Abstract
Aims/Introduction
Little information is available regarding the status of insulin resistance (IR) and insulin deficiency (ID), as well as their relationship with obesity in children using the homeostasis model assessment (HOMA) in a population‐based setting.
Materials and Methods
The study included a total of 445 ninth‐grade children participating in health check‐up programs implemented in Tsunan Town, Niigata, Japan (boys/girls, 252/193 [participation rates: 98.1/95.5%]). HOMA of insulin resistance ≥2.5 was defined as IR, and HOMA of β‐cell function <40 defined as ID.
Results
The medians (25–75th percentiles) of HOMA of insulin resistance, HOMA of β‐cell function, Disposition Index and body mass index in boys were 1.2 (0.8–1.7), 64 (44–93), 52 (43–64) and 19.2 (18.0–20.7) kg/m2, respectively, vs 1.5 (1.0–2.0), 86 (63–120), 60 (50–74) and 20.4 (18.9–22.0) kg/m2, respectively, in girls. The HOMA of insulin resistance, HOMA of β‐cell function and Disposition Index values were significantly higher in the girls (P = 0.002, P < 0.001 and P < 0.001, respectively). Those with IR accounted for a significantly higher proportion of girls than boys (15.5/8.7%; P = 0.027); those with obesity accounted for 9.9/10.7% (boys/girls); and those with IR and obesity accounted for 2.4/4.7%. Those with ID accounted for a significantly higher proportion of boys than girls (20.6/8.8%; P = 0.001), whereas those with ID and obesity accounted for a very small proportion of either group (0.4/0.5%).
Conclusions
The presence of IR was higher among the girls. In contrast, ID was more frequent among the boys. The infrequent presence of ID among children might support the presence of non‐obese type 2 diabetes adults in Japan.
Keywords: Children, Insulin deficiency, Insulin resistance
Introduction
Atherosclerosis is counted among the major causes of death in humankind, and obesity, which represents one of its risk factors, is reported to be increasing among children worldwide1, 2. In Japan, pediatric obesity has been shown to increase year by year with the Westernization of lifestyle3.
Furthermore, a series of studies consistently reported that obesity starts to develop in childhood, particularly during puberty4. In addition, children who have lower‐than‐standard body mass index (BMI) until age 2 years, but show patterns of growth characterized by an acute increase in BMI thereafter until age 11, are also at risk of future cardiovascular disease5.
These reports show that there is no doubt that obesity in puberty is a definite risk factor for future cardiovascular disease. In addition, there is also no doubt that obesity developing in the presence of metabolic syndrome is associated with insulin resistance (IR).
However, epidemiological data relating to IR in general childhood and adolescent populations is very limited6, 7, 8, 9, 10, 11, 12, 13. To date, the largest study based on the Identification and Prevention of Dietary‐ and Lifestyle‐Induced Health Effects In Children and Infants cohort (7,077 normal weight 2–10.9‐year‐old children) was reported from Europe6. They found that the median values for the homeostasis model assessment of insulin resistance (HOMA‐IR), ranged from 0.5 and 0.4 in 3‐ to <3.5‐year‐old girls and boys, to 1.7 and 1.4 in 10.5‐ to <11‐year‐old girls and boys, respectively. However, there is only one report from Asia regarding epidemiological data relating to IR in childhood and adolescent populations12, with no data available from Japan. Furthermore, little has been reported regarding insulin secretory capacity in childhood and adolescent populations13.
Thus, the present study was designed to investigate the status of IR and insulin‐secretory capacity, and their correlation with obesity in almost all ninth‐graders in the community from one of the foremost rice‐producing areas in Japan, based on their measured fasting glucose and insulin values in a population‐based setting.
Methods
Of the 459 ninth‐graders (boys/girls, 257/202) who underwent health check‐ups between 2009 and 2014 in Tsunan Town (population, approximately 12,000), one of the areas in Niigata known for longevity, the study included a total of 445 ninth‐graders (boys/girls, 252/193) whose parents consented to their participation in the study (participation rates 98.1 and 95.5%, respectively). The study participants had their fasting glucose and insulin levels measured during regular health check‐up programs implemented in April every year. None had been previously diagnosed with diabetes.
