Abstract
Aims/Introduction
There are various causes of incident bone fracture. Not only aging, low bone mineral density and history of previous fracture, but also diabetes mellitus and inflammation are regarded as risk factors for fracture. The purpose of the present study was to verify the association of glycemic control or one inflammatory marker with incident fracture in a large‐scale Japanese cohort.
Materials and Methods
The present study was carried out at the Hiroshima Atomic Bomb Casualty Council and included 6,556 participants (2,785 men and 3,771 women, aged 55–87 years) who underwent annual health examinations and were followed for 7.4 years. Information about incident fractures was collected at interviews. Participants were classified into three groups: normal, borderline and diabetes mellitus according to glycohemoglobin levels (treated diabetes patients were included in the diabetes mellitus group). Furthermore, participants were classified into four additional groups by glycemic control (diabetes mellitus or non‐diabetes mellitus) and C‐reactive protein (CRP) levels (low or high). Hazard ratios (HRs) of diabetes mellitus, CRP and their combined risk of incident fracture were evaluated.
Results
After adjusting for age, bone mineral density and previous fracture, CRP was associated with increased fracture risk (in men HR 1.04, 95% confidence interval [CI]: 1.003–1.06; in women HR 1.07, 95% CI: 1.03–1.13), and diabetes mellitus predicted fracture risk in men (HR 1.31, 95% CI: 1.02–1.51). Fracture risk was significantly higher among the diabetes mellitus with high CRP group compared with the non‐diabetes mellitus with low CRP group (in men HR 1.47, 95% CI: 1.02–1.98; in women HR 1.41, 95% CI: 1.04–1.92).
Conclusions
Among a Japanese cohort, CRP measurements were helpful to detect high fracture risk in patients with type 2 diabetes mellitus.
Keywords: C‐reactive protein, Diabetes mellitus, Incident bone fracture
Introduction
As the population ages, the number of patients with osteoporosis and bone fracture is increasing. Not only factors such as aging, low bone mineral density (BMD) or history of previous fracture, but also those like smoking, over‐consumption of alcohol, family history of fracture and insufficient exercise are considered to increase the risk of incident bone fracture1, 2, 3, 4, 5, 6.
Furthermore, the Westernization of the Japanese lifestyle is the cause of the increasing number of patients with diabetes mellitus. With the aging of society, the number of elderly diabetes mellitus patients is also increasing, and many of them suffer from complications of diabetes mellitus and fracture. According to a meta‐analysis carried out in Europe and the USA, the association between diabetes mellitus and fracture was 6.3‐fold higher in participants with type 1 diabetes mellitus, and 1.7‐fold higher in patients with type 2 diabetes mellitus than that in non‐diabetic participants7. In addition, in a large‐scale trial of an Asian cohort, patients with diabetes mellitus were at higher risk of proximal humeral fracture8, and in a Japanese cohort aged >50 years, the fracture risk in patients with type 2 diabetes mellitus was 4.7‐fold higher in men and 1.9‐fold in women9.
The systemic chronic inflammation affects not only vascular disease, but also bone metabolism. One report showed that high inflammatory markers increased the risk of osteoporosis and frailty fracture in 2,807 normal elderly women in the USA10. In addition, studies in Japan reported that high levels of inflammatory markers in 751 elderly women11 or in a general cohort of 7,283 healthy participants12 represented an increased risk of fracture.
The involvement of chronic inflammation in adipose tissue was suggested to be a cause of metabolic syndrome, and its basis is insulin resistance13. Diabetes mellitus and inflammation are considered to be fracture risks, and we hypothesized that diabetes mellitus patients with high‐level inflammation had a further risk of subsequent incident fracture. The aim of the present study was to evaluate the combined effect of diabetes mellitus and one inflammatory marker (high sensitive C‐reactive protein [hs‐CRP]) on the risk of incident fracture by following a large‐scale longitudinal cohort of approximately 7,200 participants for an average of 7.4 years.
