Abstract
Györke et al. discuss the role of sarcoplasmic reticulum Ca2+ in cardiac refractoriness and pathological implications.
Introduction
Pumping by the heart results from the synchronous contraction and relaxation of billions of cardiac cells. Immediately after each heartbeat and during relaxation, the cardiac muscle becomes functionally silent and will not respond to external electrical stimuli for a distinct time period. Although known to Italian physiologists in the 18th century, the discovery of this fundamental property of the heart is commonly attributed to the French cardiologist Eitienne-Jules Marey, who introduced the term “mechanical refractoriness” to describe this phenomenon in 1875 (Hoff, 1942). We now know that a temporary inactivation of Ca signaling underlies mechanical refractoriness and that it is likely mediated by mechanisms operating within the lumen of the SR. In this perspective, we review experimental and theoretical evidence for the regulation of SR Ca release by luminal Ca and examine how that mechanism contributes to Ca signaling refractoriness. We also discuss how alterations in this mechanism give rise to triggered arrhythmias. In particular, we summarize recent work showing how shortened refractoriness of cardiac ryanodine receptor (RyR2) channels results in synchronization of aberrant SR Ca release to cause arrhythmias.
Cardiac refractoriness
Mechanical refractory period is defined as the time required for full recovery of cardiac contractile force after contraction. Quantitative assessment of contractile restitution performed beginning from the 1950s in various cardiac muscle preparations demonstrated that, in the mammalian heart, mechanical refractoriness lasts ∼1 s (0.5–2 s, depending on species and experimental conditions; Siebens et al., 1959; Kruta and Braveny, 1963; Gibbons and Fozzard, 1975; Edman and Jóhannsson, 1976). Interestingly, this period was found to be substantially longer than the time required for the recovery of cardiac electrical excitability that initiates the mechanical activity of the heart (0.1–0.3 s; Siebens et al., 1951; Brooks et al., 1960; Van Dam et al., 1964). These early observations have led to a general conclusion that, rather than reflecting changes in electrical excitability, the bulk of mechanical restitution is determined by processes occurring at some later steps in the heartbeat generation, beyond that of electrical excitation. Some have even ascribed that refractoriness to “altered metabolism of activator Ca in the excitation–contraction coupling” (Edman and Jóhannsson, 1976).
Currently, the role of Ca in cardiac excitation–contraction (EC) coupling is well defined. Cardiac contraction results from CICR, whereby a small influx of Ca through the sarcolemma triggers the Ca release channels on the SR membrane, RyR2, allowing a release of Ca from the SR stores much greater than the triggering influx. It is that Ca release from the SR that is able to engage the contractile machinery, thereby precipitating contraction (Bers, 2001). In contrast, recycling of Ca back to the SR by the SR Ca–ATPase underlies relaxation. During that relaxation period, the functional activity of RyR2 in healthy cardiomyocytes is quenched to prevent further Ca release during diastole (Williams et al., 1992; Cheng et al., 1996). This temporary inactivation of Ca signaling opposes the positive feedback of CICR and facilitates mechanical refractoriness of the heart. Notably, impaired Ca-signaling refractoriness results in aberrant diastolic SR Ca release, a major factor underlying cardiac arrhythmias (Belevych et al., 2012; Brunello et al., 2013). Although the mechanisms of activation of SR Ca release have been well characterized (Cannell and Kong, 2012; Eisner, 2014; Hong and Shaw, 2017), the processes underlying functional inactivation of release and factors accounting for impaired refractoriness in cardiac disease remain to be fully elucidated. However, evidence accumulated during the past 10–15 yr suggests that Ca signaling and mechanical refractoriness are likely mediated by mechanisms that reside at the luminal side of the SR (for a review, see Stern and Cheng, 2004; Sobie and Lederer, 2012; Radwański et al., 2013; see Sobie et al. in this issue).
Control of SR Ca release by luminal Ca
Ca release from the SR is, in principle, a self-contained process; once initiated, it should terminate upon depletion of the Ca store. During normal EC coupling, however, only a fraction of the total stored Ca is released from the SR (Bassani et al., 1993, 1995; Shannon et al., 2003). Furthermore, as demonstrated by studies of both cell-wide and local SR Ca release, after each event of Ca release, RyR2s remain refractory to subsequent activation despite refilling of SR Ca stores (Sobie et al., 2005; Ramay et al., 2011; Belevych et al., 2012). These and similar studies led to the general view that CICR from the SR is modulated by some “restraining” mechanisms. In turn, these factors would help SR Ca release to terminate and facilitate relaxation by preventing RyR2 reopening during diastole. Importantly, such mechanisms would also contribute to the mechanical refractoriness of the heart. In contrast, impairment of these factors may result in aberrant diastolic Ca release that can precipitate cardiac arrhythmias in the diseased heart (Sobie and Lederer, 2012; Belevych et al., 2013; Radwański et al., 2013; Bers, 2014).
Based on early experiments by Fabiato (1985a) in skinned myocytes, the mechanism responsible for termination of Ca release and Ca-cycling refractoriness was believed to involve inactivation of RyR2 by cytosolic Ca. However, subsequent studies conducted either in isolated cardiomyocytes or single RyR2 channels failed to provide substantial evidence in support of that theory (Näbauer and Morad, 1990; DelPrincipe et al., 1999; Lukyanenko and Gyorke, 1999; Fill et al., 2000). Instead, work from several laboratories pointed to a different mechanism, which was most likely occurring at the luminal side of the SR.
The possibility of regulation of SR Ca release by luminal Ca was first suggested by experiments conducted in single RyR2 channels reconstituted in bilayers, which revealed that RyR2 activity is considerably diminished at lowered luminal Ca (Sitsapesan and Williams, 1994; Lukyanenko et al., 1996; Györke and Györke, 1998). However, experimental evidence in support of this mechanism in situ was obtained from cellular studies in which the buffering capacity of the SR was exogenously increased by the introduction of Ca chelators into the SR. That strategy allowed for a slower SR Ca depletion without affecting the regulatory processes at the cytosolic side of the RyR2 channel (Terentyev et al., 2002). Buffering luminal Ca markedly slowed termination of SR Ca release and its restitution thus supporting the notion that termination of Ca release and its restitution are determined by luminal Ca regulation of RyR2 gating. In particular, it was suggested that termination of Ca release and the subsequent suppression of store availability are attributable to deactivation of RyR2 caused by lowered luminal Ca level after Ca release. Important support for this deactivation mechanism also came from mathematical simulations of Ca release termination in a cardiomyocyte model (Sobie et al., 2002). That study concluded that local SR Ca depletion coupled to luminal Ca-dependent changes in RyR2 gating was sufficient to account for termination of SR Ca release and release refractoriness in cardiac cells.
The role of luminal regulation of Ca release was further examined in studies that directly assessed depletion of the Ca signal during both global and local release in cardiomyocytes (Shannon et al., 2003). These and subsequent studies with intra-SR Ca indicators demonstrated that SR Ca release leaves a substantial reserve of free Ca within the SR after both global Ca transients and local Ca sparks (Kubalova et al., 2004; Picht et al., 2006; Belevych et al., 2007; Zima et al., 2008). This, therefore, offered support for the notion that, rather than causing termination by depletion, changes in luminal Ca modulate RyR2 gating activity to regulate its release.
Modulation of RyR2 by luminal Ca appears also to be consistent with studies that have examined the dependence of SR Ca release on the SR Ca content. In general, Ca release exhibits a steep, highly nonlinear dependency on the SR Ca content in cardiac myocytes (Bassani et al., 1995; Shannon et al., 2000; Bers, 2001; Eisner et al., 2013). This behavior has been commonly attributed to an increased recruitment of neighboring Ca release units via CICR as the SR Ca content increases. In addition, it may also involve direct effects of luminal Ca on the RyR2 channel complex, which encompasses accessory regulatory proteins, such as triadin (TRD), junctin (JNT), and histidine-rich Ca-binding protein (HRC). Importantly, Bassani et al. (1995) found that SR Ca release becomes nearly extinguished when the SR Ca content is lessened only modestly to 60% of its maximal level. Absence of release is a likely indication that lowered luminal Ca has an inhibitory role during Ca cycling, particularly through deactivation of RyR2s.
