Abstract
Study Design
Cross-sectional study using T1ρ magnetic resonance imaging (MRI) of lumbar spine in healthy young adults.
Objective
To evaluate early intervertebral disc degeneration (IDD) quantified by T1ρ - and T2-weighted MRI in asymptomatic young adults and to correlate T1ρ value with Pfirrmann degenerative grade, sex, and body mass index (BMI).
Summary of Background Data
Intervertebral disc starts early to degenerate losing proteoglycan content in the nucleus pulposus (NP). A potential tool for the study of early stage of IDD is T1ρ MRI. T1ρ relaxation time of human discs has been correlated to proteoglycan content in previous studies.
Methods
T1ρ - and T2-weighted images of the lumbar spine were obtained for 63 asymptomatic young subjects (34 men and 29 women; mean age, 22.95 ± 1.8 yr), with a 1.5-T MRI scanner. T1ρ mapping and values in the NP and anulus fibrosus (n = 315) were obtained. Degenerative grade was assessed using T2-weighted images, according to the Pfirrmann scale. Differences in T1ρ value between sexes, BMI, and linear regression analyses with degenerative grade were determined.
Results
T1ρ values of NPs were significantly higher than those of anulus fibrosus at all levels. T1ρ values were significantly lower in women at L3–L4 and L4–L5 discs (P < 0.05). T1ρ values decreased linearly with degenerative grade. However, nondegenerated discs (Pfirrmann grades 1 and 2) showed a wide range of T1ρ relaxation time. No significant correlation was observed between T1ρ value and BMI.
Conclusion
The data of this study showed a significant difference in IDD onset between sexes. T1ρ values correlate with Pfirrmann degenerative grade in young adults. However, the wide distribution of T1ρ values in healthy intervertebral disc highlights the low sensitivity of Pfirrmann grade to detect the early IDD changes. T1ρ can be potentially used as a clinical tool to identify early IDD and to create a reliable quantitative scale.
Keywords: T1ρ, spine, intervertebral disc degeneration, magnetic resonance imaging, healthy young adults
Low back pain (LBP) is a common and important clinical, social, economic, and public health problem affecting the western societies, with an annual prevalence of chronic LPB ranging from 15% to 45%. 1, 2 LBP is already common among children and the prevalence increases with age. 3, 4 It has been suggested that the majority of back pain is caused by intervertebral disc degeneration (IDD). 5 Although the mechanisms by which IDD may cause LBP are not clear, the severity of IDD is associated with onset of symptoms. 6 Previous authors have suggested that physiological, that is, nontraumatic changes in the intervertebral discs (IVDs) likely begin as early as the second decade of life and progress until death. 7 Although a single initiating cause of degeneration has not been identified, early degenerative changes occur in the nucleus pulposus (NP). 8, 9 During the initial phase of IDD, the NP loses proteoglycan (PG) that accounts for up to 70% of the dry weight of the NP in the juvenile and 20% in the mature adult. PG is hydrophilic macromolecule that leads to the hydration of the NP, which accounts for the load-bearing property of the disc. 10, 11 IDD progress results in detrimental changes of disc structures and joint mechanics, which can proceed to multiple spinal disorders, such as spinal stenosis, radiculopathy, disc herniation, and instability. 5, 12 The current focus of spine surgeon is to develop new biological therapies to prevent or to stop the degenerative process of the disc. However, the current diagnostic tools used to detect early degenerative changes are not sensitive. Conventional magnetic resonance imaging (MRI) techniques provide excellent detection for late-stage morphological changes due to IDD. One of the commonly accepted grading systems for the assessment of lumbar IDD is the classification scale described by Pfirrmann et al, 13 which is based on the assessment of structure and loss of the signal intensity on T2-weighted MRI images. Among the most accepted grading systems, the Pfirrmann scale has a high clinical feasibility, whereas the Thompson classification scale 14 has more academic than clinical value because it is based on assessing gross morphology of midsagittal sections of human lumbar IVD and cannot be applied on patients. 15 However, the Pfirrmann scale does not provide reliable quantification of the degenerative grade in early IDD stages characterized by a loss of PG in an intact disc. 16, 17 A more sensitive technique is required for the quantitative measurement of biochemical changes to track the progression and regression of IDD. This technique will help evaluate new therapies for the treatment of IDD, such as recombinant growth factors injection, gene therapy, stem cell therapy and tissue engineering strategies. 18–23
