Abstract
Chronic hypertension causes vascular remodeling that is associated with an increase of periostin (postn) positive cells, including fibroblasts and smooth muscle cells. Krüppel-like factor (KLF) 5, a transcription factor, is also observed in vascular remodeling, however, it is unknown what role KLF5 plays in postn positive cells during vascular remodeling induced by deoxycorticosterone acetate (DOCA)-salt.
We used postn positive cell-specific Klf5 deficient (Klf5PostnKO: Klf5flox/flox;PostnCre/−) mice and wild type (WT: Klf5flox/flox;Postn−/−) mice. We implanted a DOCA pellet and provided drinking water that containing 0.9% NaCl for eight-weeks.
The DOCA-salt treatment induced hypertension in both genotype groups, as observed by the increase in systolic blood pressure. In WT, DOCA-salt treatment increased the aortic medial area compared to non-treated controls. Similarly, Tgf1b was overexpressed in the aortas of DOCA-salt treated WT mice compared to controls. Immunofluorescence staining revealed that fibroblast specific protein 1 (FSP1)+-α smooth muscle actin (αSMA)+ myofibroblast exist in the medial area of WT aorta after DOCA-salt intervention. Importantly, these changes were not observed in the Klf5PostnKO animals.
In conclusion, the results of this study suggest that the presence of KLF5 on postn positive cells contributes to the pathogenesis of aortic thickening induced by DOCA-salt hypertension.
Keywords: Kruppel-like factors 5, Deoxycorticosterone-acetate salt, Remodeling, Periostin
Introduction
Hypertension is the most common risk factor of cardiovascular diseases. Approximately 25% of the world’s adult population has hypertension and this is likely to increase to 29% by 2025 1. Chronic hypertension causes cardiovascular dysfunction with myocardial hypertrophy and vascular remodeling 2–9, however, the pathophysiology is yet to be elucidated.
Krüppel-like factor 5 (KLF5; also known as BTEB2 and IKLF) is one of the mediators of cardiovascular remodeling 10–15. KLF5 binds to GC boxes and SP1 site at a number of gene promoters and regulates their transcription 14, 16, 17. Previously, our group showed that KLF5 overexpression resulted in the proliferation of vascular smooth muscle cell (SMC). Gene silencing of KLF5 with RNA interference showed marked suppression of cyclin D1 expression and decreased vascular SMC growth in vitro 14. We also demonstrated that overexpression of KLF5 in rats subjected to carotid balloon injury increased neointimal formation and proliferating cell nuclear antigen-positive rate 14. Furthermore, we found that angiotensin II infusion suppressed degrees of arterial-wall thickening, angiogenesis, cardiac hypertrophy and interstitial fibrosis in Klf5-knockout mice 10.
Periostin (postn) is known as a useful marker of the noncardiomyocyte lineages, and it observed on fibroblasts 18 and SMCs 19. It has been well investigated not only in the cardiovascular system but also in the ischemic brain 20. It is not normally expressed in normal conditions, but it is induced by tissue injury, contributing to cardiac remodeling 21,22. Therefore, it is suggested that KLF5 in fibroblasts and SMCs plays a pivotal role in the vascular remodeling. Recently, we generated postn positive cell-specific Klf5 null mice 15. However, its role in fibroblasts and SMCs on vascular remodeling is still unknown. The aim of this study is to investigate the role of KLF5 in the postn positive cells on the vascular remodeling by using DOCA-salt hypertension in mice.
Methods
Generation of periostin positive cell-specific Klf5 deficient Mice
Mice containing the Klf5flox allele with mice expressing Cre recombinase under the control of the periostin (Posin) promoter were crossed to generate mice 15. The presence of the Klf5flox/flox and PostnCre/− double-transgene was determined by PCR analysis of genomic DNA from ear tips. Klf5 deletion from the fibroblasts was validated by Western blot analysis from Klf5PostnKO mice using the KLF5 monoclonal antibody hybridoma supernatant (KM1784) 15.