Recently, several studies have suggested that local depletion might be more complete than initially thought, based on measurements with SR-entrapped Ca indicators (reviewed by Sobie and Lederer, 2012; Sobie et al., 2017). Some others have pointed to mechanisms other than luminal regulation of RyR2 through which changes in SR Ca content could influence SR Ca release and its termination (see review in Cannell and Kong in this issue). For instance, Guo et al. (2012) found that, in permeabilized myocytes, large cations that reduce RyR2 channel permeability suppress Ca sparks and increase SR Ca load. The investigators concluded that the control of local Ca release by SR load is predominantly governed by the RyR2 current and not by the luminal Ca acting on intra-SR sites. Given the intrinsic propensity of SR Ca release to terminate by exhaustion of the Ca store, along with the technical difficulty of measuring or even controlling luminal Ca in cardiac cells, it is challenging to precisely define the contribution of luminal regulation to SR Ca release termination. Thus, despite substantial evidence in support of luminal Ca regulation of SR Ca release, the precise role of this mechanism in Ca release termination yet to be directly demonstrated.
Luminal Ca regulation and Ca signaling refractoriness
Similar to contraction and its refractoriness, Ca release becomes functionally suppressed after each release event. For instance, in mammalian cardiomyocytes, analogous to mechanical restitution, full recovery of the Ca transient amplitude takes ∼1 s (0.8–1.5 s depending on species; DelPrincipe et al., 1999; Szentesi et al., 2004; Belevych et al., 2012; Kornyeyev et al., 2012; Brunello et al., 2013; Fig. 1). There is a growing consensus that control by luminal Ca has a major role in that process. Based on the notion that during Ca release, lesser levels of luminal Ca deactivate RyR2, restitution of Ca release is expected to exhibit strong dependence on luminal Ca. Indeed, that notion is supported by studies that manipulated the rate of SR Ca reuptake to either slow or accelerate SR Ca refilling. For instance, interventions that delay SR Ca reuptake slow Ca signaling restitution, whereas those that facilitate those processes accelerate restitution (Terentyev et al., 2002; Szentesi et al., 2004). Importantly, restitution of SR Ca release is preceded by the recovery of Ca in the SR, thus indicating that restitution does not merely reflect Ca store refilling. As was recently demonstrated by our group by simultaneous monitoring the SR and cytosolic Ca (Belevych et al., 2012), the recovery of Ca transient amplitude was significantly delayed relative to the refilling of the SR Ca in canine ventricular myocytes (Fig. 1 B). A significant lag between SR refilling and Ca release channel availability has also been demonstrated at the subcellular level. For example, Sobie et al. (2005) demonstrated that the triggering probability for repetitive sparks lags behind spark amplitude recovery. Those authors concluded that, in addition to local SR refilling, as reflected by Ca spark amplitude recovery, the triggering probability depends on recovery of RyR2 from refractoriness.
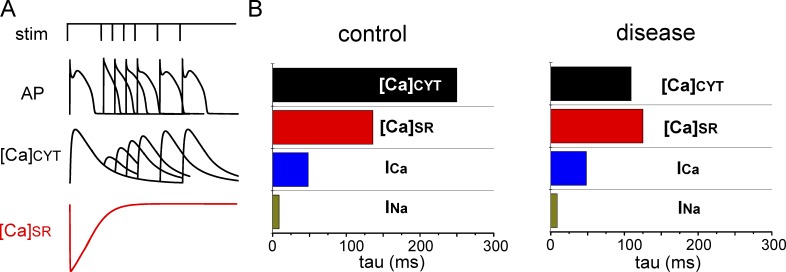
Figure 1.
Shortened Ca-signaling refractoriness in cardiac disease. (A) Schematic illustration of a two-pulse protocol experiment showing the recovery of electrically stimulated Ca release is significantly delayed in relation to the recovery of electrical excitability. (B) Exponential constants (tau) of the recovery of Ca-transient amplitude ([Ca]cyt), free SR Ca ([Ca]SR), the amplitude of L-type Ca current (ICa), and peak of voltage-gated Na current (INa), are shown for control and diseased myocytes. Data for [Ca]cyt and [Ca]SR were obtained from the two-pulse experiments in ventricular myocytes from control and diseased hearts (Belevych et al., 2011, 2012). Rates of recovery for INa and INa were obtained with the Hund and Rudy (2004) model of the canine ventricular myocyte. Note the shortened time delay between the recovery of [Ca]cyt and [Ca]SR in diseased hearts.
Further support for the role of luminal regulation in Ca-release refractoriness comes from studies of inherited arrhythmia disorders. For instance, catecholaminergic polymorphic ventricular tachycardia (CPVT), which is caused by aberrant Ca release, has also been associated with shortened refractoriness. This is particularly evident in cardiac calsequestrin (CASQ2)-associated CPVT. CASQ2 is thought to regulate the ability of RyR2 to deactivate at lower luminal Ca levels; hence, mutations in CASQ2, which decrease its affinity for the Ry2 complex, or its ablation result in impaired RyR2 deactivation and abbreviated Ca-release refractoriness (Knollmann et al., 2006; Kornyeyev et al., 2012; Brunello et al., 2013; discussed further in Modulation of Ca-release refractoriness). Of note, recent studies of CPVT have uncovered novel mutations in the cytoplasmic Ca-binding protein CaM (Xu et al., 2010; Oda et al., 2013; Yang et al., 2014; Søndergaard et al., 2015; Vassilakopoulou et al., 2015). Because CaM is known to inhibit RyR2 at elevated [Ca], it is possible that this protein also contributes to Ca-release refractoriness by inhibiting RyR2 after SR Ca release, when Ca at the cytosolic side of the channel is increased. These results, therefore, suggest that Ca-release refractoriness is a complex process that not only depends on luminal factors but also may rely on the cytosolic ones.
Collectively, these studies set the stage for our understanding of mechanisms responsible for quenching CICR in cardiac muscle. However, important questions about the specific role that luminal Ca may have in these mechanisms remain unanswered. Although strong support exists, particularly for the contribution of luminal Ca to Ca-signaling refractoriness, the role of luminal Ca in termination of Ca release remains to be clarified. Addressing the precise mechanisms by which local SR Ca depletion influences termination of SR Ca release will require advancement in luminal Ca–assessment techniques, along with novel, genetic mouse models that target the components of the RyR2 complex.
Modulation of Ca-release refractoriness and store availability
As an important factor in governing Ca cycling, Ca release refractoriness is subject to physiologic regulation. For example, β-adrenergic stimulation accelerates recovery from refractoriness at both the cellular and subcellular level (Szentesi et al., 2004; Ramay et al., 2011; Poláková et al., 2015). Combined with acceleration of SR Ca–ATPase–mediated SR Ca uptake, accelerated refractoriness serves to support the enhanced Ca cycling required to maintain increased cardiac contractility and beating rate during the adrenergic fight-or-flight response. Notably, the effects of β-adrenergic stimulation on RyR2 refractoriness are dependent on phosphorylation of RyR2-S2808 by PKA as well as Ca/calmodulin-dependent protein kinase II (CaMKII) at RyR2-S2814. Additionally, the effects of β-adrenergic stimulation on RyR2s may involve phosphorylation at -S2030, a site also implicated in enhanced RyR2 sensitivity to cytosolic and/or luminal Ca (Wehrens et al., 2004; Xiao et al., 2006).
