Abstract
Sobie et al. highlight unresolved issues concerning the regulation of sarcoplasmic reticulum calcium release in cardiac myocytes.
Introduction
In all types of muscle cells, contraction is initiated by an increase in intracellular Ca2+. Another similarity among skeletal, cardiac, and smooth muscle cells is that much of the Ca2+ responsible for contraction is released from the SR. Beyond these common features, however, molecular and subcellular differences among muscle types can frequently explain functional differences. For instance, SR Ca2+ release in cardiac myocytes occurs primarily through the type 2 RyR (RyR2), whereas different release channels are more important in other cell types. Another relevant feature in heart is that cells must contract and relax roughly once per second or faster. Thus, although skeletal and smooth muscle may experience either brief or sustained elevations of intracellular [Ca2+], rhythmic and regular heartbeats in cardiac myocytes result in continuous oscillations in intracellular [Ca2+], accompanied by regular dynamic changes in SR [Ca2+]. These concentration changes in the two compartments have been shown in recent years to convey information and regulate the release process. In this Perspective, we aim to review what has been learned about the regulatory importance of cardiac SR [Ca2+] and establish some constraints on plausible ranges for changes in SR [Ca2+] during release. In doing so, we emphasize how close coupling between experimental studies and numerical simulations has improved our understanding, and we discuss the importance of the interplay between SR [Ca2+] and diastolic [Ca2+] in the transition between stable and unstable cellular Ca2+ release. In particular, we argue that increased diastolic [Ca2+] can raise RyR2 open probability in a manner that is potentially dangerous when combined with elevated SR [Ca2+].
Dynamic changes in SR [Ca2+] during calcium cycling
Conservation of mass ensures that Ca2+ can neither appear out of thin air nor disappear into the void. Thus, when Ca2+ is released from the SR, the rise of cytosolic [Ca2+] must be accompanied by a corresponding decline in SR [Ca2+]. In skeletal muscle, because SR stores are very large, individual muscle twitches are accompanied by negligible changes in SR [Ca2+] (Launikonis et al., 2006), and special experimental conditions such as very long depolarizations are required to observe substantial SR Ca2+ depletion (Manno et al., 2017). In cardiac myocytes, however, SR Ca2+ stores are comparatively much smaller. This means that both individual cellular contractions (Shannon et al., 2003) and local release events (Brochet et al., 2005) are accompanied by substantial depletion of SR [Ca2+]. Although it is clear that these changes in SR [Ca2+] regulate the release process, the mechanisms involved in this regulation and the precise functional importance of changes in SR [Ca2+] remain intensively debated.
If the SR penetrated essentially everywhere in the cytoplasm and RyR2s released Ca2+ from all locations simultaneously, then calculating how increases in cytoplasmic [Ca2+] corresponded with decreases in SR [Ca2+] would be straightforward. For instance, in the absence of both cytosolic and SR Ca2+ buffers, the cytosolic [Ca2+] increase would be quantitatively related to the SR [Ca2+] decrease through the ratio of the two volumes. We can derive some rough estimates if we assume that the cytosol occupies 65% of the total cellular volume and the SR occupies 3% (with the remainder primarily mitochondria). If we also assume that, in a resting cell, diastolic [Ca2+] and SR [Ca2+] are 100 nM and 1 mM, respectively, then complete depletion of SR [Ca2+] will cause cytosolic [Ca2+] to increase from 100 nM to 46.3 µM, whereas 50% depletion will cause an increase in cytosolic [Ca2+] to 23.1 µM. The fact that cytosolic [Ca2+] never reaches such values, even when SR [Ca2+] is emptied, indicates that Ca2+ buffering in the cytosol is strong compared with buffering in the SR. Although the presence of Ca2+ buffers complicates this analysis such that concentration changes are difficult to calculate with a pencil and paper, buffer powers in both cytosol and SR have been measured (Berlin et al., 1994; Shannon and Bers, 1997; Trafford et al., 1999) and can be incorporated into the analysis. Fig. 1 A shows how a uniform increase in cytosolic [Ca2+] depends on the extent of SR [Ca2+] depletion, assuming realistic cytosolic and SR buffering.
Figure 1.
Hypothetical changes in SR and cytosolic [Ca2+] during SR release. (A) An increase in the fraction of total SR Ca2+ released causes a decrease in SR free [Ca2+] and a corresponding increase in cytosolic free [Ca2+]. Curves are nonlinear because of the presence of Ca2+ buffers in each compartment. Calculations assumed: (1) SR [Ca2+] and cytosolic [Ca2+] were 1,000 µM and 0.1 µM, respectively, before release; (2) cytosolic volume was 22.66 times larger than SR volume; (3) cytosolic buffering sites had maximal occupancy of 2,500 µM and a KD of 630 µM; (4) SR buffering sites had maximal occupancy of 220 µM and a KD of 0.96 µM. (B) Hypothetical profiles of SR [Ca2+] during release if Ca2+ diffusion within the SR is either extremely fast (blue) or quite slow (red). Because of blurring by confocal microscopes, attempts to record these SR [Ca2+] profiles are likely to yield similar values for the apparent extent of depletion.
