Abstract
Hepatic encephalopathy describes the array of neurological alterations that occur during acute liver failure or chronic liver injury. While key players in the pathogenesis of hepatic encephalopathy, such as increases in brain ammonia, alterations in neurosteroid levels, and neuroinflammation, have been identified, there is still a paucity in our knowledge of the precise pathogenic mechanism. This review gives a brief overview of our understanding of the pathogenesis of hepatic encephalopathy and then summarizes the significant recent advances made in clinical and basic research contributing to our understanding, diagnosis, and possible treatment of hepatic encephalopathy. A literature search using the PubMed database was conducted in May 2017 using “hepatic encephalopathy” as a keyword, and selected manuscripts were limited to those research articles published since May 2014. While the authors acknowledge that many significant advances have been made in the understanding of hepatic encephalopathy prior to May 2014, we have limited the scope of this review to the previous three years only.
Keywords: Hepatic encephalopathy, acute liver failure, chronic liver injury, neuroinflammation, pathogenesis
Introduction
Advanced liver disease is well known for its systemic consequences, notably its profound effects on brain function, in its most classic form, known as hepatic encephalopathy (HE). HE is currently defined as a brain dysfunction secondary to liver insufficiency and/or portosystemic shunting that manifests as a broad spectrum of neuropsychiatric abnormalities ranging from subclinical alterations to coma 1. It remains a diagnosis of exclusion 1. HE is frequent: overt HE is found in some 30–40% of patients with cirrhosis 1. In 2009, HE resulted in 22,931 hospitalizations with an average cost of each stay ranging from $46,663–$63,108 2. This highlights both the frequency of HE and the economic burden it currently places on the US.
The broad spectrum of HE has led to the development of multiple grading and classification systems to better categorize the severity of disease. It is currently a recommendation by the American Association for the Study of Liver Disease (AASLD) that HE should be classified according to the type of underlying disease, severity of manifestations, and precipitating factors 1. Types A, B, and C of HE define the underlying disease. Type A (acute) is due to acute liver failure (ALF), type B (bypass) is due to portosystemic shunting without intrinsic liver disease, and type C (cirrhosis) is due to cirrhosis 1. Each type has differing symptoms at presentation owing to the varying etiologies.
ALF, with the original term being fulminant hepatic failure, is defined as severe liver injury in the absence of pre-existing liver disease. The etiology of ALF is complex, ranging from viruses and drugs to genetic causes, with a vast majority remaining idiopathic 3. In developing nations, hepatitis (A, B, and C) is the most common cause of ALF, while in the US, acetaminophen toxicity accounts for 39% of ALF cases 3. Physical exam findings are often non-specific and generally reflect severe underlying liver dysfunction or complications thereof. ALF is currently a clinical diagnosis, made in the setting of acute liver injury in the context of abnormal liver tests (including dramatically elevated aminotransferases, and most importantly evidence of hepatocyte dysfunction demonstrated by a prolonged PT and increased INR 3). Unique to type A is its association with increased intracranial pressure (ICP), thus carrying a risk for cerebral herniation 1. It is believed that increased ICP results from cerebral edema secondary to hyperammonemia 3. One current proposed mechanism is that in ALF the development of hyperammonemia outpaces the compensatory mechanisms seen in chronic liver disease 3. Subsequently, ammonia increases intracellular osmolarity when it is metabolized to glutamine, resulting in cerebral edema causing increased ICP 3.
Type B HE is secondary to portosystemic bypass or bypass with no intrinsic hepatocellular disease. Precipitating factors of HE in type B are factors that are known to increase ammonia, such as azotemia, infection, GI bleed, lactulose noncompliance, and constipation 4. The most serious complication of transjugular intrahepatic portosystemic shunts (TIPS) is chronic recurrent HE that is refractory to standard treatment 4. If severe enough, shunt revision may be warranted 4.
Type C HE is associated with liver cirrhosis accompanied with either portal hypertension or portosystemic shunts 1. The causes of cirrhosis are vast, ranging from viral, autoimmune, chronic biliary disease, and fatty liver diseases to rare storage diseases such as hemochromatosis and Wilson’s disease 5. Signs and symptoms of cirrhosis are often present and include spider angioma, muscle wasting, jaundice, ascites, and gynecomastia and testicular atrophy in men. The most common form of HE in type C is minimal HE (MHE), which affects nearly 80% of cirrhotics 6. MHE affects the patient’s daily life, interfering with executive function including their working memory and orientation 6. Physical examination in MHE is often normal, and patients may present with subtle abnormalities that can be diagnosed by experts using specialized neurophysiologic tests, such as the critical flicker frequency test (CFF) 6. Currently, there is no gold standard test, and a number of studies are currently investigating the efficacy and use of them 7.
Pathophysiology
As stated above, HE is broken into three major types because of its multiple etiologies. HE presents with a broad spectrum of symptoms ranging from subclinical to comatose. This wide range of presentation is due to the multifaceted pathophysiology that underlies this complex disease. Highlighted below is the role of ammonia and inflammation in the development of HE.
Currently, ammonia is the best-characterized neurotoxin in the pathogenesis of HE and also appears to be important in the genesis of astrocyte swelling 8. In healthy individuals, nitrogenous compounds, such as proteins, are metabolized by gut microflora and transported to the liver in the form of ammonia 8. In the liver, ammonia is metabolized by the urea cycle with the majority of the subsequent urea excreted renally 8. Advanced liver disease or portosystemic shunting leads to a buildup of ammonia in the blood 8. Ammonia that builds up in the blood is then able to cross the blood–brain barrier, where it is metabolized by astrocytes into glutamine 8. Hence, levels of glutamine start to accumulate and lead to astrocyte swelling. This swelling can trigger a downward spiral leading to increase in production of reactive oxygen and nitrogen species, which can downstream target gene transcription and translation 9. Animal models of HE emphasize that astrocyte and brain swelling is also a key feature. Among these features are swelling of astrocytes, vasoconstriction of blood vessels, increase in ICP, cerebral edema, reduced cerebral perfusion, and cerebral atrophy 9. There are conflicting studies regarding the correlation of levels of ammonia and the degree of encephalopathy; however, evidence suggests that a reduction in levels of ammonia leads to reduced brain swelling 9.
In addition to the ammonia hypothesis, inflammation and cytokines are thought to be major components in the development of HE, particularly in the setting of ALF with or without sepsis 10. Septic encephalopathy is a well-documented phenomenon that closely resembles HE 10. Although sepsis and ALF have varied pathogenic mechanisms, they share the same cardinal features of encephalopathy, cardiovascular collapse, and coagulopathy 10. Both sepsis and ALF result in an upregulation of inflammatory cytokines IL-1β, IL-6, and TNF-α 10. Studies have shown that these inflammatory cytokines compromise the blood–brain barrier and disrupt the permeability of the endothelial cells 10. It is not surprising that sepsis can precipitate and worsen HE 10. Cytokines, infection, and inflammation play a significant role in the development of HE. Studies have implicated both ammonia and inflammation in the pathogenesis of HE 10. Currently, it is proposed that these pathways have a synergistic effect on each other 10.
Recent advances in understanding the pathogenesis of hepatic encephalopathy
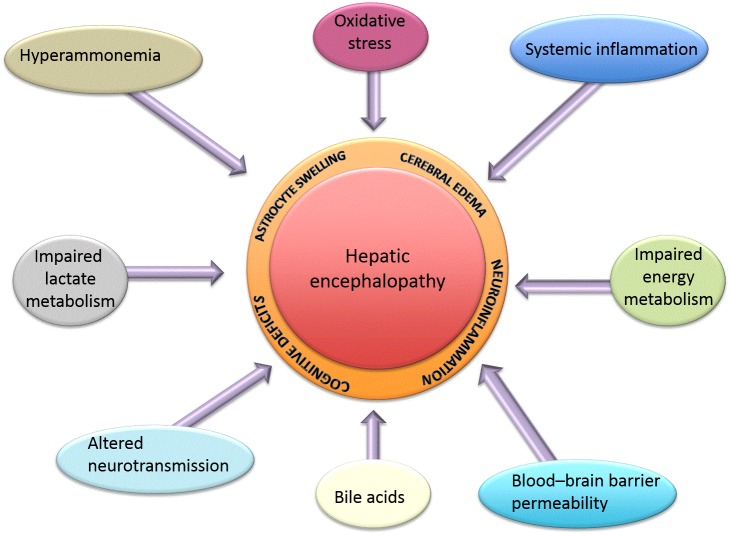
Recent advances in the understanding of the pathogenesis of HE focus on the consequences of hyperammonemia and altered neurotransmission, and new studies have aimed to examine new potential targets such as bile acid signaling and their contribution to HE. Below is a summary of the latest findings (see also Figure 1).
Figure 1. Summary of all factors known to contribute to the pathogenesis of hepatic encephalopathy.
Hyperammonemia
A major contributing factor to the development of HE is a buildup of ammonia. Liver-specific glutamine synthetase knockout (a key enzyme in ammonia metabolism) led to dramatically increased systemic hyperammonemia and subsequent cerebral oxidative stress and cognitive changes 11, consistent with the concept that the liver is integral in the maintenance of ammonia homeostasis throughout the body.