In contrast to sympathetic stimulation, parasympathetic stimulation is generally thought to act by slowing cardiac Ca cycling via activation of muscarinic receptors; however, the specific mechanisms of the parasympathetic response and their relation to luminal Ca regulation remain largely unexplored. Recently, we demonstrated (Ho et al., 2016) that, in ventricular myocytes, muscarinic receptor stimulation initiates a distinct mode of EC coupling that operates at reduced intra-SR Ca levels, characteristic of the parasympathetic resting state. In particular, despite less SR Ca content, acetylcholine increased the fraction of Ca released during the cytosolic Ca transient and reduced wasteful diastolic SR Ca leakage. Therefore, in contrast to the performance-oriented mode of EC coupling during sympathetic stimulation, the parasympathetic “efficiency” mode is poised to attain maximal Ca release at minimal SR Ca load, thereby minimizing the energy costs of Ca cycling. The effect of acetylcholine on the fractional release is dependent on phosphorylation of RyR2s at Ser-2808. That, in turn, is accomplished by activation of PKG downstream of the muscarinic receptor type 2. The inhibitory effect of acetylcholine on SR Ca leak was associated with a reduction in RyR2 phosphorylation at Ser-2814, which was mediated by muscarinic receptor type 3, and a reduction in reactive oxygen species–dependent activation of CaMKII. However, further studies are needed to elucidate the mechanisms and physiologic implications of these modulatory influences, in particular, with respect to potential changes in responsiveness to luminal Ca and restitution properties of RyR2.
Molecular basis of control by luminal Ca
Despite the aforementioned evidence for the role of luminal Ca in regulating RyR2 gating, the specific molecular steps of this process are not fully resolved. The RyR2 is complexed with several SR proteins, including CASQ2, TRD, JNT, and HRC, which could facilitate luminal Ca control of RyR2 (Györke and Terentyev, 2008; Pritchard and Kranias, 2009; Faggioni and Knollmann, 2012; Marty, 2015). The possibility of direct regulation has also been advanced in studies exploiting heterologous systems overexpressing recombinant RyR2s, as well as those in which the RyR2 was reconstituted in lipid bilayers in the absence of its auxiliary proteins (Jiang et al., 2004, 2007; Priori and Chen, 2011). These studies suggested that certain mutations in RyR2 alter its sensitivity to luminal Ca and diminish intracellular Ca oscillations caused by an increased Ca load. Recently, a point mutation in the RyR2 (RyR2-E4872A) has been proposed to abolish luminal Ca activation of RyR2 without affecting its cytosolic Ca activation (Chen et al., 2014). This mutation is localized in the S6 helix bundle–crossing region, which corresponds to the putative gate structure of RyR2. Moreover, cardiomyocytes isolated from mice, which are heterozygous for this mutation, were reportedly resistant to store overload-induced Ca waves and exhibit reduced arrhythmia burden. Based on these results it has been suggested that direct activation of RyR2 by luminal Ca, independent of accessory proteins or effects of cytosolic Ca causes the release of Ca from the SR (Jiang et al., 2004, 2007; see Jones et al. in this issue).
Alternatively, studies from several laboratories have suggested that the sensitivity of RyR2 to luminal Ca is mediated by accessory proteins; among which, is CASQ2 (Györke et al., 2004, 2009; Beard et al., 2005; Qin et al., 2008, 2009). CASQ2 has been shown to facilitate luminal Ca dependence of RyR2s reconstituted in planar lipid bilayers. However, the effects of CASQ2 are dependent on the presence of TRD and JNT (Györke et al., 2004) and are influenced by cytosolic factors, such as ATP (Tencerová et al., 2012). Additionally, the role of CASQ2 in Ca handling has been demonstrated in experiments using myocytes derived from CASQ KO mice or mice expressing CASQ2 mutants associated with CPVT (Terentyev et al., 2003; Viatchenko-Karpinski et al., 2004; Knollmann et al., 2006). These studies revealed that the absence or functional defects in CASQ2 result in dysregulated SR Ca release, which, in turn, manifests as an impaired Ca-release termination, shortened Ca-signaling refractoriness, and an enhanced predisposition to aberrant Ca release. However, the effects of CASQ2 as an intra-SR Ca buffer on luminal regulation of RyR2 cannot be completely ruled out. It is noteworthy that the arrhythmogenic effects of at least some of the CPVT-associated CASQ2 mutants (e.g., R33Q) were independent of changes in Ca buffering. For instance, myocytes that expressed both CASQ2-R33Q and WT CASQ2 exhibited unaltered Ca buffering, as indicated by a lack of change in the SR Ca content (Terentyev et al., 2006). Hence, these results are consistent with the notion that CASQ2 can regulate RyR2 function independent of its buffering function. Collectively, these results suggest that CASQ2 dynamically regulates RyR2 channels during the cardiac cycle by inhibiting the channel at the lowered luminal Ca concentration after systolic Ca release. Therefore, genetic mutations compromising this inhibitory function of CASQ2 leave CICR unrestrained, thus facilitating arrhythmogenic aberrant Ca release.
As alluded to above, in addition to regulating RyR2 activity, CASQ2 acts as a major Ca buffer within the SR. In vitro studies have demonstrated that CASQ2 monomers polymerize in a Ca-dependent manner to bind additional Ca and then depolymerize at low luminal Ca levels to release the sequestered Ca (Park et al., 2003, 2004). This raised the intriguing possibility that changes in CASQ2 polymerization, coupled to variations in the SR Ca load, regulate myocyte SR Ca release. The ability of polymerized CASQ2 to change its polymerization state in response to depletion of luminal Ca has been also demonstrated in living cells by measuring fluorescence resonance energy transfer in fibroblasts expressing GFP– and YFP–CASQ2 fusion proteins (Terentyev et al., 2008b). A recent study by Manno et al. (2017) has provided evidence for luminal Ca–dependent changes in polymerization status of CASQ1, the skeletal muscle counterpart of CASQ2, in mouse muscle fibers. Using fluorescence and electron microscopy, these authors demonstrated marked changes in CASQ1 diffusional mobility indicative of depolymerization after SR Ca depletion. These results support the potential role of CASQ in termination of Ca release through either inhibition of RyR2 channels by CASQ monomers or through promoting conformational changes in the Ca release channels thereby promoting channel closure. The slow time course characteristic of such complex protein interactions may account for the delay in restitution of RyR2s from refractoriness after refilling of the SR Ca store. Future studies will have to determine whether similar changes in CASQ2 polymerization occur in cardiac myocytes and whether the time course of those transformations is compatible with beat-to-beat luminal Ca dynamics.
As mentioned, there is evidence implicating other accessory proteins in luminal regulation of RyR2 by Ca, including TRD (Terentyev et al., 2005; Chopra et al., 2009), JNT (Altschafl et al., 2011), and HRC (Arvanitis et al., 2011). It is, therefore, through the interaction with TRD and JNT (Zhang et al., 1997; Györke and Terentyev, 2008) that CASQ appears to control the RyR2 function. In support of this intermediary role of the TRD/JNT complex, experiments in lipid bilayer with reconstituted RyR2 revealed that TRD and/or JNT were required for CASQ2-mediated, store-dependent deactivation (Györke et al., 2004). Additionally, overexpression of a decoy peptide corresponding to the CASQ2 binding domain of TRD in cardiomyocytes interfered with the ability of CASQ2 to stabilize SR Ca release (Terentyev et al., 2007). Although, to date, there is no information as to where TRD binds to the cardiac RyR (RyR2), for its skeletal counterpart (RyR1), the TRD binding region has been shown to reside at residues 4860–4917 (rabbit), near the inner part of the S6 helix bundle-crossing region. Thus, the proposed direct (e.g., Chen et al., 2014) and indirect (i.e., via TRD and CASQ2) effects of luminal Ca seem to converge on the same region, corresponding to the channel gate.
HRC is an intra-SR Ca binding protein that bears similarities to CASQ2. Just like CASQ2, HRC binds to the RyR2 complex through the same CASQ2-binding domain on the TRD (Arvanitis et al., 2011). Insight into the interaction between these two regulatory proteins comes from a study that examined the effects of genetic ablation of HRC alone or both HRC and CASQ2 (Liu et al., 2015). This study revealed that these proteins, rather than being functionally redundant, modulate RyR2 in an opposing manner determined by luminal Ca levels. Specifically, although CASQ2 stabilizes RyR2, rendering it refractory during the diastolic phase, HRC enhances RyR2 activity during that phase, thereby facilitating RyR2 recovery from refractoriness. This, then, raises the possibility of dynamic regulation RyR2 by sequential binding and unbinding of those proteins during various phases of the Ca release–reuptake cycle.