Insulin level was measured using anti‐human insulin monoclonal antibody (Kanto Kagaku Co., Tokyo, Japan). Glucose level was measured by using the G6‐PDH method (Sinotest Co., Tokyo, Japan).
The HOMA‐IR (equation: fasting insulin [mU/L] × fasting glucose [mg/dL] / 405) and homeostasis model assessment of β‐cell function (HOMA‐β; equation: 360 × fasting insulin [mU/L] / [fasting glucose (mg/dL) − 63] %) were calculated based on fasting glucose and insulin values14. We also used the fasting Disposition Index (DI; equation: HOMA‐β/HOMA‐IR).
Those with HOMA‐IR ≥2.5 were considered to be associated with IR, and those with HOMA‐β <40 to be associated with insulin deficiency (ID), and histograms were created to show their distributions.
Consistent with the globally standardized definition of obesity in adults (BMI ≥25 kg/m2), boys with BMI ≥22.96 kg/m2 and girls with BMI ≥23.66 kg/m2 (cut‐off value for 14 years‐of‐age) were defined as obese15. All values were presented as medians (25–75th percentiles), and all comparisons of variables between boys and girls were made using the Mann–Whitney test. Proportions of children with relevant findings were examined by sex, and tested for significance using the χ2‐test or Fisher's exact probability test. HOMA‐IR, HOMA‐β, DI and BMI were examined for correlation using Spearman's rank correlation coefficient. SPSS version 21 (IBM Japan, Tokyo, Japan) was used for statistical analyses.
The present study was carried out with the approval of the ethics committee of Jikei University School of Medicine, Tokyo, Japan (20‐011 [5201]).
Results
The medians (25–75th percentiles) of fasting glucose, insulin, HOMA‐IR, HOMA‐β, DI and BMI values were 93 (88–98) mg/dL, 5.4 (3.5–7.5) μIU/mL, 1.2 (0.8–1.7), 64 (44–93), 52 (43–64) and 19.2 (18.0–20.7) kg/m2, respectively, in the boys, compared with 90 (86–94) mg/dL, 6.4 (4.7–8.9) μIU/mL, 1.5 (1.0–2.0), 86 (63–120), 60 (50–74) and 20.4 (18.9–22.0) kg/m2, respectively, in the girls. A comparison of the boys and girls showed that the fasting glucose values were significantly higher in the boys (P < 0.001), whereas the fasting insulin, HOMA‐IR and HOMA‐β DI values were significantly higher in the girls (P < 0.001, P = 0.002, P < 0.001 and P < 0.001, respectively; Table 1).
Table 1.
Participant characteristics
| Boys | Girls | P‐value | |
|---|---|---|---|
| n | 252 | 193 | |
| BMI (kg/m2) | 19.2 (18.0–20.7) | 20.4 (18.9–22.0) | <0.001 |
| Fasting PG (mg/dL) | 93 (88–98) | 90 (86–94) | <0.001 |
| Fasting IRI (μIU/mL) | 5.4 (3.5–7.5) | 6.4 (4.7–8.9) | <0.001 |
| 5th, 10th, 90th, 95th percentile | 1.7, 2.2, 10.0, 12.9 | 2.0, 3.1, 12.5, 17.2 | |
| HOMA‐IR | 1.2 (0.8–1.7) | 1.5 (1.0–2.0) | 0.002 |
| 5th, 10th, 90th, 95th percentile | 0.4, 0.5, 2.3, 3.0 | 0.5, 0.7, 2.8, 3.8 | |
| HOMA‐β | 64 (44–93) | 86 (63–120) | <0.001 |
| 5,10, 90, 95 percentile | 0.4, 0.5, 2.3, 3.0 | 0.5, 0.7, 2.8, 3.8 | |
| Disposition index | 52 (43–64) | 60 (50–74) | <0.001 |
| 5,10, 90, 95 percentile | 20, 29, 127, 163 | 25, 44, 177, 241 | |
| Obesity | 25 (9.9) | 21 (10.7) | 0.787 |
| HOMA‐IR ≥2.5 | 22 (8.7) | 30 (15.5) | 0.027 |
| HOMA‐β <40 | 52 (20.6) | 17 (8.8) | 0.001 |
| Obesity + IR | 6 (2.4) | 9 (4.7) | 0.186 |
| Obesity + ID | 1 (0.4) | 1 (0.5) | 1 |
| IR + ID | 0 (0.0) | 0 (0.0) | – |
Figures in parentheses are interquartile ranges (25–75th percentiles) or %. All P‐values were calculated using the Mann–Whitney test, and all proportions were tested for significance by using χ2‐test or Fisher's exact probability test. HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; ID, homeostasis model assessment of β‐cell function <40; IR, homeostasis model assessment of insulin resistance <2.5; IRI, immunoreactive insulin; PG, plasma glucose.