DOCA Pellet Implantation
We used 8-week-old male postn positive cell-specific Klf5 deficient (Klf5PostnKO: Klf5flox/flox;PostnCre/−) mice and wild type (WT: Klf5flox/flox;Postn−/−) littermate mice. They were given a standard diet and water. This study was approved by the Animal Care and Use Committee of the Tokyo Medical and Dental University. A DOCA pellet (25 mg, Innovative Research of America, Sarasota, US) was implanted subcutaneously in the back under anesthesia as described previously 23. Mice receiving DOCA were given 0.9% NaCl to drink. Treatment with DOCA-salt continued for eight-weeks. The control groups, consisting of WT and Klf5PostnKO mice, did not receive DOCA and saline. Each group consisted of 10–12 mice. Mice were sacrificed after eight-weeks of treatment and tissue samples were collected for analysis.
Systolic Blood Pressure
Systolic blood pressure (SBP) was measured by using a tail-cuff system (BP-98A, Softron Co., Tokyo, Japan) 24,25 weekly between 10:00 am to 12:00 pm. Unanesthetized awake mice were prewarmed for 10 min at 37°C in a thermostatically controlled heating cabinet. An average of five recordings were taken at individual value.
Histopathology
Aorta was harvested immediately after the mice were sacrificed. Five transverse sections per organ were obtained for histological examination. Aortic samples were stained with Elastica van Gieson (EvG) staining 26. The areas were measured using a computerized analyzer (Scion Image beta 4.0.2).
RNA Extraction and Real-time PCR
Total RNA was extracted according to the manufacture’s protocol by the TRIsure (Bioline, Tokyo, Japan). Complementary DNA was prepared with a reverse transcriptase-polymerase chain reaction (RT-PCR) kit (QIAGEN, Tokyo, Japan) 27. The PCR was performed with a PCR-kit in the presence of oligo-primers for transforming growth factor beta 1 (Tgfb1, Mm01178820_m1) and collagen1a1 (Col1a1, Mm01302043_g1). The sequences of the PCR primers were predesigned inventoried TaqMan Gene Expression Assays (Life Technologies Japan, Tokyo, Japan). Results were obtained from 3 independent experiments (5 samples in each group).
Immunofluorescence Staining
Immunohistochemistry was performed to examine CD11b (1561-01, SouthernBiotech), α-smooth muscle actin (αSMA-FITC: F3777, Sigma) and fibroblast specific protein 1 (FSP1, also known as S100A4, ab27957, abcam) expression in the aorta. The sections were incubated overnight at 4°C with primary antibodies and washed in PBST. Secondary antibodies were then applied for 60 min at room temperature. After washing in PBST, sections were counterstained with DAPI (CS-201-06, InnoGenex).
Statistics
Statistical analysis was performed using SPSS Base System 14.0J for windows (IBM Japan, Tokyo, Japan). All data were expressed as the mean ± SE, with statistical comparisons performed using the one-way ANOVA with Tukey HSD post hoc test or one-way ANOVA repeated measures with Sidak multiple comparison test. P < 0.05 was considered statistically significant.
Results
Blood Pressure
Figure 1 shows SBP in four mice groups over an eight-week period. Despite genotype difference, control WT and Klf5PostnKO mice showed comparable SBP. Similarly, DOCA-salt treatment increased SBP between the WT and Klf5PostnKO mice. There was no difference in SBP between the WT-DOCA and Klf5PostnKO-DOCA mice.
Figure 1.
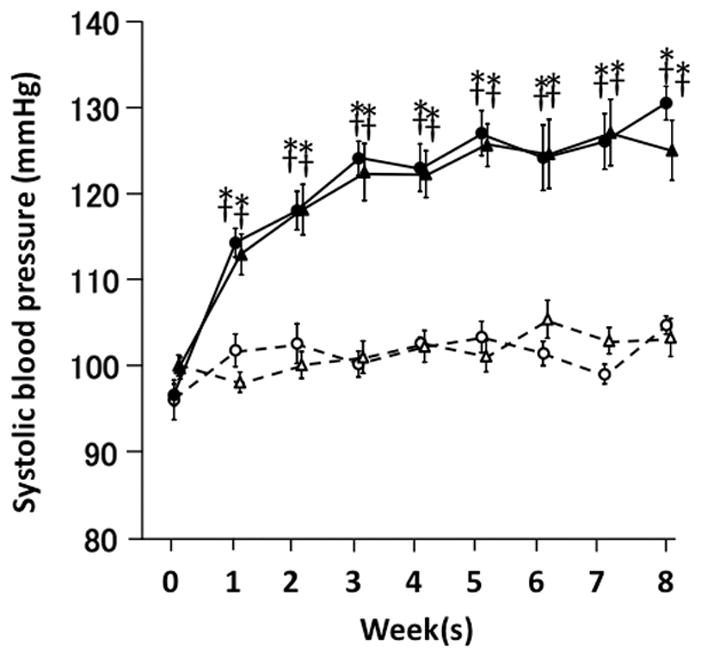
Systolic blood pressure during eight-week DOCA-salt treatment period. Open circle, WT-control mice (n=10); open triangle, Klf5PostnKO-control mice (n=12); solid circle, WT-DOCA-salt mice (n=11); solid triangle, Klf5PostnKO-DOCA-salt mice (n=10). Values are mean ± SE. *P < 0.05 vs. WT-Cont. †P < 0.05 vs. Klf5Cre/−-Cont.
