Abstract
Empaglifolzin reduces metabolic derangements such as BP (blood pressure), hyperglycemia, body weight, uric acid, increases Ht (hamtaocrit), keton bodies, and restores altered tubule‐glomerular feedback, thereby protect against diabetes‐induced caidio‐renal injuries.
![]()
Diabetic kidney disease is a leading cause of end‐stage kidney disease requiring renal transplantation or dialysis, in not only Asian countries, but also Western countries. Recent data have also implied that the prevalence of diabetic kidney disease was similar between 1988 and 2014 in cross‐sectional studies of adults aged 20 years or older with diabetes mellitus participating in National Health and Nutrition Examination Surveys1; although newly introduced treatments, such as renin–angiotensin–aldosterone system inhibitors, did affect the prevalence and clinical manifestations of diabetic kidney disease. Furthermore, the prevalence of type 2 diabetes is still increasing strikingly worldwide2, thereby the prevalence of diabetic kidney disease will increase in the future if we are not able to discover new therapeutic strategies for diabetic patients at high risk of the development and progression of kidney disease.
Sodium–glucose cotransporter 2 inhibitors, which have antihyperglycemic action with unique properties to reduce bodyweight, blood pressure and uric acid, have been approved all over the world. In addition, sodium–glucose cotransporter 2 inhibitors dose‐dependently and acutely reduce the estimated glomerular filtration rate (eGFR) by 5 mL/min/1.73 m2 and albuminuria by 30–50%, possibly through restoration of altered tubuloglomerular feedback3.
Surprisingly, the rationale, design and baseline characteristics of a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG‐OUTCOME) showed the superiority of empagliflozin vs placebo in the reduction of a three‐point major adverse cardiovascular event primary end‐point of cardiovascular death, non‐fatal heart attack or non‐fatal stroke. Furthermore, hospitalization for heart failure, cardiovascular death and all‐cause mortality were reduced by 35%, 38% and 32%, respectively4. The underlying mechanisms by which empagliflozin has improved cardiovascular disease in type 2 diabetes patients are as yet still unknown.
As aforementioned, multifactorial improvement, such as blood pressure lowering, body weight reduction, uric acid reduction, plasma volume contraction and natriuresis, could contribute to the striking results of the EMPA‐REG‐OUTCOME trial. In addition, other proposed hypotheses are summarized in Table 1.
Table 1.
Proposed hypothesis by which empagliflozin reduced cardiovascular death, hospitalization for heart failure and all‐cause mortality
| Increased ketone bodies |
| Increased hematocrit |
| Inhibition of adrenergic activity |
| Increased glucagon |
| Renin–angiotensin system‐induced angiotensin II fragments |
| Angiotensin (1–9), angiotensin (1–7) |
| Direct action on sodium–glucose cotransporter 1 in cardiomyocytes |
At baseline, the characteristics were similar between the empagliflozin and placebo groups. Regarding the stage of diabetic kidney disease, 25.5% of patients had eGFR <60 mL/min/1.73 m2, 28.7% had microalbuminuria, 11.0% had macroalbuminuria and 80.7% of patients were treated with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers at baseline in total. Prespecified renal microvascular outcomes provided evidence that the incident and worsening risk of diabetic kidney disease, as defined by the development of macroalbuminuria, a doubling of the serum creatinine level accompanied by eGFR <45 mL/min/1.73 m2, the initiation of renal replacement therapy or death from renal disease, fell by 39% in type 2 diabetes patients treated with empagliflozin as compared with those taking a placebo (P < 0.001)5. Post‐hoc renal composite outcomes defined by a doubling of the serum creatinine level accompanied by eGFR <45 mL/min/1.73 m2, the initiation of renal replacement therapy or death from renal disease fell by 46%, as compared with those taking a placebo (P < 0.001). Although the incidence of microalbuminuria from normoalbuminuria at baseline was similar between the empagliflozin and placebo groups, we now have drugs that not only lower hyperglycemia, but also have an impact on cardiorenal protection in diabetes patients. The study also examined changes in eGFR, and found that the decrease in eGFR was extremely different between the empagliflozin and placebo groups. The initial drop in the eGFR in the empagliflozin group was reversed and remained stable during the study period, whereas the eGFR in the placebo group was continuously decreasing during the study period. From week 4, the annual decrease in eGFR in the empagliflozin group was 0.19 ± 0.11 mL/min/1.73 m2, whereas that in the placebo group was 1.67 ± 0.13 mL/min/1.73 m2 (P < 0.001). Similar results were also reported in diabetes patients treated with canagliflozin or dapagliflozin, as shown in Figure 1.
Figure 1.
