Abstract
Stress is considered a potent environmental risk factor for many behavioral abnormalities, including anxiety and mood disorders1,2. Animal models can exhibit limited but quantifiable behavioral impairments resulting from chronic stress, including deficits in motivation, abnormal responses to behavioral challenges, and anhedonia3–5. The hippocampus is thought to negatively regulate the stress response and to mediate various cognitive and mnemonic aspects of stress-induced impairments2,3,5, though the neuronal underpinnings sufficient to support behavioral improvements are largely unknown. Here, we acutely rescue stress-induced, depression-related behaviors by optogenetically reactivating DG cells that were previously active during a positive experience. A brain-wide histological investigation, coupled with pharmacological and projection-specific optogenetic blockade experiments, identified glutamatergic activity in the hippocampus-amygdala-nucleus accumbens pathway as a candidate circuit supporting the acute rescue. Finally, chronically reactivating hippocampal cells associated with a positive memory resulted in a rescue of stress-induced behavioral impairments and neurogenesis at time points beyond the light stimulation. Together, our data suggest that activating positive memories artificially is sufficient to suppress depression-like behaviors and point to DG engram cells as potential therapeutic nodes for intervening with maladaptive behavioral states.
Our recent studies have demonstrated that DG cells that express c-Fos during fear or reward conditioning define an active neural population that is sufficient to elicit both aversive and appetitive responses, and that the mnemonic output elicited by these artificially reactivated cells can be updated with new information6–8. These findings raise the possibility of alleviating stress-induced behavioral impairments via a defined set of DG cells active during a positive experience. Indeed, how positive episodes interact with psychiatric disease-related behavioral states, including depression-related impairments, at the neuronal and systems level remains largely unknown, despite the promising cognitive treatments available in humans9.
To address this issue, we utilized our recently developed method that enables labelling and manipulation of memory engram cells (See Methods) 6–8. Exposing animals that were taken off Dox to a naturally rewarding experience8 (i.e. exposure to a female mouse in a modified homecage, hereafter referred to as a “positive experience” and further validated in Extended Data Fig. 1), a neutral context (hereafter referred to as a “neutral experience”), or a single bout of immobilization stress (hereafter referred to as a “negative experience”) all elicited comparable levels of ChR2-mCherry expression in the dentate gyrus (DG; Extended Data Fig. 2a–e).
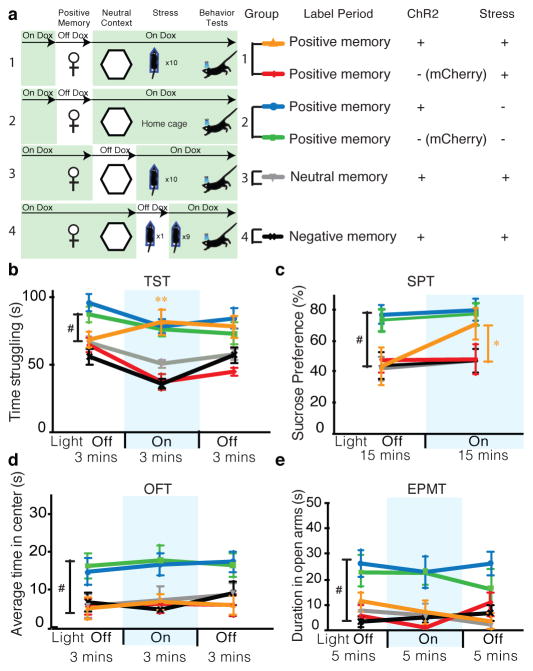
As shown in Figure 1a, mice were split into six groups (See Methods). After 10 days of chronic immobilization stress (CIS)(Extended Data Fig. 2f) or in a homecage, all groups were put through the open field test (OFT) and elevated plus maze test (EPMT) as measures of anxiety-like behaviors, as well as the tail suspension test (TST) as a measure of active/passive escape behavior in response to a challenging situation, and the sucrose preference test (SPT) for anhedonia11–15. In unstressed animals, optogenetic reactivation of cells previously active during a positive experience did not significantly change anxiety-related measures, time spent struggling, or preference for sucrose compared to unstressed mCherry controls (Fig. 1b–e). In the stressed groups, the CIS paradigm elicited a robust decrease in time struggling and preference for sucrose, as well as increased anxiogenic responses, consistent with previous reports14,15 (Fig. 1b–e).
Figure 1.
Activating positive memory engrams rescues depression-related behavior. a Behavior schedule and groups used. b–e, Optical reactivation of dentate gyrus (DG) cells that were previously active during a positive experience significantly increases time struggling in the tail suspension test (b) and preference for sucrose (c), but does not have a significant effect in anxiety-like behavior in the open field test (d) or elevated plus maze test (e). A two-way analysis of variance (ANOVA) with repeated measures revealed a group-by-light epoch interaction in the TST (F5, 294 = 21.20, P < 0.001) or SPT (F5, 196 = 6.20, P < 0.001) followed by Bonferroni post-hoc tests, which revealed significant increases in struggling or preference for sucrose in the positive memory + stress group. #P < 0.01. # used to denote significant differences between the four stressed groups (n = 18 per group) vs. the two non-stressed groups (n = 16 per group); *P<0.05, **P<0.01 (orange * and ** used to denote significant differences between the stress + positive memory group vs. the other three stressed groups). Data are means +/− SEM.
However, optically reactivating DG cells that were previously active during a positive experience, but not a neutral or a negative experience, in stressed animals acutely increased time struggling and sucrose preference to levels that matched the unstressed groups’ behavior (Fig. 1b, c). Additionally, optical reactivation of DG cells associated with a positive experience decreased the latency to feed in a novelty- suppressed feeding test15 (NSFT) (Extended Data Fig. 3a) without affecting hunger or satiety (Extended Data Fig. 3b). Once again, the CIS paradigm had an anxiogenic effect across all groups, and all groups failed to show light-induced behavioral changes in the OFT or EPMT (Fig. 1d, e). Similarly, total distance traveled was consistent across groups (Extended Data Fig. 4c). Taken together, these data argue that reactivating DG cells labeled by a positive experience is sufficient to acutely reverse the behavioral effects of stress in the TST, SPT, and NSFT.