Those with IR accounted for a significantly higher proportion of the girls (15.5%; n = 30) than the boys (8.7%; n = 22; P = 0.027); those with obesity accounted for 9.9% of the boys (n = 25) and 10.7% of the girls (n = 21); and those with IR and obesity accounted for 2.4% of the boys (n = 6) and 4.7% of the girls (n = 9), respectively.
In contrast, those with ID accounted for a significantly higher proportion of the boys than the girls (20.6 vs 8.8%; P = 0.001). Whereas those with ID and obesity accounted for a very small proportion of either group (boys/girls, 0.4% [n = 1]/0.5% [n = 1]; Table 1 and Figure 1). None of the participants had both IR and ID in the studied population.
Figure 1.
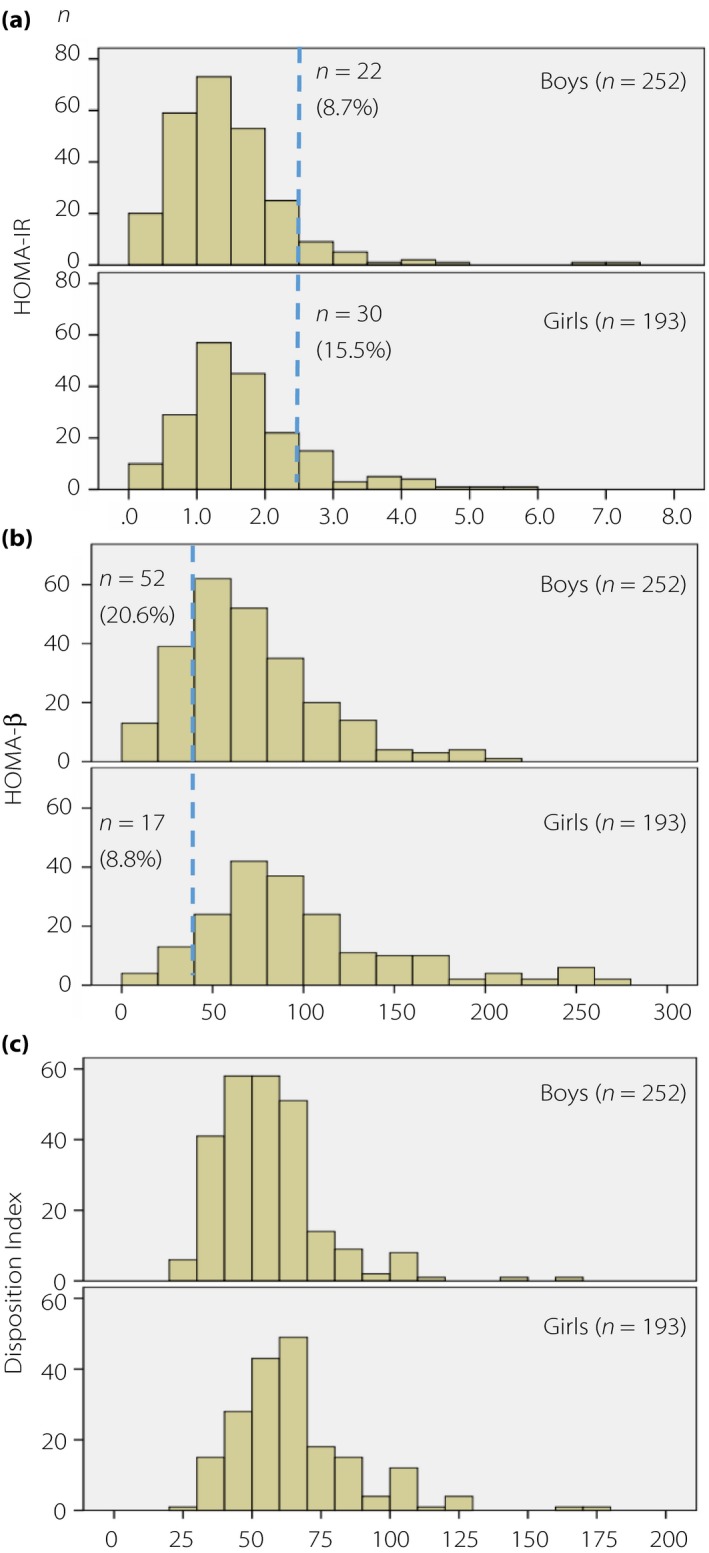
Distribution of (a) homeostasis model assessment of insulin resistance (HOMA‐IR) values and the proportion of participants with HOMA‐IR ≥2.5; (b) homeostasis model assessment of β‐cell function (HOMA‐β) values and proportion of participants with HOMA‐β <40; and (c) Disposition Index values by sex.