In the brain under hyperammonemic conditions, astrocytes rapidly convert blood-derived ammonia into glutamine, increasing glutamine levels 12. An understanding of the full array of consequences of hyperammonemia on astrocytic function in the context of HE is, as yet, unappreciated. Historically, it was thought that the effects of increased cerebral ammonia were increased oxidative stress, osmotic pressure, and subsequent astrocyte swelling. However, recent studies have demonstrated that ammonia also has effects on many signal transduction pathways 13– 15, gene expression 16, and post-translational protein modifications 17, which together lead to subsequent impairment in astrocyte function and manifest as abnormal proliferation 18, 19, neurotransmitter release 16, and even cellular senescence 18. For example, hyperammonemia was recently associated with a transient increase in astrocyte intracellular calcium release 13– 15; the precise mechanism of action may be through activation of NMDA receptor 13, the transient receptor potential channel 1 14, or the Cav1.2 L-type calcium channel 15, or, more likely, a combination of all of the above. Given that calcium-mediated effects are an early event of many different signal transduction pathways, the vast array of potential gene expression alterations due to hyperammonemia has not yet been defined.
A relatively novel mechanism by which ammonia may influence gene expression is via alterations in microRNA expression. MicroRNAs are small non-coding RNA sequences that play a role in gene silencing at the level of gene transcription as well as translation and can be regulated by oxidative stress. Exposure of astrocytes to ammonia dramatically altered microRNA profiles 19, leading to an alteration in many target genes, including heme oxygenase 1. Preventing ammonia-induced microRNA expression changes increased the expression of heme oxygenase 1 and prevented the ammonia-induced inhibition of astrocyte proliferation and prevented the associated cellular senescence 19.
Lastly, though not surprisingly, ammonia has been shown to alter the expression of genes involved in the regulation of astrocytic glutamate uptake and neurotransmitter release, such as the ephrin receptors and their ligands 16 and thrombospondin 1 20. Specifically, the treatment of astrocytes in culture with ammonia increased ephrin receptor 4 via an as-yet-undefined pathway that includes glutamine synthetase, NADPH oxidase, and nitric oxide synthase activities 16. Conversely, chronic ammonia treatment decreased thrombospondin 1 expression in astrocytes, which subsequently decreased the expression of synaptic proteins in neurons 20.
Ammonia may also contribute to the cell-to-cell crosstalk that occurs in HE. Specifically, the treatment of brain endothelial cells with ammonia, a mixture of cytokines, or lipopolysaccharide appeared to release factors that contribute to astrocyte swelling via the activation of Toll-like receptor 4 21. The understanding of cell–cell interactions and communication and the role of ammonia in HE is still quite limited. In further support of the notion that cell–cell communication is involved in ammonia-induced events during HE, strategies to uncouple the gap junctions in astrocyte cultures significantly attenuated key features of ammonia-induced neurotoxicity, such as membrane integrity, oxidative stress, and pro-inflammatory cytokine release 22.
Oxidative stress
Oxidative stress and the generation of reactive oxygen and nitrogen species in the brain has long been associated with HE. It is thought that impaired mitochondrial function and downregulation of the expression of key anti-oxidation enzymes contribute to an increase in oxidative damage to membrane lipids, protein, and DNA 23.
Recent studies have emphasized the importance of oxidative damage in the pathogenesis of HE, with various experimental treatments aimed at reducing reactive oxygen/nitrogen species 24– 31 or restoring the activity of anti-oxidative enzymes such as catalase 32, superoxide dismutase 32, thioredoxin 25, or glutathione peroxidase 25. Of particular note was an elegant study by Bosoi et al. 33, in which the authors utilized a rodent model of hyperammonemia that was also treated with an inhibitor of glutathione; they found that a synergistic relationship between systemic oxidative stress and hyperammonemia was required for the development of brain edema in HE 33.
Brain energy metabolism
The pathogenesis of HE has long been associated with impairment in cerebral energy metabolism with alterations in glucose utilization, glycolysis, and mitochondrial dysfunction (reviewed in Rama Rao and Norenberg 34). Of particular interest are the changes in cerebral lactate levels during HE. Specifically, increased blood and cerebral lactate levels have been demonstrated in patients with HE 35, in pig and rodent models of ALF 36, 37, and in acute hyperammonemia 38. However, the increase in cerebral lactate in models of type C HE is not as clear-cut. Increased lactate has been observed in the plasma of patients with cirrhosis compared to age- and sex-matched controls 39. Interestingly, there was no significant difference in plasma lactate levels between cirrhotic patients who exhibit symptoms of overt HE versus patients without overt HE, although the presence of MHE in the latter group could not be ruled out 39. A parallel change in lactate levels in the brain was not evident in a longitudinal study using 1H and 31P magnetic resonance spectroscopy in a rodent model of type C HE 12. Conversely, de novo synthesis of lactate was increased in the brain, and treatment with a lactate synthesis inhibitor attenuated HE-associated brain edema in this model 40. This disparity between these two opposing observations may lie in the methodology used to detect the differences. However, recently, it was demonstrated that the transport of lactate through connexin-containing hemichannels in astrocytes was impaired in the cerebral cortices of rats with type C HE owing, at least in part, to the actions of hyperammonemia 41. Given that the astrocyte–neuron lactate shuttle hypothesis suggests that lactate production in astrocytes is able to fuel and regulate neuronal activity 42, it was hypothesized that the impairment of lactate transport through hemichannels may be contributing to the pathogenesis of HE 41.
Neuroinflammation
Brain inflammation (i.e. “neuroinflammation”) is a key feature in common with all types of HE and appears to be predominantly modulated by microglia, the resident macrophage-like cell in the brain. Indirect clinical evidence for microglial activation has been demonstrated by an upregulation of the microglial marker ionized calcium-binding adaptor molecule 1 (Iba-1), which was found to be increased in post mortem cortical brain tissue from patients with liver cirrhosis and HE compared to cirrhotic patients without HE 43. In addition, a comprehensive gene expression profile analysis showed that markers for both the pro-inflammatory M1 and the anti-inflammatory M2 microglial phenotypes were increased, suggesting that both groups can be found in patients with HE caused by cirrhosis 44.
The activation of microglia is a delicate balance between the pro-inflammatory and anti-inflammatory signals, which in physiological conditions favors the dampening of microglia activation 45. These signals may be derived from the microglia themselves or are as a result of cell-to-cell communication derived from neurons or astrocytes. Recently, the pro-inflammatory chemokine CCL2 was demonstrated to be increased in neurons in a mouse model of type A HE 46 and a concomitant decrease in the anti-inflammatory chemokine fractalkine 45, thereby dysregulating the balance between opposing pro- and anti-inflammatory signals acting on receptors on microglia resulting in microglia activation. Strategies to either block CCL2 receptors or increase fractalkine signaling inhibited the microglia activation and attenuated the cognitive dysfunction observed in this model of HE, although the precise mechanism by which the balance between these two opposing signals is dysregulated was not identified.
Interestingly, in the hyperammonemic rat model, microglia and astrocytes were activated with a concomitant increase in the expression of pro-inflammatory cytokines IL-1β and IL-6 47, suggesting that ammonia alone is capable of inducing neuroinflammation during HE, although it is unlikely that the modulation of neuroinflammation is solely the consequence of hyperammonemia during HE.
Evidence to suggest a causal link between neuroinflammation and cognitive and motor function impairment during HE is mounting. Strategies that specifically target and dampen the neuroinflammatory signals also offer attenuation of cognitive and motor deficits 46, 48– 52, although care should be taken when interpreting data from experiments where the anti-inflammatory experimental agent is given systemically, as the mechanism of action may be via hepato-protection, thereby leading to a reduction in HE symptoms rather than as a direct modulatory effect on central neuroinflammation. While treatment strategies aimed at dampening systemic inflammation may be beneficial for both the underlying liver damage and the subsequent encephalopathy, from a basic science standpoint aimed at elucidating the pathogenic pathways associated with the development of HE, the distinction between the actions of an experimental compound on the brain versus its actions on the liver should be distinguished.
Neurotransmitter dysfunction
The cognitive and neuromuscular deficits observed during HE are ultimately the result of altered neurotransmission, regardless of the mechanism 53. Interestingly, there are opposing effects on glutamatergic neurotransmission depending upon the type of HE with increased extracellular glutamate levels observed after ALF, and a dampened glutamatergic neurotransmission observed during chronic liver disease 53. During ALF, the activation of NMDA receptors on astrocytes downregulates the expression of Kir4.1, an inward rectifying potassium channel known to regulate ion and water homeostasis 54 and contribute to neuronal dysfunction in other neurodegenerative diseases 55. The precise role for Kir4.1 in type A HE is not clear. Furthermore, strategies to block NMDA receptors in rats with ALF reduced the HE-associated changes in cerebral blood flow and brain lactate as well as increased the kidney clearance of ammonia, which collectively delayed or prevented the HE-associated mortality 56. Conversely, during MHE due to chronic liver disease, memory impairment was associated with suppression of the glutamate–nitric oxide–cyclic guanosine monophosphate (GMP) pathway, which has been demonstrated in response to hyperammonemia 57 or increased intracranial dopamine 58, and strategies to restore this pathway also restore the learning ability of rats with HE 57.
Both type A and type C HE are associated with increased GABA-ergic tone. This is due to a number of factors: increased GABA concentrations 59, increased GABA receptor expression 59, and increased neurosteroids such as allopregnanolone, known to regulate GABA-ergic neurotransmission 60. Neurosteroids are steroids produced de novo in the brain 61. Their production and function are currently under investigation. Recently, experimental compounds aimed at either inhibiting neurosteroid synthesis 62 or inhibiting the enhanced activation of GABAA receptors by neurosteroids 63 attenuated the deficits in motor co-ordination, spatial memory, and circadian rhythms observed during HE 62, 63. These data were supported by the use of an inhibitor of another neurotransmitter system thought to be dysregulated during at least MHE: the dopamine system 64. However, how this system is altered during HE remains unclear. It has been hypothesized that an increase in dopamine release from cirrhotic liver into the bloodstream contributes to the pathogenic features of MHE by inhibiting the glutamate–nitric oxide–cyclic GMP pathway in the hippocampus, thereby altering learning and memory ability 58, 64– 66. Conversely, dopamine and serotonin levels were decreased in another model of MHE 67.