Methods
The participants in the present study were 7,205 persons (3,018 men and 4,187 women, aged 55–96 years) who visited the Health Management & Promotion Center, Hiroshima Atomic Bomb Casualty Council, Hiroshima, Japan, for the purpose of undergoing health examination during the period April 2003 to March 2004. We excluded participants with a CRP value ≥3.0 mg/L or white blood cell count ≥10,000/μL, who were potentially supposed to have active inflammatory diseases. We also excluded from the study the participants already under treatment for osteoporosis, rheumatoid arthritis, collagen diseases, other inflammatory disease, all patients with type 1 diabetes mellitus (diabetes mellitus participants in this study were all diagnosed as type 2), those using corticosteroid, lower CRP drug (e.g., NSAIDs) and hormonal replacement therapy for the treatment of other diseases as far as we could ascertain in the interview (Figure S1).
The study participants answered a questionnaire about their history of previous fracture and lifestyle, and underwent height and weight measurements, physical examination, blood testing, and BMD measurements. The information about incident fragility fractures (spine, hip, proximal humeral and forearm) was collected by self‐report interview forms once a year by trained nurses. The survey on lifestyle included questions about smoking habit, alcohol intake, exercise habit, family history of fracture, history of ischemic heart disease (IHD) or cardiovascular disease (CVD) and history of previous fracture. BMD was measured by dual energy X‐ray absorptiometry method, (QDR4500A; HOLOGIC, Inc., Bedford, Massachusetts, USA) at the lumbar spine (L2–L4). The participants underwent measurements of plasma glucose, glycohemoglobin (HbA1c), serum creatinine, albumin and CRP. HbA1c values were measured by the latex‐aggregation immune nephelometry method (Roche Diagnostics K.K., Tokyo, Japan). Values of CRP were measured by the latex‐aggregation method (Roche Diagnostics K.K.), which is a high‐sensitivity assay technique.
The participants were followed by annual health examinations until the end of March 2014 (mean observation period was 7.4 years). The information about incident fracture and the period from baseline to the onset were recorded by medical interview. Cases of pathological fracture were excluded. The mortality information was ascertained from death certificates. If a participant in the present study had died or had no incident fracture, the observation period was defined as the duration from baseline to the year of death or to the date of participant's last visit for examination.
We classified participants who had not been diagnosed with diabetes mellitus into three groups by their HbA1c levels at baseline (normal: HbA1c ≤ 5.6%; borderline: 5.7% ≤ HbA1c ≤ 6.4%; diabetes mellitus: HbA1c ≥ 6.5%) in accordance with the American Diabetes Association prediabetes recommendation14. All participants already diagnosed and under treatment for diabetes mellitus were included in the diabetes mellitus group regardless of their HbA1c values at baseline. Diagnosis of diabetes mellitus was based on medical history or receiving treatment for diabetes mellitus, or HbA1c ≥ 6.5% and plasma glucose ≥126 mg/dL (7.0 mmol/L) after meal time ≥10 h, or plasma glucose ≥200 mg/dL (11.1 mmol/L) after meal time <10 h. Participants with HbA1c ≥ 6.5%, but with low plasma glucose in this criteria (eight persons), were all patients already receiving treatment for diabetes mellitus. The daily amount of alcohol intake was classified into three groups; non‐drinker, moderate (<29 g/day) and heavy (29 g/day or more)15. The participant's smoking habit, exercise habit, family history of fracture, prevalent IHD or CVD, and previous fracture were classified into two groups (yes or no). To evaluate the combined effect of diabetes mellitus and inflammation, we classified participants into four additional groups based on diabetes mellitus status (presence or absence) and CRP level. The cut‐off value of CRP was determined as 0.63 mg/L, the median value of the study participants (low: 0.02–0.62 mg/L, high: 0.63–3.0 mg/L).
The ethics committee of Hiroshima Atomic Bomb Casualty Council approved the aim and protocol for the study, and all participants completed an informed consent form for the use of their health examination data.