Histopathology of Aorta
It has been known that DOCA-salt hypertension increases aortic wall thickness 6. Thus, we examined whether DOCA-salt treatment increased the aortic medial area in the Klf5PostnKO mice (Figure 2A and B). In control groups, there was no significant difference in the aortic wall medial area between the WT and Klf5PostnKO mice. DOCA-salt treatment significantly increased the aortic wall medial area in WT animals. However, the treatment did not alter the aortic wall medial area in the Klf5PostnKO animals. This result indicates that the Klf5PostnKO mice do not histopathologically change because of eight-week DOCA-salt intervention.
Figure 2.
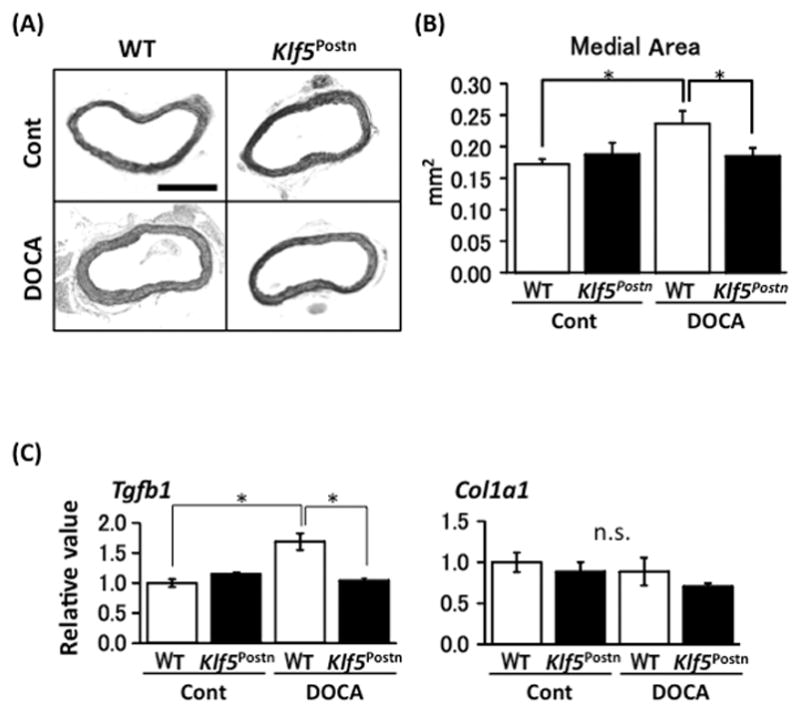
Myofibroblasts localize in thickened aortic media after eight-weeks of DOCA-salt treatment. (A) Representative EvG staining of the aorta (×100). Bar, 500 μm. (B) Aortic medial wall area. n = 10 per group. *P < 0.05. (C) mRNA expression of Tgfb1 and Col1a in aorta. n = 5 per group. Values are mean ± SE. *P < 0.05.
mRNA Expressions of Aorta
We examined whether DOCA-salt intervention alters gene expression of aorta (Figure 2C). In the control group aorta, there was no significant difference in Tgfb1 mRNA levels between the WT and Klf5PostnKO mice. DOCA-salt treatment significantly increased Tgfb1 mRNA levels in the WT animals (P < 0.001). However, the treatment did not alter Tgfb1 mRNA levels in the Klf5PostnKO animals. There was no significant difference in the mRNA levels of Col1a1 mRNA between the WT-DOCA and Klf5PostnKO-DOCA mice (P = 0.193). These observations indicate that DOCA-salt treatment increases TGFβ1 on aorta in the WT mice, but not in the Klf5PostnKO mice.