Bile acids
Elevated circulating and cranial levels of bile acids have been observed in patients with HE as early as 1977, although our understanding of the implications of this observation are only just coming to light. Specifically, total bile acid content in the cerebrospinal fluid as well as in brain tissue was elevated in patients and rodent models of type A HE 68, 69 as well as type C HE 70– 72; however, the precise role of bile acids in HE remains controversial. Increased serum bile acids have been implicated in the increased blood–brain barrier permeability observed in a rat model of chronic liver disease 70, thereby allowing access of bile acids and other signaling molecules to the brain. In mice, azoxymethane (AOM) is used as a model for type A HE due to ALF 69. Indeed, in the AOM mouse model of type A HE, increased total bile acid content was observed in the frontal cortex, and strategies to reduce circulating bile acids (e.g. cholestyramine feeding or the use of a genetically modified mouse with impaired bile acid synthesis) proved neuroprotective 69.
Bile acids can exert their effects via many different receptors that result in an array of physiological responses. FXR is a nuclear bile acid receptor, and inhibition of FXR-mediated signaling in the frontal cortex conferred partial protection against the cognitive deficit that occurs during HE 69. Furthermore, aberrant bile acid signaling in the brain can also affect neuroinflammation by increasing pro-inflammatory CCL2 expression in neurons via a sphingosine-1-phosphate receptor 2-dependent mechanism, which subsequently leads to the activation of microglia and increase in pro-inflammatory cytokine expression 73. Conversely, TGR5 is a membrane-bound G-protein-coupled bile acid receptor, the expression of which has been shown to decrease in the brain in conditions of hyperammonemia 74. Activation of this receptor with specific agonists reduced the microglia activation and pro-inflammatory cytokine production observed in a mouse model of type A HE 75.
Many therapeutic strategies to treat HE, such as lactulose and metronidazole, abolish gut microbiota with the aim of reducing ammonia production while also influencing the conjugation of secondary bile acids. By altering the bile acid pool, they generate protective effects by altering bile acid signaling. In support of this notion, feeding mice a diet enriched in particular bile acids, which does not alter the total bile acid pool but alters the balance of individual bile acids, changed the susceptibility of mice to the development of HE 69. Specifically, mice fed a diet enriched with deoxycholic acid or cholic acid had significantly quicker neurological decline compared to control-fed or ursodeoxycholic acid-fed mice 69. These data raise the possibility that the elevation of cranial bile acids as well as a change in specific bile acids in the bile acid pool contribute to the neurological decline associated with HE; further clinical studies that manipulate bile acids are necessary to determine whether these effects will be recapitulated in patients with HE.
Blood–brain barrier permeability
The role of blood–brain barrier permeability is controversial in HE. No robust evidence of its breakdown exists; however, it is highly likely that bile acids and pro-inflammatory cytokines may affect its permeability 70, 76– 79. In the AOM model of type A HE, permeability of the blood–brain barrier has been demonstrated as a late event during the development of HE 78, 79. Conversely, no changes in permeability could be detected by another group unless the mice were co-treated with trace amounts of lipopolysaccharide 80, suggesting a synergistic role for inflammation with other key HE-related features in the changes in blood–brain barrier permeability. Consistent with this notion was a study demonstrating that circulating transforming growth factor-β (TGFβ) may contribute to the increased permeability of the blood–brain barrier in ALF 78.
Evidence to suggest that blood–brain barrier permeability is increased in type C HE also exists. As stated above, increased permeability in the bile duct ligation model of HE is apparent and can be attributed, at least in part, to increased serum bile acids 70. Associated with this increased permeability was a decrease in expression of tight junction proteins 76.
Recent advances in the diagnosis of hepatic encephalopathy
HE remains a diagnosis of exclusion with a broad spectrum of clinical manifestations. The multifaceted presentation poses many limitations to the diagnosis of HE. Guidelines by the AASLD emphasize the use of specialized psychometric tests in the diagnosis of HE 81– 83. A multitude of tests exist, with some of the most studied being the psychomotor hepatic encephalopathy score (PHES), CFF, Stroop effect test, and electroencephalogram (EEG) 81, 84.
PHES is a battery of pencil and paper tests aimed at diagnosing HE 85. PHES was studied in Turkey and Romania in order to standardize a normal value in a healthy population and also assess its utility 85, 86. Age and educational status were shown to affect score 85.
Apart from PHES, a newly developed electronic number connection test (eNCT) was investigated by Wuensh et al. 87. This test flashes the numbers 1–25 on a screen and requires the participant to click them in order while being timed; those patients with HE were found to be much slower 87. This test promises to be an easier and faster method than PHES 87.
In the CFF, patients are shown a light flicker that progressively decreases in frequency 88. They must identify the frequency at which the light flickers 88. CFF can be used as a measure of cortical function and the diagnosis of HE 88. Studies show that with a cut-off value of <39 Hz, CFF has a sensitivity of 39%, specificity of 82%, and diagnostic accuracy of 70.6% for MHE 82, 88. CFF is an important and simple tool for diagnosis and should be combined with the model for end stage liver disease (MELD) score for better diagnosis 89. MELD is a scoring system used to grade the severity of liver disease 90.
A prospective study of 117 consecutive patients with cirrhosis examined the administration of the CFF to detect HE 91. The authors found that MHE was associated with a reduced 5-year survival rate in patients with cirrhosis 91. CFF could be used in the future to improve the accuracy of prognosis in patients with cirrhosis 91.
Another study focused on Stroop testing, which tests psychomotor speed, attention, and cognitive flexibility 92 in the form of an app coined EncephalApp. The app’s reliability was tested compared to the gold standard tests and was validated by multicenter and multi-national analysis in the USA with over 800 subjects 92. Another validation of the EncephalApp was done with 437 cirrhotics and 308 controls, and it was compared with PHES and the inhibitory control test (ICT) 93. When compared against the controls, the app was found to be equivalent in predicting HE, thus proving to be a convenient and validated method for the diagnosis of HE 93.
Currently, the analysis of EEG patterns in children is used for grading HE in ALF 94. Nonetheless, EEG use in HE remains controversial. One study recorded EEGs in 69 healthy controls and 113 adults with cirrhosis 95. New spectral thresholds were calculated and optimized the performance of EEG for the diagnosis of HE 95. Though improvements were made in the use of EEG for the diagnosis of HE, validation is needed along with further research to understand the pathophysiological mechanism behind the EEG changes 95– 97.
Key changes in brain function have been studied with functional magnetic resonance imaging (MRI) 98 in combination with arterial-spin labeling to enhance the detection of HE 99. Connectivity status between 90 brain regions was assessed by measuring blood flow. HE showed impairment in the ganglia–thalamo–cortical circuits, with increased blood flow in the right putamen 99.
Acknowledging the multiple etiologies of HE, researchers investigated if the cause of HE affected its presentation on imaging. Neurocognitive, biochemical, and brain MRI changes have been examined in patients with cirrhotic HE and extrahepatic portal vein obstruction (EHPVO) HE 100. Serum studies showed no change in ammonia levels while cytokine levels were higher in cirrhotic HE 100. Cirrhotic HE showed imaging changes that were absent in EHPVO HE 100. Cirrhotic HE affected multiple brain sites ranging from the frontal lobe all the way to the brainstem 100.
Spontaneous brain activity can also be measured by examining the amplitude of low-frequency fluctuation (ALFF) in the MRI 101. It can be used as a biomarker for the detection of HE 101. Upcoming research focuses on ALFF in combination with blood oxygenation level-dependent functional MRI, creating new opportunities for the diagnosis of HE 102.
Cerebral blood flow is altered in HE. Regional cerebral blood flow was studied by comparing dogs with congenital portosystemic shunt with HE to controls using nuclear imaging 103. It showed decreased perfusion of subcortical and temporal regions in dogs with HE 103. This highlights the role that nuclear imaging, specifically single photon emission computed tomography, can play in helping diagnose HE 103.
Metabolic profiling analyzes body fluids or tissues by measuring low-molecular-weight compounds using either 1H-NMR spectroscopy or mass spectrometry techniques 104. Currently, it is used in hepatocellular carcinoma; however, its use in HE had not been previously studied 104. Studies have shown that metabolic profiling can distinguish overt HE, but it cannot discriminate between differing grades of HE or according to the severity of underlying liver disease 104.
Recent research on the treatment of hepatic encephalopathy
Gut microbiome
The gut microbiome is implicated in the pathogenesis of HE 105– 108. Urease-producing bacteria predominantly lead to elevated levels of ammonia and serve as a target for many treatments 109. A cross-sectional study set out to determine the species of gut microbiota predominant in HE and suggested that an absence of Blastocystis spp. led to dysbiosis.