Statistical analysis was carried out using R 3.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Data are expressed as mean ± standard deviation or median (interquartile range) after examination of normal distribution with the Kolmogolov–Smirnov test. Values of hs‐CRP were used after logarithmic transformation in multivariate analysis. BMD comparisons according to HbA1c levels were carried out using one‐way analysis of variance with Bonferroni's method as post‐hoc multiple comparisons. The trend for values of age, spine BMD, estimated glomerular filtration rate (eGFR), albumin and CRP according to HbA1c levels were analyzed by the Jonckheere–Tarpstra test. The hazard ratios (HRs) of conventional risk factors in multivariate analysis or those of diabetes mellitus and CRP classification were assessed by Cox proportional hazards regression analysis. A P‐value of <0.05 was considered to be statistically significant.
Results
The characteristics of the study participants are shown in Table 1. Of the total 7,205 persons eligible for the study, 649 were excluded and 6,556 persons (aged 55–87 years) were ultimately selected for participation in the study (shown in supplementary material). Female participants numbered 3,771 of the total (57.5%). The mean age for men was 67.7 years and for women 68.3 years. The average spine BMD for men was 0.993 g/cm2 and for women 0.805 g/cm2. The percentages of participants categorized as the diabetes mellitus group were 14.8% in men and 10.0% in women. The median value of CRP in men was 0.67 mg/L and in women 0.62 mg/L. The rate of smoking in men was 60.0% and in women 4.1%. The rate of previous fracture in men was 30.4% and in women 25.4%. Over the follow‐up period, there were 179 incident fractures in men (6.4%) and 312 in women (8.3%).
Table 1.
Characteristics of study participants at baseline
| Men | Women | |
|---|---|---|
| n | 2,785 | 3,771 |
| Age (years) | 67.7 ± 6.7 | 68.3 ± 7.5 |
| Age at menopause (years) | – | 49.2 ± 4.8 |
| Observation period (years) | 7.3 ± 0.8 | 7.5 ± 0.7 |
| Spine BMD (g/cm2) | 0.993 ± 0.182 | 0.805 ± 0.159 |
| BMI (kg/m2) | 23.2 ± 2.9 | 22.7 ± 3.3 |
| HbA1c (%) | 5.73 ± 0.79 | 5.74 ± 0.64 |
| Normal/borderline/DM [DM %] | 2,110/262/413 [14.8%] | 3,005/387/379 [10.0%] |
| eGFR (mL/min/1.73 m2) | 54.4 ± 9.2 | 51.7 ± 8.8 |
| Albumin (g/dL) | 4.43 ± 0.21 | 4.48 ± 0.23 |
| CRP (mg/L) | 0.67 (0.38–1.32) | 0.62 (0.38–1.28) |
| Exercise, yes (%) | 1,680 (60.3%) | 2,033 (53.9%) |
| Smoking, yes (%) | 1,670 (60.0%) | 154 (4.1%) |
| Alcohol (non‐drinker/moderate/heavy) | 810/583/1,392 | 2,623/756/392 |
| Family history of fracture, yes (%) | 563 (20.2%) | 1,060 (28.1%) |
| IHD, yes (%) | 78 (2.8%) | 37 (1.0%) |
| CVD, yes (%) | 207 (7.4%) | 176 (4.7%) |
| Previous fracture, yes (%) | 846 (30.4%) | 958 (25.4%) |
| Incident fracture, yes (%) | 179 (6.4%) | 312 (8.3%) |
Data are expressed as mean ± standard deviation or median (interquartile range). Exercise, smoking, family history of fractures, ischemic heart disease (IHD), cardiovascular disease (CVD), and prevalent fracture are expressed as number and percentages of yes. Alcohol intake according daily to amount is expressed as number. BMD, bone mineral density; BMI, body mass index; CRP, C‐reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate.
As for CRP values, there was no significant difference according to generation in all study participants (Figure S2) and eligibly selected participants (Figure S3).
The characteristics according to HbA1c levels at baseline are shown in Table 2. Spine BMD tended to increase according to bad glycemic control. Values of CRP also increased with bad glycemic control (P < 0.05 for trend). As for incidence rates of fracture, there were no significant differences between the three glycemic control groups.
Table 2.
Classification according to glycohemoglobin levels
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Normal | Borderline | DM | Normal | Borderline | DM | |
| HbA1c (%) | 5.4 ± 0.2 | 6.1 ± 0.1 | 7.2 ± 1.3 | 5.5 ± 0.2 | 6.1 ± 0.1 | 7.3 ± 1.2 |
| n | 2,110 | 262 | 413 | 3,005 | 387 | 379 |
| Age (years) | 67.6 ± 6.7 | 68.4 ± 7.2 | 68.2 ± 6.4 | 68.0 ± 7.6 | 69.0 ± 7.4 | 70.1 ± 7.6 |
| Spine BMD (g/cm2)* | 0.981 ± 0.180 | 1.014 ± 0.183 | 1.038 ± 0.182** | 0.803 ± 0.126 | 0.808 ± 0.154 | 0.824 ± 0.152** |
| BMI (kg/m2)* | 23.1 ± 2.8 | 23.4 ± 3.0 | 23.9 ± 2.9 | 22.5 ± 3.2 | 23.6 ± 3.2 | 23.7 ± 3.5 |
| eGFR (mL/min/1.73 m2) | 54.5 ± 9.5 | 53.5 ± 9.0 | 55.1 ± 10.1 | 51.9 ± 8.5 | 51.2 ± 8.2 | 51.3 ± 10.1 |
| Albumin (g/dL)* | 4.43 ± 0.25 | 4.44 ± 0.19 | 4.46 ± 0.21 | 4.47 ± 0.20 | 4.50 ± 0.18 | 4.51 ± 0.24 |
| CRP (mg/L)* | 0.66 (0.47–1.45) | 0.78 (0.55–1.55) | 0.89 (0.38–2.29) | 0.62 (0.42–1.65) | 0.77 (0.55–1.54) | 0.90 (0.37–2.19) |
| Previous fracture (%) | 30.9 | 23.9 | 32.0 | 25.9 | 21.0 | 25.1 |
| Incident fracture (%) | 6.6 | 4.6 | 6.9 | 8.8 | 5.7 | 6.8 |
Data are expressed as mean ± standard deviation or median (interquartile range) unless otherwise indicated. *P < 0.05 for trend for glycemic control in both men and women. **P < 0.05 vs normal by using one‐way analysis of variance with Bonferroni's method. BMD, bone mineral density; BMI, body mass index; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate.
HRs for incident fracture after adjustment for multivariate factors are shown in Table 3. In both men and women, aging, low BMD, previous fracture and high CRP level had a significant association with fracture. Furthermore, in men, diabetes mellitus was a significant risk factor after adjusting for multivariate factors (age, BMD, CRP, eGFR, albumin, exercise, smoking, alcohol, family history of fracture, IHD, CVD and previous fracture). Female smokers had a higher risk of fracture.
Table 3.
Hazard ratios for incident fracture
| Variables | Men | Women | ||
|---|---|---|---|---|
| Age (+1 year) | 1.03 | (1.01–1.05)* | 1.05 | (1.01–1.07)* |
| Spine BMD (−0.1 g/cm2) | 1.14 | (1.07–1.23)* | 1.14 | (1.06–1.18)* |
| HbA1c | ||||
| Normal vs Borderline | 1.05 | (0.75–1.41) | 1.01 | (0.54–1.28) |
| Normal vs DM | 1.31 | (1.02–1.51)* | 1.14 | (0.82–1.25) |
| eGFR (+1.0 mL/min/1.73 m2) | 0.99 | (0.97–1.03) | 0.97 | (0.94–1.04) |
| Albumin (+1.0 g/dL) | 0.63 | (0.33–1.42) | 0.76 | (0.40–1.40) |
| CRP (+1.0 mg/L) | 1.04 | (1.003–1.06)* | 1.07 | (1.03–1.13)* |
| Exercise (yes/no) | 0.70 | (0.59–1.25) | 0.99 | (0.85–1.46) |
| Smoking (yes/no) | 1.34 | (0.81–1.54) | 1.80 | (1.15–2.43)** |
| Alcohol | ||||
| Non‐drinker vs Moderate | 0.76 | (0.54–1.37) | 0.97 | (0.80–1.60) |
| Non‐drinker vs Heavy | 1.02 | (0.63–1.44) | 1.02 | (0.43–1.43) |
| Family history of fracture (yes/no) | 1.14 | (0.80–1.68) | 1.53 | (0.94–1.70) |
| IHD (yes/no) | 0.94 | (0.49–1.62) | 0.99 | (0.55–1.47) |
| CVD (yes/no) | 1.10 | (0.82–1.52) | 1.33 | (0.82–1.51) |
| Previous fracture (yes/no) | 1.72 | (1.51–3.82)** | 2.90 | (2.20–5.65)** |
Data are expressed as hazard ratio (95% confidence interval) evaluated by Cox proportional hazards regression analysis after adjustment for multivariate factors shown above. C‐reactive protein (CRP) value was normalized by logarithmic conversion before analysis. *P < 0.05, **P < 0.01. BMD, bone mineral density; BMI, body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IHD, ischemic heart disease.