A potential tool for studying the early stage of IDD is the T1ρ time constant obtained by spin-lock MRI. 24 The relaxation that occurs during the application of the spin-lock pulse is referred to as spin-lattice relaxation in the rotating frame, or T1ρ relaxation. The T1ρ time constant is related to slow motional interactions between macromolecules of extracellular matrix and bulk water. 25 In articular cartilage, T1ρ is strongly correlated with PG content and has been shown to detect early degenerative changes. 26–28 Previous studies demonstrated that the T1ρ relaxation time was directly correlated to PG content of human lumbar IVDs ex vivo 29, 30 and correlated significantly with disc degeneration in vivo 31 as well as clinical symptoms. 32–34
IDD is known to be common among asymptomatic young adults, and few studies on young population cohort demonstrate that degenerative MRI findings are also present in the lumbar spine among young adults. 7, 35 There are no studies to our knowledge that evaluate early degenerative changes in lumbar spine of young adults, using functional MRI techniques sensible to early degenerative changes and the correlation with potential risks for IDD progress such as sex and body mass index (BMI).
The purpose of this study was to evaluate early IDD in the lumbar spine quantified by T1ρ and T2-weighted MRI in a population of asymptomatic young adults by correlating T1ρ value with Pfirrmann degenerative grade, sex, and BMI.
MATERIALS AND METHODS
Study Sample
The study was approved by the ethics committee of Campus Bio-Medico University of Rome, and all participants provided written informed consent prior to enrollment. The study population consisted of 63 Italian volunteers (Table 1; 34 men and 29 women; mean age, 22.95 ± 1.8 yr; age range, 19–25 yr). All patients underwent standard clinical and T1ρ MRI of the lumbar spine. Inclusion criteria consisted of age younger than 25 years, no history of chronic back pain, no prior back surgery, no trauma history of the spine, no metabolic disease, no autoimmune disease, no osteoarticular disease, and no morphological alteration. Subjects were excluded if they failed to meet the above criteria or if they had any indicative suggestive of sciatica or neurological symptoms, including leg weakness, numbness, or tingling. BMI of each volunteer was calculated from height and weight. The study evaluated discs segments between L1 and S1 for a total of 315 discs.
TABLE 1.
Degenerative Grade According to Pfirrmann Scale, Sex, and Disc Level of the Lumbar Intervertebral Discs of Healthy Young Adults Analyzed in Study
| Degeneration Grades | Sex | Disc Levels | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | L1–L2 | L2–L3 | L3–L4 | L4–L5 | L5–S1 | ||
| 1 | 87 | 71 | 30 | 30 | 38 | 32 | 28 | 158 |
| 2 | 54 | 54 | 25 | 29 | 20 | 16 | 18 | 108 |
| 3 | 20 | 17 | 8 | 4 | 4 | 9 | 12 | 37 |
| 4 | 8 | 3 | 0 | 0 | 1 | 5 | 5 | 11 |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total | 170 | 145 | 63 | 63 | 63 | 63 | 63 | 315 |
MR Image Acquisition
Each participant underwent MRI on a clinical 1.5-T scanner (Simens Magneton Avanto 76 × 18; Campus Bio-Medico University Hospital of Rome, Rome, Italy) according to 2 protocols. First, conventional T1- and T2-weighted images were acquired using a standard spin-echo imaging sequence with the following parameters: T2-weighted: field of view (FoV) = 320 mm, slice thickness = 4 mm, acquisition matrix = 514, and time echo time/repetition time (TE/TR) = 83 ms/5560 ms; inversion time (TI) = 150 ms, T1-weighted images were acquired TE/TR = 8.1 ms/400 ms, turbo spin echo (TSE), FoV = 400 mm, slice thickness = 4 mm, and matrix = 384. Next, a series of T1ρ -weighted images was acquired using a TSE pulse sequence with fat suppression, with the following parameters: FoV = 280 mm, slice thickness = 4 mm, acquisition matrix = 358, flip angle = 30°, TE/TR = 5.24 ms/3030.96 ms, and TI = 1700 ms. Spin-lock durations ranged from 15 to 75 ms with a spin-lock pulse amplitude set to correspond to a spin-lock frequency of 400 Hz. Both axial and sagittal images were acquired. Total time for each examination was about 15 minutes each.