Immunofluorescence Staining
Figure 3 shows representative immunofluorescence staining. CD11b+ lymphocyte infiltration was observed only on tunica adventitia. Following this, immunofluorescence staining showed that αSMA was equally expressed in the aortic media in all groups (Figure 3B). In contrast, FSP1 was observed in the aortic media of WT-DOCA mice compared with WT-Cont, Klf5PostnKO-Cont, and Klf5PostnKO-DOCA mice. These results show that FSP1 cells co-express with αSMA in the aortic media.
Figure 3.
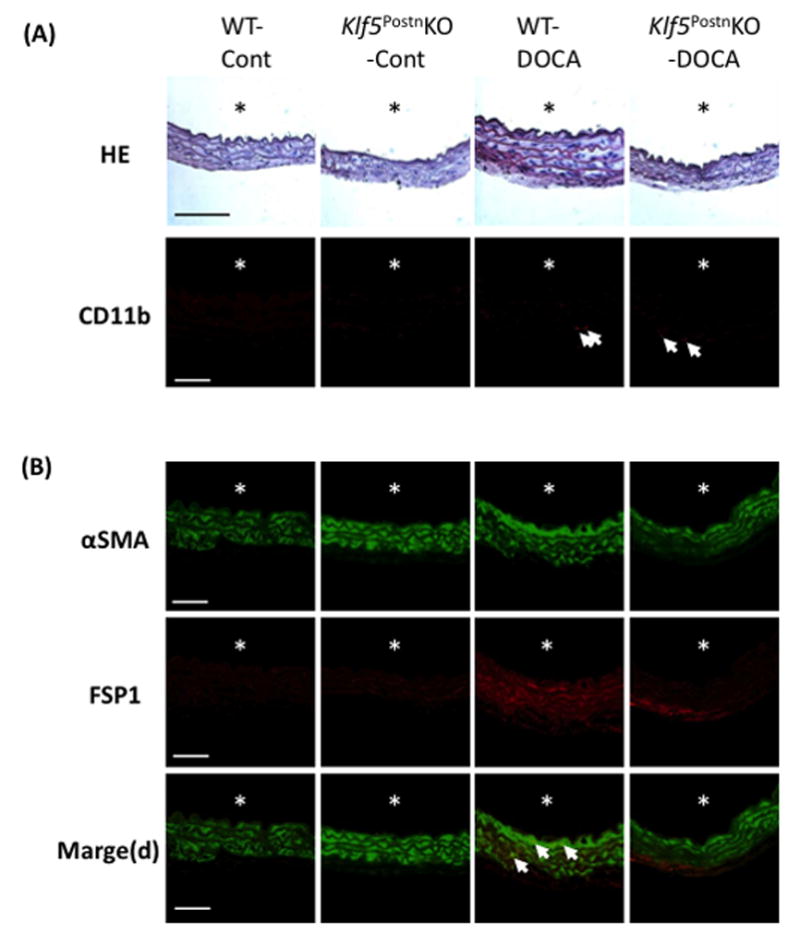
DOCA-salt treatment induced myofibroblast transition in aortic media of WT, but not Klf5PostnKO mice. (A) Representative EvG (Scale, ×400; bar, 100μm) and immunofluorescence staining (Scale, ×600; bar, 50μm) of aorta. Arrows point to CD11b+ cells. (B) Immunofluorescence staining of aorta revealed an increased number of αSMA+ FSP1+ cells in WT-DOCA-salt mice. Scale, ×600; Bar, 50μm. The asterisks indicate the lumen.
Discussion
The present study revealed that only the aortic medial area in WT mice increased, while DOCA-salt treatment similarly increased SBP between the WT and Klf5PostnKO. Also, our data revealed that many FSP1+ αSMA+ myofibroblasts are in thickening aortic media during DOCA-salt treatment. Additionally, in contrast to WT murine aorta, Klf5PostnKO exhibited lower FSP1 protein expression induction by DOCA-salt treatment. These results suggest that KLF5 involves myofibroblast conversion and/or migration to the aortic media induced by DOCA-salt induced hypertension.