In Figure 1, HRs for incident fracture of the four groups categorized by diabetes mellitus (presence or absence) and CRP levels (low or high) are shown. After adjustment was made for multivariate factors (age, BMD, eGFR, albumin, exercise, smoking, alcohol, family history of fracture, IHD, CVD and previous fracture), the non‐diabetes mellitus with high CRP group had a fracture risk of 1.11‐fold in men and 1.16‐fold in women compared with the non‐diabetes mellitus with low CRP group (reference), a marginally significant difference (P = 0.06 for men, and 0.08 for women). In addition, even with diabetes mellitus, fracture incidence in the low CRP group was not statistically significant, but the fracture risk of the diabetes mellitus with high CRP group was 1.47‐fold in men and 1.41‐fold in women, significantly higher than those of the reference group.
Figure 1.
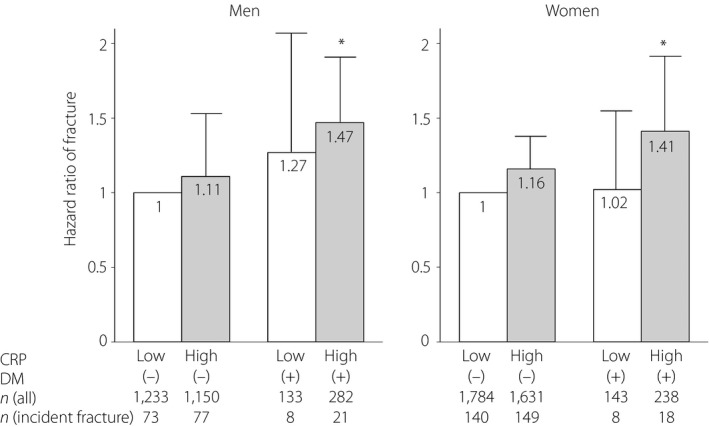
Hazard ratio of fracture according to the presence of diabetes mellitus (DM) and C‐reactive protein (CRP) level in men and women. Hazard ratios were evaluated by Cox proportional hazards regression analysis after adjustment for age, bone mineral density, estimated glomerular filtration rate, albumin, exercise, smoking, alcohol, family history of fracture, ischemic heart disease, cerebrovascular disease and previous fracture. White and gray bars represent low CRP and high CRP respectively. Error bars represent 95% confidence interval of hazard ratios compared with the reference group. *P < 0.05 vs DM(–) with low CRP (reference group).
Discussion
To our knowledge, this is the first report showing that a combination of type 2 diabetes mellitus and a high inflammatory marker (hs‐CRP) had a significant association with the incidence rates of bone fracture among a large‐scale Japanese cohort. In the present study, in both men and women, fracture risk was associated with aging, low BMD, previous fracture and CRP. Male participants with type 2 diabetes mellitus had a high risk of incident fracture after adjustment for other confounders. Participants with type 2 diabetes mellitus and high CRP level had a significantly increased risk of incident fracture in both men and women.
CRP was a significant risk factor of incident fracture in both men and women. Some reports show CRP and other inflammatory markers are associated with not only atherosclerotic diseases, but also bone fracture11, 12. In animal studies, inflammatory cytokines, such as tumor necrosis factor‐α and interleukin‐1, were proven to increase bone resorption by activating osteoclasts16, 17. Kami et al.18 investigated the effect of inflammatory markers on hip fracture in a cohort of 1,171 Caucasian elderly women, and showed that a high value of soluble tumor necrosis factor‐α and interleukin‐6 receptors increased fracture risk dependent on their concentration.
After adjusting for multivariate risk factors, type 2 diabetes mellitus represents a significant fracture risk in men, but not in women. The reason for this result was unclear. To verify the statistical difference by sex, we carried out an additional analysis to assess an interaction for sex and diabetes mellitus. As a result, we found there was no significant interaction in these 6,556 participants. Another larger sample size study might be effective to assert that there was truly a difference between men and women.
After dividing the participants into diabetes mellitus and non‐diabetes mellitus groups, the effect of inflammation on bone fracture was not significant in the non‐diabetes mellitus group. Even in the diabetes mellitus group, low levels of inflammation did not result in a significant risk for fracture. However, the diabetes mellitus and high levels of inflammation group had a significant risk of fracture. In this cohort, CRP values were not different according to generation (Figure S2 and S3). These results suggest that, in type 2 diabetes mellitus patients with high CRP, the combined effects of high blood glucose and high level of inflammation brought about increased bone fracture risk.
In the classification according to HbA1c levels, the diabetes mellitus group had a significantly higher BMD level than that of the normal HbA1c group. According to meta‐analysis, BMD decreases in type 1 diabetes mellitus, but increases in type 2 diabetes mellitus19. Insulin is a growth factor similar to insulin‐like growth factor‐1, and receptors of both insulin and insulin‐like growth factor‐1 express in osteoblasts. They promote the proliferation of osteoblasts20. In type 1 diabetes mellitus patients, absolute loss of insulin production and decrease of blood insulin‐like growth factor‐1 level21 are considered as the reasons for BMD decrease. Conversely, in type 2 diabetes mellitus, BMD level is maintained as a result of the obesity and hyperinsulinemia accompanied by insulin resistance22, 23.
BMD explains 70% of bone strength24, and decreased bone quality is considered as another factor. In diabetes mellitus patients, the accumulation of advanced glycation end‐products as a result of constant high blood glucose levels and oxidative stress has been confirmed in bone tissue. Non‐physiological and frail cross‐linking of pentosidine, one type of advanced glycation end‐product, leads to deteriorated bone quality and exacerbated bone frailty25, 26. In another study among patients with type 2 diabetes mellitus using high‐resolution computed tomography technology, the BMD of the cortical bone surface was low in long bones, such as the radius and the tibia, contrary to high BMD in cancellous bone27. Therefore, despite high BMD levels, patients with type 2 diabetes mellitus tend to have weaker bones due to various risk factors. In addition, vision impairment caused by diabetic retinopathy and gait disorder caused by diabetic neuropathy can lead to increased frequency of falls28, 29. These factors are supposed to be one cause of the increased fracture risk in patients with diabetes mellitus.
The participants of the present study included atomic bomb survivors in Hiroshima and Nagasaki, but several reports have suggested that the level of radiation exposure had no significant association with thoracic, lumber spine or hip fracture30, 31, 32, 33.
A large‐scale Japanese cohort participated in the present survey, and they were followed longitudinally (approximately a 7.4‐year observation period), which is the strength of this study. However, the present study had several limitations. We used self‐reported information of fracture by medical interview at every visit, but not morphological examination using X‐ray images. In addition, the association between thiazolidine and fracture risk has been pointed out in Europe, the USA34 and Japan35. However, as we did not completely grasp the information about the use of oral hypoglycemic agents (including thiazolidine), we were unable to adjust for this effect. Additionally, the change of CRP values and the onset of inflammatory disease in the observation period were unknown. We could not thoroughly analyze whether the onset or condition of inflammatory disease had any effect on incident fracture.
In conclusion, the present study of a large‐scale Japanese cohort showed that CRP was a predictive factor in incident bone fracture. Independent of BMD, type 2 diabetes mellitus patients with high CRP were at an approximately 40% higher risk for fracture compared with non‐diabetes mellitus with low CRP participants. These results suggest that measurement of not only BMD but also CRP might detect high fracture risk in patients with type 2 diabetes mellitus.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Selection process of study participants for this study.