Thus, it appears that regulation of Ca release and refractoriness by the Ca level within the SR involves multiple molecular pathways that include sites on the RyR2 protein itself as well as a system of interacting proteins comprising the RyR2 complex. That system, moreover, appears to interact with regulatory mechanisms residing on the cytosolic side of RyR2, which include Ca-dependent interaction with CaM as well as posttranslational modification of RyR2 (e.g., 2808, 2814, and 2030). Support for that intricate interaction of regulatory mechanisms comes from structural analysis of the RyR2. In particular, recent cryo-electron microscopy studies performed at near-atomic resolution demonstrated that RyR2 is composed of several distinct domains. Those domains constitute a network of superhelical scaffolds for protein binding, as well as offering support for propagation of conformational changes to the RyR2 and facilitating long-range allosteric regulation of the channel activity (Peng et al., 2016). However, additional functional and structural studies are needed to further delineate the cross talk between these regulatory influences in normal and diseased hearts.
Pathological implications of impaired, altered luminal Ca regulation
Impaired regulation of SR Ca release is an important factor contributing to various forms of cardiac disease, including cardiac arrhythmias. The role of dysregulated SR Ca release in arrhythmogenesis was first demonstrated in studies examining the mechanisms of arrhythmias associated with digoxin intoxication (Wasserstrom and Aistrup, 2005). The discovery of CPVTs, cardiac arrhythmias caused by genetic mutations in the RyR2 complex (e.g., RyR2, CASQ2, JNT) established a direct relation between RyR2 dysfunction and arrhythmias (Lahat et al., 2001; Laitinen et al., 2001; Priori et al., 2001; Roux-Buisson et al., 2012). Parallel studies using various models of ischemic and nonischemic cardiac disease have demonstrated the role of posttranslational modifications in RyR2 in acquired cardiac arrhythmias (Marx et al., 2000; Ai et al., 2005; Zhang et al., 2005; Mochizuki et al., 2007; Terentyev et al., 2008a; Curran et al., 2010; Shan et al., 2010; Belevych et al., 2011, 2012, 2017; Respress et al., 2012). In all these pathological settings, it has been shown that arrhythmogenesis involves aberrant diastolic Ca release from the SR, which activates depolarizing currents via the electrogenic NCX, which, in turn, leads to afterdepolarizations and extrasystolic action potentials. Important aspects of Ca-dependent arrhythmogenesis, however, remain to be elucidated. The main areas of uncertainty include the mechanisms of initiation of aberrant Ca release and how impaired regulation by luminal Ca contributes to this arrhythmogenic processes. Furthermore, it is unclear how aberrant Ca-release events are synchronized within a cardiomyocyte and then within the cardiac tissue to elicit triggered activity in the entire myocardium. Growing evidence suggests that these pathologic processes involve shortened Ca-signaling refractoriness from impaired regulation of the RyR2s by luminal Ca.
Generation of aberrant, diastolic SR Ca release
Fabiato (1985b) first demonstrated in mechanically skinned, cardiac myocytes that Ca release from the SR can occur spontaneously, independent of an external Ca trigger, when the Ca content of the SR is elevated. This phenomenon of aberrant Ca release has been investigated in many subsequent studies (reviewed in Izu et al., 2013). It has been demonstrated that the propensity of myocytes toward generation of aberrant Ca release is enhanced by increasing the SR Ca content. These results led to the concept of a SR Ca-content threshold, which postulates that aberrant Ca release occurs when the SR Ca content reaches a certain critical level (Orchard et al., 1995; Díaz et al., 1997). Moreover, this apparent threshold for aberrant Ca release was reported to be lower in settings of cardiac disease associated with genetic and acquired defects in RyR2s (Jiang et al., 2004; Kubalova et al., 2005; Terentyev et al., 2008b). These results have been interpreted in light of the direct activation of RyR2 by luminal Ca (Priori and Chen, 2011). However, recent evidence suggests that the notion of an SR Ca content threshold for aberrant Ca release needs to be reexamined.
Specifically, most studies of aberrant Ca release have been either performed in resting, unstimulated cardiac myocytes or in heterologous systems allowing for reconstitution of RyR2s. Those conditions may not be directly applicable to physiologic Ca cycling because that process occurs in the dynamic system of a working heart. Recent experiments with direct, intra-SR monitoring in healthy and postinfarction canine cardiomyocytes demonstrated that aberrant Ca release does not start immediately after luminal Ca reaches its diastolic level (Belevych et al., 2012). Instead, a distinct time delay separates the onset of aberrant Ca release from the time of completion of SR refilling, thus arguing against the possibility of threshold-mediated aberrant Ca release. Most of that delay in healthy cardiomyocytes can be attributed to RyR2 refractoriness, which is markedly abbreviated in cardiac disease (Fig. 1 B). After the complete functional recovery of RyR2s, the onset of aberrant diastolic Ca release is preceded by a brief idle period. Based on theoretical studies (Maltsev et al., 2011; Nivala et al., 2013), that period reflects the time delay required for temporal alignment of stochastic Ca-release sites that exit from refractoriness. That, in turn leads to the generation of global, aberrant Ca release via self-amplifying CICR. An abbreviated refractory period in arrhythmic, postinfarction myocytes permits aberrant Ca release to occur within the diastolic window. A similar abbreviation in RyR2 refractoriness associated with enhanced aberrant Ca release was described in paced myocytes as well as intact hearts from CPVT mice deficient in CASQ2 (Kornyeyev et al., 2012; Brunello et al., 2013). Thus, rather than being activated directly from within the SR, aberrant Ca release seems to arise because of a CICR-based probabilistic process in which luminal Ca has a regulatory role.
Another important factor in arrhythmogenesis revealed in dynamic studies using physiologically relevant pacing is synchronization of aberrant Ca release. In unstimulated cells, Ca release arises and then propagates as a wave of CICR from one site via a fire-diffuse-fire mechanism. Consequently, Ca wave propagation is relatively slow (20–150 µm/s). On the other hand, in paced myocytes, each action potential-induced systolic-release event presents a synchronizing influence that places all release sites into the same functional state, and hence, temporally aligns their recovery from refractoriness and their stochastic firing. Therefore, shortened refractoriness results in abbreviated latency and exquisitely synchronized firing of Ca-release sites, thus facilitating transition to a form of global release that has characteristics of a Ca phase wave (Izu et al., 2013). Such synchronization of spontaneous release sites in settings of shortened RyR2 refractoriness have been described in both ventricular and atrial myocytes derived from CASQ2-deficient mice phenocopying CPVT (Brunello et al., 2013; Lou et al., 2015; Radwański et al., 2015).
Synchronization of aberrant, diastolic Ca release in myocardium
Although the link between aberrant Ca release and ectopic electrical activity is considered to be well established in isolated myocytes, the mechanism underlying the cross talk between aberrant Ca handling and electrical activity in tissue-wide excitation remains unclear. A fundamental feature of the myocardium, which is lost upon isolation of individual cardiomyocytes, is that within the myocardium, each cell is electrically coupled with neighboring cells. Because of that electrical continuity, the Ca-dependent depolarizing currents generated in any given cell are readily absorbed by the neighboring cells (a phenomenon referred to as source–sink mismatch). Consequently, should a Ca wave arise in any particular cell, its depolarization will be far too small to reach the threshold for generation of an action potential (AP). Computer simulations have suggested that a very large number of cells (>6 × 105) must synchronously release Ca to generate a sufficient current to overcome that source–sink mismatch and to generate an ectopic beat (Xie et al., 2010). Thus, whether, and in what manner, Ca waves synchronize within the myocardium is a key issue for understanding cardiac arrhythmogenesis. The notion of synchronization of Ca release, as outlined in the previous section for isolated cardiomyocytes, offers a conceptual framework for resolving that issue. Specifically, temporarily aligned Ca release on a cellular level in multiple myocytes across the myocardium may precipitate triggered electrical activity simultaneously in those cells to cause an increase in tissue-wide, premature ventricular beat.