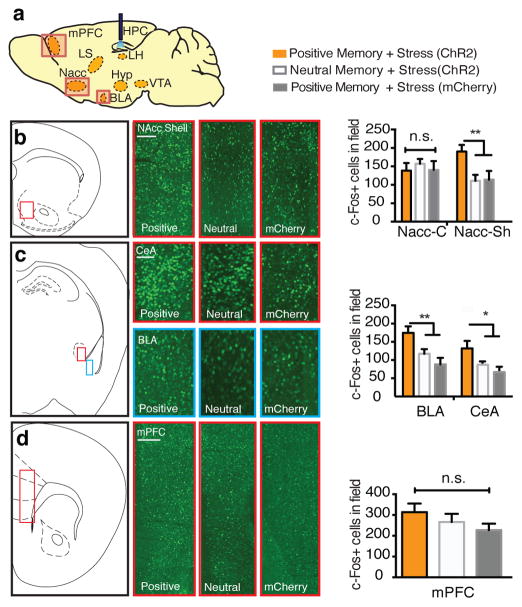
To identify potential neural loci that mediate the light-induced reversal of the stress-induced behaviors observed in our experiments, all subjects first underwent the CIS protocol and then were exposed to the TST while DG cells previously active during a positive experience were optically reactivated. We then performed a brain- wide mapping of c-Fos expression in areas activated by this treatment (Fig. 2a). Optical reactivation of DG cells labeled by a positive experience correlated with a robust increase of c-Fos expression in several brain areas, including the nucleus accumbens shell (NAC-Sh), lateral septum (LS), basolateral amygdala (BLA), central amygdala (CeA), as well as the dorsomedial, ventromedial, and lateral hypothalamus (Fig. 2b–i and Extended Data Fig. 5a, b), but not in the medial prefrontal cortex (mPFC) (Fig. 2j–m) or in several other loci (Extended Data Fig. 5c–e). Furthermore, we monitored single-unit activity in the BLA of mice while simultaneously activating DG positive memory-engram cells with blue light and found that ~8% of cells (9/106; n = 3 mice) had excitatory (8/9 cells) or inhibitory (1/9 cells) responses (Extended Data Fig. 4a). A parallel set of experiments in which unstressed animals received optical stimulation of DG cells revealed mostly similar patterns of c-Fos expression (Extended Data Fig. 6).
Figure 2.
Positive memory reactivation increases c-Fos expression in the nucleus accumbens shell and the amygdala. a, Brain diagram illustrating target areas analyzed. b, Activation of a positive memory, but not a neutral memory or mCherry only, in the DG during the TST elicits robust c-Fos expression in the nucleus accumbens shell (b), basolateral amygdala, and central amygdala (c), but not in the medial prefrontal cortex (d). For histological data, a one-way ANOVA followed by a Bonferroni post-hoc test revealed a significant increase of c-Fos expression in the positive memory + stress group relative to controls in the NAcc and amygdala, but not the mPFC (NAcc Shell: F2, 30 = 15.2, P < 0.01; BLA: F2, 30 = 11.71, P < 0.01; CeA: F2, 30 = 11.45, P < 0.05; mPFC: F2, 30 = 1.33, P = 0.294. n = 6 per group, 3–5 slices per animal). n.s., not significant, *P<0.05, **P<0.01,***P < 0.001. Data are means +/− SEM. Scale bars correspond to 100μm.
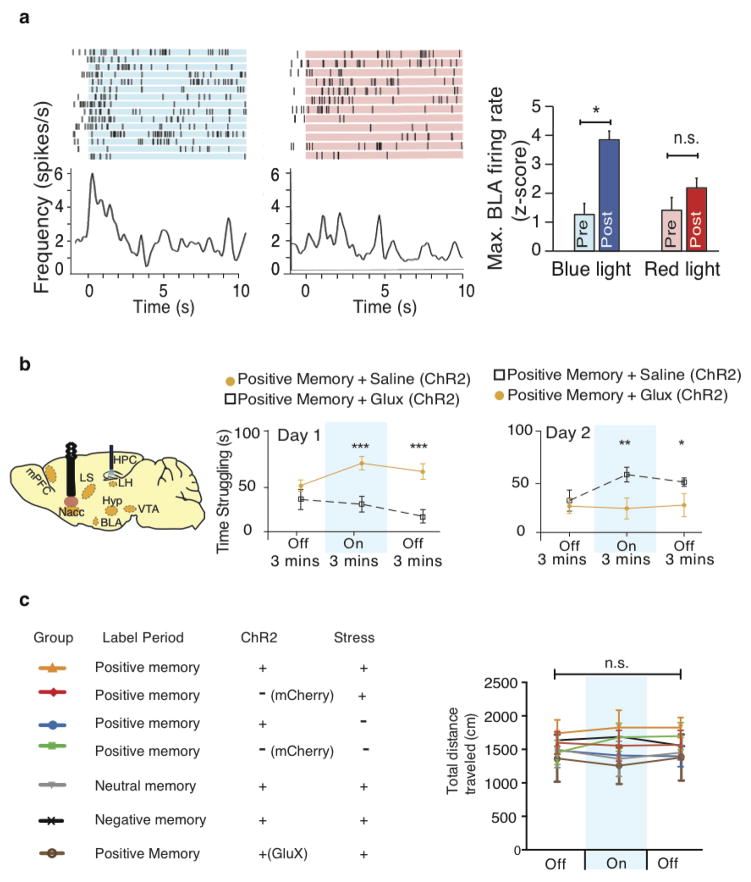
The NAC has been heavily implicated in stress responses, mood disorders, and processing natural rewards 2,5,11–13,16–21. Moreover, pathophysiological dysfunction of the NAcc in response to various stressors has been implicated in anhedonia and reward conditioning18–21. Our within-subject experiments revealed that, in the TST, the behavioral effects of optically reactivating DG cells labeled by a positive experience were blocked in the group of mice that concurrently received the glutamate receptor antagonists NBQX and AP5 in the NAcc, but not in the group that received saline, without altering basal locomotion (Extended Data Fig. 4b, c). Blocking dopaminergic activity yielded a similar blockade of the DG light-induced effects (Extended Data Fig. 7a).