Additionally, the correlation between HOMA‐IR and BMI values was shown to be significant among the boys (r = 0.207; P = 0.001), but non‐significant among the girls (r = 0.132; P = 0.067). The correlation between HOMA‐β and BMI values was shown to be significant both among the boys (r = 0.229; P < 0.001) and girls (r = 0.170; P = 0.018), whereas the correlation was stronger among the boys. No significant correlation was observed between DI and BMI in either sex (Figure 2).
Figure 2.
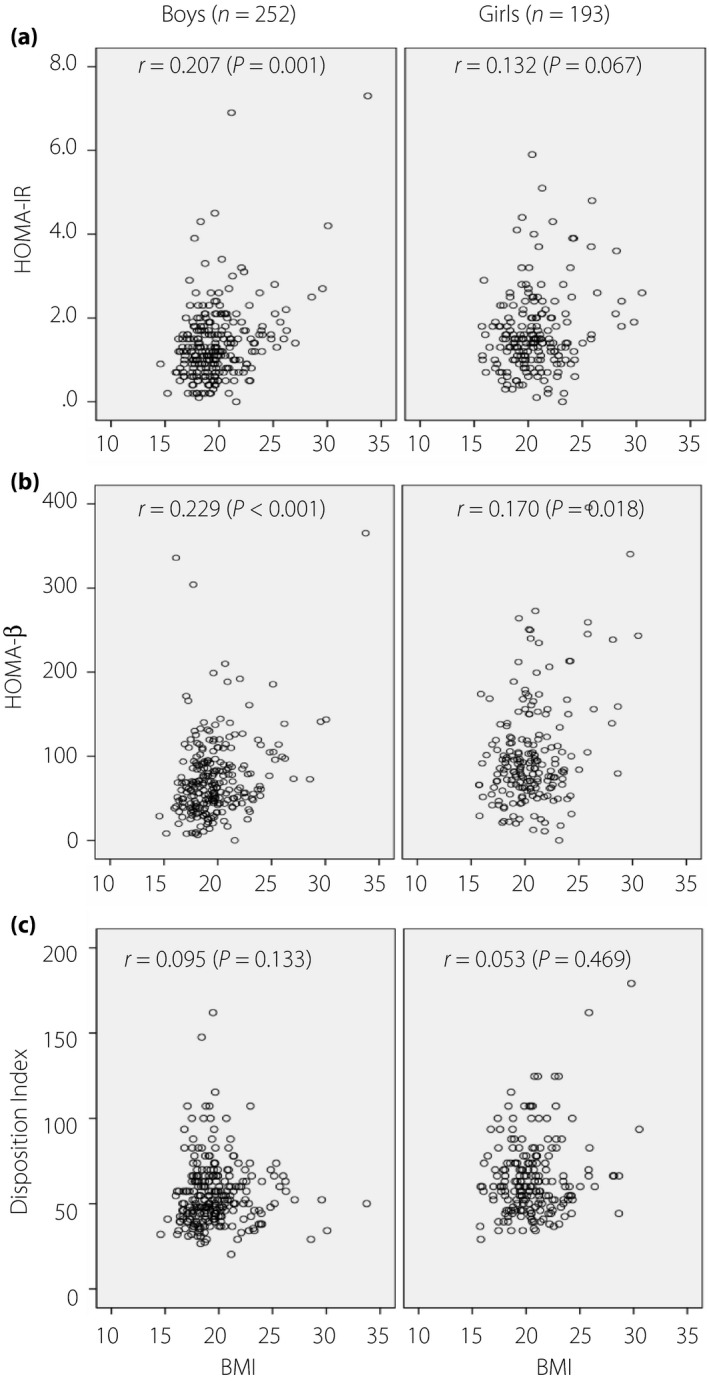
Correlation between (a) homeostasis model assessment of insulin resistance (HOMA‐IR) values and body mass index (BMI), between (b) homeostasis model assessment of β‐cell function (HOMA‐β) values and BMI, and between (c) Disposition Index values and BMI by sex. r, Spearman's rank correlation coefficient.