Proton pump inhibitors (PPIs), a major treatment for gastroesophageal reflux, have been shown to be related to HE 110– 112. While the exact mechanism underlying this association is not known, it is currently thought to be due to alterations in the gut microbiome. PPIs have a known association with gut dysbiosis 110– 112. A case-control study of one million people in Taiwan followed for an average of nearly 14 years demonstrated that in patients with both cirrhosis and HE, 38% used a PPI 112; all categories of PPIs, except rabeprazole, led to an increased risk of HE 112. It is crucial to recognize the irreversible consequences that can occur with non-specific selection of treatment for reflux. Likewise, a meta-analysis showed that those with liver dysfunction who took a PPI had a higher risk of developing HE compared to those who did not take a PPI 110.
Recognizing the importance of the gut microbiome hypotheses has led to study of the role of probiotics in HE 113, 114. A meta-analysis of randomized trials suggested that probiotics are more effective in decreasing hospitalization rates, improving MHE, and preventing progression to overt HE 115. Similarly, a further meta-analysis by the Cochrane collaboration revealed that when compared to placebo, probiotics mildly improved recovery, decreased overt HE, improved quality of life, and mildly decreased plasma ammonia 116– 118. Of note, probiotics have no effect on mortality 116– 118. Because of the low quality of evidence, no direct conclusions can be made, as more research is needed 116– 118. A double-blind randomized placebo-controlled clinical trial studied the effectiveness of VSL#3 119 in Chandigarh, India. This study demonstrated reductions in hospitalizations and severity of breakthrough episodes of HE as well as improvement in PHES and liver function in patients receiving VSL#3 119.
Diet changes have been studied to determine their effects on cognitive function in patients with MHE with cirrhosis 120. One study in 2016 compared patients prescribed a diet with a caloric restriction of 30–35 kcal/kg and a protein intake of 1.0–1.5 g of vegetable protein/kg to a control group for six months 120. PHES was used to diagnose HE. They found that a higher proportion of patients on the prescribed diet had reversal of HE (71.1% versus 22.9%, p=0.001) along with a decreased incidence of overt HE in the treatment group (10% versus 21.7%, p=0.4) 120.
Alteration in the gut–brain axis in HE has led to investigations in determining the effectiveness of gut-selective antibiotics. Modulating the gut microbiota with the use of antibiotics such as rifaximin may improve cognitive performance in cirrhotics 121– 126. This hypothesis was tested in a study at the Hunter Holmes McGuire VA Medical Center 127. Patients with confirmed HE were prescribed rifaximin 550 mg oral twice a day over a period of eight weeks 127. Compared to the placebo group, the treatment group had cognitive improvement, decreased endotoxemia, and a modest decrease in bacteria of the small bowel 127.
A retrospective, observational, cross-sectional study examined the efficacy of different rifaximin dosing (400 mg three times daily versus 550 mg twice daily) 128. There was no difference in readmission rates between the two treatment groups 128. The long-term cost effectiveness of lactulose monotherapy versus lactulose plus rifaximin was studied in France 129. Despite having no effect on hospital length of stay, combination therapy led to lower readmission rates at 180 days compared to lactulose alone 130. The authors concluded that rifaximin was a cost-effective and affordable treatment for patients suffering from at least two prior events of HE 129.
Hyperammonemia
As stated above, numerous studies have shown that ammonia is key in the pathogenesis of HE. Currently, the gold-standard treatment for the reduction of ammonia during HE is lactulose 131, although a number of other experimental drugs aimed at lowering ammonia have been trialed including ornithine phenylacetate (OP) 9, 132– 134 and L-ornithine L-aspartate (LOLA) 135– 137. A major breakthrough came with an investigation of bacterial-DNA translocation effects on hyperammonemia and neurocognitive scores in patients with HE after the use of lactulose 131. The authors showed that neurocognitive scores improved with lactulose use and also led to reduced bacterial-DNA translocation 131.
Evidence is mounting that the use of polyethylene glycol (PEG), with or without lactulose, is effective. A randomized controlled trial in 40 patients compared the use of lactulose versus combined lactulose and PEG in cirrhotic patients admitted with HE 138. There was a statistically significant decrease in length of hospital stay (in women) 138. Surprisingly, there was no effect on ammonia levels 138. Likewise, the HELP randomized trial found that, compared to lactulose, PEG led to more rapid HE resolution in the hospital and concluded that it was superior to standard lactulose therapy 139.
Studies are investigating albumin transfusion as a possible treatment for HE 140. A prospective randomized controlled trial investigated lactulose alone versus lactulose plus albumin 140. Combination treatment of lactulose plus albumin led to a decrease in levels of ammonia, IL-6, IL-18, and TNF-α and other endotoxins 140.
Studies are also investigating branched chain amino acid (BCAA) supplementation in advanced liver disease. The ratio of normal BCAAs to aromatic amino acids becomes reduced in HE 141. Randomized controlled studies show that BCAA supplementation does not prevent the development of HE but may slow down the progression of hepatic failure 141, 142. Despite BCAAs having no effect on mortality, 16 randomized controlled clinical trials have shown that BCAAs decrease the signs and symptoms of HE 143. Conflicting studies exist, with some stating BCAAs have no significant effect on HE 144.
Recent studies have examined the use of corticosteroid administration in patients with acute liver injury and ALF 145. A prospective observational study of 469 patients from 2004 to 2015 showed that high-dose therapy decreased the risk of HE, but the time to initiate therapy has yet to be established 145.
Surgical treatment
As might be predicted, liver transplantation has been shown to improve the brain activity and cognitive function of patients with cirrhosis with or without HE 146. Zhang et al. studied the effect of liver transplant on resting-state brain activity by quantizing the ALFF before and one month after transplant. Cognitive function improved in both groups with and without encephalopathy, showing transplant as a viable treatment option for HE 146.
In a case report, a 73-year-old male presented with new-onset HE and was found to have a congenital portosystemic shunt with a normal liver histology. Correction of the shunt led to full reversal of his symptoms 147. This highlights a surgically correctable cause of acute HE without underlying liver disease.
An increase in shunt blood bypassing the liver owing to portal hypertension can result in HE. Surgical interventions to close these shunts have been studied as a possible treatment for HE 148. Percutaneous transhepatic obliteration (PTO) and percutaneous transhepatic sclerotherapy (PTS) are two methods used to close shunts in the setting of emergent variceal hemorrhage when other methods have failed 148. PTO is performed by placing metallic coils in the afferent veins to reduce blood flow, while PTS is performed by injecting a sclerosing agent 148. Both methods utilize an invasive transcutaneous intrahepatic puncture 148. A study of 37 patients with variceal hemorrhage and intractable HE underwent PTO/PTS 148. Blood ammonia levels greatly improved, dropping from 135 mg/dL to 65 mg/dL at six months, and there was also an improvement in HE 148.
TIPS is a procedure that places a stent between the hepatic vein and portal vein to alleviate portal hypertension 149. However, allowing blood to bypass the liver can worsen HE 149, 150. The size of the stent in TIPS has been associated with HE 151, 152. One study showed that the use of a larger diameter stent improved ascites without a significant change in HE 151, 152. Additionally, the consumption of a low-protein diet after TIPS can reduce the incidence of HE 153. Interestingly, antegrade embolization of spontaneous splenorenal shunt has also been employed to decrease the incidence of post-TIPS HE and has decreased the rate of variceal bleeding 154.
Balloon-occluded retrograde transvenous obliteration (BRTO) is an alternative or adjuvant to TIPS in the management of gastric varices 155. It works by abolishing gastrorenal or splenorenal shunts 155. A retrospective record-based study of patients who underwent BRTO for gastric variceal bleeding or refractory HE showed great improvement in grade of encephalopathy after BRTO 155.
Conclusions
This review summarizes the advances made in basic and clinical research over the last three years that have furthered our understanding of the pathophysiology of HE and possible treatments. Recent developments in basic research have focused on the role of hyperammonemia, oxidative stress, neuroinflammation, neurotransmitter function, bile acids, and the blood–brain barrier. More research is needed in these areas to fully establish the mechanism behind these various components and the role they play in the pathophysiology of HE. The diagnosis of HE is being investigated and is expected to become more precise by optimizing and validating current scoring systems. It appears that at least some of these will be amenable to modern app technology. Research in non-invasive imaging is also active and promises to aid in the diagnosis of HE. Strides have been made in the treatment of HE by specifically targeting the gut microbiome and hyperammonemia.
Abbreviations
AASLD, American Association for the Study of Liver Disease; ALF, acute liver failure; ALFF, amplitude of low-frequency fluctuation; AOM, azoxymethane; BCAA, branched chain amino acid; BRTO, balloon-occluded retrograde transvenous obliteration; CFF, critical flicker frequency test; EEG, electroencephalogram; EHPVO, extrahepatic portal vein obstruction; GMP, guanosine monophosphate; HE, hepatic encephalopathy; ICP, intracranial pressure; MELD, model for end stage liver disease; MHE, minimal hepatic encephalopathy; MRI, magnetic resonance imaging; PEG, polyethylene glycol; PHES, psychomotor hepatic encephalopathy score; PPI, proton pump inhibitor; PTO, percutaneous transhepatic obliteration; PTS, percutaneous transhepatic sclerotherapy; TIPS, transjugular intrahepatic portosystemic shunts.