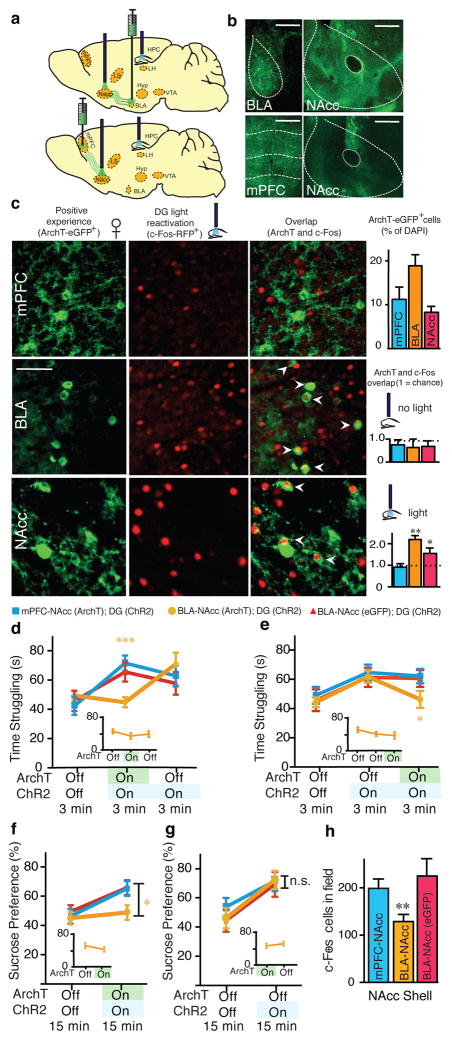
The BLA is known to have robust glutamatergic inputs to the NAcc20, and previous studies have implicated BLA projections to the NAcc in enabling reward- seeking behavior20. We therefore investigated whether the hippocampus (DG)-BLA- NAcc functional pathway is crucial for the real-time light-induced rescue of depression-related behavior. The c-fos-tTA mice were bilaterally injected with TRE- ArchT-eGFP into the BLA to allow for activity-dependent ArchT-eGFP labeling of axonal terminals from the BLA to the NAcc in response to a positive experience10 (Fig. 3a, b). Optic fibers were bilaterally placed over the NAcc and the DG to allow for real-time inhibition of these terminals originating from ~18% (Fig. 3c) of BLA neurons and simultaneous activation of ChR2-mCherry-positive DG cells, respectively, in stressed mice. At the neuronal level, light-induced reactivation of DG cells previously activated by a positive experience also reactivated BLA8 and NAcc19, but not mPFC, cells (i.e. endogenous c-Fos+ cells, red) previously activated by the same positive experience (i.e. Arch T-eGFP+ cells, green) (Fig. 3c). These results suggest that the DG engram cells are functionally connected to BLA engram cells and NAcc engram cells. At the behavioral level, inhibition of BLA terminals onto the NAcc blocked the DG light-induced rescue in both the TST and SPT (Fig. 3d–g). Within the same behavioral session for the TST, and across two days for the SPT, when ArchT-mediated inhibition was released (i.e. the green light was turned off), the rescue effects of reactivating DG cells previously active during a positive experience were rapidly observed in all groups (Fig. 3d–g). Arch-T-mediated inhibition of BLA- NAcc terminals alone did not negatively affect behavior in the TST or SPT beyond the levels of the stressed animals (Fig. 3d–g insets). The specificity of the hippocampus (DG)-BLA-NAcc pathway for the rescue was supported by an analogous experiment conducted with bilateral injections of TRE-ArchT-eGFP into the mPFC. Although the mPFC is also known to provide robust glutamatergic input to the NAcc20, the induction of c-Fos expression in this area upon optogenetic activation of DG cells associated with a positive experience was not significantly higher than that observed with a neutral experience (Fig. 2m), and mPFC cells reactivated by DG cell reactivation was at chance level (Fig. 3c). Correspondingly, inhibition of terminals originating from ~12% of the mPFC (Fig. 3c) onto the NAcc did not block the DG light-induced rescue in either the TST or SPT (Fig. 3d–g). Moreover, inhibition of BLA, but not mPFC, terminals onto the NAcc partially inhibited the DG- mediated, light-induced increase of c-Fos+ cells observed in the NAcc shell (Fig. 3h), supporting the conclusion that the hippocampal DG-BLA-NAcc pathway of positive engrams plays a crucial role in the rescue of depression-related behavioral phenotypes.
Figure 3.
The antidepressant effects of an optically activated positive memory require real-time terminal activity from the BLA to the NAcc. a, Brain diagram illustrating target areas manipulated. b, Representative coronal slices showing TRE-ArchT-eGFP-positive cells in the BLA or mPFC, as well as their corresponding terminals in the NAcc. Scale bars: BLA and mPFC: 500μm, NAcc, 200μm. c, Animals were taken off Dox and initially exposed to a positive experience, which caused labeling of corresponding BLA (~18%), mPFC (~12%), or NAcc (~9%) cells with eGFP derived from AAV9-TRE-ArchT-eGFP (green, halo-like expression). Light-activation of a positive memory engram in the DG preferentially reactivated the BLA and NAcc shell cells, as measured by endogenous c-Fos expression (red, nucleus-localized), that were originally labeled by the same positive experience, while no-light stimulation groups showed levels of overlap not significantly different from chance. Arrowheads indicate double-stained cells. Scale bar: 5 μm. d–g, ArchT-mediated inhibition of BLA, but not mPFC, terminals in the NAcc prevents the DG-mediated light-induced increases in struggling (d, e) or preference for sucrose (f, g), while inhibition of BLA terminals in the NAcc without DG stimulation does not affect behavior (insets). h, ArchT- mediated inhibition of BLA, but not mPFC, terminals prevents the DG-mediated light-induced increase of c-Fos expression in the NAcc. For behavioral data, a two- way ANOVA with repeated measures followed by Bonferroni post-hoc test revealed a group-by-light epoch interaction and significant ArchT-mediated attenuation of struggling in the TST (d: F2, 99 = 7.30, P < 0.001; e: F2, 99 = 6.61, P < 0.01) or preference for sucrose water in the the SPT (f: F2, 66 = 10.66, P < 0.01). n = 12 per behavioral group. *P<0.05, **P<0.01, ***P<0.001; orange ** and *** used to denote significant differences between the stress + positive memory group vs. all other groups. For histological data, one-sample t-tests against chance overlap were performed (n = 4 per group, 3–5 slices per animal). n.s., not significant. Data are means +/− SEM.