Recently, it was demonstrated that diastolic Ca waves can indeed occur in a highly synchronized manner in multicellular cardiac preparations (trabeculae) in settings of CPVT and are capable of generating triggered APs (Brunello et al., 2013). That study, along with others (Wasserstrom et al., 2010; Kornyeyev et al., 2012; Lou et al., 2015), also identified key factors contributing to synchronization of aberrant Ca release. Similar to isolated myocytes, synchronization of aberrant Ca release in tissue depends on (1) the presence of synchronizing events (systolic APs), which temporally align release-site recovery from refractoriness, and (2) shortened refractoriness that abbreviates the latencies and increases the temporal alignment of spontaneous Ca release events within and between myocytes. Importantly, synchronized, aberrant Ca release can be disrupted by pharmacologic stabilization of RyR2 to prolong Ca-release refractoriness (Brunello et al., 2013). Thus, targeting abbreviated Ca-release refractoriness could provide a curative strategy for arrhythmia.
Conclusion and future perspective
In recent years, Ca-signaling refractoriness has emerged as an important concept for understanding myocyte Ca cycling and the rhythmic activity of the heart (Fig. 2). Importantly, it furnishes a possible explanation for the classic phenomenon of mechanical refractoriness. In essence, it presents a mechanism for dynamic control throughout the cardiac cycle of CICR, a self-perpetuating process with a tendency for “run-away” activation. That process seems to rely mainly on diastolic deactivation of RyR2, driven by a reduction in SR Ca; however, it may be also modulated by the intrinsic and extrinsic factors on the cytosolic side that include Ca activation, Ca-dependent inactivation through CaM, as well as posttranslational modification of the RyR2 itself through phosphorylation and oxidation. As for the molecular mechanisms of luminal regulation, there is evidence for both direct modulation of luminal Ca of the RyR2 and for the role of intermediate luminal Ca sensor molecules, such as CASQ2 or HRC. Moreover, the process appears to be more complex because it may also involve an orchestrated molecular rearrangement of CASQ2 associated with its polymerization and depolymerization based on luminal Ca concentration. Defining the precise molecular and structural basis for regulation by luminal Ca is an important direction for future research. At the same time, Ca signaling refractoriness presents a conceptual framework for understanding dysregulated Ca cycling in cardiac disease. Growing evidence suggests that impairment in RyR2 refractoriness is a unifying mechanism for various forms of inherited and acquired arrhythmias. Specifically, shortened RyR2 refractoriness facilitates synchronous, aberrant, diastolic SR Ca release across the myocardium to induce triggered activity and the resultant malignant arrhythmias. Thus, prolonging RyR2 refractoriness and thereby desynchronizing aberrant Ca release is a logical, next therapeutic step in management of cardiac arrhythmias.
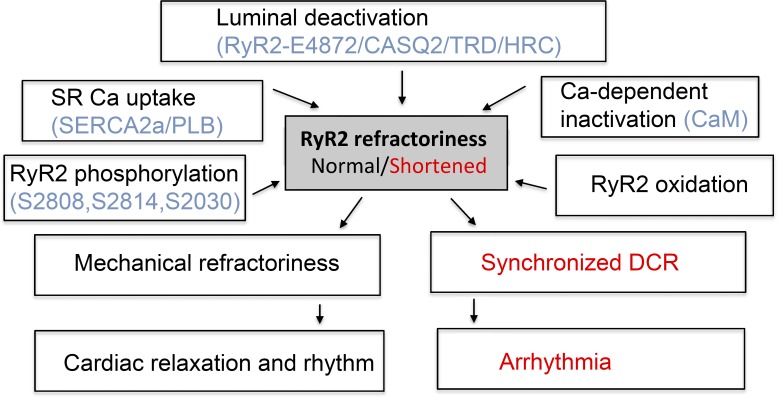
Figure 2.
Mechanistic and molecular factors determining RyR2 refractoriness and its role in healthy cardiac function and arrhythmogenesis. RyR2 refractoriness is primarily determined by effects on RyR2 of luminal Ca, i.e., luminal deactivation, and modulated by other factors, including Ca-dependent inhibition of RyR2 by CaM, RyR2 phosphorylation and oxidation, and changes in SR Ca uptake. RyR2 refractoriness is essential for healthy cardiac relaxation and rhythm. Genetic and acquired defects in RyR2 complex result in shortened RyR2 refractoriness, synchronized aberrant Ca cycling, and arrhythmia.
Acknowledgments
This work was supported by National Institutes of Health (grants HL074045 and HL063043 to S. Györke) and by the Russian Science Foundation (N15-15-20008 to S. Györke).
The authors declare no competing financial interests.
Eduardo Ríos served as editor.
Footnotes
Abbreviations used:
- AP
- action potential
- CPVT
- catecholaminergic polymorphic ventricular tachycardia
- EC
- excitation–contraction
- HRC
- histidine-rich Ca-binding protein
- JNT
- junctin
- TRD
- triadin
References
- Ai X., Curran J.W., Shannon T.R., Bers D.M., and Pogwizd S.M.. 2005. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 97:1314–1322. 10.1161/01.RES.0000194329.41863.89 [DOI] [PubMed] [Google Scholar]
- Altschafl B.A., Arvanitis D.A., Fuentes O., Yuan Q., Kranias E.G., and Valdivia H.H.. 2011. Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. J. Physiol. 589:6063–6080. 10.1113/jphysiol.2011.215988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis D.A., Vafiadaki E., Sanoudou D., and Kranias E.G.. 2011. Histidine-rich calcium binding protein: The new regulator of sarcoplasmic reticulum calcium cycling. J. Mol. Cell. Cardiol. 50:43–49. 10.1016/j.yjmcc.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J.W., Bassani R.A., and Bers D.M.. 1993. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am. J. Physiol. 265:C533–C540. [DOI] [PubMed] [Google Scholar]
- Bassani J.W., Yuan W., and Bers D.M.. 1995. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol. 268:C1313–C1319. [DOI] [PubMed] [Google Scholar]
- Beard N.A., Casarotto M.G., Wei L., Varsányi M., Laver D.R., and Dulhunty A.F.. 2005. Regulation of ryanodine receptors by calsequestrin: Effect of high luminal Ca2+ and phosphorylation. Biophys. J. 88:3444–3454. 10.1529/biophysj.104.051441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A., Kubalova Z., Terentyev D., Hamlin R.L., Carnes C.A., and Györke S.. 2007. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys. J. 93:4083–4092. 10.1529/biophysj.107.114546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A.E., Terentyev D., Terentyeva R., Nishijima Y., Sridhar A., Hamlin R.L., Carnes C.A., and Györke S.. 2011. The relationship between arrhythmogenesis and impaired contractility in heart failure: Role of altered ryanodine receptor function. Cardiovasc. Res. 90:493–502. 10.1093/cvr/cvr025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A.E., Terentyev D., Terentyeva R., Ho H.-T., Gyorke I., Bonilla I.M., Carnes C.A., Billman G.E., and Györke S.. 2012. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ. Res. 110:569–577. 10.1161/CIRCRESAHA.111.260455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A.E., Radwański P.B., Carnes C.A., and Györke S.. 2013. ‘Ryanopathy’: Causes and manifestations of RyR2 dysfunction in heart failure. Cardiovasc. Res. 98:240–247. 10.1093/cvr/cvt024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A.E., Ho H.-T., Bonilla I.M., Terentyeva R., Schober K.E., Terentyev D., Carnes C.A., and Györke S.. 2017. The role of spatial organization of Ca2+ release sites in the generation of arrhythmogenic diastolic Ca2+ release in myocytes from failing hearts. Basic Res. Cardiol. 112:44 10.1007/s00395-017-0633-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Bers D.M. 2001. Excitation–Contraction Coupling and Cardiac Contractile Force. Second edition Kluwer Academic Publishers, Boston: pp. [Google Scholar]
- Bers D.M. 2014. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu. Rev. Physiol. 76:107–127. 10.1146/annurev-physiol-020911-153308 [DOI] [PubMed] [Google Scholar]
- Brooks C.M., Gilbert J.L., Greenspan M.E., Lange G., and Mazzella H.M.. 1960. Excitability and electrical response of ischemic heart muscle. Am. J. Physiol. 198:1143–1147. [DOI] [PubMed] [Google Scholar]