What complicates the calculation more than buffers, however, is the fact that the SR does not release Ca2+ at all locations simultaneously. Instead, RyR2s are clustered, and spatial segregation of these RyR2 clusters is a critical feature responsible for the stability of SR Ca2+ release (Niggli and Lederer, 1990; Stern, 1992; Cannell et al., 1995). This spatial segregation necessarily means that during SR Ca2+ release, cytosolic [Ca2+] is greater in the immediate vicinity of the RyR2s, and lower further from the channels. By the same logic, SR Ca2+ depletion must be greater right next to the RyR2s compared with farther away. Spatial nonuniformities in cytosolic [Ca2+] during SR Ca2+ release have long been appreciated, and considerable effort has been devoted to estimating the extent of the heterogeneity. Indeed, several mathematical modeling studies, dating back more than 30 yr, have calculated spatial changes in cytosolic [Ca2+] (Cannell and Allen, 1984; Langer and Peskoff, 1996; Soeller and Cannell, 1997), and novel experimental methods have been developed to derive better estimates of [Ca2+] in the immediate vicinity of RyR2s (Despa et al., 2014; Shang et al., 2014). It has been technically more challenging, however, to determine spatial nonuniformity in SR [Ca2+], and as a result comparatively less effort has been devoted to understanding the luminal side of the SR membrane, even though the same principles apply.
Spatial heterogeneity of SR [Ca2+] during local Ca2+ release events
During local Ca2+ release, by how much does SR Ca2+ content deplete? Mathematical modeling studies, beginning with ours in 2002 (Sobie et al., 2002), have suggested that during a Ca2+ spark, in which release lasts from 10–20 ms, [Ca2+] in the junctional SR, or JSR, will deplete by 80–90% (i.e., from a resting level of 1000 µM to 100–200 µM). This initial estimate was based on a relatively simple, phenomenological model of local Ca2+ release, but more recent models, which represent geometrical details more explicitly, have obtained nearly identical calculations of the extent of depletion during sparks (Hake et al., 2012; Cannell et al., 2013; Walker et al., 2014). Experimental studies, however, using Ca2+ indicators localized in the SR, such as fluo-5N, have consistently recorded local depletion signals, or Ca2+ blinks, with a nadir of roughly 50–60% of the initial value (Brochet et al., 2005, 2011; Zima et al., 2008b; Picht et al., 2011). To understand this discrepancy, a critical point to note is the limited spatial resolution of the experimental recordings. With a typical confocal microscope, an infinitesimally small source of light will be imaged to the shape of a prolate spheroid. The dimensions of this spheroid, measured as full width at half maximal intensity, will be ∼0.5 µm in the two directions in the plane of focus and 1 µm in the direction perpendicular to the plane of focus, yielding a “recording volume” of roughly 150 aL (atto = 10−18). In contrast, a typical region of JSR containing RyR2s can be approximated as a disk with diameter ∼300 nm and a height of ∼35 nm, yielding a volume of 2.5 aL. This means that, when measuring Ca2+ blinks, the microscope records from a volume that is ∼60 times larger than the JSR volume that is being interrogated. Given that the local [Ca2+] right near the site of release will necessarily be lower than the concentration away the site of release, it is clear that a typical blink will represent a weighted mean of more extensive depletion near the RyR2s and less extensive depletion further away from the channels.
When trying to relate the experimental blink signal to the true extent of Ca2+ depletion immediately near the RyR2s, a critical factor is the spatial scale over which [Ca2+] varies within the SR, and this in turn depends on the speed of Ca2+ movement in the SR. Here, references to SR Ca2+ movement include both diffusion within the network SR (NSR) and Ca2+ transfer from NSR to JSR. Two hypothetical possibilities are illustrated in Fig. 1 B. If Ca2+ movement is extremely fast, [Ca2+] will quickly adjust to a relatively constant value throughout the SR during release (Fig. 1 B, blue curve). On the other hand, if movement is slow, in particular if a “bottleneck” exists between the JSR and the NSR, then JSR concentration during release will be dramatically lower than the concentration in the surrounding NSR (Fig. 1 B, red curve). The important point is that because of blurring by the confocal microscope, both scenarios will produce identical Ca2+ blink nadirs.
We believe the latter scenario is more likely than the former, for several reasons. First, as noted above, several mathematical models, developed by us and other groups (Sobie et al., 2002; Hake et al., 2012; Cannell et al., 2013; Walker et al., 2014), have predicted that JSR [Ca2+] depletes to 10–20% of its initial value during Ca2+ sparks. Beyond predicting the extent of Ca2+ depletion in the immediate vicinity of the RyR2s, modeling studies have also demonstrated that an apparent depletion of 50% estimated from experimental blink measurements is consistent with a true depletion of 80–90% near RyR2s (Williams et al., 2011; Hake et al., 2012; Kong et al., 2013). Second, related computational studies have shown that extremely fast SR Ca2+ diffusion may lead to unrealistic behavior. When developing a model of the Ca2+ spark, a critical parameter that must be chosen involves the speed of Ca2+ movement from NSR to JSR. In our studies, we have constrained these parameters on the basis of data showing that, after an initial Ca2+ spark, the amplitude of a second spark from the same site recovers with a time constant of 70–90 ms (Sobie et al., 2005; Ramay et al., 2011; Guo et al., 2012b; Poláková et al., 2015). This recovery of Ca2+ spark amplitude is broadly consistent with the wide range of Ca2+ blink recovery time constants, 30–150 ms, that have been measured (Brochet et al., 2005; Zima et al., 2008b; Picht et al., 2011). In general, we would expect spark amplitude to be proportional to total JSR [Ca2+] (free Ca2+ plus Ca2+ bound to buffers), a quantity that should recover more quickly than the free JSR [Ca2+] that is detected in blink measurements. When NSR-to-JSR transfer rates are chosen to match these experimental data, Ca2+ sparks terminate and recover normally (Ramay et al., 2011; Cannell et al., 2013; Stern et al., 2013). However, when the rate of Ca2+ transfer from NSR to JSR is made dramatically faster, Ca2+ sparks can fail to terminate, leading to the emergence of so-called metastable sparks (Stern et al., 2013; Sato et al., 2016; Song et al., 2016).
