
Funding: Supported by intramural funds at Division of Pediatric Cardiology - Rady Children’s Hospital and by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health (Z01-HL006040 to RJL).
ABBREVIATIONS AND ACRONYMS
- 3D
3-dimensional
- CT
computed tomography
- NYHA
New York Heart Association
- PA
pulmonary artery
- SVC
superior vena cava
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Transmural Systems: collaborator (KR, RJL), advisor (JWM); RR, SRH, HE: no disclosures
References
- 1.Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation. 2007;115:800–12. doi: 10.1161/CIRCULATIONAHA.105.592378. [DOI] [PubMed] [Google Scholar]
- 2.Blalock A, Taussig HB. Landmark article May 19, 1945: The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock and Helen B. Taussig JAMA. 1984;251:2123–38. doi: 10.1001/jama.251.16.2123. [DOI] [PubMed] [Google Scholar]
- 3.Muller WH, Jr, Danimann JF., Jr The treatment of certain congenital malformations of the heart by the creation of pulmonic stenosis to reduce pulmonary hypertension and excessive pulmonary blood flow; a preliminary report. Surg Gynecol Obstet. 1952;95:213–9. [PubMed] [Google Scholar]
- 4.Glenn WW. Circulatory bypass of the right side of the heart. IV. Shunt between superior vena cava and distal right pulmonary artery; report of clinical application. N Engl J Med. 1958;259:117–20. doi: 10.1056/NEJM195807172590304. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RA, Armstrong BE, Serwer GA, Peterson RJ, Oldham HN., Jr Physiological rationale for a bidirectional cavopulmonary shunt. A versatile complement to the Fontan principle. J Thorac Cardiovasc Surg. 1985;90:391–8. [PubMed] [Google Scholar]
- 6.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TM, Aiyagari R, Hirsch JC, Ohye RG, Bove EL, Devaney EJ. Risk factor analysis for second-stage palliation of single ventricle anatomy. Ann Thorac Surg. 2012;93:614–8. doi: 10.1016/j.athoracsur.2011.10.012. discussion 619. [DOI] [PubMed] [Google Scholar]
- 8.Scheurer MA, Hill EG, Vasuki N, et al. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. J Thorac Cardiovasc Surg. 2007;134:82–9. 89 e1–2. doi: 10.1016/j.jtcvs.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, Kanter KR. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237–42. doi: 10.1016/j.jtcvs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Kogon B, Oster M. Assessing surgical risk for adults with congenital heart disease: are pediatric scoring systems appropriate? J Thorac Cardiovasc Surg. 2014;147:666–71. doi: 10.1016/j.jtcvs.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorf G, Schneider M, Konertz W. Surgical preconditioning and completion of total cavopulmonary connection by interventional cardiac catheterisation: a new concept. Heart. 1996;75:403–9. doi: 10.1136/hrt.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi DS, Danon S, Gordon B, et al. Creation of transcatheter aortopulmonary and cavopulmonary shunts using magnetic catheters: feasibility study in swine. Pediatr Cardiol. 2009;30:397–403. doi: 10.1007/s00246-009-9422-5. [DOI] [PubMed] [Google Scholar]
- 13.Ratnayaka K, Rogers T, Schenke WH, et al. Magnetic Resonance Imaging-Guided Transcatheter Cavopulmonary Shunt. JACC Cardiovasc Interv. 2016;9:959–70. doi: 10.1016/j.jcin.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halabi M, Ratnayaka K, Faranesh AZ, Chen MY, Schenke WH, Lederman RJ. Aortic access from the vena cava for large caliber transcatheter cardiovascular interventions: preclinical validation. J Am Coll Cardiol. 2013;61:1745–6. doi: 10.1016/j.jacc.2013.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum AB, Babaliaros VC, Chen MY, et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J Am Coll Cardiol. 2017;69:511–21. doi: 10.1016/j.jacc.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederman RJ, Greenbaum AB, Rogers T, Khan JM, Fusari M, Chen MY. Anatomic Suitability for Transcaval Access Based on Computed Tomography. JACC Cardiovasc Interv. 2017;10:1–10. doi: 10.1016/j.jcin.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation. 2015;131:1884–931. doi: 10.1161/CIR.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 18.Vida VL, Berggren H, Brawn WJ, et al. Risk of surgery for congenital heart disease in the adult: a multicentered European study. Ann Thorac Surg. 2007;83:161–8. doi: 10.1016/j.athoracsur.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Putman LM, van Gameren M, Meijboom FJ, et al. Seventeen years of adult congenital heart surgery: a single centre experience. Eur J Cardiothorac Surg. 2009;36:96–104. doi: 10.1016/j.ejcts.2009.01.046. discussion 104. [DOI] [PubMed] [Google Scholar]
- 20.Vogt MO, Horer J, Grunewald S, et al. Independent risk factors for cardiac operations in adults with congenital heart disease: a retrospective study of 543 operations for 500 patients. Pediatr Cardiol. 2012;33:75–82. doi: 10.1007/s00246-011-0093-7. [DOI] [PubMed] [Google Scholar]
- 21.Kwiatkowski DM, Price E, Axelrod DM, et al. Incidence, risk factors, and outcomes of acute kidney injury in adults undergoing surgery for congenital heart disease. Cardiol Young. 2016:1–8. doi: 10.1017/S1047951116002067. [DOI] [PubMed] [Google Scholar]
- 22.Metton O, Calvaruso D, Stos B, Ben Ali W, Boudjemline Y. A new surgical technique for transcatheter Fontan completion. Eur J Cardiothorac Surg. 2011;39:81–5. doi: 10.1016/j.ejcts.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt B, Sabi TM, Sigler M, Berger F, Ewert P. Upper cavo-pulmonary anastomosis by transcatheter technique. Catheter Cardiovasc Interv. 2012;80:93–9. doi: 10.1002/ccd.23458. [DOI] [PubMed] [Google Scholar]
- 24.Mehta C, Jones T, De Giovanni JV. Percutaneous transcatheter communication between the pulmonary artery and atrium following an extra-cardiac Fontan: an alternative approach to fenestration avoiding conduit perforation. Catheter Cardiovasc Interv. 2008;71:936–9. doi: 10.1002/ccd.21453. [DOI] [PubMed] [Google Scholar]
- 25.AboulHosn J, Danon S, Levi D, Castellon Y, Child J, Moore J. Regression of pulmonary arteriovenous malformations after transcatheter reconnection of the pulmonary arteries in patients with unidirectional Fontan. Congenit Heart Dis. 2007;2:179–84. doi: 10.1111/j.1747-0803.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 26.Esch JJ, Shah PB, Cockrill BA, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013;32:381–7. doi: 10.1016/j.healun.2013.01.1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