- Brunello L., Slabaugh J.L., Radwanski P.B., Ho H.-T., Belevych A.E., Lou Q., Chen H., Napolitano C., Lodola F., Priori S.G., et al. 2013. Decreased RyR2 refractoriness determines myocardial synchronization of aberrant Ca2+ release in a genetic model of arrhythmia. Proc. Natl. Acad. Sci. USA. 110:10312–10317. 10.1073/pnas.1300052110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., and Kong C.H.T.. 2012. Local control in cardiac E–C coupling. J. Mol. Cell. Cardiol. 52:298–303. 10.1016/j.yjmcc.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Cannell M.B., and Kong C.H.T.. 2017. Quenching the spark: Termination of CICR in the submicroscopic space of the dyad. J. Gen. Physiol. 149 10.1085/jgp.201711807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Wang R., Chen B., Zhong X., Kong H., Bai Y., Zhou Q., Xie C., Zhang J., Guo A., et al. 2014. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat. Med. 20:184–192. 10.1038/nm.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer M.R., Lederer W.J., and Cannell M.B.. 1996. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270:C148–C159. [DOI] [PubMed] [Google Scholar]
- Chopra N., Yang T., Asghari P., Moore E.D., Huke S., Akin B., Cattolica R.A., Perez C.F., Hlaing T., Knollmann-Ritschel B.E.C., et al. 2009. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation–contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA. 106:7636–7641. 10.1073/pnas.0902919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Brown K.H., Santiago D.J., Pogwizd S., Bers D.M., and Shannon T.R.. 2010. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+–calmodulin-dependent protein kinase II. J. Mol. Cell. Cardiol. 49:25–32. 10.1016/j.yjmcc.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelPrincipe F., Egger M., and Niggli E.. 1999. Calcium signalling in cardiac muscle: Refractoriness revealed by coherent activation. Nat. Cell Biol. 1:323–329. 10.1038/14013 [DOI] [PubMed] [Google Scholar]
- Díaz M.E., Trafford A.W., O’Neill S.C., and Eisner D.A.. 1997. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J. Physiol. 501:3–16. 10.1111/j.1469-7793.1997.003bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K.A., and Jóhannsson M.. 1976. The contractile state of rabbit papillary muscle in relation to stimulation frequency. J. Physiol. 254:565–581. 10.1113/jphysiol.1976.sp011247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. 2014. Calcium in the heart: From physiology to disease. Exp. Physiol. 99:1273–1282. 10.1113/expphysiol.2013.077305 [DOI] [PubMed] [Google Scholar]
- Eisner D., Bode E., Venetucci L., and Trafford A.. 2013. Calcium flux balance in the heart. J. Mol. Cell. Cardiol. 58:110–117. 10.1016/j.yjmcc.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1985a Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 85:247–289. 10.1085/jgp.85.2.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. 1985b Spontaneous versus triggered contractions of “calcium-tolerant” cardiac cells from the adult rat ventricle. Basic Res. Cardiol. 80(Suppl 2):83–87. [PubMed] [Google Scholar]
- Faggioni M., and Knollmann B.C.. 2012. Calsequestrin 2 and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 302:H1250–H1260. 10.1152/ajpheart.00779.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M., Zahradníková A., Villalba-Galea C.A., Zahradník I., Escobar A.L., and Györke S.. 2000. Ryanodine receptor adaptation. J. Gen. Physiol. 116:873–882. 10.1085/jgp.116.6.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W.R., and Fozzard H.A.. 1975. Slow inward current and contraction of sheep cardiac Purkinje fibers. J. Gen. Physiol. 65:367–384. 10.1085/jgp.65.3.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Gillespie D., and Fill M.. 2012. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ. Res. 111:28–36. 10.1161/CIRCRESAHA.112.265652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I., and Györke S.. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810. 10.1016/S0006-3495(98)77723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., and Terentyev D.. 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77:245–255. 10.1093/cvr/cvm038 [DOI] [PubMed] [Google Scholar]
- Györke I., Hester N., Jones L.R., and Györke S.. 2004. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86:2121–2128. 10.1016/S0006-3495(04)74271-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Stevens S.C.W., and Terentyev D.. 2009. Cardiac calsequestrin: Quest inside the SR. J. Physiol. 587:3091–3094. 10.1113/jphysiol.2009.172049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.-T., Belevych A.E., Liu B., Bonilla I.M., Radwański P.B., Kubasov I.V., Valdivia H.H., Schober K., Carnes C.A., and Györke S.. 2016. Muscarinic stimulation facilitates sarcoplasmic reticulum Ca release by modulating ryanodine receptor 2 phosphorylation through protein kinase G and Ca/calmodulin-dependent protein kinase II. Hypertension. 68:1171–1178. 10.1161/HYPERTENSIONAHA.116.07666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H.E. 1942. The history of the refractory period: A neglected contribution of Felice Fontana. Yale J. Biol. Med. 14:635–672. [PMC free article] [PubMed] [Google Scholar]
- Hong T., and Shaw R.M.. 2017. Cardiac T-tubule microanatomy and function. Physiol. Rev. 97:227–252. 10.1152/physrev.00037.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund T.J., and Rudy Y.. 2004. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 110:3168–3174. 10.1161/01.CIR.0000147231.69595.D3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu L.T., Xie Y., Sato D., Bányász T., and Chen-Izu Y.. 2013. Ca2+ waves in the heart. J. Mol. Cell. Cardiol. 58:118–124. 10.1016/j.yjmcc.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Xiao B., Yang D., Wang R., Choi P., Zhang L., Cheng H., and Chen S.R.W.. 2004. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA. 101:13062–13067. 10.1073/pnas.0402388101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Chen W., Wang R., Zhang L., and Chen S.R.. 2007. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl. Acad. Sci. USA. 104:18309–18314. 10.1073/pnas.0706573104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.P., Guo W., and Chen S.R.W.. 2017. Control of cardiac ryanodine receptor by sarcoplasmic reticulum luminal Ca2+. J. Gen. Physiol. 149 10.1085/jgp.201711805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann B.C., Chopra N., Hlaing T., Akin B., Yang T., Ettensohn K., Knollmann B.E.C., Horton K.D., Weissman N.J., Holinstat I., et al. 2006. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 116:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyeyev D., Petrosky A.D., Zepeda B., Ferreiro M., Knollmann B., and Escobar A.L.. 2012. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J. Mol. Cell. Cardiol. 52:21–31. 10.1016/j.yjmcc.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruta V., and Braveny P.. 1963. Rate of restitution and self-regulation of contractility in mammalian heart muscle. Nature. 197:905–906. 10.1038/197905a0 [DOI] [PubMed] [Google Scholar]
- Kubalova Z., Györke I., Terentyeva R., Viatchenko-Karpinski S., Terentyev D., Williams S.C., and Györke S.. 2004. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J. Physiol. 561:515–524. 10.1113/jphysiol.2004.073940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z., Terentyev D., Viatchenko-Karpinski S., Nishijima Y., Györke I., Terentyeva R., da Cuñha D.N., Sridhar A., Feldman D.S., Hamlin R.L., et al. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 102:14104–14109. 10.1073/pnas.0504298102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H., Pras E., Olender T., Avidan N., Ben-Asher E., Man O., Levy-Nissenbaum E., Khoury A., Lorber A., Goldman B., et al. 2001. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am. J. Hum. Genet. 69:1378–1384. 10.1086/324565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen P.J., Brown K.M., Piippo K., Swan H., Devaney J.M., Brahmbhatt B., Donarum E.A., Marino M., Tiso N., Viitasalo M., et al. 2001. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 103:485–490. 10.1161/01.CIR.103.4.485 [DOI] [PubMed] [Google Scholar]