Acknowledgements
This work was completed with support from the Veterans Health Administration and with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Kevin Mullen, Case Western Reserve University, Cleveland, OH, USA
Christopher Rose, University of Montreal, Montreal, Canada
Manuela Merli, Department of Clinical Medicine, Sapienza University of Rome, Rome, Italy
Don Rockey, Medical University of South Carolina, Charleston, SC, USA
Funding Statement
This study was funded by an NIH R01 award (DK082435) and a VA Merit award (BX002638) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service to Sharon DeMorrow.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. Vilstrup H, Amodio P, Bajaj J, et al. : Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35. 10.1002/hep.27210 [DOI] [PubMed] [Google Scholar]
- 2. Stepanova M, Mishra A, Venkatesan C, et al. : In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–41.e1. 10.1016/j.cgh.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 3. Bernal W, Wendon J: Acute liver failure. N Engl J Med. 2013;369(26):2525–34. 10.1056/NEJMra1208937 [DOI] [PubMed] [Google Scholar]
- 4. Riggio O, Nardelli S, Moscucci F, et al. : Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16(1):133–46. 10.1016/j.cld.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 5. Wiegand J, Berg T: The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013;110(6):85–91. 10.3238/arztebl.2013.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu PP, Shah NJ: Clinical and Neurologic Manifestation of Minimal Hepatic Encephalopathy and Overt Hepatic Encephalopathy. Clin Liver Dis. 2015;19(3):461–72. 10.1016/j.cld.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 7. Flamm SL: Covert Hepatic Encephalopathy: Who Should Be Tested and Treated? Clin Liver Dis. 2015;19(3):473–85. 10.1016/j.cld.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 8. Parekh PJ, Balart LA: Ammonia and Its Role in the Pathogenesis of Hepatic Encephalopathy. Clin Liver Dis. 2015;19(3):529–37. 10.1016/j.cld.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 9. Romero-Gómez M, Montagnese S, Jalan R: Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62(2):437–47. 10.1016/j.jhep.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 10. Aldridge DR, Tranah EJ, Shawcross DL: Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5(Suppl 1):S7–S20. 10.1016/j.jceh.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qvartskhava N, Lang PA, Görg B, et al. : Hyperammonemia in gene-targeted mice lacking functional hepatic glutamine synthetase. Proc Natl Acad Sci U S A. 2015;112(17):5521–6. 10.1073/pnas.1423968112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rackayova V, Braissant O, McLin VA, et al. : 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: in vivo longitudinal measurements of brain energy metabolism. Metab Brain Dis. 2016;31(6):1303–14. 10.1007/s11011-015-9715-8 [DOI] [PubMed] [Google Scholar]
- 13. Haack N, Dublin P, Rose CR: Dysbalance of astrocyte calcium under hyperammonemic conditions. PLoS One. 2014;9(8):e105832. 10.1371/journal.pone.0105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang C, Du T, Zhou J, et al. : Ammonium increases Ca 2+ signalling and up-regulates expression of TRPC1 gene in astrocytes in primary cultures and in the in vivo brain. Neurochem Res. 2014;39(11):2127–35. 10.1007/s11064-014-1406-z [DOI] [PubMed] [Google Scholar]
- 15. Wang F, Du T, Liang C, et al. : Ammonium increases Ca 2+ signalling and upregulates expression of Ca v1.2 gene in astrocytes in primary cultures and in the in vivo brain. Acta Physiol (Oxf). 2015;214(2):261–74. 10.1111/apha.12500 [DOI] [PubMed] [Google Scholar]
- 16. Sobczyk K, Jördens MS, Karababa A, et al. : Ephrin/Ephrin receptor expression in ammonia-treated rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Neurochem Res. 2015;40(2):274–83. 10.1007/s11064-014-1389-9 [DOI] [PubMed] [Google Scholar]
- 17. Karababa A, Görg B, Schliess F, et al. : O-GlcNAcylation as a novel ammonia-induced posttranslational protein modification in cultured rat astrocytes. Metab Brain Dis. 2014;29(4):975–82. 10.1007/s11011-013-9454-7 [DOI] [PubMed] [Google Scholar]
- 18. Görg B, Karababa A, Shafigullina A, et al. : Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia. 2015;63(1):37–50. 10.1002/glia.22731 [DOI] [PubMed] [Google Scholar]
- 19. Oenarto J, Karababa A, Castoldi M, et al. : Ammonia-induced miRNA expression changes in cultured rat astrocytes. Sci Rep. 2016;6: 18493. 10.1038/srep18493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayakumar AR, Tong XY, Curtis KM, et al. : Decreased astrocytic thrombospondin-1 secretion after chronic ammonia treatment reduces the level of synaptic proteins: in vitro and in vivo studies. J Neurochem. 2014;131(3):333–47. 10.1111/jnc.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jayakumar AR, Tong XY, Curtis KM, et al. : Increased toll-like receptor 4 in cerebral endothelial cells contributes to the astrocyte swelling and brain edema in acute hepatic encephalopathy. J Neurochem. 2014;128(6):890–903. 10.1111/jnc.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bobermin LD, Arús BA, Leite MC, et al. : Gap Junction Intercellular Communication Mediates Ammonia-Induced Neurotoxicity. Neurotox Res. 2016;29(2):314–24. 10.1007/s12640-015-9581-5 [DOI] [PubMed] [Google Scholar]
- 23. Lemberg A, Fernández MA: Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol. 2009;8(2):95–102. [PubMed] [Google Scholar]
- 24. Milewski K, Hilgier W, Albrecht J, et al. : The dimethylarginine (ADMA)/nitric oxide pathway in the brain and periphery of rats with thioacetamide-induced acute liver failure: Modulation by histidine. Neurochem Int. 2015;88:26–31. 10.1016/j.neuint.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 25. Ruszkiewicz J, Albrecht J: Changes of the thioredoxin system, glutathione peroxidase activity and total antioxidant capacity in rat brain cortex during acute liver failure: modulation by L-histidine. Neurochem Res. 2015;40(2):293–300. 10.1007/s11064-014-1417-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bobermin LD, Wartchow KM, Flores MP, et al. : Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase 1. Neurotoxicology. 2015;49:28–35. 10.1016/j.neuro.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 27. Chang CC, Lee WS, Chuang CL, et al. : Effects of raloxifene on portal hypertension and hepatic encephalopathy in cirrhotic rats. Eur J Pharmacol. 2017;802:36–43. 10.1016/j.ejphar.2017.02.039 [DOI] [PubMed] [Google Scholar]
- 28. Ghiassy B, Rahimi N, Javadi-Paydar M, et al. : Nitric oxide mediates effects of acute, not chronic, naltrexone on LPS-induced hepatic encephalopathy in cirrhotic rats. Can J Physiol Pharmacol. 2017;95(1):16–22. 10.1139/cjpp-2016-0188 [DOI] [PubMed] [Google Scholar]
- 29. Jamshidzadeh A, Heidari R, Abasvali M, et al. : Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother. 2017;86:514–20. 10.1016/j.biopha.2016.11.095 [DOI] [PubMed] [Google Scholar]
- 30. Milewski K, Hilgier W, Fręśko I, et al. : Carnosine Reduces Oxidative Stress and Reverses Attenuation of Righting and Postural Reflexes in Rats with Thioacetamide-Induced Liver Failure. Neurochem Res. 2016;41(1–2):376–84. 10.1007/s11064-015-1821-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu YB, Zhang L, Li WT, et al. : Artesunate restores spatial learning of rats with hepatic encephalopathy by inhibiting ammonia-induced oxidative damage in neurons and dysfunction of glutamate signaling in astroglial cells. Biomed Pharmacother. 2016;84:972–8. 10.1016/j.biopha.2016.09.104 [DOI] [PubMed] [Google Scholar]
- 32. Mladenović D, Petronijević N, Stojković T, et al. : Finasteride Has Regionally Different Effects on Brain Oxidative Stress and Acetylcholinesterase Activity in Acute Thioacetamide-Induced Hepatic Encephalopathy in Rats. PLoS One. 2015;10(8):e0134434. 10.1371/journal.pone.0134434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosoi CR, Tremblay M, Rose CF: Induction of systemic oxidative stress leads to brain oedema in portacaval shunted rats. Liver Int. 2014;34(9):1322–9. 10.1111/liv.12414 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Rama Rao KV, Norenberg MD: Brain energy metabolism and mitochondrial dysfunction in acute and chronic hepatic encephalopathy. Neurochem Int. 2012;60(7):697–706. 10.1016/j.neuint.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh TS, McLellan S, Mackenzie SJ, et al. : Hyperlactatemia and pulmonary lactate production in patients with fulminant hepatic failure. Chest. 1999;116(2):471–6. 10.1378/chest.116.2.471 [DOI] [PubMed] [Google Scholar]
- 36. Rose C, Ytrebø LM, Davies NA, et al. : Association of reduced extracellular brain ammonia, lactate, and intracranial pressure in pigs with acute liver failure. Hepatology. 2007;46(6):1883–92. 10.1002/hep.21877 [DOI] [PubMed] [Google Scholar]
- 37. Zwingmann C, Chatauret N, Leibfritz D, et al. : Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [H-C] nuclear magnetic resonance study. Hepatology. 2003;37(2):420–8. 10.1053/jhep.2003.50052 [DOI] [PubMed] [Google Scholar]
- 38. Fitzpatrick SM, Hetherington HP, Behar KL, et al. : Effects of acute hyperammonemia on cerebral amino acid metabolism and pH i in vivo, measured by 1H and 31P nuclear magnetic resonance. J Neurochem. 1989;52(3):741–9. 10.1111/j.1471-4159.1989.tb02517.x [DOI] [PubMed] [Google Scholar]