Discussion
In the present study involving nearly all ninth‐graders from a typically rural area in Japan, obesity was reported in approximately 10% of the boys and girls, whereas the diagnosis of obesity was shown to be not always consistent with the presence of IR in these children.
The present study also showed that increased BMI was significantly correlated with increased IR in boys, suggesting that increased BMI might signal the presence of insulin resistance among boys, but not among girls.
Therefore, these findings suggest that: (i) increases in BMI could have far more diverse implications for girls than for boys (e.g., sites of fat accumulation can vary in a subpopulation of girls); and (ii) IR might be present in a subpopulation of girls without increased BMI, as clearly shown in the present study, which demonstrated that IR was seen in only half of the girls who met the diagnosis of obesity.
In a study of 7,074 non‐obese European children aged 3–11 years, Peplies et al.6 reported that the median HOMA‐IR value was 1.39 and 1.70 in the boys and girls, respectively, values that are closely consistent with those reported in the present study (1.2 and 1.5). However, given that the HOMA‐IR was shown to increase with increasing age in the Caucasian children6, 7, 8, 9, 10, 11, an age‐matched comparison might show that HOMA‐IR values might be lower in Japanese children.
A report from Korea based on the Korea National Health and Nutrition Examination Survey showed that the median HOMA‐IR value for ages 14–15 years was 2.70 and 2.60 in boys and girls, respectively, which are much higher compared with those reported in the present study (1.2 and 1.5).
Regarding the HOMA‐IR cut‐off values used for determining IR, the upper 95th percentiles have been shown in previous studies to be 3.38 in boys and 4.25 in girls in the 10.5‐ to <11‐year‐old group6; 3.35 in boys and 3.20 in girls in 14‐year‐old Mexican adolescents10; and 5.01 in boys and 4.15 in girls in a 14–15‐year‐old Korean cohort12. The results of the current study, 3.0 in boys and 3.8 in girls, are similar to previous reports, other than those from Korea. Therefore, further Asian studies are required to establish a database, and to determine the cut‐off values for HOMA‐IR in children and adolescents.
ID was shown to be more frequent among boys than girls in the present study. Given that type 2 diabetes is clearly more frequent among males worldwide16, and this is also true in Japan17, it could be that some determinants of epidemiological findings; for example, higher susceptibility to ID among males, might have been present and observable in our adolescent population.
Of note, the aforementioned European study6 also showed that insulin values were consistently lower in boys than in girls, across all age brackets, which the authors concluded might have been related to the onset of puberty in boys vs girls.
Furthermore, significant correlation was seen between ID and obesity in the children of the present study, whereas it was also shown that the lower the BMI, the lower the insulin‐secretory capacity tended to be. It is known that there exists a sizable population of non‐obese type 2 diabetes patients in Japan18. Therefore, it remains to be clarified in long‐term studies whether or not the presence of ID in early life might predict the onset of diabetes in later years. Again, little has been reported regarding insulin secretory capacity evaluated by HOMA‐β13, or especially by Disposition Index, in childhood and adolescent populations; in‐depth and long‐term follow up of the children in the present study is warranted to account for the sex differences in ID observed in this study.
A limitation of the present study was that it was an observational, cross‐sectional study, and therefore a prospective study is warranted to follow up on changes in IR and ID among the study participants. As 90% of the participants of the current study were non‐obese, therefore, HOMA‐IR in this population might not represent true insulin resistance. Insulin resistance is related to the levels of physical activity. Unfortunately, information regarding physical activity was not available in the current study.
In conclusion, in this study involving nearly all ninth‐graders dwelling in one of Japan's foremost agricultural areas, both HOMA‐IR and HOMA‐β values were shown to be higher among girls, whereas the presence of IR was not related with obesity in this group.