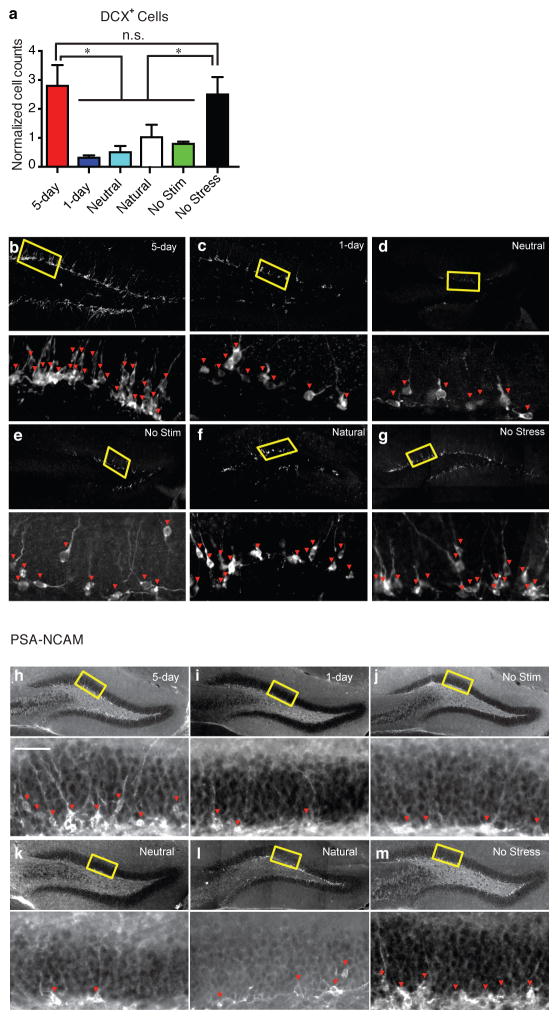
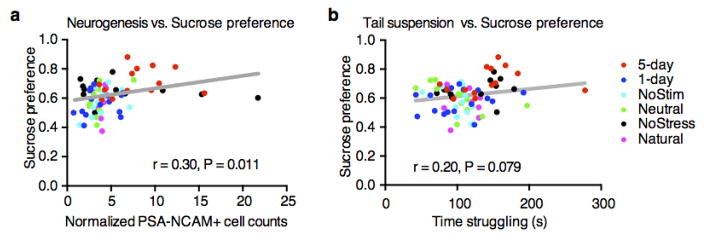
Recent meta-analyses have suggested that treating psychiatric disorders through prescribed medication or cognitive interventions are capable of producing symptom remission when administered chronically21, though the neural underpinnings inducing and correlating with long-lasting rescues are poorly understood21–23. The aforementioned acute intervention did not induce enduring behavioral changes (Extended Data Fig. 7b). We therefore investigated whether chronic reactivation of DG engram cells could have an attenuation of depression-related behaviors that outlasted acute optical stimulation following the protocol depicted in Figure 4A (See Methods). The positive experience and 5-day stimulation group, but not 1-day or no stimulation group, showed a reversal of the stress-induced behavioral deficits measured in the TST and SPT that was not significantly different from an unstressed control group (Fig. 4b, c). A neutral experience and 5 day group stimulation group were reactivated for 5-days did not show such effects, nor did a group that was exposed to a natural social reward for 5-days (Fig. 4b–d). Histological analyses revealed decreased levels of neurogenesis as measured both by the polysialylated neuronal cell adhesion molecule (PSA-NCAM) and doublecortin (DCX)—often considered markers of developing and migrating neurons24,25—in all stressed groups except for the positive experience and 5-day stimulation group, and the unstressed control group (Fig. 4d, Extended Data Fig. 8). This increase in adult-born neurons positively correlated with the degree to which each group preferred sucrose in the SPT (Extended Data Fig. 9a); moreover, performance levels on the SPT and TST positively correlated with one another on an animal-by-animal basis (Extended Data Fig. 9b).
Figure 4.