- 39. Dabos KJ, Parkinson JA, Sadler IH, et al. : 1H nuclear magnetic resonance spectroscopy-based metabonomic study in patients with cirrhosis and hepatic encephalopathy. World J Hepatol. 2015;7(12):1701–7. 10.4254/wjh.v7.i12.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bosoi CR, Zwingmann C, Marin H, et al. : Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol. 2014;60(3):554–60. 10.1016/j.jhep.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 41. Hadjihambi A, de Chiara F, Hosford PS, et al. : Ammonia mediates cortical hemichannel dysfunction in rodent models of chronic liver disease. Hepatology. 2017;65(4):1306–18. 10.1002/hep.29031 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Magistretti PJ: Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–11. 10.1242/jeb.02208 [DOI] [PubMed] [Google Scholar]
- 43. Zemtsova I, Görg B, Keitel V, et al. : Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011;54(1):204–15. 10.1002/hep.24326 [DOI] [PubMed] [Google Scholar]
- 44. Görg B, Bidmon HJ, Häussinger D: Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology. 2013;57(6):2436–47. 10.1002/hep.26265 [DOI] [PubMed] [Google Scholar]
- 45. McMillin M, Grant S, Frampton G, et al. : Fractalkine suppression during hepatic encephalopathy promotes neuroinflammation in mice. J Neuroinflammation. 2016;13(1):198. 10.1186/s12974-016-0674-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McMillin M, Frampton G, Thompson M, et al. : Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation. 2014;11:121. 10.1186/1742-2094-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hernández-Rabaza V, Cabrera-Pastor A, Taoro-González L, et al. : Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane. J Neuroinflammation. 2016;13:41. 10.1186/s12974-016-0505-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Agusti A, Hernández-Rabaza V, Balzano T, et al. : Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone, and improves motor in-coordination in rats with hepatic encephalopathy. CNS Neurosci Ther. 2017;23(5):386–94. 10.1111/cns.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernandez-Rabaza V, Agusti A, Cabrera-Pastor A, et al. : Sildenafil reduces neuroinflammation and restores spatial learning in rats with hepatic encephalopathy: underlying mechanisms. J Neuroinflammation. 2015;12:195. 10.1186/s12974-015-0420-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hernandez-Rabaza V, Cabrera-Pastor A, Taoro-Gonzalez L, et al. : Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation. 2016;13(1):83. 10.1186/s12974-016-0549-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Agusti A, Dziedzic JL, Hernandez-Rabaza V, et al. : Rats with minimal hepatic encephalopathy due to portacaval shunt show differential increase of translocator protein (18 kDa) binding in different brain areas, which is not affected by chronic MAP-kinase p38 inhibition. Metab Brain Dis. 2014;29(4):955–63. 10.1007/s11011-013-9461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McMillin M, Galindo C, Pae HY, et al. : Gli1 activation and protection against hepatic encephalopathy is suppressed by circulating transforming growth factor β1 in mice. J Hepatol. 2014;61(6):1260–6. 10.1016/j.jhep.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Llansola M, Montoliu C, Agusti A, et al. : Interplay between glutamatergic and GABAergic neurotransmission alterations in cognitive and motor impairment in minimal hepatic encephalopathy. Neurochem Int. 2015;88:15–9. 10.1016/j.neuint.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 54. Obara-Michlewska M, Ruszkiewicz J, Zielińska M, et al. : Astroglial NMDA receptors inhibit expression of K ir4.1 channels in glutamate-overexposed astrocytes in vitro and in the brain of rats with acute liver failure. Neurochem Int. 2015;88:20–5. 10.1016/j.neuint.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 55. Tong X, Ao Y, Faas GC, et al. : Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. 2014;17(5):694–703. 10.1038/nn.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cauli O, González-Usano A, Cabrera-Pastor A, et al. : Blocking NMDA receptors delays death in rats with acute liver failure by dual protective mechanisms in kidney and brain. Neuromolecular Med. 2014;16(2):360–75. 10.1007/s12017-013-8283-5 [DOI] [PubMed] [Google Scholar]
- 57. Gonzalez-Usano A, Cauli O, Agusti A, et al. : Pregnenolone sulfate restores the glutamate-nitric-oxide-cGMP pathway and extracellular GABA in cerebellum and learning and motor coordination in hyperammonemic rats. ACS Chem Neurosci. 2014;5(2):100–5. 10.1021/cn400168y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ding S, Huang W, Ye Y, et al. : Elevated intracranial dopamine impairs the glutamate‑nitric oxide‑cyclic guanosine monophosphate pathway in cortical astrocytes in rats with minimal hepatic encephalopathy. Mol Med Rep. 2014;10(3):1215–24. 10.3892/mmr.2014.2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Palomero-Gallagher N, Zilles K: Neurotransmitter receptor alterations in hepatic encephalopathy: a review. Arch Biochem Biophys. 2013;536(2):109–21. 10.1016/j.abb.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 60. Butterworth RF: Neurosteroids in hepatic encephalopathy: Novel insights and new therapeutic opportunities. J Steroid Biochem Mol Biol. 2016;160:94–7. 10.1016/j.jsbmb.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 61. Tsutsui K, Haraguchi S: Breakthrough in neuroendocrinology by discovering novel neuropeptides and neurosteroids: 2. Discovery of neurosteroids and pineal neurosteroids. Gen Comp Endocrinol. 2014;205:11–22. 10.1016/j.ygcen.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 62. Mladenović D, Hrnčić D, Petronijević N, et al. : Finasteride improves motor, EEG, and cellular changes in rat brain in thioacetamide-induced hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2014;307(9):G931–40. 10.1152/ajpgi.00463.2013 [DOI] [PubMed] [Google Scholar]
- 63. Johansson M, Agusti A, Llansola M, et al. : GR3027 antagonizes GABA A receptor-potentiating neurosteroids and restores spatial learning and motor coordination in rats with chronic hyperammonemia and hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G400–9. 10.1152/ajpgi.00073.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ding S, Liu L, Jing H, et al. : Dopamine from cirrhotic liver contributes to the impaired learning and memory ability of hippocampus in minimal hepatic encephalopathy. Hepatol Int. 2013;7(3):923–36. 10.1007/s12072-013-9431-6 [DOI] [PubMed] [Google Scholar]
- 65. Ding S, Hu J, Yang J, et al. : The inactivation of JAK2/STAT3 signaling and desensitization of M1 mAChR in minimal hepatic encephalopathy (MHE) and the protection of naringin against MHE. Cell Physiol Biochem. 2014;34(6):1933–50. 10.1159/000366391 [DOI] [PubMed] [Google Scholar]
- 66. Ding S, Wang W, Wang X, et al. : Dopamine Burden Triggers Neurodegeneration via Production and Release of TNF-α from Astrocytes in Minimal Hepatic Encephalopathy. Mol Neurobiol. 2016;53(8):5324–43. 10.1007/s12035-015-9445-2 [DOI] [PubMed] [Google Scholar]
- 67. Dhanda S, Sandhir R: Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav Brain Res. 2015;286:222–35. 10.1016/j.bbr.2015.01.042 [DOI] [PubMed] [Google Scholar]
- 68. Bron B, Waldram R, Silk DB, et al. : Serum, cerebrospinal fluid, and brain levels of bile acids in patients with fulminant hepatic failure. Gut. 1977;18(9):692–6. 10.1136/gut.18.9.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McMillin M, Frampton G, Quinn M, et al. : Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure. Am J Pathol. 2016;186(2):312–23. 10.1016/j.ajpath.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Quinn M, McMillin M, Galindo C, et al. : Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46(6):527–34. 10.1016/j.dld.2014.01.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tripodi V, Contin M, Fernández MA, et al. : Bile acids content in brain of common duct ligated rats. Ann Hepatol. 2012;11(6):930–4. [PubMed] [Google Scholar]
- 72. Weiss N, Barbier Saint Hilaire P, Colsch B, et al. : Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016;65(6):1120–30. 10.1016/j.jhep.2016.07.046 [DOI] [PubMed] [Google Scholar]
- 73. Wright G, Swain M, Annane D, et al. : Neuroinflammation in liver disease: sessional talks from ISHEN. Metab Brain Dis. 2016;31(6):1339–54. 10.1007/s11011-016-9918-7 [DOI] [PubMed] [Google Scholar]
- 74. Keitel V, Görg B, Bidmon HJ, et al. : The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58(15):1794–805. 10.1002/glia.21049 [DOI] [PubMed] [Google Scholar]
- 75. McMillin M, Frampton G, Tobin R, et al. : TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015;135(3):565–76. 10.1111/jnc.13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dhanda S, Sandhir R: Blood-Brain Barrier Permeability Is Exacerbated in Experimental Model of Hepatic Encephalopathy via MMP-9 Activation and Downregulation of Tight Junction Proteins. Mol Neurobiol. 2017. 10.1007/s12035-017-0521-7 [DOI] [PubMed] [Google Scholar]
- 77. Weiss N, Rosselli M, Mouri S, et al. : Modification in CSF specific gravity in acutely decompensated cirrhosis and acute on chronic liver failure independent of encephalopathy, evidences for an early blood-CSF barrier dysfunction in cirrhosis. Metab Brain Dis. 2017;32(2):369–76. 10.1007/s11011-016-9916-9 [DOI] [PubMed] [Google Scholar]