Third, as discussed in more detail below, solid evidence indicates that during regenerative Ca2+ waves, in which Ca2+ sparks trigger additional sparks in a chain reaction that moves through the cell, SR [Ca2+] seems to transiently increase at sites that have not yet been triggered (Maxwell and Blatter, 2012, 2017). We would only expect such behavior if Ca2+ movement between JSR and NSR were relatively slow; extremely fast diffusion would create a flat profile of [Ca2+] within the SR and make it impossible for Ca2+ to accumulate at untriggered sites. This group of observations, collected under different experimental conditions by several different groups, supports the idea that within the SR lumen, each release unit is mostly disconnected from its neighbors.
The physiological importance of local SR depletion
Why should we care about local decreases in SR [Ca2+]? The short answer is that compelling evidence, gathered over roughly the past two decades, indicates that local reductions in SR [Ca2+] have important regulatory roles under normal and pathological conditions. In 2002, a stochastic mathematical model of the Ca2+ spark (Sobie et al., 2002) and an experimental study published soon afterward (Terentyev et al., 2002) independently postulated that substantial local depletion of SR [Ca2+] is required for release termination. In the years following, important confirmatory evidence was gathered by several groups. Many of these studies are described more extensively in the Perspectives by Cannell and Kong and Györke et al. in this issue and are therefore not discussed in detail here. In brief, results obtained in these studies included the following: (1) changes in SR Ca2+ buffering due to overexpression or knockdown of calsequestrin affect the duration of sparks (Terentyev et al., 2003); (2) partial blockade of RyR2s can dramatically prolong Ca2+ sparks (Zima et al., 2008a,b), presumably by slowing the rate at which the JSR depletes; and (3) refilling of SR [Ca2+] controls the time course of Ca2+ release refractoriness at both the Ca2+ spark (Sobie et al., 2005; Ramay et al., 2011) and cellular (Szentesi et al., 2004; Kornyeyev et al., 2012) levels.
Despite the results that clearly indicated an important role for SR [Ca2+] depletion in release termination, some weaknesses of the early mathematical model (Sobie et al., 2002) should be mentioned. In particular, the limited experimental data available in 2002 required our group to make assumptions that have subsequently been questioned. First, on the basis of two compelling studies investigating RyR gating (Marx et al., 1998, 2001), we assumed that the opening and closing of each RyR2 influenced the behavior of its neighbors through allosteric interactions, or so-called coupled gating. This feature, however, has not been confirmed in experiments from other groups (Xiao et al., 2007) and is virtually impossible to either prove or refute in intact cells. Consequently, the potential importance of coupled gating remains indeterminate. Second, in 2002 we assumed that changes in SR [Ca2+] had a relatively large effect on RyR2 gating, on the basis of somewhat limited data (Györke and Györke, 1998; Ching et al., 2000). Data gathered subsequently, which paint a more complete picture of how SR [Ca2+] influences RyR2 gating, seem to indicate that the effect is not as strong as originally implemented (Qin et al., 2008), and descendants of the 2002 model have updated formulations to reflect these new data (Ramay et al., 2011; Williams et al., 2011; Wescott et al., 2016).
In addition, mathematical modeling results have demonstrated that RyR2 gating does not need to depend on SR [Ca2+] in order for Ca2+ sparks to terminate robustly (Cannell et al., 2013; Laver et al., 2013; Stern et al., 2013; Walker et al., 2014). In this scenario, the important feature is the fact that when [Ca2+] in the JSR decreases, the Ca2+ current flowing through each open RyR2 will decline, and each RyR2 opening will be less likely to reopen any neighbors that stochastically close, until the spark terminates when all RyR2s are closed simultaneously (Cannell and Kong, 2017). This termination mechanism has been named “induction decay” (Cannell et al., 2013; Laver et al., 2013) and, in a less quantitative presentation of the same notion, “pernicious attrition” (Gillespie and Fill, 2013). These recent studies are important because they have demonstrated that regulation of RyR2 gating by SR [Ca2+] is not essential, and they have brought renewed attention to the importance of the Ca2+ current flowing through each open RyR2 (Guo et al., 2012a). It is important to note, however, that the ability of an RyR2 to trigger its neighbors, and the dependence of this on SR [Ca2+], has been implicitly included in all previous models that simulate realistic depletion of JSR [Ca2+].
The exact physiological role for changes in SR [Ca2+] to modulate RyR2 gating, therefore, remains somewhat in question, given that this mechanism is not required for Ca2+ spark termination. Recent modeling studies have proposed reasonable hypotheses, specifically that SR [Ca2+] regulation of RyR2 gating may enable a narrow distribution of Ca2+ spark durations (Stern et al., 2013) and that this feature contributes substantially to the nonlinear relationship between SR [Ca2+] and release triggering (Walker et al., 2014). Thus, the mathematical modeling has helped to clarify hypotheses, but many of these ideas remain to be conclusively confirmed or refuted.
Unstable Ca2+ release and the role of SR [Ca2+]
Under pathological conditions, Ca2+ release through clusters of RyR2s is not necessarily spatially constrained but instead can propagate between RyR2 clusters such that spontaneous release occurs throughout the entire cell. In this mechanism, the Ca2+ released from one RyR2 cluster diffuses in the cytosol to raise [Ca2+] in the vicinity of neighboring clusters. When the diffusing Ca2+ is sufficient to activate the opening of RyR2s in these nearby clusters, a propagating, regenerative Ca2+ wave can result.