- Liu B., Ho H.-T., Brunello L., Unudurthi S.D., Lou Q., Belevych A.E., Qian L., Kim D.H., Cho C., Janssen P.M.L., et al. 2015. Ablation of HRC alleviates cardiac arrhythmia and improves abnormal Ca handling in CASQ2 knockout mice prone to CPVT. Cardiovasc. Res. 108:299–311. 10.1093/cvr/cvv222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Q., Belevych A.E., Radwański P.B., Liu B., Kalyanasundaram A., Knollmann B.C., Fedorov V.V., and Györke S.. 2015. Alternating membrane potential/calcium interplay underlies repetitive focal activity in a genetic model of calcium-dependent atrial arrhythmias. J. Physiol. 593:1443–1458. 10.1113/jphysiol.2014.280784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., and Gyorke S.. 1999. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 521:575–585. 10.1111/j.1469-7793.1999.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke I., and Györke S.. 1996. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 432:1047–1054. 10.1007/s004240050233 [DOI] [PubMed] [Google Scholar]
- Maltsev A.V., Maltsev V.A., Mikheev M., Maltseva L.A., Sirenko S.G., Lakatta E.G., and Stern M.D.. 2011. Synchronization of stochastic Ca2+ release units creates a rhythmic Ca2+ clock in cardiac pacemaker cells. Biophys. J. 100:271–283. 10.1016/j.bpj.2010.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C., Figueroa L.C., Gillespie D., Fitts R., Kang C., Franzini-Armstrong C., and Rios E.. 2017. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. USA. 114:E638–E647. 10.1073/pnas.1620265114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty I. 2015. Triadin regulation of the ryanodine receptor complex. J. Physiol. 593:3261–3266. 10.1113/jphysiol.2014.281147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., and Marks A.R.. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 101:365–376. 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Yano M., Oda T., Tateishi H., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., and Matsuzaki M.. 2007. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 49:1722–1732. 10.1016/j.jacc.2007.01.064 [DOI] [PubMed] [Google Scholar]
- Näbauer M., and Morad M.. 1990. Ca2+-induced Ca2+ release as examined by photolysis of caged Ca2+ in single ventricular myocytes. Am. J. Physiol. 258:C189–C193. [DOI] [PubMed] [Google Scholar]
- Nivala M., Ko C.Y., Nivala M., Weiss J.N., and Qu Z.. 2013. The emergence of subcellular pacemaker sites for calcium waves and oscillations. J. Physiol. 591:5305–5320. 10.1113/jphysiol.2013.259960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Yang Y., Nitu F.R., Svensson B., Lu X., Fruen B.R., Cornea R.L., and Bers D.M.. 2013. In cardiomyocytes, binding of unzipping peptide activates ryanodine receptor 2 and reciprocally inhibits calmodulin binding. Circ. Res. 112:487–497. 10.1161/CIRCRESAHA.111.300290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C.H., Mustafa M.R., and White E.. 1995. Oscillations and waves of intracellular [Ca2+] in cardiac muscle cells. Chaos Solitons Fractals. 5:447–458. 10.1016/0960-0779(93)E0036-B [DOI] [Google Scholar]
- Park H., Wu S., Dunker A.K., and Kang C.. 2003. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 278:16176–16182. 10.1074/jbc.M300120200 [DOI] [PubMed] [Google Scholar]
- Park H., Park I.Y., Kim E., Youn B., Fields K., Dunker A.K., and Kang C.. 2004. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: A proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 279:18026–18033. 10.1074/jbc.M311553200 [DOI] [PubMed] [Google Scholar]
- Peng W., Shen H., Wu J., Guo W., Pan X., Wang R., Chen S.R.W., and Yan N.. 2016. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science. 354:aah5324 10.1126/science.aah5324 [DOI] [PubMed] [Google Scholar]
- Picht E., DeSantiago J., Blatter L.A., and Bers D.M.. 2006. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ. Res. 99:740–748. 10.1161/01.RES.0000244002.88813.91 [DOI] [PubMed] [Google Scholar]
- Poláková E., Illaste A., Niggli E., and Sobie E.A.. 2015. Maximal acceleration of Ca2+ release refractoriness by β-adrenergic stimulation requires dual activation of kinases PKA and CaMKII in mouse ventricular myocytes. J. Physiol. 593:1495–1507. 10.1113/jphysiol.2014.278051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S.G., and Chen S.R.. 2011. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108:871–883. 10.1161/CIRCRESAHA.110.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S.G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V., and Danieli G.A.. 2001. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 103:196–200. 10.1161/01.CIR.103.2.196 [DOI] [PubMed] [Google Scholar]
- Pritchard T.J., and Kranias E.G.. 2009. Junctin and the histidine-rich Ca2+ binding protein: Potential roles in heart failure and arrhythmogenesis. J. Physiol. 587:3125–3133. 10.1113/jphysiol.2009.172171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Valle G., Nani A., Nori A., Rizzi N., Priori S.G., Volpe P., and Fill M.. 2008. Luminal Ca2+ regulation of single cardiac ryanodine receptors: Insights provided by calsequestrin and its mutants. J. Gen. Physiol. 131:325–334. 10.1085/jgp.200709907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Valle G., Nani A., Chen H., Ramos-Franco J., Nori A., Volpe P., and Fill M.. 2009. Ryanodine receptor luminal Ca2+ regulation: Swapping calsequestrin and channel isoforms. Biophys. J. 97:1961–1970. 10.1016/j.bpj.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwański P.B., Belevych A.E., Brunello L., Carnes C.A., and Györke S.. 2013. Store-dependent deactivation: Cooling the chain-reaction of myocardial calcium signaling. J. Mol. Cell. Cardiol. 58:77–83. 10.1016/j.yjmcc.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwański P.B., Brunello L., Veeraraghavan R., Ho H.-T., Lou Q., Makara M.A., Belevych A.E., Anghelescu M., Priori S.G., Volpe P., et al. 2015. Neuronal Na+ channel blockade suppresses arrhythmogenic diastolic Ca2+ release. Cardiovasc. Res. 106:143–152. 10.1093/cvr/cvu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramay H.R., Liu O.Z., and Sobie E.A.. 2011. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc. Res. 91:598–605. 10.1093/cvr/cvr143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respress J.L., van Oort R.J., Li N., Rolim N., Dixit S.S., deAlmeida A., Voigt N., Lawrence W.S., Skapura D.G., Skårdal K., et al. 2012. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ. Res. 110:1474–1483. 10.1161/CIRCRESAHA.112.268094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux-Buisson N., Cacheux M., Fourest-Lieuvin A., Fauconnier J., Brocard J., Denjoy I., Durand P., Guicheney P., Kyndt F., Leenhardt A., et al. 2012. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum. Mol. Genet. 21:2759–2767. 10.1093/hmg/dds104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Betzenhauser M.J., Kushnir A., Reiken S., Meli A.C., Wronska A., Dura M., Chen B.X., and Marks A.R.. 2010. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J. Clin. Invest. 120:4375–4387. 10.1172/JCI37649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.R., Ginsburg K.S., and Bers D.M.. 2000. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 78:334–343. 10.1016/S0006-3495(00)76596-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.R., Guo T., and Bers D.M.. 2003. Ca2+ scraps: Local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 93:40–45. 10.1161/01.RES.0000079967.11815.19 [DOI] [PubMed] [Google Scholar]