Data Processing
The degenerative grade of each lumbar disc (n = 315) was assessed from T2-weighted images according to the classification system described by Pfirrmann et al 13 scale as grade 1 (normal shape, no horizontal bands, and distinction of annulus fibrosus [AF] and NP is clear), grade 2 (nonhomogeneous shape with horizontal bands and some blurring between NP and AF), grade 3 (nonhomogeneous shape with blurring between NP and AF, AF shape is still recognizable), grade 4 (nonhomogeneous shape with hypointensity, AF shape is not intact and distinction between NP and AF is impossible, and disc height is usually decreased), and grade 5 (same as grade 4 but collapsed disc space). Grading was performed by 3 independent operators: 2 musculoskeletal radiologists (S.B., F.M.M) 1 orthopedic surgeon (G.V.).
T1ρ values were calculated on a pixel-by-pixel basis by a linear regression of intensity data to an exponential decay function:
Values were used to create 3-dimensional spatial maps of T1ρ using MATLAB (Mathworks, Cupertino, CA). A circular region of interest of 5-mm diameter was manually positioned by 2 independent investigators in the center of the NP of the map, and mean T1ρ value was computed within that region. Moreover, T1ρ values of the NP and the AF of the IVD were also calculated by manually segmenting the different region of the disc using custom-written software in MATLAB (http://cmroi.med.upenn.edu/index.php/t1rho-t2-processing).
Statistic Analysis
We compared median T1ρ values of men and women of both NP and AF according to the anatomic level of each disc, using the MannWhitney test. The Jonckheere-Terpstra test, a nonparametric test for trends in ordered groups, was used to evaluate the decreasing trend in the relationship between NP (and AF) and the level of the discs. Scatter plots were mapped and Spearman rank correlation coefficient was applied to determine relationships between NP and Pfirrmann grade. Two-sided tests were used (except for the Jonckheere-Terpstra test which is 1-tailed). A P value of <0.05 was considered statistically significant. Linear regressions between T1ρ values and BMI were performed.
Statistical analyses were done with Stata software, version 10.0 (StataCorp. 2007. Stata Statistical Software: Release 10; StataCorp LP, College Station, TX).
RESULTS
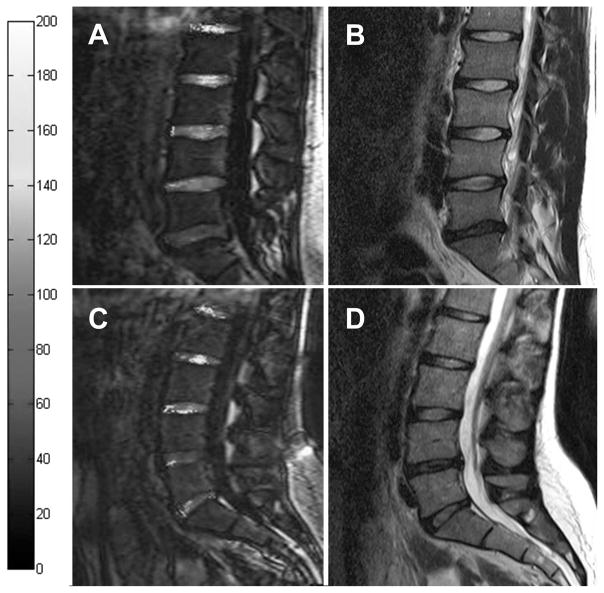
Table 1 shows the characteristics of all analyzed discs sorted by their Pfirrmann degenerative grade. Sagittal T2-weighted images and corresponding quantitative T1ρ -weighted images were used to evaluate the degenerative grade and the T1ρ value, respectively. Representative sagittal T2-weighted images and corresponding quantitative T1ρ maps are shown in Figure 1, reporting the degenerative grade as Pfirrmann grading and the T1ρ value. The T1ρ map is a graphical representation of the quantitative T1ρ parameter calculated at each pixel location.
Figure 1.