- 78. McMillin MA, Frampton GA, Seiwell AP, et al. : TGF β1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Invest. 2015;95(8):903–13. 10.1038/labinvest.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shimojima N, Eckman CB, McKinney M, et al. : Altered expression of zonula occludens-2 precedes increased blood-brain barrier permeability in a murine model of fulminant hepatic failure. J Invest Surg. 2008;21(3):101–8. 10.1080/08941930802043565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chastre A, Bélanger M, Nguyen BN, et al. : Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014;34(3):353–61. 10.1111/liv.12252 [DOI] [PubMed] [Google Scholar]
- 81. De Rui M, Montagnese S, Amodio P: Recent developments in the diagnosis and treatment of covert/minimal hepatic encephalopathy. Expert Rev Gastroenterol Hepatol. 2016;10(4):443–50. 10.1586/17474124.2016.1141675 [DOI] [PubMed] [Google Scholar]
- 82. Sharma P: Critical flicker frequency: A stethoscope for minimal hepatic encephalopathy evaluation. Turk J Gastroenterol. 2017;28(3):155–6. 10.5152/tjg.2017.050101 [DOI] [PubMed] [Google Scholar]
- 83. Moretti R, Gazzin S, Crocè LS, et al. : Rapid identification system of frontal dysfunction in subclinical hepatic encephalopathy. Ann Hepatol. 2016;15(4):559–67. [PubMed] [Google Scholar]
- 84. Bajaj JS: Diagnosing minimal hepatic encephalopathy: from the ivory tower to the real world. Gastroenterology. 2015;149(6):1330–3. 10.1053/j.gastro.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 85. Coskun B, Ozen M, Gursoy S, et al. : Normalization of the psychometric hepatic encephalopathy score for diagnosis of minimal hepatic encephalopathy in Turkey. Niger J Clin Pract. 2017;20(4):421–6. [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Badea MA, Drug VL, Dranga M, et al. : Diagnosis of minimal hepatic encephalopathy in a tertiary care center from eastern Romania: validation of the psychometric hepatic encephalopathy score (PHES). Metab Brain Dis. 2016;31(6):1463–71. 10.1007/s11011-016-9878-y [DOI] [PubMed] [Google Scholar]
- 87. Wuensch T, Ruether DF, Zöllner C, et al. : Performance characterization of a novel electronic number connection test to detect minimal hepatic encephalopathy in cirrhotic patients. Eur J Gastroenterol Hepatol. 2017;29(4):456–63. 10.1097/MEG.0000000000000806 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Özel Coşkun BD, Özen M: Critical flicker frequency test for diagnosing minimal hepatic encephalopathy in patients with cirrhosis. Turk J Gastroenterol. 2017;28(3):191–6. 10.5152/tjg.2017.16618 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Greinert R, Ripoll C, Hollenbach M, et al. : Stepwise diagnosis in covert hepatic encephalopathy: critical flicker frequency and MELD-score as a first-step approach. Aliment Pharmacol Ther. 2016;44(5):514–21. 10.1111/apt.13721 [DOI] [PubMed] [Google Scholar]
- 90. Martel-Laferrière V, Homberger C, Bichoupan K, et al. : MELD score and antibiotics use are predictors of length of stay in patients hospitalized with hepatic encephalopathy. BMC Gastroenterol. 2014;14:185. 10.1186/1471-230X-14-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ampuero J, Simón M, Montoliú C, et al. : Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology. 2015;149(6):1483–9. 10.1053/j.gastro.2015.07.067 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Bajaj JS: Adventures in Developing an App for Covert Hepatic Encephalopathy. Clin Transl Gastroenterol. 2017;8(4):e85. 10.1038/ctg.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Allampati S, Duarte-Rojo A, Thacker LR, et al. : Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol. 2016;111(1):78–86. 10.1038/ajg.2015.377 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Press CA, Morgan L, Mills M, et al. : Spectral Electroencephalogram Analysis for the Evaluation of Encephalopathy Grade in Children With Acute Liver Failure. Pediatr Crit Care Med. 2017;18(1):64–72. 10.1097/PCC.0000000000001016 [DOI] [PubMed] [Google Scholar]
- 95. Jackson CD, Gram M, Halliday E, et al. : New spectral thresholds improve the utility of the electroencephalogram for the diagnosis of hepatic encephalopathy. Clin Neurophysiol. 2016;127(8):2933–41. 10.1016/j.clinph.2016.03.027 [DOI] [PubMed] [Google Scholar]
- 96. Olesen SS, Gram M, Jackson CD, et al. : Electroencephalogram variability in patients with cirrhosis associates with the presence and severity of hepatic encephalopathy. J Hepatol. 2016;65(3):517–23. 10.1016/j.jhep.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 97. Schiff S, Casa M, Di Caro V, et al. : A low-cost, user-friendly electroencephalographic recording system for the assessment of hepatic encephalopathy. Hepatology. 2016;63(5):1651–9. 10.1002/hep.28477 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Scheau C, Dinu R, Tarta-Arsene E, et al. : Current Stance of Magnetic Resonance Imaging in the Diagnosis and Monitoring of Hepatic Encephalopathy. Maedica (Buchar). 2015;10(3):243–7. [PMC free article] [PubMed] [Google Scholar]
- 99. Li Y, Liu H, Yang J, et al. : Combining arterial-spin labeling with functional magnetic resonance imaging measurement for characterizing patients with minimal hepatic encephalopathy. Hepatol Res. 2017;47(9):862–71. 10.1111/hepr.12827 [DOI] [PubMed] [Google Scholar]
- 100. Yadav SK, Goel A, Saraswat VA, et al. : Evaluation of cognitivity, proinflammatory cytokines, and brain magnetic resonance imaging in minimal hepatic encephalopathy induced by cirrhosis and extrahepatic portal vein obstruction. J Gastroenterol Hepatol. 2016;31(12):1986–94. 10.1111/jgh.13427 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 101. Chen HJ, Zhang L, Jiang LF, et al. : Identifying minimal hepatic encephalopathy in cirrhotic patients by measuring spontaneous brain activity. Metab Brain Dis. 2016;31(4):761–9. 10.1007/s11011-016-9799-9 [DOI] [PubMed] [Google Scholar]
- 102. Zhong WJ, Zhou ZM, Zhao JN, et al. : Abnormal spontaneous brain activity in minimal hepatic encephalopathy: resting-state fMRI study. Diagn Interv Radiol. 2016;22(2):196–200. 10.5152/dir.2015.15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Or M, Peremans K, Martlé V, et al. : Regional cerebral blood flow assessed by single photon emission computed tomography (SPECT) in dogs with congenital portosystemic shunt and hepatic encephalopathy. Vet J. 2017;220:40–2. 10.1016/j.tvjl.2016.12.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. McPhail MJ, Montagnese S, Villanova M, et al. : Urinary metabolic profiling by 1H NMR spectroscopy in patients with cirrhosis may discriminate overt but not covert hepatic encephalopathy. Metab Brain Dis. 2017;32(2):331–41. 10.1007/s11011-016-9904-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Betrapally NS, Gillevet PM, Bajaj JS: Gut microbiome and liver disease. Transl Res. 2017;179:49–59. 10.1016/j.trsl.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shawcross DL: Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy? Expert Rev Gastroenterol Hepatol. 2015;9(5):539–42. 10.1586/17474124.2015.1035257 [DOI] [PubMed] [Google Scholar]
- 107. Dam G, Vilstrup H, Watson H, et al. : Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64(4):1265–72. 10.1002/hep.28737 [DOI] [PubMed] [Google Scholar]
- 108. Lachar J, Bajaj JS: Changes in the Microbiome in Cirrhosis and Relationship to Complications: Hepatic Encephalopathy, Spontaneous Bacterial Peritonitis, and Sepsis. Semin Liver Dis. 2016;36(4):327–30. 10.1055/s-0036-1593881 [DOI] [PubMed] [Google Scholar]
- 109. Rai R, Saraswat VA, Dhiman RK: Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(Suppl 1):S29–36. 10.1016/j.jceh.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bian J, Wang A, Lin J, et al. : Association between proton pump inhibitors and hepatic encephalopathy: A meta-analysis. Medicine (Baltimore). 2017;96(17):e6723. 10.1097/MD.0000000000006723 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Khan MA, Cholankeril G, Howden CW: Proton Pump Inhibitors and the Possible Development of Hepatic Encephalopathy in Cirrhotic Patients: True Association or Residual Confounding? Gastroenterology. 2017;152(8):2076. 10.1053/j.gastro.2016.10.050 [DOI] [PubMed] [Google Scholar]
- 112. Tsai CF, Chen MH, Wang YP, et al. : Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152(1):134–41. 10.1053/j.gastro.2016.09.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 113. Sharma BC, Singh J: Probiotics in management of hepatic encephalopathy. Metab Brain Dis. 2016;31(6):1295–301. 10.1007/s11011-016-9826-x [DOI] [PubMed] [Google Scholar]
- 114. Zhao LN, Yu T, Lan SY, et al. : Probiotics can improve the clinical outcomes of hepatic encephalopathy: An update meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(6):674–82. 10.1016/j.clinre.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 115. Saab S, Suraweera D, Au J, et al. : Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials. Liver Int. 2016;36(7):986–93. 10.1111/liv.13005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 116. Dalal R, McGee RG, Riordan SM, et al. : Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. 10.1002/14651858.CD008716.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 117. Pratap Mouli V, Benjamin J, Bhushan Singh M, et al. : Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial. Hepatol Res. 2015;45(8):880–9. 10.1111/hepr.12429 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Shavakhi A, Hashemi H, Tabesh E, et al. : Multistrain probiotic and lactulose in the treatment of minimal hepatic encephalopathy. J Res Med Sci. 2014;19(8):703–8. [PMC free article] [PubMed] [Google Scholar]