- Siebens A.A., Hoffman B.F., Gilbert J.L., and Suckling E.E.. 1951. Effect of rate on excitability of dog’s ventricle. Am. J. Physiol. 166:610–618. [DOI] [PubMed] [Google Scholar]
- Siebens A.A., Hoffman B.F., Cranefield P.F., and Brooks C.M.. 1959. Regulation of contractile force during ventricular arrhythmias [legacy content]. Am. J. Physiol. 197:971–977. [Google Scholar]
- Sitsapesan R., and Williams A.J.. 1994. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+. J. Membr. Biol. 137:215–226. 10.1007/BF00232590 [DOI] [PubMed] [Google Scholar]
- Sobie E.A., and Lederer W.J.. 2012. Dynamic local changes in sarcoplasmic reticulum calcium: Physiological and pathophysiological roles. J. Mol. Cell. Cardiol. 52:304–311. 10.1016/j.yjmcc.2011.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Dilly K.W., dos Santos Cruz J., Lederer W.J., and Jafri M.S.. 2002. Termination of cardiac Ca2+ sparks: An investigative mathematical model of calcium-induced calcium release. Biophys. J. 83:59–78. 10.1016/S0006-3495(02)75149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Song L.S., and Lederer W.J.. 2005. Local recovery of Ca2+ release in rat ventricular myocytes. J. Physiol. 565:441–447. 10.1113/jphysiol.2005.086496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Williams G.S.B., and Lederer W.J.. 2017. Ambiguous interactions between diastolic and SR Ca2+ in the regulation of cardiac Ca2+ release. J. Gen. Physiol. 149 10.1085/jgp.201711814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard M.T., Tian X., Liu Y., Wang R., Chazin W.J., Chen S.R.W., and Overgaard M.T.. 2015. Arrhythmogenic calmodulin mutations affect the activation and termination of cardiac ryanodine receptor-mediated Ca2+ release. J. Biol. Chem. 290:26151–26162. 10.1074/jbc.M115.676627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., and Cheng H.. 2004. Putting out the fire: What terminates calcium-induced calcium release in cardiac muscle? Cell Calcium. 35:591–601. 10.1016/j.ceca.2004.01.013 [DOI] [PubMed] [Google Scholar]
- Szentesi P., Pignier C., Egger M., Kranias E.G., and Niggli E.. 2004. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ. Res. 95:807–813. 10.1161/01.RES.0000146029.80463.7d [DOI] [PubMed] [Google Scholar]
- Tencerová B., Zahradníková A., Gaburjáková J., and Gaburjáková M.. 2012. Luminal Ca2+ controls activation of the cardiac ryanodine receptor by ATP. J. Gen. Physiol. 140:93–108. 10.1085/jgp.201110708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Valdivia H.H., Escobar A.L., and Györke S.. 2002. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91:414–420. 10.1161/01.RES.0000032490.04207.BD [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Györke I., Volpe P., Williams S.C., and Györke S.. 2003. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA. 100:11759–11764. 10.1073/pnas.1932318100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Cala S.E., Houle T.D., Viatchenko-Karpinski S., Gyorke I., Terentyeva R., Williams S.C., and Gyorke S.. 2005. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ. Res. 96:651–658. 10.1161/01.RES.0000160609.98948.25 [DOI] [PubMed] [Google Scholar]
- Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalova Z., Gyorke I., Terentyeva R., Vedamoorthyrao S., Blom N.A., Valle G., et al. 2006. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 98:1151–1158. 10.1161/01.RES.0000220647.93982.08 [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Vedamoorthyrao S., Oduru S., Györke I., Williams S.C., and Györke S.. 2007. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J. Physiol. 583:71–80. 10.1113/jphysiol.2007.136879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., et al. 2008a Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 103:1466–1472. 10.1161/CIRCRESAHA.108.184457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Kubalova Z., Valle G., Nori A., Vedamoorthyrao S., Terentyeva R., Viatchenko-Karpinski S., Bers D.M., Williams S.C., Volpe P., and Gyorke S.. 2008b Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: Effects of CASQ2 mutations linked to sudden cardiac death. Biophys. J. 95:2037–2048. 10.1529/biophysj.107.128249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam R.T., Hoffman B.F., and Stuckey J.H.. 1964. Recovery of excitability and of impulse propagation in the in situ canine conduction system. Am. J. Cardiol. 14:184–192. 10.1016/0002-9149(64)90131-6 [DOI] [PubMed] [Google Scholar]
- Vassilakopoulou V., Calver B.L., Thanassoulas A., Beck K., Hu H., Buntwal L., Smith A., Theodoridou M., Kashir J., Blayney L., et al. 2015. Distinctive malfunctions of calmodulin mutations associated with heart RyR2-mediated arrhythmic disease. Biochim. Biophys. Acta. 1850:2168–2176. 10.1016/j.bbagen.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S., Terentyev D., Györke I., Terentyeva R., Volpe P., Priori S.G., Napolitano C., Nori A., Williams S.C., and Györke S.. 2004. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ. Res. 94:471–477. 10.1161/01.RES.0000115944.10681.EB [DOI] [PubMed] [Google Scholar]
- Wasserstrom J.A., and Aistrup G.L.. 2005. Digitalis: New actions for an old drug. Am. J. Physiol. Heart Circ. Physiol. 289:H1781–H1793. 10.1152/ajpheart.00707.2004 [DOI] [PubMed] [Google Scholar]
- Wasserstrom J.A., Shiferaw Y., Chen W., Ramakrishna S., Patel H., Kelly J.E., O’Toole M.J., Pappas A., Chirayil N., Bassi N., et al. 2010. Variability in timing of spontaneous calcium release in the intact rat heart is determined by the time course of sarcoplasmic reticulum calcium load. Circ. Res. 107:1117–1126. 10.1161/CIRCRESAHA.110.229294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens X.H., Lehnart S.E., Reiken S.R., and Marks A.R.. 2004. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 94:e61–e70. 10.1161/01.RES.0000125626.33738.E2 [DOI] [PubMed] [Google Scholar]
- Williams D.A., Delbridge L.M., Cody S.H., Harris P.J., and Morgan T.O.. 1992. Spontaneous and propagated calcium release in isolated cardiac myocytes viewed by confocal microscopy. Am. J. Physiol. 262:C731–C742. [DOI] [PubMed] [Google Scholar]
- Xiao B., Zhong G., Obayashi M., Yang D., Chen K., Walsh M.P., Shimoni Y., Cheng H., Ter Keurs H., and Chen S.R.. 2006. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem. J. 396:7–16. 10.1042/BJ20060116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Sato D., Garfinkel A., Qu Z., and Weiss J.N.. 2010. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys. J. 99:1408–1415. 10.1016/j.bpj.2010.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yano M., Uchinoumi H., Hino A., Suetomi T., Ono M., Tateishi H., Oda T., Okuda S., Doi M., et al. 2010. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem. Biophys. Res. Commun. 394:660–666. 10.1016/j.bbrc.2010.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Guo T., Oda T., Chakraborty A., Chen L., Uchinoumi H., Knowlton A.A., Fruen B.R., Cornea R.L., Meissner G., and Bers D.M.. 2014. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ. Res. 114:295–306. 10.1161/CIRCRESAHA.114.302857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Kelley J., Schmeisser G., Kobayashi Y.M., and Jones L.R.. 1997. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272:23389–23397. 10.1074/jbc.272.37.23389 [DOI] [PubMed] [Google Scholar]
- Zhang R., Khoo M.S., Wu Y., Yang Y., Grueter C.E., Ni G., Price E.E. Jr., Thiel W., Guatimosim S., Song L.S., et al. 2005. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 11:409–417. 10.1038/nm1215 [DOI] [PubMed] [Google Scholar]
- Zima A.V., Picht E., Bers D.M., and Blatter L.A.. 2008. Termination of cardiac Ca2+ sparks: Role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ. Res. 103:e105–e115. 10.1161/CIRCRESAHA.107.183236 [DOI] [PMC free article] [PubMed] [Google Scholar]