Representative T1ρ map overlaid on T1ρ image (A and C) and T2-weighted images (B and D) from a 23-year-old man (A and B) and a 24-year-old woman (C and D). The L1–L2 and L2–L3 discs are nondegenerative with relatively high T1ρ relaxation times in both sexes. In contrast, the L3–L4 discs show a normal T2-signal intensity on T2-weighted images and a significant decrease of T1ρ value in both subjects. The L4 and L5–S1 show a distinct loss of signal intensity on T2 imaging and a dramatic decrease in T1ρ time in women and men, respectively.
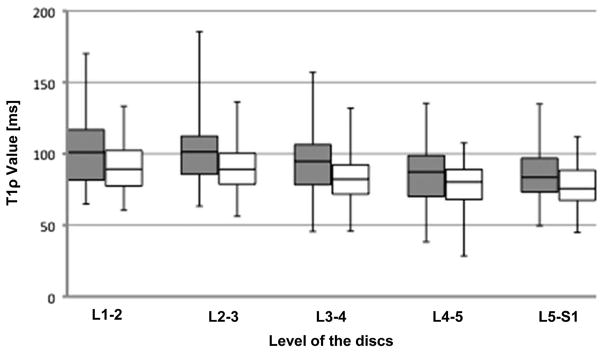
In Table 2, the T1ρ values of both NP and AF are listed according to the anatomic level of each disc and sex. For both sexes, the T1ρ values of the NP were significantly higher compared with the AF values in all levels. T1ρ value decreased as one proceeded caudally down the spinal column (Figure 2).
TABLE 2.
T1ρ Value of Male and Female Both Nucleus Pulposus (NP) and Annulus Fibrosus (AF) According to the Anatomic Level of Each Disc Expressed as Median and Interquartile Range
| Disc Levels | T1ρ Value [ms] of the NP | P | T1ρ Value [ms] of the AF | P | ||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| L1–L2 | 97.6 (81.3–115.7) | 107.4 (84.1–116.9) | 86.6 (78.7–99.9) | 94.3 (77.4–108.0) | ||
| L2–L3 | 107.8 (90.7–119.4) | 98.7 (82.1–110.0) | 92.5 (78.7–106.1) | 88.4 (83.6–93.4) | ||
| L3–L4 | 96.7 (86.0–116.2) | 86.4 (69.4–98.1) | < 0.01 | 87.2 (78.4–93.9) | 76.7 (64.6–85.5) | < 0.05 |
| L4–L5 | 91.6 (75.0–104.0) | 81.2 (60.0–93.1) | < 0.05 | 84.8 (71.4–89.0) | 78.3 (67.3–81.4) | |
| L5–S1 | 83.7 (75.7–103.4) | 82.9 (73.0–95.5) | 76.5 (69.5–88.8) | 75.3 (66.7–84.9) | ||
Figure 2.
Box plots of T1ρ value of both nucleus pulposus (gray) and annulus fibrosus (white) according to the anatomic level of each disc. The boxes represent the median and the interquartile range, with the vertical lines showing the range.
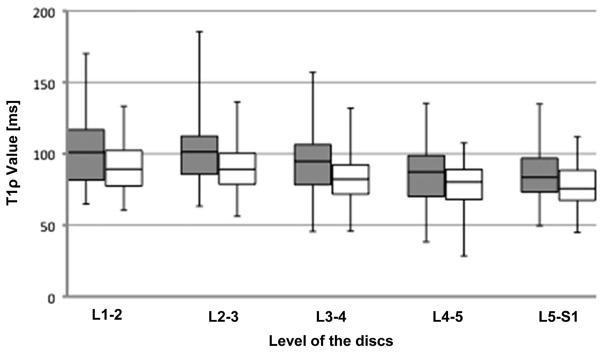
Evaluation of the sex difference in T1ρ value at different levels showed a significant lower value in the female discs at L3–L4 (P < 0.01) and L4–L5 discs (P < 0.05) in T1ρ value of the NP and a significant lower value in the female discs at L3–L4 (P < 0.05) in T1ρ value of the AF (graph not shown). Figure 3 shows the box plot for NP T1ρ values for men and women at the different lumbar levels. A significant decreasing trend in T1ρ values of both NP and AF, from the upper to the lower lumbar level, was found in men and women (trend P values were <0.0001, according to the Jonckheere-Terpstra test).