TGFβ1 expression is known to be induced by DOCA and endothelin-1 28,29. Consistent with previous observations, DOCA-salt hypertension is associated with endothelin-1-dependence 30–33. An earlier study demonstrated that endothelin-1 also directly up-regulates KLF5 expression 13. Additionally, we have reported that Tgfb1 expression is significantly lower in the hearts of systemic Klf5 knockout mice than in those of WT mice following angiotensin II infusion, suggesting that TGFβ1 lies downstream from KLF5 10. Therefore, DOCA-salt treatment enhances the expression of KLF5 via an endothelin-1 mechanism within myofibroblasts; KLF5 on myofibroblasts might be involved in this aortic thickening via a Tgfb1 expression pathway.
Our group previously showed that pressure overload using transverse aortic constriction (TAC) suppressed degrees of cardiac hypertrophy and interstitial fibrosis in the Klf5-knockout mice 15. In this study, we used a DOCA-salt induced hypertension model in mice, however, there was no difference in heart weight between WT and Klf5PostnKO mice (data not shown). A reason for the differences in both studies may depend on pressure intensity. Indeed, systolic blood pressure was about 130 mmHg in the present study. Although these studies used different models (DOCA-salt or TAC), the results suggest that KLF5 is necessary for pressure load-induced cardiovascular remodeling.
Myofibroblasts are involved with wound healing and tissue repair 34. Results demonstrate that most myofibroblasts express αSMA and that the expression of αSMA and collagen type I in these cells is regulated coordinately by TGFβ1 35. However, results of the present study show that DOCA-induced aorta and myofibroblast did not increase Col1a1 gene expression. This result suggests that DOCA-treated myofibroblasts tend toward being contraction type rather than extracellular-matrix producing type.
A study showed that transient receptor potential melastatin 7 promotes vascular adventitial remodeling in TAC rats 36, while the present study suggests that KLF5 involves the aortic medial wall. Zhang et al. demonstrated that KLF5 expression increased with phenotypic switch of vascular smooth muscle cells from a contractile to a proliferative state in atherosclerotic aortas in clinical aortic wall samples. They also showed that significantly increased KLF5 gene and protein expression in cultured vascular smooth muscle cells from atherosclerotic donors. These results suggest that a more proliferative state of vascular smooth muscle cells from patients with atherosclerosis may be associated with higher expression of KLF-5 37. Endothelin-1 is known to have an especially prominent role in DOCA-salt hypertension 38. One important effect of its situation is to increase total peripheral resistance by contracting arteries and arterioles 39. Prepro-endothelin-1 mRNA expression and immunoreactive endothelin-1 content of aorta are increased in DOCA-salt rats 40–42, suggesting that one mechanism of endothelin-1-induced arterial constriction in hypertension is an increased level of peptide around arterial smooth muscles. However, there is no direct evidence from endothelin-1 to the aortic SMC proliferation via KLF-5 at this moment. Recently, Courboulin et al. demonstrated that endothelin-1 triggered KLF-5 activation in pulmonary artery smooth muscle cells. They showed that the pulmonary artery smooth muscle cells with enhanced KLF-5 were implicated in the pro-proliferative phenotype 43. Thus, DOCA-endothelin-1-KLF5 pathway may be critical in this pathophysiology. Because arterial remodeling includes different pathological phenomena, further investigation is needed to clarify the detailed mechanism.
In conclusion, the results of this study suggest that the presents of KLF5 on periostin positive cells contribute to the pathogenesis of the aortic thickening induced by DOCA-salt treated hypertension.
Acknowledgments
We would like to thank Ms. Noriko Tamura and Ms. Yasuko Matsuda for their excellent technical assistance.
Grants: This study was supported by the Japan Society for the Promotion of Science (JSPS) through its “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program).”
Footnotes
Conflict of Interest: None declared.