- 119. Dhiman RK, Rana B, Agrawal S, et al. : Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327–37.e3. 10.1053/j.gastro.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 120. Maharshi S, Sharma BC, Sachdeva S, et al. : Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2016;14(3):454–460.e3; quiz e33. 10.1016/j.cgh.2015.09.028 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. Bajaj JS, Barrett AC, Bortey E, et al. : Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41(1):39–45. 10.1111/apt.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 122. Tapper EB: Rifaximin for the prevention of readmissions for patients with hepatic encephalopathy - the price is right. Liver Int. 2016;36(9):1252–4. 10.1111/liv.13130 [DOI] [PubMed] [Google Scholar]
- 123. Whitehouse JT, Berni E, Conway P, et al. : Evaluation of The Cost Effectiveness and Societal Impact of Rifaximin-Á 550mg In The Reduction of Recurrence of Overt Hepatic Encephalopathy In The Netherlands. Value Health. 2015;18(7):A629. 10.1016/j.jval.2015.09.2219 26533529 [DOI] [Google Scholar]
- 124. Berni E, Connolly M, Conway P, et al. : Evaluation of The Cost Effectiveness of Rifaximin-Á In The Reduction of Recurrence of Overt Hepatic Encephalopathy In Belgium. Value Health. 2015;18(7):A628. 10.1016/j.jval.2015.09.2216 26533525 [DOI] [Google Scholar]
- 125. Berni E, Poole CD, Conway P, et al. : Cost Effectiveness of Rifaximin-Á 550mg In The Reduction of Recurrence of Overt Hepatic Encephalopathy In United Kingdom. Value Health. 2015;18(7):A626. 10.1016/j.jval.2015.09.2206 26533513 [DOI] [Google Scholar]
- 126. Poole CD, Berni E, Conway P, et al. : Evaluation of The Cost Effectiveness of Rifaximin-á 550mg In The Reduction of Recurrence of Overt Hepatic Encephalopathy In Sweden. Value Health. 2015;18(7):A626. 10.1016/j.jval.2015.09.2202 26533517 [DOI] [Google Scholar]
- 127. Bajaj JS, Heuman DM, Sanyal AJ, et al. : Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. 10.1371/journal.pone.0060042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lyon KC, Likar E, Martello JL, et al. : Retrospective Cross-Sectional Pilot Study of Rifaximin Dosing for the Prevention of Recurrent Hepatic Encephalopathy. J Gastroenterol Hepatol. 2017. 10.1111/jgh.13759 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 129. Kabeshova A, Ben Hariz S, Tsakeu E, et al. : Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France. Therap Adv Gastroenterol. 2016;9(4):473–82. 10.1177/1756283X16644249 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. Courson A, Jones GM, Twilla JD: Treatment of Acute Hepatic Encephalopathy: Comparing the Effects of Adding Rifaximin to Lactulose on Patient Outcomes. J Pharm Pract. 2016;29(3):212–7. 10.1177/0897190014566312 [DOI] [PubMed] [Google Scholar]
- 131. Moratalla A, Ampuero J, Bellot P, et al. : Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2017;37(2):212–23. 10.1111/liv.13200 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 132. Ventura-Cots M, Arranz JA, Simón-Talero M, et al. : Safety of ornithine phenylacetate in cirrhotic decompensated patients: an open-label, dose-escalating, single-cohort study. J Clin Gastroenterol. 2013;47(10):881–7. 10.1097/MCG.0b013e318299c789 [DOI] [PubMed] [Google Scholar]
- 133. Ventura-Cots M, Concepción M, Arranz JA, et al. : Impact of ornithine phenylacetate (OCR-002) in lowering plasma ammonia after upper gastrointestinal bleeding in cirrhotic patients. Therap Adv Gastroenterol. 2016;9(6):823–35. 10.1177/1756283X16658252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kristiansen RG: Current state of knowledge of hepatic encephalopathy (part I): newer treatment strategies for hyperammonemia in liver failure. Metab Brain Dis. 2016;31(6):1357–8. 10.1007/s11011-016-9908-9 [DOI] [PubMed] [Google Scholar]
- 135. Sharma K, Pant S, Misra S, et al. : Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol. 2014;20(4):225–32. 10.4103/1319-3767.136975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Grover VP, McPhail MJ, Wylezinska-Arridge M, et al. : A longitudinal study of patients with cirrhosis treated with L-ornithine L-aspartate, examined with magnetization transfer, diffusion-weighted imaging and magnetic resonance spectroscopy. Metab Brain Dis. 2017;32(1):77–86. 10.1007/s11011-016-9881-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sidhu SS, Sharma BC, Goyal O, et al. : L-ornithine L-aspartate in bouts of overt hepatic encephalopathy. Hepatology. 2017. 10.1002/hep.29410 [DOI] [PubMed] [Google Scholar]
- 138. Naderian M, Akbari H, Saeedi M, et al. : Polyethylene Glycol and Lactulose versus Lactulose Alone in the Treatment of Hepatic Encephalopathy in Patients with Cirrhosis: A Non-Inferiority Randomized Controlled Trial. Middle East J Dig Dis. 2017;9(1):12–9. 10.15171/mejdd.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Rahimi RS, Singal AG, Cuthbert JA, et al. : Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–33. 10.1001/jamainternmed.2014.4746 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 140. Sharma BC, Singh J, Srivastava S, et al. : Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32(6):1234–9. 10.1111/jgh.13666 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. Tajiri K, Shimizu Y: Branched-chain amino acids in liver diseases. World J Gastroenterol. 2013;19(43):7620–9. 10.3748/wjg.v19.i43.7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vergara M, Castro-Gutiérrez V, Rada G: Do branched chain amino acids improve hepatic encephalopathy in cirrhosis? Medwave. 2016;16(Suppl5):e6795. 10.5867/medwave.2016.6795 [DOI] [PubMed] [Google Scholar]
- 143. Gluud LL, Dam G, Les I, et al. : Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;5:CD001939. 10.1002/14651858.CD001939.pub4 [DOI] [PubMed] [Google Scholar]
- 144. Ruiz-Margáin A, Macías-Rodríguez RU, Ríos-Torres SL, et al. : Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol Mex. 2017; pii: S0375-0906(17)30060-5. 10.1016/j.rgmx.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 145. Kakisaka K, Kataoka K, Suzuki Y, et al. : Appropriate timing to start and optimal response evaluation of high-dose corticosteroid therapy for patients with acute liver failure. J Gastroenterol. 2017;52(8):977–85. 10.1007/s00535-017-1306-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 146. Zhang G, Cheng Y, Shen W, et al. : The short-term effect of liver transplantation on the low-frequency fluctuation of brain activity in cirrhotic patients with and without overt hepatic encephalopathy. Brain Imaging Behav. 2016;1–13. 10.1007/s11682-016-9659-6 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 147. Brader RA, Kim KR: Transhepatic embolization of a congenital intrahepatic portosystemic shunt for the treatment of hepatic encephalopathy in a noncirrhotic patient using Amplatzer vascular plug device. Radiol Case Rep. 2017;12(2):318–22. 10.1016/j.radcr.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ishikawa T, Imai M, Ko M, et al. : Percutaneous transhepatic obliteration and percutaneous transhepatic sclerotherapy for intractable hepatic encephalopathy and gastric varices improves the hepatic function reserve. Biomed Rep. 2017;6(1):99–102. 10.3892/br.2016.811 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 149. Routhu M, Safka V, Routhu SK, et al. : Observational cohort study of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS). Ann Hepatol. 2017;16(1):140–8. 10.5604/16652681.1226932 [DOI] [PubMed] [Google Scholar]
- 150. Wang CM, Li X, Fu J, et al. : Construction of Transjugular Intrahepatic Portosystemic Shunt: Bare Metal Stent/Stent-graft Combination versus Single Stent-graft, a Prospective Randomized Controlled Study with Long-term Patency and Clinical Analysis. Chin Med J (Engl). 2016;129(11):1261–7. 10.4103/0366-6999.182830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Miraglia R, Maruzzelli L, Tuzzolino F, et al. : Transjugular Intrahepatic Portosystemic Shunts in Patients with Cirrhosis with Refractory Ascites: Comparison of Clinical Outcomes by Using 8- and 10-mm PTFE-covered Stents. Radiology. 2017;284(1):281–8. 10.1148/radiol.2017161644 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 152. Borghol S, Perarnau JM, Pucheux J, et al. : Short- and long-term evolution of the endoluminal diameter of underdilated stents in transjugular intrahepatic portosystemic shunt. Diagn Interv Imaging. 2016;97(11):1103–7. 10.1016/j.diii.2016.06.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 153. Luo L, Fu S, Zhang Y, et al. : Early diet intervention to reduce the incidence of hepatic encephalopathy in cirrhosis patients: post-Transjugular Intrahepatic Portosystemic Shunt (TIPS) findings. Asia Pac J Clin Nutr. 2016;25(3):497–503. 10.6133/apjcn.092015.14 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 154. Doshi M, Pereira K, Carrion A, et al. : Antegrade embolization of spontaneous splenorenal shunt for post-transjugular intrahepatic portosystemic shunt refractory hepatic encephalopathy. Hepatology. 2016;64(1):314–5. 10.1002/hep.28343 [DOI] [PubMed] [Google Scholar]
- 155. Mukund A, Deogaonkar G, Rajesh S, et al. : Safety and Efficacy of Sodium Tetradecyl Sulfate and Lipiodol Foam in Balloon-Occluded Retrograde Transvenous Obliteration (BRTO) for Large Porto-Systemic Shunts. Cardiovasc Intervent Radiol. 2017;40(7):1010–6. 10.1007/s00270-017-1593-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation