Abstract
Peripheral neuropathy is a major cause of disability worldwide. Diabetes is the most common cause of neuropathy, accounting for 50% of cases. Over half of people with diabetes develop neuropathy, and diabetic peripheral neuropathy (DPN) is a major cause of reduced quality of life due to pain, sensory loss, gait instability, fall‐related injury, and foot ulceration and amputation. Most patients with non‐diabetic neuropathy have cryptogenic sensory peripheral neuropathy (CSPN). A growing body of literature links prediabetes, obesity and metabolic syndrome to the risk of both DPN and CSPN. This association might be particularly strong in type 2 diabetes patients. There are no effective medical treatments for CSPN or DPN, and aggressive glycemic control is an effective approach to neuropathy risk reduction only in type 1 diabetes. Several studies suggest lifestyle‐based treatments that integrate dietary counseling with exercise might be a promising therapeutic approach to early DPN in type 2 diabetes and CSPN associated with prediabetes, obesity and metabolic syndrome.
Keywords: Metabolic syndrome, Peripheral neuropathy, Prediabetes
Introduction
Nearly 50% of diabetes patients develop diabetic peripheral neuropathy (DPN), and 20% of type 2 diabetes patients have DPN at diabetes presentation. According to the World Health Organization, the number of people with diabetes has risen from 108 million in 1980 to 422 million in 20141. Peripheral neuropathy is a major cause of disability and reduced quality of life as a result of sensory loss, pain, gait disturbance, fall‐related injury, and foot ulceration and amputation. Globally, healthcare expenditure for diabetes totaled USD 673 billion in 2015, equivalent to 12% of total health spending2. Furthermore, diabetes strikes earlier in India and China as compared with Western Europe and the USA, placing further strain on economies. Despite its prevalence, the pathogenesis of DPN remains unclear, although the mechanisms underlying neuropathy in type 1 diabetes and type 2 diabetes are overlapping but different. A growing literature links obesity and metabolic syndrome (MetS) to neuropathy risk in type 1 diabetes and type 2 diabetes, although this relationship might be particularly robust in the latter. Furthermore, several studies show that patients with MetS and prediabetes have an elevated risk of cryptogenic sensory polyneuropathy (CSPN) before the onset of frank diabetes, and that CSPN patients have an elevated risk of MetS and its component metabolic abnormalities. The goal of the present review is to provide an overview of the relationship between prediabetes, obesity and MetS, and peripheral neuropathy, including a review of disease pathogenesis and potential therapeutic approaches. For the sake of clarity, CSPN associated with MetS, prediabetes and obesity will be referred to as ‘CSPN‐MetS‘ in this review. CSPN‐MetS and early DPN share clinical features, and it is probable that they also share disease mechanisms, and that promising therapies could work for both.
Diagnostic Criteria for Prediabetes and MetS
Prediabetes represents the earliest stage of glucose dysregulation, and precedes the development of overt type 2 diabetes. Prediabetes can be divided into impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). The American Diabetes Association defines prediabetes as a hemoglobin A1c of 5.7–6.4%. Prediabetes can be separated into IFG and IGT using a 2‐h oral glucose tolerance test (OGTT). After measurement of a fasting plasma glucose early in the morning (ideally before 09.00 hours), the patient is given 75 g of oral anhydrous dextrose, and the glucose is repeated 2 h later. A fasting plasma glucose of 100–125 mg/dL (5.6–6.9 mmol/L) defines IFG, and a 2‐h oral glucose of 140–199 mg/dL (7.8–11.0 mmol/L) defines IGT. The diagnosis of diabetes requires a hemoglobin A1c of >6.5%, a fasting glucose >126 mg/dL (7 mmol/L) or a 2‐h glucose of >200 mg/dL (11.1 mmol/L; Table 1). It is worth noting, nevertheless, that the 2‐h OGTT has been noted to have reduced reproducibility in diagnosing patients with IGT (just 49%) as compared with diabetes patients (73%) and normoglycemic patients (93%)4. Repeat testing should be considered to confirm the diagnosis. The prevalence of IFG and IGT varies internationally by ethnicity, with IFG being generally more common among men. It is expected that up to 472 million adults worldwide will have IGT by 2030, with the greatest anticipated rise in southeast Asia and the western Pacific2.
Table 1.
Diagnostic criteria for prediabetes and diabetes3
| Diagnosis | Fasting plasma glucose | 2‐h OGTT | Hemoglobin A1C |
|---|---|---|---|
| Normal | <100 mg/dl (5.6 mmol/L) | <140 mg/dL (7.8 mmol/L) | <5.7% |
| Prediabetes | 100–125 mg/dL (5.6‐6.9 mmol/L) | 140–199 mg/dL (7.8–11 mmol/L) | 5.7–6.4% |
| Diabetes | >126 mg/dL (7 mmol/L) | >200 mg/dL (11.1 mmol/L) | >6.5% |
OGTT, oral glucose tolerance test.
MetS is the aggregation of dyslipidemia, particularly elevated serum triglycerides and reduced high‐density lipoprotein cholesterol, central obesity, insulin resistance (diabetes or prediabetes), and hypertension (Table 2). There are several sets of criteria for MetS, which generally require three of these five criteria be present. MetS is a major worldwide problem, with 22% of the adult USA population fulfilling formal MetS criteria. The prevalence increases with age; over 43% of Americans over the age of 60 years have MetS6. Worldwide, the problem is similarly burdensome, with figures ranging from 20–30% in countries such as India, Iran and Turkey7, to nearly 30% in China8, 9. MetS is associated with an elevated risk of macrovascular outcomes.
Table 2.
Criteria for clinical diagnosis of metabolic syndrome
| Criterion | Definition |
|---|---|
| Abdominal obesity | Waist circumference ≥102 cm (40 in) in men and ≥88 cm (35 in) in women |
| Hypertriglyceridemia | Triglyceride level ≥150 mg/dL (1.7 mmol/L) or taking drug for elevated triglycerides |
| Reduced HDL cholesterol | HDL cholesterol <40 mg/dL (1.03 mmol/L) in men or <50 mg/dL (1.3 mmol/L) in women or taking drug for reduced HDL |
| Hypertension | Systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or taking antihypertensive agent in a patient with history of hypertension |
| Hyperglycemia (insulin resistance) | Fasting glucose ≥100 mg/dL or taking drug for elevated glucose |
The 2005 American Heart Association and National Heart, Lung, Blood Institute Criteria for MetS require three of five specific criterion be fulfilled5. HDL, high‐density lipoprotein.
Clinical Phenotype of Distal Symmetric Peripheral Neuropathy
Peripheral neuropathy is a broad term that refers to damage to various components of the peripheral nerve, extending from the cell body (the dorsal root ganglion or anterior horn cell) to the cell projection itself, with its myelin outer coating and axonal projection. Peripheral neuropathy can involve motor, sensory or autonomic fibers depending on the underlying cause. Diabetes is associated with a wide spectrum of peripheral nerve complications. The most common are distal symmetric polyneuropathy and autonomic neuropathy.
DPN causes a length‐dependent ‘stocking‘ or ‘stocking glove‘ pattern of sensory loss. DPN can affect large‐diameter myelinated A‐beta somatic fibers (‘large fibers‘), which are responsible for joint position, vibration and touch/protective sensation. Clinically, patients whose neuropathy affects large fibers complain of numbness, tingling and often aching discomfort. Gait instability is common, particularly when walking in the dark or on uneven ground. Most patients with DPN have large fiber involvement, particularly those with longstanding neuropathy. Large fiber neuropathy can lead to loss of protective sensation in the absence of sensory symptoms, placing the patient at significant risk of ulceration and ultimately amputation. Nerve conduction studies (NCS) are commonly used to evaluate large fiber neuropathy.
A significant minority of DPN patients has preferential injury to small‐diameter thinly myelinated A‐delta fibers, which carry cold and mechanical pain information, and unmyelinated C somatic fibers, which carry thermal mechanical and chemical pain signals (‘small fibers‘). Patients with small fiber DPN have reduced thermal and pain sensation with relative sparing of vibration and proprioceptive sensation. Neuropathic pain is particularly common, described as burning, stinging, pins and needles, and aching. NCS are frequently normal in small fiber neuropathy. Diagnosis can be confirmed using skin biopsy for assessment of intra‐epidermal nerve fiber density (IENFD; Figure 1)10. Biopsies are typically carried out at the lateral distal leg 10 cm above the medial malleolus and at the proximal thigh level. Reproducibility of IENFD has been established11, and normative values are available12. Skin biopsy is well tolerated, and can be repeated over time to assess neuropathy progression or response to therapy. IENFD is an established surrogate measure for small fiber injury that is commonly used in natural history studies and clinical trials. More recently, in vivo confocal corneal microscopy (CCM) has been used to evaluate unmyelinated C fibers on the surface of the cornea (Figure 2)13. CCM estimates of nerve fiber density and nerve fiber length are reproducible14, and might be sensitive to early diabetic neuropathy15.
Figure 1.

(a) Skin biopsy stained with PGP 9.5 shows normal intra‐epidermal nerve fiber density at the distal leg in a control participant. (b) Intra‐epidermal nerve fiber density is significantly reduced in a patient with distal symmetric polyneuropathy and type 2 diabetes mellitus. Images courtesy of the University of Utah Cutaneous Nerve Laboratory.
Figure 2.
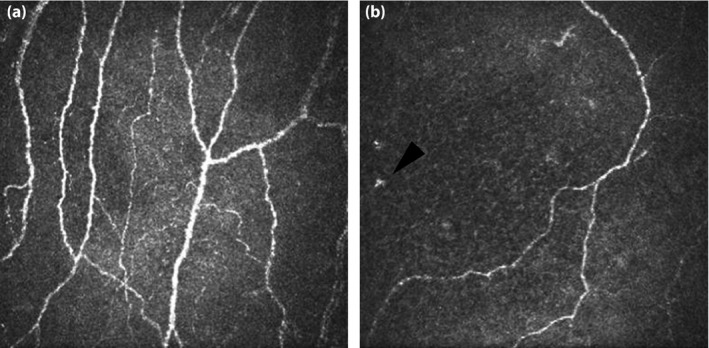
(a) Corneal confocal microscopy from a normal participant shows a normal nerve fiber density of 66.7 fibers/mm2 and nerve fiber length of 37.8 mm/mm2. (b) Images from a patient with distal symmetric polyneuropathy show reduced nerve fiber density (12.0 fibers/mm2) and nerve fiber length (12.0 mm/mm2). There is also proliferation of dendritic cells (Langerhans cells), similar to what is observed in skin biopsies of patients with diabetic neuropathy (arrowhead).
Both CSPN‐MetS and early DPN are associated with preferential injury to small fibers16. In light of the epidemiological data linking MetS to both DPN and CSPN, this clinical similarity suggests a common underlying pathophysiology. Although the reason for early small fiber injury is not fully elucidated, it is likely that epidermal C fibers are particularly prone to injury. Myelination supports a fast conduction velocity (70 m/s), and provides some degree of axonal protection that is lacking in unmyelinated nociceptive axons. Although epidermal C fibers might be particularly prone to metabolic, vascular and mechanical injury, they are uniquely capable of regeneration17. It is this robust regenerative capacity that might ideally suit small fibers to the treacherous distal epidermal environment18. Furthermore, the epidermis is constantly remodeling, necessitating continuous axonal regeneration. This physiology could explain the early small fiber injury in metabolic neuropathies, and it also explains why biomarkers sensitive to small fiber injury, such as IENFD and CCM, might be particularly suitable as diagnostic tests and clinical trial end‐points in this patient population.
Patients with prediabetes and MetS also have an increased risk of developing autonomic neuropathy, particularly cardiovagal dysfunction, even in the absence of frank diabetes19, 20. Investigators have also shown that the presence of early parasympathetic autonomic neuropathy in type 2 diabetes (5 years from diagnosis) was associated with an unfavorable metabolic profile and negative health outcomes21. Autonomic neuropathy can present with varied symptoms including lightheadedness, dyspnea with minimal exertion, gastrointestinal dysmotility and erectile dysfunction. The autonomic reflex screen is used to assess the cardiac adrenergic, cardiac vagal, vascular adrenergic and sympathetic post‐ganglionic sudomotor components of the autonomic nervous system. The Quantitative Sudomotor Axon Reflex Test (QSART) component of the autonomic reflex screen, which evaluates sympathetic post‐ganglionic sudomotor innervation to sweat glands (C autonomic fibers), also serves as a useful surrogate measure of somatic epidermal C fiber function.
The diagnosis of DPN and CSPN‐MetS is based on the recognition of neuropathy symptoms and signs, and might be supported by confirmatory diagnostic testing, as per the Toronto Consensus Criteria22. In addition to a thorough history and examination, a number of validated measures have also been developed to both capture and track neuropathy severity, such as the Utah Early Neuropathy Scale23 and the Michigan Neuropathy Screening Instrument (MNSI)24. Although not all patients with suspected CSPN‐MetS or DPN require NCS, electrodiagnostic testing can be helpful in confirming a diagnosis of large fiber neuropathy25. However, NCS are not sensitive to small fiber neuropathy, and thus cannot be used to exclude early DPN or CSPN‐MetS. When diagnostic confirmation is necessary in this setting, skin biopsy for IENFD assessment is an appropriate test. QSART or other measures of sudomotor function might also be helpful in confirming small fiber injury. CCM is also a promising diagnostic modality, and several ongoing studies are prospectively and rigorously examining its diagnostic utility.
Epidemiology Linking Prediabetes, MetS and CSPN
A growing literature shows that CSPN risk is increased in patients with prediabetes. A large population‐based evaluation of type 2 diabetes patients in the San Luis Valley, Colorado, USA, in 1984–1986 formally assessed the incidence of DSP in prediabetics, diabetics and control participants26. Patients were classified as having either definite or possible neuropathy based on history, deep tendon reflex assessment and temperature sensation. A vibration stimulator was used for independent validation of neuropathy status. A glucose tolerance test was used to confirm glycemic status. Ultimately, 279 diabetics, 89 patients with IGT and 488 controls were enrolled. The results showed an age‐adjusted prevalence of neuropathy of 25.8% in diabetic patients, 11.2% in those with IGT and 3.9% in control participants. More recently, the MONICA/KORA study showed that neuropathy was more common in patients with IGT than controls27. In that population‐based study, 195 patients with physician‐confirmed diabetes or taking antidiabetic medications were compared with 198 controls, who underwent OGTT to confirm their status. Of the 198 controls, 81 were normoglycemic, 71 had IFG and 46 had IGT. Neuropathic pain (evaluated using the MNSI) was detected in 13.3% of diabetes patients, 8.7% of patients with IGT, 4.2% of patients with IFG and 1.2% of patients with normoglycemia, consistent with preferential small fiber involvement in patients with prediabetes. In addition, bodyweight, peripheral arterial disease and age were risk factors for neuropathic pain development amongst diabetes patients. The more recent Prospective Metabolism and Islet Cell Evaluation (PROMISE) study evaluated a longitudinal cohort of 467 patients for peripheral neuropathy as detected by an MNSI >2. Vibration perception thresholds were also incorporated to evaluate for the severity of large fiber dysfunction. Patients were recruited based on risk factors for diabetes, such as family history, hypertension, obesity and gestational diabetes. At 3‐year follow up, patients were tested for diabetes along with other measures of MetS, and evaluated for the presence of neuropathy. The prevalence of neuropathy was highest in patients who, in those 3 years, progressed to diabetes at 3‐year follow up (50%) followed by those who progressed to prediabetes (49%)28. Those with normoglycemia had a lower incidence (29%). Interestingly, the prevalence of neuropathy at 3‐year follow up was comparable between those with baseline prediabetes and diabetes (49% for both) when progression status was excluded. Prediabetic patients also had higher MNSI scores and vibration perception thresholds than normoglycemic controls. One case–control study in Olmsted county, Minnesota, USA, however, noted a neuropathy incidence of 2% in both prediabetes and control patients compared with 7.8% in diabetes patients, although that study was limited by failure to include a measure of small fiber neuropathy29.
Parallel literature shows that patients with CSPN have an increased risk for prediabetes. This observation was first brought to attention by clinicians caring for patients with CSPN who observed that patients who did not have diabetes shared many phenotypic characteristics including obesity, hypertension and dyslipidemia. Several studies have shown that patients with CSPN are at higher risk of having IGT and/or MetS. Up to 40–50% of CSPN patients have prediabetes30, 31. In one study31, 73 patients with confirmed CSPN were subjected to an oral glucose tolerance test; 41 (56%) had abnormal glucose metabolism, 15 (21%) of whom had diabetes and 26 (36%) of whom had IGT. Those with IGT had predominantly small fiber involvement, whereas diabetes patients had large fiber involvement.
CSPN patients also have an elevated risk of MetS, independent of glycemic status. MetS has also been shown to accelerate the rate of DPN progression in patients with established diabetes. Among 249 CSPN patients and 709 controls, 55% of CSPN patients fulfilled MetS criteria compared with 34% of control participants (P < 0.001)32. In addition, multivariate analysis showed an increased prevalence of hypertension and obesity in CSPN patients than controls. There was no difference in the prevalence of IFG between patients and controls, although an OGTT was not carried out. Another study of 219 sequential participants with proven CSPN found a significantly elevated risk of MetS compared with published population controls. Interestingly, although 86% of prediabetic patients had MetS, 54% of those without prediabetes had MetS, suggesting an epidemiological relationship independent of glycemic status33. One case–control study identified hypertriglyceridemia as a risk factor for the development of peripheral neuropathy34. Elevated triglycerides have been shown to correlate with loss in sural nerve myelin fiber density in another study35. Elevated serum triglyceride, one of the five features of MetS, has also been shown to be an independent stepwise risk factor for lower extremity amputation among diabetes patients36. MetS and obesity have also been linked to the risk of chemotherapy‐induced peripheral neuropathy37, 38, 39. A prospective study of chemotherapy‐induced peripheral neuropathy in women with invasive breast cancer who received taxane chemotherapy showed a lower incidence of chemotherapy‐induced peripheral neuropathy in patients who engaged in moderate‐to‐vigorous physical activity (low: <2.5 h/week; medium: 2.5–5 h/week; high: >5 h/week)39.
MetS is also a risk factor for DPN among patients with established diabetes. The Utah Diabetic Neuropathy Study enrolled 218 patients with type 2 diabetes without DPN symptoms, or with symptoms of <5 years' duration. Obesity, hypertriglyceridemia and MetS were independent risk factors for early DPN (risk ratios 2.1 [P < 0.03], 2.9 [P < 0.02] and 3.0 [P < 0.004], respectively)40. This finding was independent of glycemic control. Obesity and triglycerides correlated with loss in IENFD (small unmyelinated axons), whereas elevated hemoglobin A1C correlated with motor conduction velocity slowing on NCS (large myelinated axons), suggesting obesity and MetS might preferentially injure small fibers, whereas hyperglycemia might differentially impact large fibers. In a separate Italian study, investigators carried out a nationwide, multicenter, clinic‐based, cross‐sectional survey of type 1 diabetes and type 2 diabetes, evaluating for MetS and diabetes‐related complications, using either the American Heart Association and the National Heart, Lung, and Blood Institute criteria or the International Diabetes Federation criteria to define MetS. Using a multivariate model, MetS was found to be an independent risk factor for DPN in both type 1 diabetes and type 2 diabetes according to both criteria (American Heart Association/National Heart, Lung, and Blood Institute criteria – odds ratios of 1.75 [P = 0.021] for type 1 diabetes and 1.24 [P < 0.0001] for type 2 diabetes; International Diabetes Federation criteria – odds ratios of 1.76 [P = 0.020] for type 1 diabetes and 1.62 [P < 0.0093] for type 2 diabetes)41.
Pathophysiology of CSPN‐MetS
The pathophysiology of neuropathy associated with prediabetes and MetS (CSPN‐MetS) is likely similar to that of the early DPN in type 2 diabetes. Although intensive glycemic control appears to arrest progression in type 1 diabetes, as shown in the Diabetes Control and Complications Trial, the same does not hold true for type 2 diabetes. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD)42 and the Veterans Affairs Diabetes Study43 showed no mitigation of clinically‐defined peripheral neuropathy in patients receiving intensive vs conventional glycemic control. In combination with the epidemiological data suggesting differential risk of small vs large fiber injury in patients with prediabetes and early diabetes, as opposed to those with longstanding type 1 diabetes, these data suggest the pathophysiology of peripheral nerve injury differs between type 1 diabetes and type 2 diabetes. In line with the strong epidemiological evidence linking MetS and CSPN in humans, several animal models have also lent support to this finding, and helped further our understanding of the underlying pathophysiology. Animal models have shown that diet‐induced obesity in normoglycemic animals causes microvascular injury and peripheral nerve dysfunction44. Obesity and insulin resistance result in a self‐reinforcing cascade of metabolic and inflammatory effects ultimately leading to microvascular and peripheral nerve injury (Figure 3). In the setting of decreased skeletal muscle sensitivity to insulin, adipocytes start to take up glucose, which then stimulates the release of free fatty acids and triglycerides45. This, in turn, stimulates hepatic gluconeogenesis, and produces a state of hyperglycemia. Oxidized lipoproteins, especially in the form of low‐density lipoprotein, can bind to extracellular toll‐like receptors and trigger a downstream cascade of additional oxidative stress. Other relevant pathophysiological elements include formation of advanced glycation end‐products and release of a variety of pro‐inflammatory mediators.
Figure 3.
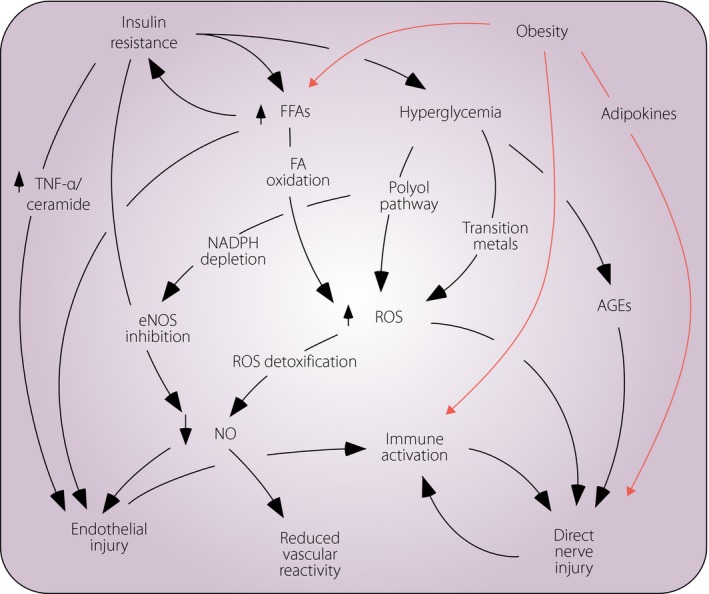
Diabetic neuropathy (DPN) and cryptogenic sensory peripheral neuropathy associated with metabolic syndrome likely share common disease mechanisms. Insulin resistance is a core metabolic feature of type 2 diabetes. Obesity and insulin resistance each lead to overlapping and self‐reinforcing mechanisms that converge on direct axonal injury, as well as endothelial injury and abnormal vascular reactivity, each of which in turn lead to axonal injury. AGEs, advanced glycosylation end‐products FA, fatty acid; FFA, free fatty acids; eNOS, endothelial nitric oxides synthase; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen; NO, nitric oxide; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha.
Several studies suggest that CSPN‐MetS and early DPN might respond to exercise‐based lifestyle modification strategies (see below). Data from an animal model of non‐diabetic obesity also suggest that DPN might respond to exercise. Groover et al. showed that C57BL/6 mice fed a high‐fat diet developed a neuropathy characterized by cutaneous and visceral hypersensitivity with altered cutaneous innervation and neurotrophin expression. Although IENFD did not differ between high‐fat fed mice and controls, those fed the high‐fat diet had increased nerve growth factor expression and upregulation of its high‐affinity receptor, TrKA, on epidermal axons, which correlated with the development of mechanical allodynia. After baseline evaluation, high‐fat fed mice and controls were separated into sedentary or exercise groups (the latter were given a running wheel). The high‐fat fed mice in the exercise group experienced improvement in neuropathy symptoms and normalization of epidermal innervation, whereas sedentary mice did not46. These findings link obesity to neuropathy, imply that alteration of epidermal growth factor biology could contribute to neuropathic pain, and suggest that exercise is a promising therapeutic strategy that, when used early in the disease course, might result in neuropathy improvement, not merely slowed progression.
Pathways for Treatment
MetS, particularly hypertriglyceridemia and obesity, play a central role in peripheral nerve injury as illustrated above. The most obvious therapeutic strategy is treatment of the underlying metabolic changes. As noted above, aggressive treatment of hyperglycemia is not effective in type 2 diabetes, and is thus unlikely to be so in patients with CSPN‐MetS. The timing of treatment is also likely to be important. Once established, neuropathy is difficult to reverse. For example, data from pancreatic transplantation studies among patients with DPN in type 1 diabetes show normalization of blood glucose after transplantation results in DPN stability, but not improvement47. Treatment must extend beyond treatment of hyperglycemia, and should be started as early in the disease course as possible. CSPN‐MetS is often painful, facilitating early recognition. Furthermore, unmyelinated C fibers regenerate with much greater ease than large diameter fibers. Therefore, this population represents an ideal opportunity to explore potential therapeutic approaches.
The effect of treating multiple metabolic risk factors on DPN has been explored. The Steno‐2 study randomized patients with type 2 diabetes to a multifactorial approach addressing hyperglycemia, hypertension, dyslipidemia and smoking cessation or simply to conventional therapy. After 8 years of follow up, patients in the intervention arm had more marked reduction in hemoglobin A1c, systolic and diastolic blood pressure, serum cholesterol and triglycerides, and urine albumin excretion, with a nearly 50% reduction in the risk of cardiovascular and microvascular events. Those in the conventional therapy group were also twice as likely to develop autonomic neuropathy (assessed by examination of heart rate response to deep breathing) compared with those in the treatment cohort48.
An alternative approach to pharmacological treatment of multiple individual risk factors is implementation of lifestyle‐based strategies aimed at improving diet, increasing exercise and reducing weight. The Diabetes Prevention Program randomized 3,234 patients with prediabetes to a placebo, metformin or a lifestyle modification program integrating diet and exercise. The lifestyle intervention reduced the risk of progression to diabetes over an average follow up of 2.8 years by 58% compared with a placebo, compared with just 31% for metformin49. The Impaired Glucose Tolerance Neuropathy Study utilized a similar lifestyle‐based intervention among 32 patients with CSPN and IGT. All 32 participants received counseling with target weight loss of 7% and increased weekly exercise of at least 150 min for 1 year per the Diabetes Prevention Program protocol. Dietary counseling was provided quarterly, fitness was tracked using a 6‐min walk test and participants adjusted their home exercise effort based on a perceived exertion scale. Both objective (QSART and IENFD) and subjective (visual analog pain scale) measures showed that metabolic improvement was associated with small nerve fiber improvement. At baseline, all participants had morphological abnormalities on baseline skin biopsy evaluation, with IENFD reduction seen in 83% of patients. After 1 year of therapy, there was a significant improvement in IENFD and foot sweat volume using QSART. The degree of improvement in IENFD was significantly correlated with improvement in neuropathic pain16.
Reversing established neuropathy is likely to be very difficult. An alternative approach is to target treatment to patients at highest risk for neuropathy development. The Utah Diabetic Neuropathy Study carried out a proof of concept neuropathy prevention trial by randomizing patients with diabetes in the absence of symptoms or signs of DPN to either a lifestyle modification regimen similar to the Impaired Glucose Tolerance Neuropathy program or standard of care counseling. Those in the standard of care group received quarterly sessions addressing diet and carrying out moderate home exercise. The intervention group completed a baseline 3‐day food diary and received individualized counseling on dietary habits, and participated in supervised weekly exercise (30–90 min) with added home exercises, using a balance of aerobic and resistance training. After 1 year, there was a significant improvement in distal leg and proximal thigh IENFD, whereas there was no change in the standard of care group50. Interestingly, there was no difference in glycemic control, although there was a trend towards improvement in high‐density lipoprotein cholesterol. These results support the potential role of diet and exercise in neuropathy prevention, as well as early therapy.
Because the distal axonal environment is constantly remodeling, the beneficial impact of exercise‐based treatments could either be due to a reduction in degenerative pressure or enhancement of axonal regenerative capacity. Nerve regeneration can be studied in vivo using a capsaicin axotomy technique. At low doses with brief exposure, capsaicin (the heat‐inducing ingredient in chili peppers) stimulates TRPV1‐expressing nociceptive axons, resulting in painful sensation. At higher doses, or with more prolonged exposure, capsaicin induces axonal degeneration of these same fibers. Over time, cutaneous axons regenerate. The rate of axonal regeneration can be determined by carrying out a baseline skin biopsy for IENFD determination, then repeating the biopsy 48 h after capsaicin application using an occlusive dressing to show denervation, and again periodically to measure the rate of regeneration (Figure 4). Patients with diabetes have a reduced rate of axon regeneration, which is even further reduced among those with DPN17. This technique may be used to study the impact of experimental treatment by either randomizing patients undergoing the axotomy experiment to receive active drug or placebo, or in a within individual comparison. A prospective trial of lifestyle modification in patients with MetS used the latter technique. A total of 67 patients with MetS (45 of whom had diabetes) without symptoms or signs of CSPN‐MetS or DPN underwent baseline assessment of nerve regenerative capacity. A 3‐mm punch biopsy was obtained from the distal lateral thigh 10‐cm proximal to the patella for IENFD determination. Afterwards, a 5 × 5‐cm gauze bandage containing 1.8 g of 0.1% capsaicin was applied 5‐cm lateral to the biopsy site. The bandage was removed after 48 h, and a repeat biopsy was carried out to document the degree of axonal degeneration. A third biopsy was carried out 1 month later to measure the rate of axonal regeneration. After baseline assessment of the nerve regeneration rate, 36 participants (19 with diabetes) completed a 6‐month regimen of lifestyle counseling and twice‐weekly supervised exercise sessions.
Figure 4.
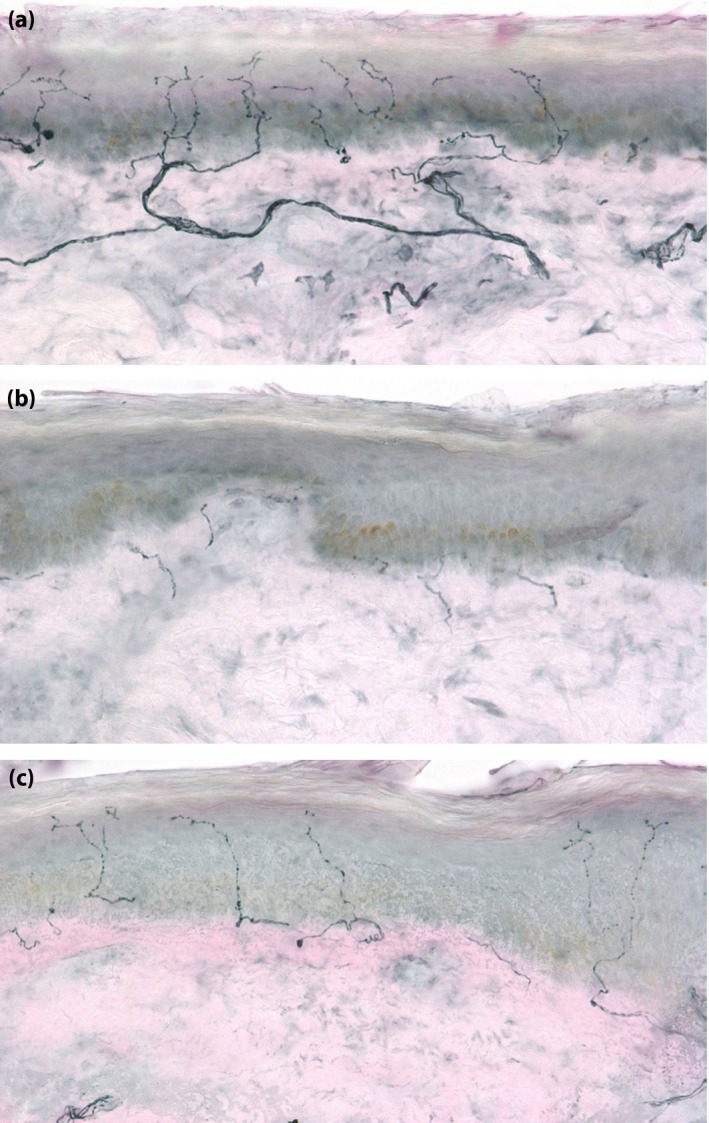
The capsaicin axotomy technique can be used to assess peripheral nerve regenerative capacity in vivo. (a) At baseline, a 3‐mm punch biopsy is obtained from the distal lateral thigh. The baseline intra‐epidermal nerve fiber density is 21.1 fibers/mm (×40 magnification). (b) Topical capsaicin is placed for 48 h and a biopsy is repeated from the area treated. The post‐capsaicin intra‐epidermal nerve fiber density is markedly reduced (1.0 fibers/mm2). (c) One month after capsaicin application, a skin biopsy is repeated adjacent to the prior biopsies, showing significant nerve fiber regeneration (intra‐epidermal nerve fiber density 10.4 fibers/mm).
At baseline, patients with MetS were found to have reduced IENFD and regenerative capacity comparable with patients with diabetes. After the lifestyle modification regimen, there was a significant improvement in axon regeneration rate (from 0.51 to 0.72 fibers/mm, P < 0.002). Those who achieved improvement in more MetS criteria experienced a greater degree of improvement51. These findings suggest that lifestyle modification exerts its positive effect at least in part through improvement in the ability of axons to regenerate.
Despite the remarkable efficacy of intensive exercise therapy, it is difficult to sustain. Patients might compensate for intensive exercise by increasing caloric intake after bouts of exercise or by becoming more sedentary when not exercising52. Sedentary lifestyle is a major source of morbidity and a contributor to all‐cause mortality in the general population. It is associated with increased waist circumference, elevated lipid and cholesterol levels, and decreased glycemic control53, 54. Prolonged sitting contributes to insulin resistance, even in the setting of reduced caloric intake55. Furthermore, the limited use and contraction of postural support muscles also leads to reduced glucose uptake, unbalanced regulation of lipoprotein lipase, increased free fatty acid formation and toxic lipid secretion by the liver56, 57. For this reason, an alternative approach to exercise‐based lifestyle modification is to integrate strategies to reduce sedentary behavior. It is possible that such an integrated approach might be easier for patients to sustain over prolonged periods of time. The Activity for Diabetic Polyneuropathy (ADAPT) Study is an ongoing two‐site, National Institutes of Health‐funded, randomized, single‐blinded controlled trial comparing the efficacy of supervised exercise training and individual dietary counseling coupled with actigraphy‐based counseling to reduce sedentary behavior with standard of care counseling in reducing the rate of progression of symptomatic DPN58. Participants randomized to the intervention group are provided counseling based on 7‐day actigraphy data. On at least a monthly basis, patients receive vibrotactile stimulation from the actigraphy device to remind them to move if they sit or lie down for more than 20 min. IENFD at the distal thigh and the Norfolk Quality of Life‐Diabetic Neuropathy score are co‐primary outcome measures.
Although development of a sustainable behavioral modification approach to DPN and CSPN‐MetS that integrates activity with exercise would be a major advance, not all patients would be able to participate because of medical problems limiting mobility. Therefore, development of a pharmacological approach is a priority. Given the consistent benefit of exercise in DPN and CSPN‐MetS studies, a medical (rather than behavioral) approach to weight loss and improved insulin sensitivity is a promising alternative approach. The anti‐epileptic drug, topiramate, has been shown to improve neuropathy symptoms, quality of life and epidermal innervation in patients with diabetic neuropathy59, 60. Topiramate is consistently associated with a degree of weight loss that parallels that observed in the Impaired Glucose Tolerance Neuropathy Study61. An ongoing National Institutes of Health‐funded multicenter clinical trial aims to determine if topiramate can impact the natural history of CSPN‐MetS (not merely control the symptoms)62. The Topiramate as a Disease Altering Therapy for Cryptogenic Sensory Neuropathy (TopCSPN) Study is randomizing patients with CSPN‐MetS and obesity to 100 mg daily of topiramate or a matched placebo. The co‐primary outcome measures are IENFD and the Norfolk Quality of Life – Diabetic Neuropathy scale. The study will be considered positive if there is treatment efficacy for one outcome measure, and non‐inferiority in the other. The TopCSPN study will be the first major clinical trial of an agent intended to alter the natural history of CSPN. With ongoing therapeutic development for DPN, it is hoped that additional therapeutic strategies can be identified for future study in patients with early DPN and CSPN‐MetS.
Conclusion
The initial observation of an epidemiological link between prediabetes and CSPN was controversial. However, there can be no doubt about the association between MetS and CSPN. Patients with MetS and prediabetes are at elevated risk for CSPN, and CSPN patients are at increased risk for MetS and prediabetes. Animal models of non‐diabetic obesity replicate the human condition, and suggest a common underlying pathophysiology with type 2 diabetes. It has been suggested that CSPN‐MetS and early DPN associated with type 2 diabetes represent a common disorder, which might be termed ‘metabolic neuropathy‘45. Although no pharmacological agent has been found to alter the natural history of DPN or CSPN‐MetS, exercise‐based lifestyle modification regiments have consistently shown promising results, likely because of enhanced peripheral nerve regenerative capacity. Although this approach might not be sustainable for many patients, integrating strategies to reduce sedentary behavior represent a promising alternative approach, as does pharmacological treatment aimed at weight loss and improved insulin sensitivity.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
R01DK064814 (AGS), DP3DK104394 (AGS) and U01NS095388 (AGS) were made possible through the support of the National Institutes of Health (NIH), while ADA 7‐11‐AEC23 (AGS) was made possible through support of the American Diabetes Association (ADA). Both AG Smith and A Stino have no industrial links or affiliations. We thank the University of Utah Cutaneous Nerve Laboratory for its assistance with the preparation of select images for this article.
J Diabetes Investig 2017; 8: 646–655
References
- 1. Global report on diabetes. World Health Organization, Geneva, 2016. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/ Accessed January 3, 2017. [Google Scholar]
- 2. International Diabetes federation . IDF Diabetes Atlas, 5th edn Brussels: International Diabetes Federation, 2011. [Google Scholar]
- 3. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balion CM, Raina PS, Gerstein HC, et al Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin Chem Lab Med 2007; 45: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 5. Grundy SM, Cleeman JI, Daniels SR, et al Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 7. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 2004; 33: 351–375. [DOI] [PubMed] [Google Scholar]
- 8. Xu WH, Ruan XN, Fu XJ, et al Prevalence of the metabolic syndrome in Pudong New Area of Shanghai using three proposed definitions among Chinese adults. BMC Public Health 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuo H, Shi Z, Hu X, et al Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism 2009; 58: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 10. Holland NR, Stocks A, Hauer P, et al Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology 1997; 48: 708–711. [DOI] [PubMed] [Google Scholar]
- 11. Smith AG, Howard JR, Kroll R, et al The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci 2005; 228: 65–69. [DOI] [PubMed] [Google Scholar]
- 12. Lauria G, Bakkers M, Schmitz C, et al Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010; 15: 202–207. [DOI] [PubMed] [Google Scholar]
- 13. Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non‐invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp 2011; 3: e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith AG, Kim G, Porzio M, et al Corneal confocal microscopy is efficient, well‐tolerated, and reproducible. J Peripher Nerv Syst 2013; 18: 54–58. [DOI] [PubMed] [Google Scholar]
- 15. Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. The Lancet 2005; 366: 1340–1343. [DOI] [PubMed] [Google Scholar]
- 16. Smith AG, Russell J, Feldman EL, et al Lifestyle intervention for pre‐diabetic neuropathy. Diabetes Care 2006; 29: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 17. Polydefkis M, Hauer P, Sheth S, et al The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004; 127: 1606–1615. [DOI] [PubMed] [Google Scholar]
- 18. Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia 2008; 56: 1518–1531. [DOI] [PubMed] [Google Scholar]
- 19. Laitinen T, Lindström J, Eriksson J, et al Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance. Diabet Med 2011; 28: 699–704. [DOI] [PubMed] [Google Scholar]
- 20. Stein PK, Barzilay JI, Domitrovich PP, et al The relationship of heart rate and heart rate variability to non‐diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med 2007; 24: 855–863. [DOI] [PubMed] [Google Scholar]
- 21. Gottsäter A, Ahmed M, Fernlund P, et al Autonomic neuropathy in Type 2 diabetic patients is associated with hyperinsulinaemia and hypertriglyceridaemia. Diabet Med 1999; 16: 49–54. [DOI] [PubMed] [Google Scholar]