It has been well established over many years that these Ca2+ waves tend to occur under conditions of elevated SR [Ca2+]. In fact, some studies have observed a sharp transition from localized local spontaneous release (Ca2+ sparks) to regenerative waves, such that a “threshold SR Ca2+ content” can be determined (Díaz et al., 1997; Eisner et al., 2009). From these data and related work examining the consequences of RyR2 mutations, some investigators have proposed the concept of SOICR, or store overload–induced Ca2+ release (Jiang et al., 2004, 2005). However, although it is clear that increases in SR [Ca2+] contribute to the generation of spontaneous Ca2+ waves, it is also clear that these waves propagate through a mechanism of CICR in the cytosol. This is illustrated most clearly by the observation that cell-wide waves can readily be terminated by adding buffers such as EGTA or BAPTA to the cytosol. Under these conditions, elevated SR [Ca2+] continues to encourage spontaneous Ca2+ release, but this release occurs only in the form of localized Ca2+ sparks, not in the form of cell-wide Ca2+ waves (Lukyanenko et al., 2001; Loughrey et al., 2002).
To better understand the practical distinction between CICR and a purely SR-mediated SOICR mechanism, we can consider a single cluster of RyR2, with an associated JSR volume. The mechanism of action of CICR is inherently a positive feedback process. When an individual RyR2 within the cluster opens stochastically, the efflux of Ca2+ from the JSR raises local [Ca2+] on the cytosolic side of the SR membrane. This higher local cytosolic [Ca2+] increases the likelihood that adjacent RyR2 channels will open. If a second RyR2 opens, the additional Ca2+ efflux from the JSR will increase local cytosolic [Ca2+] further, and a Ca2+ spark can be produced when sufficient RyR2s are activated. In contrast, SOICR is intrinsically a negative feedback mechanism. In this case, a single RyR2 that opens stochastically because of the high [Ca2+] on the luminal side of the SR membrane will pass Ca2+ ions that will act to decrease local SR [Ca2+]. This decrease of SR [Ca2+] would then make it less likely for a mechanism based on luminal activation to trigger the opening of additional RyR2s within that cluster. Thus SOICR, acting in isolation, would not constitute a self-sustaining, regenerative process that produces Ca2+ sparks and Ca2+ waves. For these reasons, it is more appropriate characterize increased SR [Ca2+] as encouraging or enabling spontaneous regenerative Ca2+ release by CICR rather than directly triggering or inducing Ca2+release.
A question that remains intriguing concerns the spatiotemporal changes in SR [Ca2+] that occur on a time scale of tens of milliseconds during waves. It is clear that at the local site of Ca2+ release, SR [Ca2+] must decrease at the same time that local cytosolic [Ca2+] increases. What remain less clear, however, are the changes that occur at neighboring clusters of RyR2s. Cytosolic [Ca2+] will increase at these untriggered clusters because of diffusion of Ca2+ from the initial site of release, but what will happen to SR [Ca2+]? This quantity could theoretically either decrease if the SR is well-connected between neighboring release units, or it could increase when Ca2+ that has diffused in the cytosol is pumped into the SR by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) at locations immediately adjacent to the untriggered cluster. A further complication concerns how these concentration changes might be altered by increased SERCA pump activity, for instance as occurs during β-adrenergic activation. Because an increase in SERCA activity would presumably decrease cytosolic [Ca2+] at untriggered clusters while potentially increasing SR [Ca2+] at these locations, the overall effects on Ca2+ wave propagation remain ambiguous.
A decade ago, an important experimental study observed that sudden inhibition of the SERCA pump led to an immediate decrease in the velocity of propagating Ca2+ waves (Keller et al., 2007). On the basis of this finding, the authors speculated that SERCA pumps take up Ca2+ near untriggered sites, and the resulting localized increase in SR [Ca2+] sensitizes the RyR2s ahead of a propagating Ca2+ wave. A subsequent mathematical modeling investigation showed that this hypothesis is feasible, but only if Ca2+ diffusion in the SR is relatively slow compared with Ca2+ diffusion in the cytosol (Ramay et al., 2010). Soon thereafter, an experimental study showed that localized increases in SR [Ca2+] do in fact occur during Ca2+ waves in ventricular cells (Maxwell and Blatter, 2012), and more recent work suggests that this sensitization mechanism is critical to the propagation of SR Ca2+ release in atrial myocytes (Maxwell and Blatter, 2017).
This particular issue is notable for a couple of reasons. First, the timeline illustrates the usefulness of close interplay between experiments and numerical simulations, even when the studies are performed by different groups. A hypothesis was proposed on the basis of indirect evidence, as a way to explain surprising results (Keller et al., 2007), numerical simulations demonstrated the feasibility of the hypothesis, as long as certain conditions hold (Ramay et al., 2010), then subsequent experiments provided direct support (Maxwell and Blatter, 2012). Second, the changes in SR [Ca2+] that are possible during waves relate closely to the nonuniformity of SR [Ca2+] during blinks and in turn to how well the experimental blink signal reflects the true extent of SR depletion. In this case, simulations showed that increases in SR [Ca2+] ahead of Ca2+ waves are possible only when SR Ca2+ diffusion is rather slow. From this inference, it follows that JSR units in cardiac myocytes must be relatively isolated from one another and, in turn, that SR [Ca2+] during local release must be quite nonuniform (e.g., closer to the red than the blue profile in Fig. 1 B).