References
- 1.Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis. 2010;55(3):590–598. doi: 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Hagiwara M, Bledsoe G, Yang ZR, Smith RS, Jr, Chao L, Chao J. Intermedin ameliorates vascular and renal injury by inhibition of oxidative stress. Am J Physiol Renal Physiol. 2008;295(6):F1735–1743. doi: 10.1152/ajprenal.90427.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolinsky H. Long-term effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Morphological and chemical studies. Circ Res. 1972;30(3):301–309. doi: 10.1161/01.res.30.3.301. [DOI] [PubMed] [Google Scholar]
- 4.Levy BI, Michel JB, Salzmann JL, Azizi M, Poitevin P, Safar M, Camilleri JP. Effects of chronic inhibition of converting enzyme on mechanical and structural properties of arteries in rat renovascular hypertension. Circ Res. 1988;63(1):227–239. doi: 10.1161/01.res.63.1.227. [DOI] [PubMed] [Google Scholar]
- 5.Simko F, Matuskova J, Luptak I, Krajcirovicova K, Kucharska J, Gvozdjakova A, Babal P, Pechanova O. Effect of simvastatin on remodeling of the left ventricle and aorta in L-NAME-induced hypertension. Life Sci. 2004;74(10):1211–1224. doi: 10.1016/j.lfs.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Watts SW, Rondelli C, Thakali K, Li X, Uhal B, Pervaiz MH, Watson RE, Fink GD. Morphological and biochemical characterization of remodeling in aorta and vena cava of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292(5):H2438–2448. doi: 10.1152/ajpheart.00900.2006. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima T, Umemoto S, Yoshimura K, Matsuda S, Itoh S, Murata T, Fukai T, Matsuzaki M. TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res. 2015;38(10):649–655. doi: 10.1038/hr.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102(6):720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Chen J, Guan W, Han Y, Wang WE, Wang X, Wang H, Jose PA, Zeng C. Activation of the D4 dopamine receptor attenuates proliferation and migration of vascular smooth muscle cells through downregulation of AT1a receptor expression. Hypertens Res. 2015;38(9):588–596. doi: 10.1038/hr.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8(8):856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 11.Nagai R, Shindo T, Manabe I, Suzuki T, Kurabayashi M. KLF5/BTEB2, a Kruppel-like zinc-finger type transcription factor, mediates both smooth muscle cell activation and cardiac hypertrophy. Adv Exp Med Biol. 2003;538:57–65. doi: 10.1007/978-1-4419-9029-7_5. discussion 66. [DOI] [PubMed] [Google Scholar]
- 12.Fujiu K, Manabe I, Ishihara A, Oishi Y, Iwata H, Nishimura G, Shindo T, Maemura K, Kagechika H, Shudo K, Nagai R. Synthetic retinoid Am80 suppresses smooth muscle phenotypic modulation and in-stent neointima formation by inhibiting KLF5. Circ Res. 2005;97(11):1132–1141. doi: 10.1161/01.RES.0000190613.22565.13. [DOI] [PubMed] [Google Scholar]
- 13.Cullingford TE, Butler MJ, Marshall AK, Tham el L, Sugden PH, Clerk A. Differential regulation of Kruppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: effects of endothelin-1, oxidative stress and cytokines. Biochim Biophys Acta. 2008;1783(6):1229–1236. doi: 10.1016/j.bbamcr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Sawaki D, Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J Biol Chem. 2009;284(14):9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120(1):254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sogawa K, Imataka H, Yamasaki Y, Kusume H, Abe H, Fujii-Kuriyama Y. cDNA cloning and transcriptional properties of a novel GC box-binding protein, BTEB2. Nucleic Acids Res. 1993;21(7):1527–1532. doi: 10.1093/nar/21.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66(16):2691–2706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105(10):934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208(2):358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimamura M, Taniyama Y, Nakagami H, Katsuragi N, Wakayama K, Koriyama H, Kurinami H, Tenma A, Tomioka H, Morishita R. Long-term expression of periostin during the chronic stage of ischemic stroke in mice. Hypertens Res. 2014;37(6):494–499. doi: 10.1038/hr.2014.36. [DOI] [PubMed] [Google Scholar]