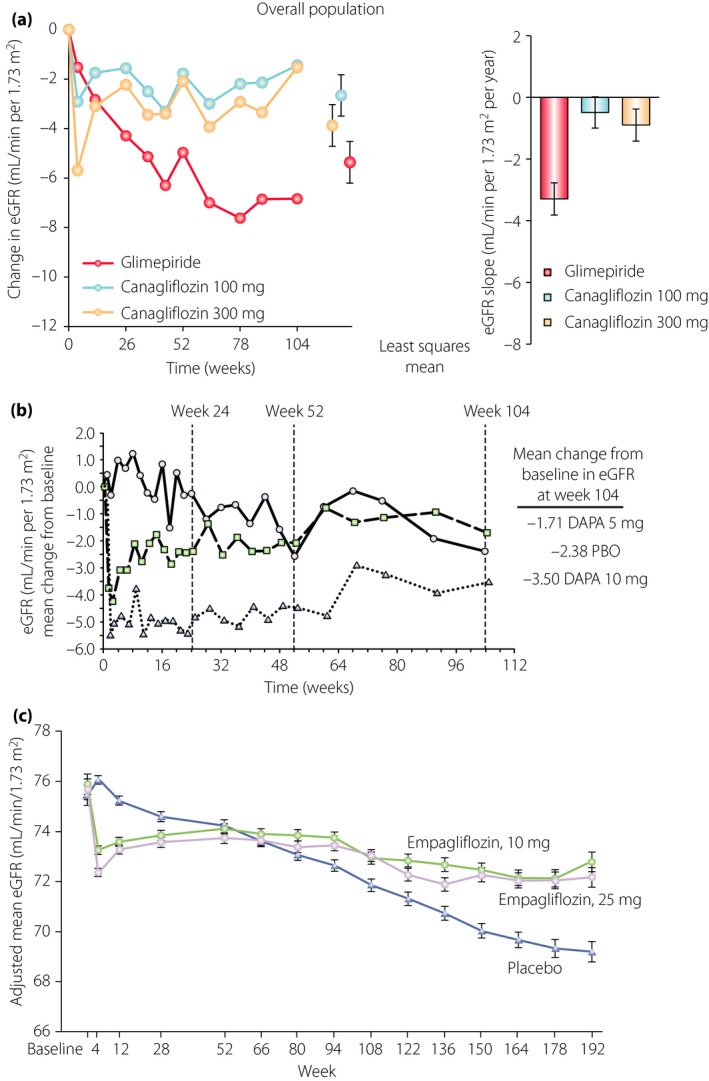
Effects of sodium–glucose cotransporter 2 inhibitors on estimated glomerular filtration rate (eGFR). (a) The effects of canagliflozin (100 mg daily, light blue circles; 300 mg daily, blue circles) vs glimepiride (red circles) in type 2 diabetes patients with preserved renal function (an eGFR of ≥55 mL/min/1.73 m2 or ≥60 mL/min/1.73 m2 if based on restriction of metformin use in local label). The eGFR decline was 2.7 mL/min/1.73 m2/year (95% confidence interval −3.3 to −2.1) in the glimepiride group, 0.1 mL/min/1.73 m2/year (95% confidence interval −0.5 to 0.6) in the canagliflozin 100 mg group (P < 0.001 vs glimepiride) and 0.1 mL/min/1.73 m2/year (95% confidence interval −0.5 to 0.6) in the canagliflozin 300 mg group (P < 0.001 vs glimepiride). Reproduced from Heerspink et al. with permission of Copyright © 2016 from the American Society of Nephrology. (b) Dapagliflozin (DAPA; 5 mg daily, squares; 10 mg daily, triangles) vs placebo (PBO) in type 2 diabetes patients with chronic kidney disease (eGFR values of 30–59 mL/min/1.73 m2.) The eGFR was decreased dose‐dependently at week 1, and remained stable during the study period in the dapagliflozin groups while it was continuously decreased in the placebo group. Reproduced from Kohan et al. with permission from the publisher. Copyright © 2014, International Society of Nephrology. Published by Elsevier Inc. (c) Empagliflozin 10 mg daily and 25 mg daily vs a placebo in type 2 diabeties patients enrolled in a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG‐OUTCOME; (eGFR of at least 30 mL/min/1.73 m2. Patients with eGFR <60 mL/min/1.73 m2 were 25.9%, and those with eGFR >60 mL/min/1.73 m2 were 74.1%). The eGFR was decreased dose‐dependently at week 4 and remained stable during the study period in the empagliflozin groups, whereas it was continuously decreased in the placebo group. Reproduced from Wanner et al. with permission from the publisher. Copyright © 2016 Massachusetts Medical Society.
Are there any special mechanisms by which empagliflozin combats the incident and progression of diabetic kidney disease in addition to the aforementioned hypotheses? One plausible mechanism caused by empaglifolozin treatment is the normalization of tubuloglomerular feedback, which is altered in the diabetic condition, thereby reducing diabetes‐induced glomerular hyperfiltration and higher intraglomerular pressure. Diabetes‐induced glomerular hyperfiltration and higher intraglomerular pressure have been well‐known to damage kidneys, resulting in the development and progression of diabetic kidney disease. There could be another mechanism by which empagliflozin protects against diabetic kidney disease. Our hypothesis is the direct toxicity of reabsorbed glucose and Na+ overload in the proximal tubular cells through sodium–glucose cotransporter 2, thereby causing tubulointersitial fibrosis and nephron loss.
Diabetic kidney disease continues to be a major complication for diabetic patients, and represents the major cause of end‐stage renal disease globally. Although intensified blood glucose and blood pressure control with inhibition of the renin–angiotensin system are critical for reducing albuminuria and preserving or slowing the decline of renal function in diabetes patients, empagliflozin, as mentioned here, sheds light on renoprotection against the development and progression of diabetic kidney disease. Future studies are urgently required to identify which mechanisms are responsible for the cardiorenal protection qualities of empagliflozin.
Disclosure
DK received lecture fees from Boehringer Ingelheim, Eli Lilly and Mitsubishi Tanabe Pharma. Boehringer Ingelheim, Eli Lilly and Mitsubishi Tanabe Pharma donated to Kanazawa Medical University, and were not directly associated with this project. Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Taisho Toyama Pharma, Kirinkyowahakkou Pharma and Ono Pharmaceutical contributed to establishing the Division of Anticipatory Molecular Food Science and Technology.
References
- 1. Afkarian M, Zelnick LR, Hall YN, et al Clinical manifestations of kidney disease among US Adults with diabetes, 1988‐2014. JAMA 2016; 316: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas, 7th edn. Brussels: International Diabetes Federation, 2015. http://www.diabetesatlas.org. [Google Scholar]
- 3. Heerspink HJ, Perkins BA, Fitchett DH, et al Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 5. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]