The semi-neglected factor of diastolic [Ca2+]
With considerable recent research energy focused on determining the regulatory importance of changes in SR [Ca2+], an additional important factor has been relatively neglected, namely, diastolic [Ca2+], that is, the minimum cytosolic [Ca2+] reached in myocytes between contractions. Given that CICR is the commonly accepted mechanism discussed in textbooks, it seems surprising that the importance of cytosolic [Ca2+] could be somewhat overlooked. However, small changes in diastolic [Ca2+] can be challenging to track in experiments, and investigators naturally focus on the more interesting dynamic time courses rather than on changes in the baseline level. Examining diastolic [Ca2+], however, is important because of the factors that modify it. Conditions that lead to increased SR [Ca2+], such as rapid pacing and β-adrenergic stimulation, are frequently accompanied by an increase in diastolic [Ca2+]. When this occurs, the rate at which RyR2s spontaneously open at rest should be increased in a nonlinear manner. Mathematical models based on planar lipid bilayer data generally assume that the RyR2 opening rate depends on local cytosolic [Ca2+] raised to a power between 2 and 4 (Ramay et al., 2011; Sato and Bers, 2011; Williams et al., 2011; Walker et al., 2014; Wescott et al., 2016). Thus a 50% increase in diastolic [Ca2+] will not cause a 50% increase in the spontaneous RyR2 opening rate but instead will increase the opening rate up to fivefold. These more frequent RyR2 openings will not only increase the rate of Ca2+ sparks and the “leak” that is seen in resting cells (Bovo et al., 2011) but will also produce many more potential triggers for regenerative Ca2+ waves. Under such conditions, elevated diastolic and SR [Ca2+] can synergize in a potentially dangerous way. Diastolic [Ca2+] will increase the number of Ca2+ sparks that can potentially initiate waves, and the elevated SR [Ca2+] will increase the probability that an individual event will trigger release from neighboring RyR2 clusters. Together these effects can greatly increase the risk of unstable Ca2+ release.
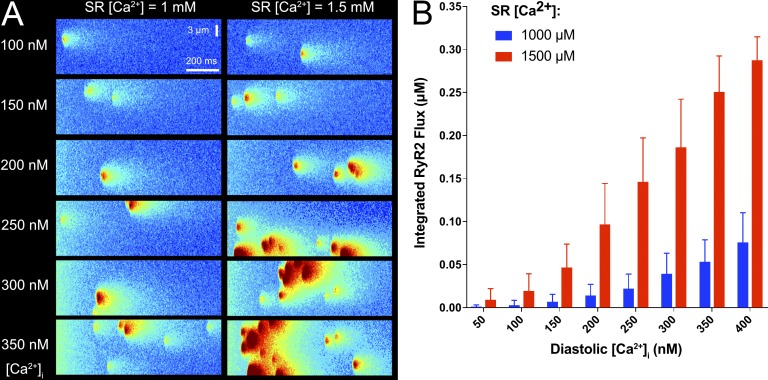
To illustrate this idea, we performed simulations using a recently published stochastic mathematical model of a cell containing multiple, spatially distributed clusters of RyR2s (Wescott et al., 2016). By using the model, we are able to independently vary the initial values of SR [Ca2+] and diastolic [Ca2+], an intervention that is challenging to perform in experiments. Fig. 2 A shows sample results from these stochastic simulations. In these spatiotemporal images, analogous to those that would be obtained experimentally by a confocal microscope in line-scan mode, we observe both Ca2+ sparks, reflecting release from an individual RyR2 cluster, and larger events reflecting release from multiple clusters. The results show that an increase in SR [Ca2+] can, by itself, increase the probability that individual Ca2+ sparks will trigger neighboring sites and produce larger release events. However, when SR [Ca2+] is increased and diastolic [Ca2+] remains low, these multisite events remain relatively uncommon. In contrast, when diastolic [Ca2+] and SR [Ca2+] are simultaneously elevated, both the frequency of Ca2+ release events and their spatial extent are substantially increased. These effects are quantified in Fig. 2 B, which plots the integrated Ca2+ release flux, or Ca2+ release “mass,” as a function of SR and diastolic [Ca2+]. The potentially dangerous interplay between these two factors can be readily seen. Thus, experimental and mathematical modeling studies exploring the transition between stable and unstable Ca2+ release need to carefully consider the effects of both diastolic and SR [Ca2+].
Figure 2.
Contributions of increased SR and diastolic [Ca2+] to unstable Ca2+release. (A) Simulated line-scan images of typical stochastic simulations of Ca2+ sparks and waves. Simulations were performed with a mathematical model of Ca2+ release, as recently described (Wescott et al., 2016). The horizontal dimension represents time (1 s), and the vertical dimension represents transverse distance (14 µm). Labels indicate initial values of cytosolic and SR [Ca2+]. Each SR Ca2+ release unit contains a cluster of 50 stochastically gating RyR2s (see Wescott et al. [2016] for more details), and RyR2 clusters are randomly distributed, with a mean intersite distance of 700 nm. Large SR Ca2+ release events involving multiple RyR2 clusters are frequently observed when both SR [Ca2+] and diastolic [Ca2+] are increased (bottom right images). (B) Integrated RyR2 Ca2+release flux as a function of initial diastolic [Ca2+]i when initial SR [Ca2+] is either 1 mM (blue) or 1.5 mM (red). Flux is integrated over each 1-s simulation and averaged over 50 trials. Error bars show standard deviation.
Although the above discussion focuses primarily on the role of Ca2+ release in ventricular myocytes, we should note that diastolic and SR [Ca2+] can also potentially synergize in other cell types, such as sinoatrial nodal cells, atrial myocytes (Blatter in this issue), and Purkinje cells. In sinoatrial nodal cells, considerable recent evidence suggests that spontaneous Ca2+ release events, in the form of sparks triggering additional sparks, drives the late phase of diastolic depolarization when released Ca2+ is extruded from the cell through Na+-Ca2+ exchange (Stern et al., 2014; Yaniv et al., 2015). In atrial and Purkinje cells, Ca2+ release is initiated only at the cell periphery and propagates into the cell center under some conditions (Blatter et al., 2003; Lee et al., 2011). Both of these phenomena are likely to depend on diastolic as well as SR [Ca2+], emphasizing the importance of considering the interplay between these two factors.
Conclusions and outlook
It is clear that numerous studies performed over the past two decades have yielded substantial insight into the importance of SR [Ca2+] in regulating Ca2+ release. When considering the published literature as a whole, a common theme that emerges is the difficult-to-predict interplay among overall SR [Ca2+] content, local changes in free SR [Ca2+], the potential regulation of RyR2 gating by SR [Ca2+], and the changes in diastolic [Ca2+] that frequently accompany altered SR [Ca2+]. The fact that these factors sometimes work in opposite directions highlights the need to integrate experimental studies with mechanistic mathematical modeling, and, indeed, many of the advances described above came from such efforts.
Going forward, experimental and computational advances are likely to help to address the remaining unresolved questions and provide additional insight. First, technical advances such as knock-in mice, localized Ca2+ probes, and super-resolution microscopy will presumably help to fully resolve issues such as the spatial nonuniformity of SR [Ca2+] during release and the importance of SR [Ca2+] in regulating RyR2 gating. Second, even though most experimental studies focus on the average behavior of events such as sparks and blinks, and most models are developed on the basis of repeating, uniform units, it is clear that considerable heterogeneity exists both within a cell and between cells. For instance, both structural parameters such as the number of RyR2 per cluster (Baddeley et al., 2009) and physiological variables such as blink time constants (Zima et al., 2008b) exhibit wide distributions. Modeling studies have shown how heterogeneity between RyR2 clusters can influence both Ca2+ spark properties (Lee et al., 2013) and the emergence of intracellular Ca2+ waves (Nivala et al., 2013), but more work clearly needs to be done to understand the consequences of heterogeneity. However, with recently developed tools in hand to address these unresolved issues, it is clear that the next several years will bring significant additional insight into the roles of SR and diastolic [Ca2+] in regulating cardiac myocyte function.
Acknowledgments
Research in E.A. Sobie’s laboratory is supported by the National Institutes of Health (U54 HG008098 and U01 HL136297), the National Science Foundation (MCB 1615677), and the American Heart Association (15GRNT25490006). W.J. Lederer is supported by National Institutes of Health grants R01 HL106056, R01 HL105239, and U01 HL116321. G.S.B. Williams is supported by National Institutes of Health award K25 HL125762.
The authors declare no competing financial interests.
Eduardo Ríos served as editor.
Footnotes
Abbreviations used:
- JSR
- junctional SR
- NSR
- network SR
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- SOICR
- store overload–induced Ca2+ release
References
- Baddeley D., Jayasinghe I.D., Lam L., Rossberger S., Cannell M.B., and Soeller C.. 2009. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA. 106:22275–22280. 10.1073/pnas.0908971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin J.R., Bassani J.W., and Bers D.M.. 1994. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys. J. 67:1775–1787. 10.1016/S0006-3495(94)80652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L.A. 2017. The intricacies of atrial calcium cycling during excitation–contraction coupling. J. Gen. Physiol. 149 10.1085/jgp.201711809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter L.A., Kockskämper J., Sheehan K.A., Zima A.V., Hüser J., and Lipsius S.L.. 2003. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J. Physiol. 546:19–31. 10.1113/jphysiol.2002.025239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovo E., Mazurek S.R., Blatter L.A., and Zima A.V.. 2011. Regulation of sarcoplasmic reticulum Ca2+ leak by cytosolic Ca2+ in rabbit ventricular myocytes. J. Physiol. 589:6039–6050. 10.1113/jphysiol.2011.214171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet D.X., Yang D., Di Maio A., Lederer W.J., Franzini-Armstrong C., and Cheng H.. 2005. Ca2+ blinks: Rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 102:3099–3104. 10.1073/pnas.0500059102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet D.X., Xie W., Yang D., Cheng H., and Lederer W.J.. 2011. Quarky calcium release in the heart. Circ. Res. 108:210–218. 10.1161/CIRCRESAHA.110.231258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., and Allen D.G.. 1984. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys. J. 45:913–925. 10.1016/S0006-3495(84)84238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., and Kong C.H.T.. 2017. Quenching the spark: Termination of CICR in the submicroscopic space of the dyad. J. Gen. Physiol. 149 10.1085/jgp.201711807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Cheng H., and Lederer W.J.. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049. 10.1126/science.7754384 [DOI] [PubMed] [Google Scholar]
- Cannell M.B., Kong C.H., Imtiaz M.S., and Laver D.R.. 2013. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys. J. 104:2149–2159. 10.1016/j.bpj.2013.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching L.L., Williams A.J., and Sitsapesan R.. 2000. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 87:201–206. 10.1161/01.RES.87.3.201 [DOI] [PubMed] [Google Scholar]