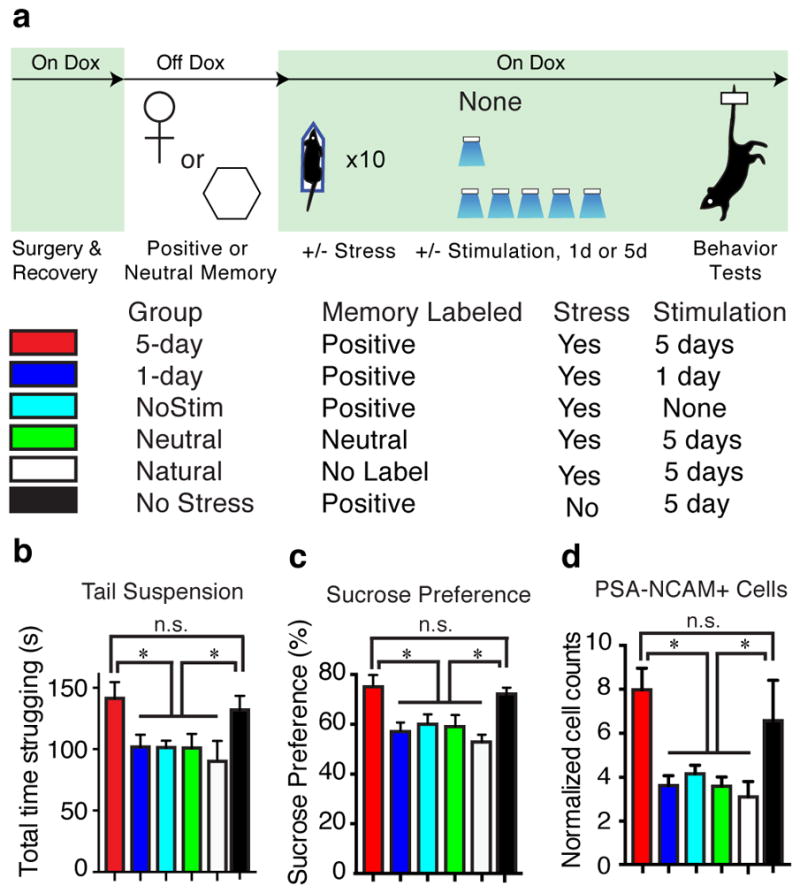
Chronic activation of a positive memory elicits a long-lasting rescue of depression-related behavior. a, Behavioral schedule and groups utilized. b, c, Animals in which a positive memory was reactivated twice a day for five days showed increased struggling in a 6-minute tail suspension test (F5, 78 = 3.34, P < 0.05) (b) and increased preference for sucrose measured over 24 hours (F5, 84 = 6.25, P < 0.01) (c). d, The 5-day positive memory stimulation group showed a significant increase of adult new-born cells in the DG as measured by PSA-NCAM+ cells (F5, 72 = 4.65, P < 0.01; See Extended Data Fig. 8 for doublecortin data and PSA-NCAM images). For these data (b–d), a one-way ANOVA revealed a significant interaction of the experimental-group factor and stimulation-condition factor and was followed by a Bonferroni post-hoc test. n = 14 per TST behavioral group, n = 15 per SPT behavioral group, n = 5 slices per animal for data appearing in (d). Scale bars in expanded images correspond to 5 μm. *P<0.05. Data are means +/− SEM.
Our data demonstrate that the depression-related readouts of active/passive coping-like behavior and anhedonia, as measured in the TST and SPT, respectively, can be ameliorated by activating cells in the hippocampus associated with a positive memory, while anxiety-related behaviors measured by the OFT and EPMT remained unchanged. Differential regulation of depression- and anxiety-related behavior could have been achieved by leveraging the functional segregation present along the hippocampus dorsal-ventral axis; for instance, activation of ventral hippocampal DG engram cells could reveal heterogeneous, behaviorally relevant roles in the emotional regulation of anxiety and stress responses that our dorsal hippocampus manipulations presumably did not access26,27. To that end, we speculate that, at the engram level, the circuitry sufficient to modulate anxiety-related behavior relies more heavily on a synaptic dialogue within the amygdala, its bidirectional connections with the ventral hippocampus, and its effects on downstream mesolimbic and cortical structures11,12,26,27.
Depression is diagnosed as a constellation of heterogeneous symptoms; their complex etiology and pathophysiology underscore the varied responses to currently available treatments. While most psychopharmacological treatments take weeks to achieve effects, other alternative treatments such as deep-brain stimulation (DBS) and the NMDA antagonist ketamine have been reported to have rapid effects in a subset of patients28. In rodents, optogenetic stimulation of mPFC neurons, mPFC to raphe projections, and VTA dopaminergic neurons achieved a rapid reversal of stress- induced maladaptive behaviors4,11,12.. We speculate that our acute behavioral changes reflect the degree to which directly stimulating positive memory engram-bearing cells might bypass the plasticity that normally takes antidepressants weeks or months to achieve, thereby temporarily suppressing the depression-like state. In support, we observed that the effects of optically stimulating a positive memory are contingent on active glutamatergic projections from the amygdala to the NAcc in real-time, as well as intra-NAcc dopamine activity19. Our data dovetail with this circuit’s proposed role of relaying BLA stimulus-reward associations to a ventral striatal motor-limbic interface. This interface is thought to be capable of coalescing such information with motivational states and finally translating such activity into behaviorally relevant outputs5,18–20.
Moreover, our chronic stimulation data reveal that repeatedly activating DG engram cells associated with a positive experience elicits an enduring reversal of stress-induced behavioral abnormalities and an increase in neurogenesis. While future experiments are required to identify the causal link between chronically reactivated positive memory engrams and the corresponding rescue of behaviors, many tantalizing hypotheses surface, including a normalization of VTA firing rates29, epigenetic and differential modification of effector proteins (e.g. CREB, BDNF) in areas upstream and downstream of the hippocampus30, and a reversal of neural atrophy in areas such as CA3 and mPFC or hypertrophy in BLA26. The aforementioned molecular and homeostatic mechanisms—in addition to our observed increase of adult-born neurons in the 5-day stimulation group—could be partly realized in a hormone- or neuromodulator-mediated manner (Extended Data Fig. 5). Finally, our data demonstrate that exposing stressed subjects to a natural positive experience repeatedly is not effective, while repeated direct reactivations of DG engram cells associated with a previously acquired positive memory (Fig. 4b–d). We speculate that invasively stimulating these DG cells is effective in activating both the internal contextual representation associated with a positive experience as well as associated downstream areas, while exposure to natural exogenous positive cues may not be able to access similar neural pathways in subjects displaying depression-like symptoms such as passive behavior in challenging situations and anhedonia (Fig. 4b–d).
Collectively, the data described here build a novel experimental bridge between memory engrams in the brain and animal models of psychiatric disorders. We propose that direct activation of DG engram cells associated with a positive memory offers a potential therapeutic node for alleviating a subset of depression-related behaviors and, more generally, that directly activating endogenous neuronal processes may be an effective means to correct maladaptive behaviors.