Figure 3.
Box plots of T1ρ value of nucleus pulposus by sex according to the anatomic level of each disc. Gray = Male; white = Female. The boxes represent the median and the interquartile range, with the vertical lines showing the range. The graph shows a significant lower T1ρ value in the female discs at L3–L4 and L4–L5 discs than that in the male discs. * P < 0.05.
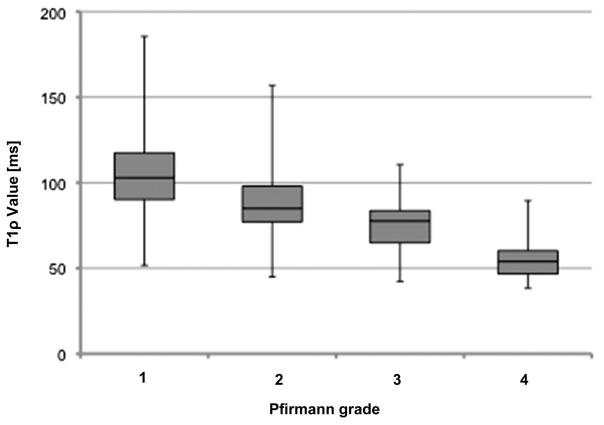
The box plot for the 2 variables T1ρ values and Pfirmann grades, as shown in Figure 4, indicates that the 2 variables were inversely and linearly related. The correlation between T1ρ values and Pfirmann grades was − 0.51 (P < 0.0001). However, the interquartile range shows the wide heterogeneity distribution of T1ρ value, especially for the nondegenerated disc as evaluated according to the Pfirrmann scale; in fact, the T1ρ value of all the population ranged from 51.7 to 185.5 ms for the 158 discs of grade 1, from 45.0 to 156.9 ms for the 108 discs of grade 2, and from 42.3 to 110.5 ms for the 36 discs of grade 3.
Figure 4.
Box plots of T1ρ value of nucleus pulposus according to the grade of Pfirmann. The boxes represent the median and the interquartile range, with the vertical lines showing the range. One case with Pfirmann grade 5 (T1ρ = 51.7) was excluded.
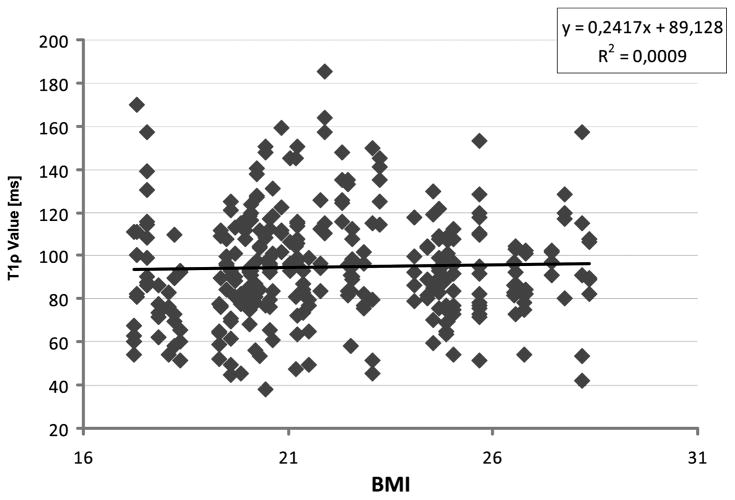
No significant linear association was observed between the T1ρ value of the NP and the BMI of each subject (Figure 5).
Figure 5.
Correlation between T1ρ relaxation time and BMI of all discs was analyzed. No correlation was found. BMI indicates body mass index.
DISCUSSION
Restoring PG content during the early stage of IDD is at the basis of new biological strategies for IDD prevention and disc regeneration. 36 Translational research in this field will be based on clinical trials where noninvasive quantitative analysis on water and PG content of the disc are required. T1ρ -weighted MRI technique has been shown to be a promising tool for PG detection and early stage of IDD evaluation 29; therefore, it might be used for translational research focusing on reversing IDD.