- Despa S., Shui B., Bossuyt J., Lang D., Kotlikoff M.I., and Bers D.M.. 2014. Junctional cleft [Ca2+]i measurements using novel cleft-targeted Ca2+ sensors. Circ. Res. 115:339–347. 10.1161/CIRCRESAHA.115.303582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M.E., Trafford A.W., O’Neill S.C., and Eisner D.A.. 1997. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J. Physiol. 501:3–16. 10.1111/j.1469-7793.1997.003bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D.A., Kashimura T., O’Neill S.C., Venetucci L.A., and Trafford A.W.. 2009. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J. Mol. Cell. Cardiol. 46:474–481. 10.1016/j.yjmcc.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Gillespie D., and Fill M.. 2013. Pernicious attrition and inter-RyR2 CICR current control in cardiac muscle. J. Mol. Cell. Cardiol. 58:53–58. 10.1016/j.yjmcc.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Gillespie D., and Fill M.. 2012a Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ. Res. 111:28–36. 10.1161/CIRCRESAHA.112.265652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Zhang T., Ginsburg K.S., Mishra S., Brown J.H., and Bers D.M.. 2012b CaMKIIδC slows [Ca]i decline in cardiac myocytes by promoting Ca sparks. Biophys. J. 102:2461–2470. 10.1016/j.bpj.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I., and Györke S.. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810. 10.1016/S0006-3495(98)77723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Belevych A.E., Liu B., Kubasov I.V., Carnes C.A., and Radwański P.B.. 2017. The role of luminal Ca regulation in Ca signaling refractoriness and cardiac arrhythmogenesis. J. Gen. Physiol. 149 10.1085/jgp.201711808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake J., Edwards A.G., Yu Z., Kekenes-Huskey P.M., Michailova A.P., McCammon J.A., Holst M.J., Hoshijima M., and McCulloch A.D.. 2012. Modelling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit. J. Physiol. 590:4403–4422. 10.1113/jphysiol.2012.227926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Xiao B., Yang D., Wang R., Choi P., Zhang L., Cheng H., and Chen S.R.. 2004. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA. 101:13062–13067. 10.1073/pnas.0402388101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Wang R., Xiao B., Kong H., Hunt D.J., Choi P., Zhang L., and Chen S.R.. 2005. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ. Res. 97:1173–1181. 10.1161/01.RES.0000192146.85173.4b [DOI] [PubMed] [Google Scholar]
- Keller M., Kao J.P.Y., Egger M., and Niggli E.. 2007. Calcium waves driven by “sensitization” wave-fronts. Cardiovasc. Res. 74:39–45. 10.1016/j.cardiores.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Kong C.H., Laver D.R., and Cannell M.B.. 2013. Extraction of sub-microscopic Ca fluxes from blurred and noisy fluorescent indicator images with a detailed model fitting approach. PLOS Comput. Biol. 9:e1002931 10.1371/journal.pcbi.1002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyeyev D., Petrosky A.D., Zepeda B., Ferreiro M., Knollmann B., and Escobar A.L.. 2012. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J. Mol. Cell. Cardiol. 52:21–31. 10.1016/j.yjmcc.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G.A., and Peskoff A.. 1996. Calcium concentration and movement in the diadic cleft space of the cardiac ventricular cell. Biophys. J. 70:1169–1182. 10.1016/S0006-3495(96)79677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., Zhou J., Royer L., Shannon T.R., Brum G., and Ríos E.. 2006. Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc. Natl. Acad. Sci. USA. 103:2982–2987. 10.1073/pnas.0511252103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D.R., Kong C.H., Imtiaz M.S., and Cannell M.B.. 2013. Termination of calcium-induced calcium release by induction decay: An emergent property of stochastic channel gating and molecular scale architecture. J. Mol. Cell. Cardiol. 54:98–100. 10.1016/j.yjmcc.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Dun W., Boyden P.A., and Sobie E.A.. 2011. Complex and rate-dependent beat-to-beat variations in Ca2+ transients of canine Purkinje cells. J. Mol. Cell. Cardiol. 50:662–669. 10.1016/j.yjmcc.2010.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Liu O.Z., Hwang H.S., Knollmann B.C., and Sobie E.A.. 2013. Parameter sensitivity analysis of stochastic models provides insights into cardiac calcium sparks. Biophys. J. 104:1142–1150. 10.1016/j.bpj.2012.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrey C.M., MacEachern K.E., Neary P., and Smith G.L.. 2002. The relationship between intracellular [Ca2+] and Ca2+ wave characteristics in permeabilised cardiomyocytes from the rabbit. J. Physiol. 543:859–870. 10.1113/jphysiol.2002.021519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Viatchenko-Karpinski S., Smirnov A., Wiesner T.F., and Györke S.. 2001. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys. J. 81:785–798. 10.1016/S0006-3495(01)75741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C., Figueroa L.C., Gillespie D., Fitts R., Kang C., Franzini-Armstrong C., and Rios E.. 2017. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. USA. 114:E638–E647. 10.1073/pnas.1620265114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Ondrias K., and Marks A.R.. 1998. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science. 281:818–821. 10.1126/science.281.5378.818 [DOI] [PubMed] [Google Scholar]
- Marx S.O., Gaburjakova J., Gaburjakova M., Henrikson C., Ondrias K., and Marks A.R.. 2001. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ. Res. 88:1151–1158. 10.1161/hh1101.091268 [DOI] [PubMed] [Google Scholar]
- Maxwell J.T., and Blatter L.A.. 2012. Facilitation of cytosolic calcium wave propagation by local calcium uptake into the sarcoplasmic reticulum in cardiac myocytes. J. Physiol. 590:6037–6045. 10.1113/jphysiol.2012.239434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J.T., and Blatter L.A.. 2017. A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes. J. Physiol. 595:3835–3845. 10.1113/JP273611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E., and Lederer W.J.. 1990. Voltage-independent calcium release in heart muscle. Science. 250:565–568. 10.1126/science.2173135 [DOI] [PubMed] [Google Scholar]