Extended Data
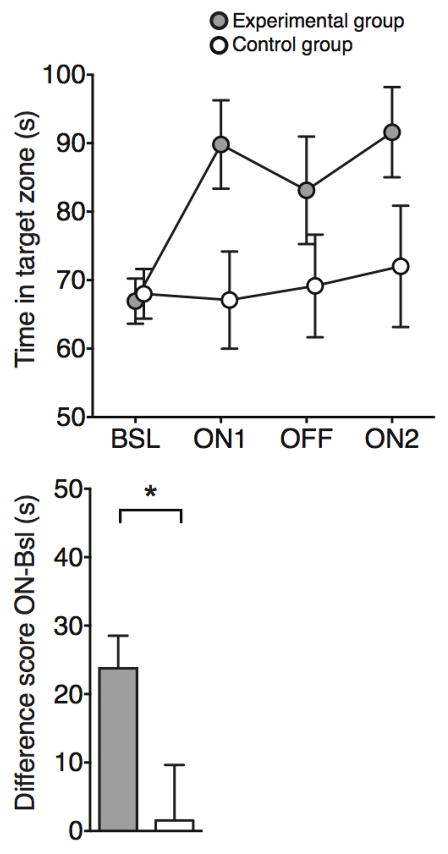
Extended Data Figure 1. Male mice spend more time around an object associated with females.
a, Time spent in the target zone where the object associated with females is introduced in the ON phases. Female-object mice (experimental group) spend more time in the target zone during the ON phases than the Neutral-object mice (control group; two-way ANOVA with multiple comparisons, ON1 t88 = 2.41; p < 0.05, ON2 t88 = 2.08; p < 0.05). b, Difference score (avg of ON phases – Bsl) also shows the increased preference for the target zone in the female-object group compared to neutral-object group (t22 = 2.37; p < 0.05). n = 12 per group. See Supplementary Information for detailed methods.
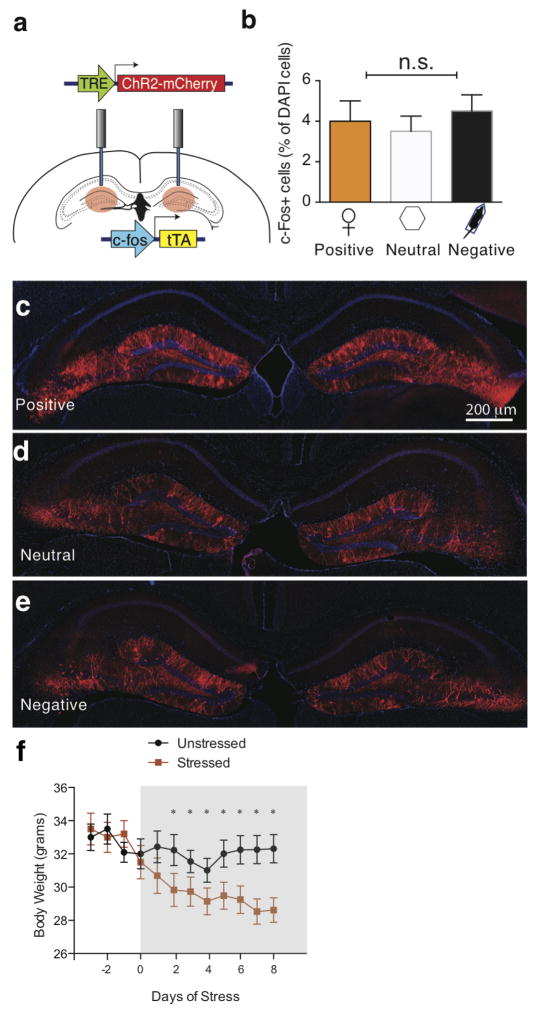
Extended Data Figure 2. Positive, neutral, or negative experiences label a similar proportion of dentate gyrus cells with ChR2; stress prevents weight gain over 10 days.
a, The c-Fos mice were bilaterally injected with AAV9-TRE-ChR2-mCherry and implanted with optical fibers targeting DG. b–e, Histological quantifications reveal that, while off Dox, a similar proportion of DG cells are labeled by ChR2-mCherry in response to positive (c), neutral (d), or negative experience (e). All animals were sacrificed a day after completing the CIS protocol. One-way ANOVA followed by Bonferroni post-hoc test, P > 0.05, n.s. not significant. f, Animals were chronically immobilized for 10 days, during which they lost a significant amount of weight compared to an unstressed group (one-way ANOVA followed by Bonferroni post-hoc test, *P<0.05, n = 9 per group). Data are means +/− SEM.
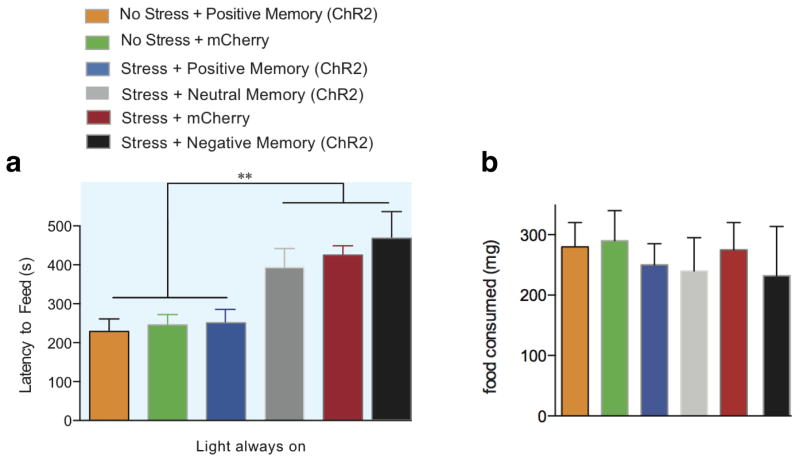
Extended Data Figure 3. Reactivation of a positive memory decreases latency to feed in a novelty suppressed feeding paradigm.
a, All groups were food deprived for 24 hours and then underwent a novelty suppressed feeding protocol. While chronic immobilization increased the latency to feed, light-reactivation of a positive memory significantly decreased the latency to feed and at levels that matched the unstressed groups. b, Upon completion of the novelty suppressed feeding test, all groups were returned to their home cage and food intake was measured after 5 minutes (one-way ANOVA followed by Bonferroni post-hoc test, **P<0.01, n = 16 per group). Data are means +/− SEM.