- 22. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singleton JR, Bixby B, Russell JW, et al The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst 2008; 13: 218–227. [DOI] [PubMed] [Google Scholar]
- 24. Feldman EL, Stevens MJ, Thomas PK, et al A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 25. Smith AG, Singleton JR. Diabetic neuropathy. Continuum 2012; 18: 60–84. [DOI] [PubMed] [Google Scholar]
- 26. Franklin GM, Kahn LB, Baxter J, et al Sensory neuropathy in non‐insulin‐dependent diabetes mellitus The San Luis Valley Diabetes Study. Am J Epidemiol 1990; 131: 633–643. [DOI] [PubMed] [Google Scholar]
- 27. Ziegler D, Rathmann W, Dickhaus T, et al Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009; 10: 393–400. [DOI] [PubMed] [Google Scholar]
- 28. Lee CC, Perkins BA, Kayaniyil S, et al Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Diabetes Care 2015; 38: 793–800. [DOI] [PubMed] [Google Scholar]
- 29. Dyck PJ, Clark VM, Overland CJ, et al Impaired Glycemia and Diabetic Polyneuropathy The OC IG Survey. Diabetes Care 2012; 35: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001; 24: 1229–1331. [DOI] [PubMed] [Google Scholar]
- 31. Sumner CJ, Sheth S, Griffin JW, et al The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003; 60: 108–111. [DOI] [PubMed] [Google Scholar]
- 32. Visser NA, Vrancken AF, Van Der Schouw YT, et al Chronic idiopathic axonal polyneuropathy is associated with the metabolic syndrome. Diabetes Care 2013; 36: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci 2008; 273: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hughes RA, Umapathi T, Gray IA, et al A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004; 127: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 35. Wiggin TD, Sullivan KA, Pop‐Busui R, et al Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009; 58: 1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Callaghan BC, Feldman E, Liu J, et al Triglycerides and Amputation Risk in Patients with Diabetes Ten‐year follow‐up in the DISTANCE study. Diabetes Care 2011; 34: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ottaiano A, Nappi A, Tafuto S, et al Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with Capecitabine and Oxaliplatin adjuvant chemotherapy. Oncology 2016; 90: 36–42. [DOI] [PubMed] [Google Scholar]
- 38. Bao T, Basal C, Seluzicki C, et al Long‐term chemotherapy‐induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016; 159: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenlee H, Hershman DL, Shi Z, et al BMI, lifestyle factors and taxane‐induced neuropathy in breast cancer patients: the Pathways Study. J Natl Cancer Inst 2017; 109: djw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications 2013; 27: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Metascreen Writing Committee . The Metabolic Syndrome Is a Risk Indicator of Microvascular and Macrovascular Complications in Diabetes Results from Metascreen, a multicenter diabetes clinic–based survey. Diabetes Care 2006; 29: 2701–2707. [DOI] [PubMed] [Google Scholar]
- 42. Ismail‐Beigi F, Craven T, Banerji MA, et al Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. The Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duckworth W, Abraira C, Moritz T, et al Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 44. Davidson EP, Coppey LJ, Calcutt NA, et al Diet‐induced obesity in Sprague‐Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Rev 2010; 26: 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Callaghan B, Feldman E. The metabolic syndrome and neuropathy: therapeutic challenges and opportunities. Ann Neurol 2013; 74: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Groover AL, Ryals JM, Guilford BL, et al Exercise‐mediated improvements in painful neuropathy associated with prediabetes in mice. Pain 2013; 154: 2658–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navarro X, Sutherland DE, Kennedy WR. Long‐term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol 1997; 42: 727–736. [DOI] [PubMed] [Google Scholar]
- 48. Gæde P, Vedel P, Larsen N, et al Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393. [DOI] [PubMed] [Google Scholar]
- 49. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 2002: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singleton JR, Marcus RL, Jackson JE, et al Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann Clin Transl Neurol 2014; 1: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singleton JR, Marcus RL, Lessard MK, et al Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol 2015; 77: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Praet SF, Van Rooij ES, Wijtvliet A, et al Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2008; 51: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cooper AR, Sebire S, Montgomery AA, et al Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia 2012; 55: 589–599. [DOI] [PubMed] [Google Scholar]
- 54. Cooper AJ, Brage S, Ekelund U, et al Association between objectively assessed sedentary time and physical activity with metabolic risk factors among people with recently diagnosed type 2 diabetes. Diabetologia 2014; 57: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stephens BR, Granados K, Zderic TW, et al Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 2011; 60: 941–949. [DOI] [PubMed] [Google Scholar]
- 56. Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low‐intensity activity. J Physiol 2003; 551: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007; 56: 2655–2667. [DOI] [PubMed] [Google Scholar]
- 58. Kluding PM, Singleton JR, Pasnoor M, et al Activity for Diabetic Polyneuropathy (ADAPT): Study Design and Protocol for a 2‐Site Randomized Controlled Trial. Phys Ther 2017; 97: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Donofrio PD, Raskin P, Rosenthal NR, et al Safety and effectiveness of topiramate for the management of painful diabetic peripheral neuropathy in an open‐label extension study. Clin Ther 2005; 27: 1420–1431. [DOI] [PubMed] [Google Scholar]
- 60. Boyd AL, Barlow PM, Pittenger GL, et al Topiramate improves neurovascular function, epidermal nerve fiber morphology, and metabolism in patients with type 2 diabetes mellitus. Diabetes Metab Rev 2010; 3: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kramer CK, Leitao CB, Pinto LC, et al Efficacy and safety of topiramate on weight loss: a meta‐analysis of randomized controlled trials. Obes Rev 2011; 12: 338–347. [DOI] [PubMed] [Google Scholar]
- 62. Smith AG. Topiramate for Cryptogenic Sensory Peripheral Neuropathy (TopCSPN). www.clinicaltrials.gov. NCT02878798