This study evaluated quantitatively the early degenerative grade of the lumbar discs of asymptomatic young adults. T1ρ value measured in this study was lower at caudal lumbar levels in both the NP and the AF, with an increasing trend of T1ρ values proceeding cranially up the spinal column. This finding is in accordance with observations by others with different evaluations techniques. 7, 35, 37–39 It reflects that mechanical loads are involved in early PG loss of young discs because upper and midlumbar spine are subjected to lower mechanical loads than the caudal lumbar spine.
T1ρ values resulted higher in NP than in AF. These reflect the different composition of their extracellular matrix. The AF is a multilamellar fibrocartilage with PG precipitates in the interlamellar space that resist the tensile forces generated during bending or twisting, whereas the NP has a more random organization rich in PG that provides the osmotic properties needed for the discs to resist compressive loads. 36
A new finding arises by the sex comparison, because the T1ρ values of NP at L3–L4 and L4–L5 discs were significantly lower in women. These results suggest that PG loss of the NP may begin first in women and then in men. A straightforward comparison with earlier research is not possible due to lack of studies that used T1ρ relaxation time as method to evaluate IDD. A cross-sectional MRI study of Takatalo et al 7 investigated the prevalence of degenerative findings of the lumbar spine among young Finnish adults aged 20 to 22 years. These authors reported that women had significantly higher prevalence of annular tears whereas men had significantly higher prevalence of IDD and disc herniations. However, these data were based on Pfirrmann degenerative grade whereas grades 1 to 2 were classified as normal discs, while grades 3 to 5 were defined as degenerated, with 13% prevalence in their cohort. 7 According to these data, we observed 15% of grade 3 to 5 degenerated discs, while the sex difference evaluation we performed was based on T1ρ values of both normal and degenerated discs. In a cross-sectional cohort study, Kjaer et al 35 described the associations between lumbar MRI findings and LBP in 13-year-old children. These authors observed differences between sexes; degenerative disc changes in the upper lumbar spine were more strongly associated with LBP in boys, whereas disc abnormalities in the lower lumbar spine (L3–L4, L4–L5, and L5–S1) were more strongly associated with LBP in girls. 35 Thus, our findings are in accordance with the results from these Danish population–based cohorts.
Degenerative spondylolisthesis is the only disorder of the adult spine in which a distinct difference between sexes has been reported. It is approximately 4 times more common in women than in men and occurs more frequently at L4–L5. 40 This reason has not been explained, although factors, such as the lumbosacral junction anatomy (sacral horizontal angle and/or lumbar lordosis angle), facet joint orientation, ligamentous laxity, previous pregnancy, and hormonal factors have been suggested as etiological factors. 41
A number of lifestyle and social demographic factors have been linked with LBP. 42, 43 BMI has been correlated with LBP of obese people; however, this association has been considered controversial and causal link has not been demonstrated. 44 Although the population described in this study includes very few obese subjects, a correlation between T1ρ value of the NP and BMI has not been observed.
We observed that T1ρ MRI was linearly correlated to the Pfirrmann degenerative grade. However, nondegenerated discs (Pfirrmann grades 1 and 2) showed a wide range of T1ρ relaxation time (Figure 4). Indeed, the grading system based on T2-weighted images assesses disc water content and other morphological features and is able to give information on biochemical changes that characterize IDD. Sowa et al 45 examined the changes observed during normal aging of the IVD in an animal model and showed modest age-related MRI changes assessed with T2-weighted images compared with the dramatic histological changes analyzed on the same discs. These data suggested that conventional MRI techniques are relatively insensitive measures of IDD. 45
T1ρ weighted MRI may have greater utility in demonstrating age- and degeneration-related disc changes that correlate with PG content, and it could provide spatial and quantitative measurements, which may be more closely correlated with biochemical changes in disc. Moreover, Nguyen et al 30 demonstrated that T1ρ weighted MRI is directly correlated with isometric swelling pressure of cadaveric discs and suggested that it may be a valuable quantitative biomarker of the mechanical function of the NP. Furthermore, Blumenkrantz et al 32 evaluated the association between T1ρ and clinical finding as quantified by 36-Item Short Form Health Survey and Oswestry Disability Index in 16 patients observing a correlation. Therefore, T1ρ -weighted MRI may be sensitive to early degenerative changes and clinical symptoms in IDD, and it could be used to define a new quantification IDD scale with higher specificity than T2-weighted MRI-based Pfirrmann scales.