In contrast, ID was shown to be more frequent among boys, with a significant and strong correlation seen between ID and lower BMI values in this group. Again, this appears to warrant long‐term follow up of those with ID to clarify whether they might constitute a high‐risk group for future onset of diabetes.
Disclosure
RN received a speaker's fee from Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd., Eli Lily Japan K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd. and Sanofi K.K. NT received a speaker's fee from Astellas Pharma Inc., MSD K.K., Kissei Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Ltd., Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd. and Novo Nordisk Pharma Ltd. KU received research support from Kyowa, Ono and Taishyo, and has participated in speakers bureau/advisory panels for Astellas, Astra Zeneca and Sanofi. All other authors declare no conflict of interest.
Acknowledgments
We thank all study participants; their parents or guardians; all the members of the Tsunan Town Hall and Tsunan Town Hospital, Niigata Prefecture; Kazuko Takahashi; Ryoichi Fujinoki; and Emiko Fujinoki. This study was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Kiban [c] 23590815 and Kiban [c] 19590600).
J Diabetes Investig 2017; 8: 672–676
References
- 1. Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr 2002; 75: 971–977. [DOI] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Curtin LR, et al Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295: 1549–1555. [DOI] [PubMed] [Google Scholar]
- 3. Matsushita Y, Yoshiike N, Kaneda F, et al Trends in childhood obesity in Japan over the last 25 years from the national nutrition survey. Obes Res 2004; 12: 205–214. [DOI] [PubMed] [Google Scholar]
- 4. Whitaker RC, Wright JA, Pepe MS, et al Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997; 337: 869–873. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ, Osmond C, Forsen TJ, et al Trajectories of growth among children who have coronary events as adults. N Engl J Med 2005; 353: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 6. Peplies J, Jiménez‐Pavón D, Savva SC, et al Percentiles of fasting serum insulin, glucose, HbA1c and HOMA‐IR in pre‐pubertal normal weight European children from the IDEFICS cohort. Int J Obes 2014; 38: S39–S47. [DOI] [PubMed] [Google Scholar]
- 7. Atabek ME, Pirgon O. Assessment of insulin sensitivity from measurements in fasting state and during an oral glucose tolerance test in obese children. J Pediatr Endocrinol Metab 2007; 20: 187–195. [DOI] [PubMed] [Google Scholar]
- 8. Kurtoglu S, Hatipoglu N, Mazicioglu M, et al Insulin resistance in obese children and adolescents: HOMA‐IR cut‐off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol 2010; 2: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allard P, Delvin EE, Paradis G, et al Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin Chem 2003; 49: 644–649. [DOI] [PubMed] [Google Scholar]
- 10. Aradillas‐Garcia C, Rodriguez‐Moran M, Garay‐Sevilla ME, et al Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. Eur J Endocrinol 2012; 166: 301–306. [DOI] [PubMed] [Google Scholar]
- 11. Lee JM, Okumura MJ, Davis MM, et al Prevalence and determinants of insulin resistance among U.S. adolescents: a population‐based study. Diabetes Care 2006; 29: 2427–2432. [DOI] [PubMed] [Google Scholar]
- 12. Yi KH, Hwang JS, Kim EY, et al Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population‐based study. Diabetes Res Clin Pract 2014; 103: 106–113. [DOI] [PubMed] [Google Scholar]
- 13. d'Annunzio G, Vanelli M, Pistorio A, et al Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed 2009; 80: 21–28. [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 15. Cole TJ, Bellizzi MC, Flegal KM, et al Establishing a standard definition for childhood overweight and obesity worldwide: international survey. BMJ 2000; 320: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Diabetes Federation . Available from: http://www.diabetesatlas.org/ Accessed February 28, 2017.
- 17. Oya J, Nakagami T, Yamamoto Y, et al Effects of age on insulin resistance and secretion in subjects without diabetes. Intern Med 2014; 53: 941–947. [DOI] [PubMed] [Google Scholar]
- 18. Tajima N, Nishimura R, Izumi K, et al A large‐scale, observational study to investigate the current status of diabetes complications and their prevention in Japan: research outline and baseline data for type 2 diabetes—JDCP study 1. Diabetol Int 2015; 6: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]