Extended Data Figure 4. Activation of a positive memory elicits BLA spiking activity, requires NAcc glutamatergic activity in the tail suspension test, but does not alter locomotor activity in the open field test.
a, Raster plots and peri-stimulus time histograms (PSTH) illustrating a transient excitatory response from a single BLA neuron out of the 9 neurons responsive to DG positive memory activation during 10 seconds of blue light stimulation, but not in response to 10 seconds of red light as a control. Blue bar plots on the right illustrate maximum BLA neural firing rate before (Pre) and after (Post) blue light stimulation in the DG (paired t-test, t(7) = 6.91, P = 0.023. Red bar plots show the maximum neural activity for the same neurons after red light stimulation in the DG that serves as a control (paired t-test, t(7) = 1.62, P = 0.15). b, Brain diagram illustrating target areas manipulated. Within-subjects experiments revealed that glutamatergic antagonists (Glux), but not saline, in the accumbens shell blocked the light-induced effects of a positive memory in stressed subjects. c, All groups failed to show significant changes in locomotor activity within a session of open field exploration during either light off or light on epochs, though any trends towards decreases in locomotion are consistent with stress-induced behavioral impairments For behavioral data, a two-way ANOVA with repeated measures followed by Bonferroni post-hoc test revealed a group-by-light epoch interaction on day 1 (F1, 90 = 28.39, P < 0.001; n = 16 per group) and day 2 of testing (F1, 90 = 8.28, P < 0.01). Data are means +/− SEM.
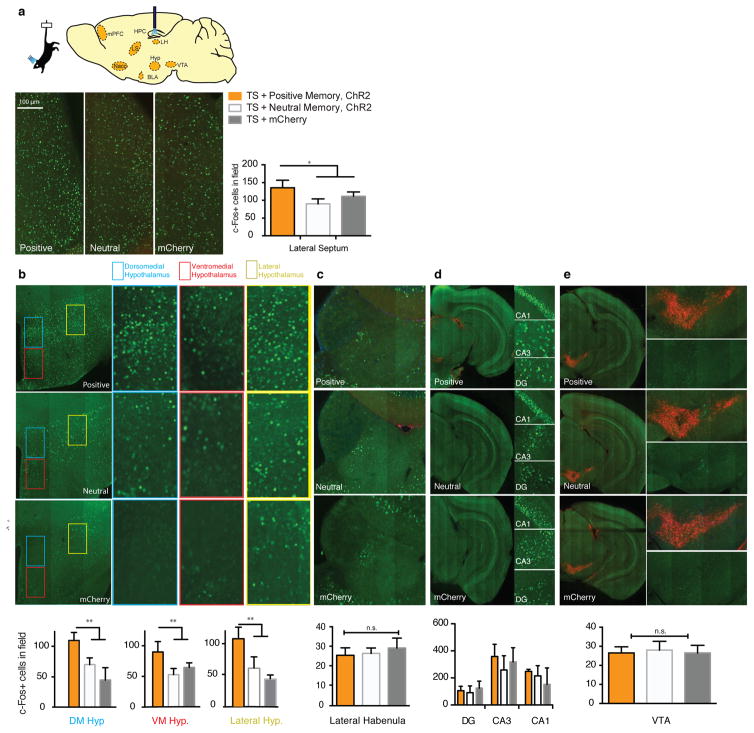
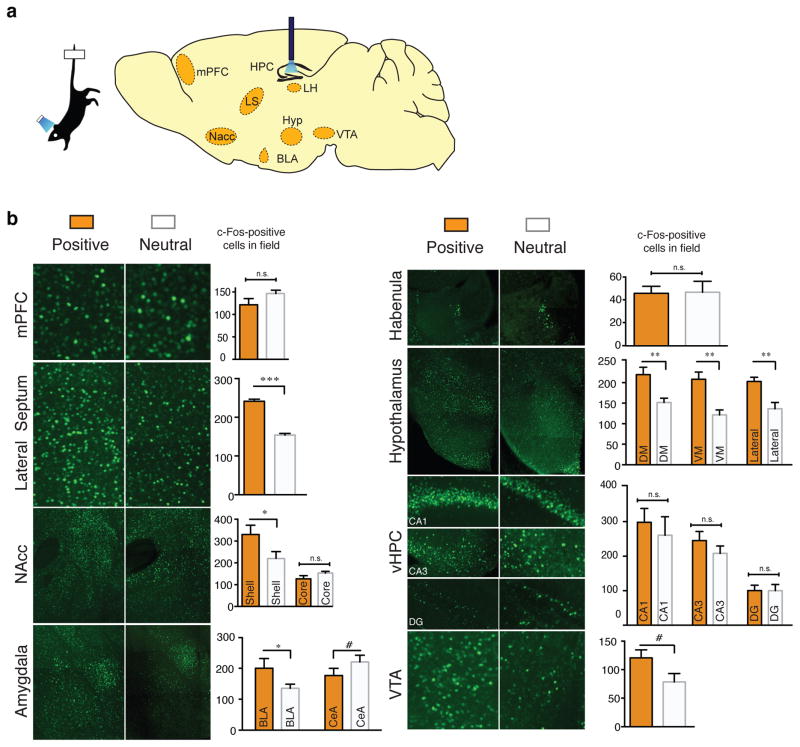
Extended Data Figure 5. Activating a positive memory in the dentate gyrus produces an increase in c-Fos expression in the lateral septum and hypothalamus, but not the lateral habenula, ventral hippocampus, or VTA.
a, Diagram of regions analyzed. b, c-Fos expression significantly increased in the lateral septum (b) and subregions of the hypothalamus (c) in the positive memory group but not in a group in which a neutral memory was stimulated or in a group expressing mCherry alone. c–e, c-Fos expression did not significantly increase in the lateral habenula (c), various ventral hippocampus subregions (d), or VTA, identified by tyrosine hydroxylase staining (red) stainings in the images expanded on the right (e) (one-way ANOVA followed by Bonferroni post-hoc test *P<0.05, **P<0.01, ***P<0.001, n = 5 per group, 3–5 slices per animal). TS, tail suspension. Data are means +/− SEM.
Extended Data Figure 6. Activating a positive memory through the dentate gyrus of unstressed animals increases c-Fos expression in various downstream regions.
a, Diagram of regions analyzed. b, In the positive compared to the neutral memory group, c-Fos expression is significantly increased in the lateral septum, NAcc shell, BLA, dorsomedial, ventromedial, and lateral hypothalamus, but not in the mPFC, NAcc core, habenula, or ventral hippocampus. Trends were observed in the CeA and VTA. Each brain region was analyzed using an unpaired Student’s t test, n = 5 per group, 3–5 slices per animal; #P= 0.17 for CeA and P=0.09 for VTA; *P<0.05, **P<0.01, ***P<0.001, n.s. not significant. Data are means +/− SEM.
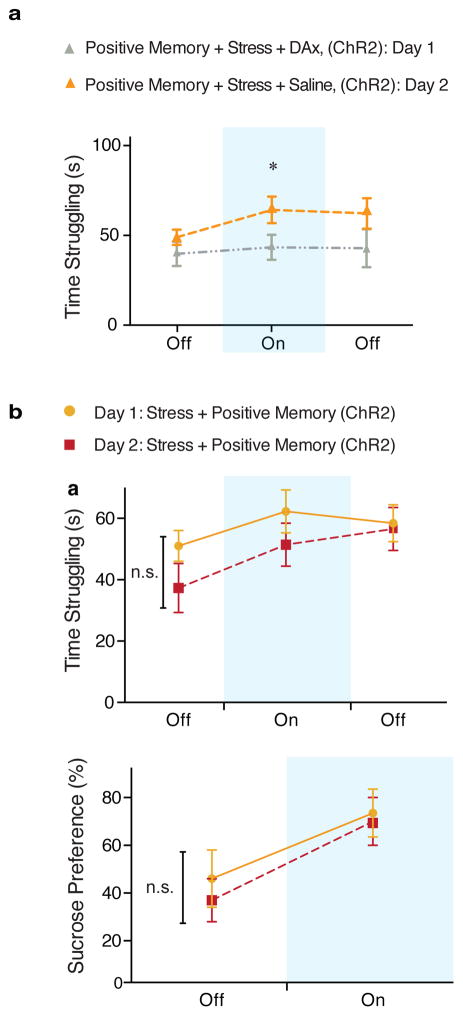
Extended Data Figure 7. Dopamine receptor antagonists block the light-induced effects of positive memory activation; a single session of activating a positive memory in the dentate gyrus does not produce long-lasting antidepressant-like effects.
a, Administration of a cocktail of dopamine receptor antagonists prevented the light-induced increases in struggling during the tail suspension test. When animals were tested again on day 2 and infused with saline, the behavioral effects of optically reactivating a positive memory were observed (two-way ANOVA with repeated measures followed by Bonferroni post-hoc test, *P<0.05, n = 9 per group). b, Animals in which a positive memory was optically activated during the tail suspension test or sucrose preference test showed acute increases in time struggling or preference for sucrose; this change in behavior did not persist when tested again on day 2 (within subjects ANOVA followed by Bonferroni post-hoc test), n = 9. n.s. not significant. Data are means +/− SEM.
Extended Data Figure 8. Chronic activation of a positive memory prevents stress-induced decreases in neurogenesis.
a, The 5-day positive memory stimulation group showed a significant increase of adult new-born cells in the DG as measured by doublecortin (DCX)-positive cells (one-way ANOVA followed by Bonferroni post-hoc test, F5, 72 = 7.634, P < 0.01) relative to control groups.. b–g, Representative images of DCX-positive cells in the DG for the 5-day (b), 1-day (c), Neutral (d), No Stimulation (e), Natural (f), and No Stress (g). h–m, Representative PSA-NCAM images corresponding to data appearing in Figure 4d. n = 5 slices per animal, 13 animals per group for data appearing in (a and b). *P<0.05, n.s. not significant. Data are means +/− SEM.
Extended Data Figure 9. Behavioral and neuronal correlations.
a, Performance levels in the SPT and the number of adult-born neurons as measured by PSA-NCAM are positively correlated on an animal-by-animal basis. b, Performance levels between the TST and SPT show strong positive correlation trends on an animal-by-animal basis. n = 14 per TST behavioral group, n = 15 per SPT behavioral group.
Acknowledgments
We thank B. Chen, D.S. Roy, and J. Kim for help with the experiments, T.J. Ryan and T. Kitamura for the TRE-ArchT-eGFP construct, J. Sarinana, and E. Hueske for comments and extensive discussions on the manuscript, and all the members of the Tonegawa lab for their support. This work was supported by RIKEN Brain Science Institute and Howard Hughes Medical Institute.
Footnotes
Author Contributions:
S.R., X.L., A.M., J.Z., R.L.R. and S.T. contributed to the study design. S.R., X.L., A.M., J.Z., C.M., and R.L.R. contributed to the data collection and interpretation. X.L. cloned all constructs. S.R., X.L., J.Z., and A.M. conducted the surgeries, behavior experiments, and histological analyses. S.R., X.L., and S.T. wrote the paper. All authors discussed and commented on the manuscript.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References and Notes
- 1.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE. Revitalizing psychiatric therapeutics. Neuropsychopharmacology. 2014;39:220–229. doi: 10.1038/npp.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covington HE, III, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. The Journal of neuroscience. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- 8.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seligman MEP. Am Psychol. 2006;61:774–788. doi: 10.1037/0003-066X.61.8.774. [DOI] [PubMed] [Google Scholar]
- 10.Han X, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Frontiers in systems neuroscience. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tye KM, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warden MR, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deisseroth K. Nature. 2014;505:309–317. doi: 10.1038/nature12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaepfer TE, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 19.Xiu J, et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci. 2014;17:1552–1559. doi: 10.1038/nn.3813. [DOI] [PubMed] [Google Scholar]
- 20.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versis medication. Nat Rev Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brody AL, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy. Arch Gen Psych. 2001;58:631–649. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 23.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;318:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 24.Seki XT, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–8. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 26.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 27.Felix-Ortiz AC, et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psych. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 29.Friedman AK, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neursoci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]