- 21.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101(3):313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Current genomics. 2008;9(8):548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed SF, Ohtani T, Korinek J, Lam CS, Larsen K, Simari RD, Valencik ML, Burnett JC, Jr, Redfield MM. Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via “nongenomic effects”. Circulation. 2010;122(4):370–378. doi: 10.1161/CIRCULATIONAHA.109.915215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail. 2007;9(2):152–159. doi: 10.1016/j.ejheart.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Liao Y, Ishikura F, Beppu S, Asakura M, Takashima S, Asanuma H, Sanada S, Kim J, Ogita H, Kuzuya T, Node K, Kitakaze M, Hori M. Echocardiographic assessment of LV hypertrophy and function in aortic-banded mice: necropsy validation. Am J Physiol Heart Circ Physiol. 2002;282(5):H1703–1708. doi: 10.1152/ajpheart.00238.2001. [DOI] [PubMed] [Google Scholar]
- 26.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, Garcia-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol. 2014;307(8):H1204–1215. doi: 10.1152/ajpheart.00246.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashigaki N, Suzuki J, Ogawa M, Watanabe R, Aoyama N, Kobayashi N, Hanatani T, Sekinishi A, Zempo H, Tada Y, Takamura C, Wakayama K, Hirata Y, Nagai R, Izumi Y, Isobe M. Periodontal bacteria aggravate experimental autoimmune myocarditis in mice. Am J Physiol Heart Circ Physiol. 2013;304(5):H740–748. doi: 10.1152/ajpheart.00634.2012. [DOI] [PubMed] [Google Scholar]
- 28.Sarzani R, Brecher P, Chobanian AV. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ammarguellat F, Larouche I, Schiffrin EL. Myocardial fibrosis in DOCA-salt hypertensive rats: effect of endothelin ET(A) receptor antagonism. Circulation. 2001;103(2):319–324. doi: 10.1161/01.cir.103.2.319. [DOI] [PubMed] [Google Scholar]
- 30.Di Zhang A, Nguyen Dinh Cat A, Soukaseum C, Escoubet B, Cherfa A, Messaoudi S, Delcayre C, Samuel JL, Jaisser F. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension. 2008;52(6):1060–1067. doi: 10.1161/HYPERTENSIONAHA.108.117531. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension. 2003;42(3):316–321. doi: 10.1161/01.HYP.0000084853.47326.F2. [DOI] [PubMed] [Google Scholar]
- 32.Karam H, Heudes D, Hess P, Gonzales MF, Loffler BM, Clozel M, Clozel JP. Respective role of humoral factors and blood pressure in cardiac remodeling of DOCA hypertensive rats. Cardiovasc Res. 1996;31(2):287–295. [PubMed] [Google Scholar]
- 33.Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42(4):811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- 34.Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19(5):471–476. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- 35.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Jiang H, Ruan C, Zhong J, Gao P, Zhu D, Niu W, Guo S. The interaction of transient receptor potential melastatin 7 with macrophages promotes vascular adventitial remodeling in transverse aortic constriction rats. Hypertens Res. 2014;37(1):35–42. doi: 10.1038/hr.2013.110. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YN, Xie BD, Sun L, Chen W, Jiang SL, Liu W, Bian F, Tian H, Li RK. Phenotypic switching of vascular smooth muscle cells in the ‘normal region’ of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med. 2016 Mar 15; doi: 10.1111/jcmm.12825. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiffrin EL. Endothelin: potential role in hypertension and vascular hypertrophy. Hypertension. 1995;25(6):1135–1145. doi: 10.1161/01.hyp.25.6.1135. [DOI] [PubMed] [Google Scholar]
- 39.Day R, Lariviere R, Schiffrin EL. In situ hybridization shows increased endothelin-1 mRNA levels in endothelial cells of blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Am J Hypertens. 1995;8(3):294–300. doi: 10.1016/0895-7061(95)96213-4. [DOI] [PubMed] [Google Scholar]
- 40.Lariviere R, Day R, Schiffrin EL. Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1993;21(6 Pt 2):916–920. doi: 10.1161/01.hyp.21.6.916. [DOI] [PubMed] [Google Scholar]
- 41.Lariviere R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993;21(3):294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- 42.Schiffrin EL, Lariviere R, Li JS, Sventek P. Enhanced expression of the endothelin-1 gene in blood vessels of DOCA-salt hypertensive rats correlation with vascular structure. J Vasc Res. 1996;33(3):235–248. doi: 10.1159/000159151. [DOI] [PubMed] [Google Scholar]
- 43.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C, Provencher S, Bonnet S. Krüppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]