- Nivala M., Ko C.Y., Nivala M., Weiss J.N., and Qu Z.. 2013. The emergence of subcellular pacemaker sites for calcium waves and oscillations. J. Physiol. 591:5305–5320. 10.1113/jphysiol.2013.259960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht E., Zima A.V., Shannon T.R., Duncan A.M., Blatter L.A., and Bers D.M.. 2011. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ. Res. 108:847–856. 10.1161/CIRCRESAHA.111.240234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poláková E., Illaste A., Niggli E., and Sobie E.A.. 2015. Maximal acceleration of Ca2+ release refractoriness by β-adrenergic stimulation requires dual activation of kinases PKA and CaMKII in mouse ventricular myocytes. J. Physiol. 593:1495–1507. 10.1113/jphysiol.2014.278051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Valle G., Nani A., Nori A., Rizzi N., Priori S.G., Volpe P., and Fill M.. 2008. Luminal Ca2+ regulation of single cardiac ryanodine receptors: Insights provided by calsequestrin and its mutants. J. Gen. Physiol. 131:325–334. 10.1085/jgp.200709907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramay H.R., Jafri M.S., Lederer W.J., and Sobie E.A.. 2010. Predicting local SR Ca2+ dynamics during Ca2+ wave propagation in ventricular myocytes. Biophys. J. 98:2515–2523. 10.1016/j.bpj.2010.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramay H.R., Liu O.Z., and Sobie E.A.. 2011. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc. Res. 91:598–605. 10.1093/cvr/cvr143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D., and Bers D.M.. 2011. How does stochastic ryanodine receptor-mediated Ca leak fail to initiate a Ca spark? Biophys. J. 101:2370–2379. 10.1016/j.bpj.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D., Shannon T.R., and Bers D.M.. 2016. Sarcoplasmic reticulum structure and functional properties that promote long-lasting calcium sparks. Biophys. J. 110:382–390. 10.1016/j.bpj.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W., Lu F., Sun T., Xu J., Li L.L., Wang Y., Wang G., Chen L., Wang X., Cannell M.B., et al. . 2014. Imaging Ca2+ nanosparks in heart with a new targeted biosensor. Circ. Res. 114:412–420. 10.1161/CIRCRESAHA.114.302938 [DOI] [PubMed] [Google Scholar]
- Shannon T.R., and Bers D.M.. 1997. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys. J. 73:1524–1531. 10.1016/S0006-3495(97)78184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.R., Guo T., and Bers D.M.. 2003. Ca2+ scraps: Local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 93:40–45. 10.1161/01.RES.0000079967.11815.19 [DOI] [PubMed] [Google Scholar]
- Sobie E.A., Dilly K.W., dos Santos Cruz J., Lederer W.J., and Jafri M.S.. 2002. Termination of cardiac Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys. J. 83:59–78. 10.1016/S0006-3495(02)75149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Song L.S., and Lederer W.J.. 2005. Local recovery of Ca2+ release in rat ventricular myocytes. J. Physiol. 565:441–447. 10.1113/jphysiol.2005.086496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C., and Cannell M.B.. 1997. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys. J. 73:97–111. 10.1016/S0006-3495(97)78051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Karma A., Weiss J.N., and Qu Z.. 2016. Long-lasting sparks: multi-metastability and release competition in the calcium release unit network. PLOS Comput. Biol. 12:e1004671 10.1371/journal.pcbi.1004671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D. 1992. Theory of excitation-contraction coupling in cardiac muscle. Biophys. J. 63:497–517. 10.1016/S0006-3495(92)81615-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Ríos E., and Maltsev V.A.. 2013. Life and death of a cardiac calcium spark. J. Gen. Physiol. 142:257–274. 10.1085/jgp.201311034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Maltseva L.A., Juhaszova M., Sollott S.J., Lakatta E.G., and Maltsev V.A.. 2014. Hierarchical clustering of ryanodine receptors enables emergence of a calcium clock in sinoatrial node cells. J. Gen. Physiol. 143:577–604. 10.1085/jgp.201311123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P., Pignier C., Egger M., Kranias E.G., and Niggli E.. 2004. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ. Res. 95:807–813. 10.1161/01.RES.0000146029.80463.7d [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Valdivia H.H., Escobar A.L., and Györke S.. 2002. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91:414–420. 10.1161/01.RES.0000032490.04207.BD [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Györke I., Volpe P., Williams S.C., and Györke S.. 2003. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA. 100:11759–11764. 10.1073/pnas.1932318100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A.W., Díaz M.E., and Eisner D.A.. 1999. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Arch. 437:501–503. 10.1007/s004240050808 [DOI] [PubMed] [Google Scholar]
- Walker M.A., Williams G.S., Kohl T., Lehnart S.E., Jafri M.S., Greenstein J.L., Lederer W.J., and Winslow R.L.. 2014. Superresolution modeling of calcium release in the heart. Biophys. J. 107:3018–3029. 10.1016/j.bpj.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescott A.P., Jafri M.S., Lederer W.J., and Williams G.S.. 2016. Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling. J. Mol. Cell. Cardiol. 92:82–92. 10.1016/j.yjmcc.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.S., Chikando A.C., Tuan H.T., Sobie E.A., Lederer W.J., and Jafri M.S.. 2011. Dynamics of calcium sparks and calcium leak in the heart. Biophys. J. 101:1287–1296. 10.1016/j.bpj.2011.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Tian X., Jones P.P., Bolstad J., Kong H., Wang R., Zhang L., Duff H.J., Gillis A.M., Fleischer S., et al. . 2007. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J. Biol. Chem. 282:34828–34838. 10.1074/jbc.M707423200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv Y., Lakatta E.G., and Maltsev V.A.. 2015. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 6:28 10.3389/fphys.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima A.V., Picht E., Bers D.M., and Blatter L.A.. 2008a Partial inhibition of sarcoplasmic reticulum Ca release evokes long-lasting Ca release events in ventricular myocytes: role of luminal Ca in termination of Ca release. Biophys. J. 94:1867–1879. 10.1529/biophysj.107.114694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima A.V., Picht E., Bers D.M., and Blatter L.A.. 2008b Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ. Res. 103:e105–e115. 10.1161/CIRCRESAHA.107.183236 [DOI] [PMC free article] [PubMed] [Google Scholar]