The strength of this study is the large homogeneous asymptomatic population of young adults analyzed with unconventional versus standard MRI techniques. Because early IDD changes start by the second decade of an individual’s life, this is the first study that used T1ρ-weighted MRI allowing a spatial and quantitative assessment of IDD in such population. Early degenerative changes were quantitatively assessed by T1ρ relaxation time revealing a difference between sexes in the lower disc levels. Moreover, IDD changes assessed by T2-weighted MRI Pfirrmann grading (grades 3–5) were observed in 15% of the subjects, and the nondegenerated discs (grade 1) were just in the 45.8%. Although IDD is known to be common among asymptomatic patients, 7, 46 the prevalence of MRI findings among young adults is virtually unknown.
This cross-sectional study is limited by a relatively small sample size due to the complexity of the assessment and the costs of magnetic resonance examination of healthy young volunteers that limited the number of participant enrolled. This study is also limited by the lack of further advanced imaging techniques such as T2 mapping 47 to compare with T1ρ
Future T1ρ MRI spine studies will include a large patient population consisting of both symptomatic and asymptomatic subjects and may lead to a better understanding of the pathophysiology of IDD. Prospective studies on subjects with high prevalence of IDD, such as heavy workers, athletes, and obese people, may provide further information on preventive strategies. The use of T1ρ -weighted images in clinical protocols may be a useful guide to enroll and to evaluate the patients who may be treated with emerging technologies developed to treat early IDD, such as stem cell therapy.
Key Points.
Early IDD was evaluated in asymptomatic young adults with the new T1ρMRI.
T1ρ values were significantly lower in women at L3–L4 and L4–L5 discs, showing a difference in IDD onset between sexes.
The data of the study show that T1ρ value correlates with Pfirrmann degenerative grade highlighting the low sensitivity of Pfirrmann grade to detect the early IDD changes.
T1ρ can be potentially used as a clinical tool to identify early IDD and to create a reliable quantitative scale.
Footnotes
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
No funds were received in support of this work.
References
- 1.Manchikanti L. Epidemiology of low back pain. Pain Phys. 2000;3:167–92. [PubMed] [Google Scholar]
- 2.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31:2724–7. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 3.Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents. A nationwide, cohortbased questionnaire survey in Finland. Spine (Phila Pa 1976) 1997;22:1132–6. doi: 10.1097/00007632-199705150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Wedderkopp N, Leboeuf-Yde C, Andersen LB, et al. Back pain reporting pattern in a Danish population-based sample of children and adolescents. Spine (Phila Pa 1976) 2001;26:1879–83. doi: 10.1097/00007632-200109010-00012. [DOI] [PubMed] [Google Scholar]
- 5.Boni M, Denaro V. Anatomo-clinical correlations in cervical spondylosis. In: Kehr P, Weidner A, editors. Cervical Spine I. New York: Springer- Verlag; 1987. pp. 3–20. [Google Scholar]
- 6.Peterson CK, Bolton JE, Wood AR. A cross-sectional study correlating lumbar spine degeneration with disability and pain. Spine (Phila Pa 1976) 2000;25:218–23. doi: 10.1097/00007632-200001150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Takatalo J, Karppinen J, Niinimaki J, et al. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine (Phila Pa 1976) 2009;34:1716–21. doi: 10.1097/BRS.0b013e3181ac5fec. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 10.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 1988;13:179–87. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Mimura M, Panjabi MM, Oxland TR, et al. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976) 1994;19:1371–80. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JP, Pearce RH, Schechter MT, et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15:411–5. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kettler A, Wilke HJ. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur Spine J. 2006;15:705–18. doi: 10.1007/s00586-005-0954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luoma K, Vehmas T, Riihimaki H, et al. Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine (Phila Pa 1976) 2001;26:680–6. doi: 10.1097/00007632-200103150-00026. [DOI] [PubMed] [Google Scholar]
- 17.Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(suppl 4):441–51. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadala G, Sowa GA, Kang JD. Gene therapy for disc degeneration. Expert Opin Biol Ther. 2007;7:185–96. doi: 10.1517/14712598.7.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Vadala G, Studer RK, Sowa G, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33:870–6. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 21.Vadala G, Denaro V, Kang JD. Stem cell therapy for intervertebral disc degeneration. US Musculoskel Rev. 2009;4:2–6. [Google Scholar]
- 22.Vadala G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–55. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 23.Vadala G, Mozetic P, Rainer A, et al. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J. 2012;21(suppl 1):20–6. doi: 10.1007/s00586-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Auerbach JD, Witschey WR, et al. Advances in magnetic resonance imaging for the assessment of degenerative disc disease of the lumbar spine. Semin Spine Surg. 2007;19:65–71. doi: 10.1053/j.semss.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–23. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 26.Borthakur A, Mellon E, Niyogi S, et al. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Han ET, Ma CB, et al. In vivo 3T spiral imaging based multislice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–36. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 28.Lozano J, Li X, Link TM, et al. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–52. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 29.Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rhoweighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31:1253–7. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen AM, Johannessen W, Yoder JH, et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1rhoweighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auerbach JD, Johannessen W, Borthakur A, et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15(suppl 3):S338–44. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenkrantz G, Zuo J, Li X, et al. In vivo 3. 0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn Reson Med. 2010;63:1193–200. doi: 10.1002/mrm.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borthakur A, Maurer PM, Fenty M, et al. T1ρ magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa 1976) 2011;36:2190–6. doi: 10.1097/BRS.0b013e31820287bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo J, Joseph GB, Li X, et al. In-vivo intervertebral disc characterization using magnetic resonance spectroscopy and T1rho imaging: association with discography and Oswestry Disability Index and SF-36. Spine (Phila Pa 1976) 2011 doi: 10.1097/BRS.0b013e3182294a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjaer P, Leboeuf-Yde C, Sorensen JS, et al. An epidemiologic study of MRI and low back pain in 13-year-old children. Spine (Phila Pa 1976) 2005;30:798–806. doi: 10.1097/01.brs.0000157424.72598.ec. [DOI] [PubMed] [Google Scholar]
- 36.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 37.Braithwaite I, White J, Saifuddin A, et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363–8. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weishaupt D, Zanetti M, Hodler J, et al. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661–6. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- 39.Siemionow K, An H, Masuda K, et al. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976) 2011;36:1333–9. doi: 10.1097/BRS.0b013e3181f2a177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen IR, Wei TS. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine (Phila Pa 1976) 2009;34:1402–9. doi: 10.1097/BRS.0b013e31817b8fbd. [DOI] [PubMed] [Google Scholar]
- 41.Love TW, Fagan AB, Fraser RD. Degenerative spondylolisthesis. Developmental or acquired? J Bone Joint Surg Br. 1999;81:670–4. doi: 10.1302/0301-620x.81b4.9682. [DOI] [PubMed] [Google Scholar]
- 42.Harkness EF, Macfarlane GJ, Nahit ES, et al. Risk factors for newonset low back pain amongst cohorts of newly employed workers. Rheumatology (Oxford) 2003;42:959–68. doi: 10.1093/rheumatology/keg265. [DOI] [PubMed] [Google Scholar]
- 43.Harreby M, Kjer J, Hesselsoe G, et al. Epidemiological aspects and risk factors for low back pain in 38-year-old men and women: a 25-year prospective cohort study of 640 school children. Eur Spine J. 1996;5:312–8. doi: 10.1007/BF00304346. [DOI] [PubMed] [Google Scholar]
- 44.Leboeuf-Yde C, Kjaer P, Bendix T, et al. Self-reported hard physical work combined with heavy smoking or overweight may result in so-called Modic changes. BMC Musculoskelet Disord. 2008;9:5. doi: 10.1186/1471-2474-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sowa G, Vadala G, Studer R, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008;33:1821–8. doi: 10.1097/BRS.0b013e31817e2ce3. [DOI] [PubMed] [Google Scholar]
- 46.Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 47.Marinelli NL, Haughton VM, Anderson PA. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. AJNR Am J Neuroradiol. 2010;31:1278–82. doi: 10.3174/ajnr.A2080. [DOI] [PMC free article] [PubMed] [Google Scholar]