Figure S2 | C‐reactive protein, white blood cell levels and inflammatory diseases according to sex or generation in all study participants (n = 7,205).
Figure S3 | C‐reactive protein levels and inflammatory diseases according to sex or generation in all eligible participants (n = 6,556).
Acknowledgments
This study was supported by examinee of annual health examination at the Health Management & Promotion Center, Hiroshima Atomic Bomb Casualty Council. We thank all of the study participants who cooperated in this longitudinal study.
J Diabetes Investig 2017; 8: 709–715
References
- 1. Johnell O, Kanis JA, Oden A, et al Predictive value of BMD for hip and other fractures. J Bone Miner Res 2005; 20: 1185–1194. [DOI] [PubMed] [Google Scholar]
- 2. Klotzbuecher CM, Ross PD, Landsman PB, et al Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000; 15: 721–739. [DOI] [PubMed] [Google Scholar]
- 3. Kanis JA, Johnell O, Oden A, et al Smoking and fracture risk: a meta‐analysis. Osteoporos Int 2005; 16: 155–162. [DOI] [PubMed] [Google Scholar]
- 4. Kanis JA, Johansson H, Johnell O, et al Alcohol intake as a risk factor for fracture. Osteoporos Int 2005; 16: 737–742. [DOI] [PubMed] [Google Scholar]
- 5. Kanis JA, Johansson H, Oden A, et al A family history of fracture and fracture risk: a meta‐analysis. Bone 2004; 35: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 6. Joakimsen RM, Magnus JH, Fønnebø V. Physical activity and predisposition for hip fractures: a review. Osteoporos Int 1997; 7: 503–513. [DOI] [PubMed] [Google Scholar]
- 7. Janghorbani M, Van Dam RM, Willett WC, et al Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- 8. Chen HF, Ho CA, Li CY. Increased risks of hip fracture in diabetic patients of Taiwan: a population‐based study. Diabetes Care 2008; 31: 75–80. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto M, Yamaguchi T, Yamauchi M, et al Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2008; 24: 702–709. [DOI] [PubMed] [Google Scholar]
- 10. Ganesan K, Teklehaimanot S, Tran TH, et al Relationship of C‐reactive protein and bone mineral density in community‐dwelling elderly females. J Natl Med Assoc 2005; 97: 329–333. [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura K, Saito T, Kobayashi R, et al C‐reactive protein predicts incident fracture in community‐dwelling elderly Japanese women: the Muramatsu study. Osteoporos Int 2011; 22: 2145–2150. [DOI] [PubMed] [Google Scholar]
- 12. Tomiyama H, Okazaki R, Koji Y, et al Elevated C‐reactive protein: a common marker for atherosclerotic cardiovascular risk and subclinical stages of pulmonary dysfunction and osteopenia in a healthy population. Atherosclerosis 2005; 178: 187–192. [DOI] [PubMed] [Google Scholar]
- 13. Fuentes E, Fuentes F, Vilahur G, et al Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm 2013; 2013: 136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care 2016; 39 (Suppl 1): S13–S22. [DOI] [PubMed] [Google Scholar]
- 15. Berg KM, Kunins HV, Jackson JL, et al Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008; 121: 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol 2010; 54: 123–132. [DOI] [PubMed] [Google Scholar]
- 17. Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 2005; 208: 207–227. [DOI] [PubMed] [Google Scholar]
- 18. Kami E, Lui LY, Ensrud KE, et al Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res 2014; 29: 2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes‐a meta‐analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 20. Kawaguchi H. Molecular backgrounds of age‐related osteoporosis from mouse genetics approaches. Rev Endocr Metab Disord 2006; 7: 17–22. [DOI] [PubMed] [Google Scholar]
- 21. Palta M, LeCaire TJ, Sadek‐Badawi M, et al The trajectory of IGF‐1 across age and duration of type 1 diabetes. Diabetes Metab Res Rev 2014; 30: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leslie WD, Rubin MR, Schwartz AV, et al Type 2 diabetes and bone. J Bone Miner Res 2012; 27: 2231–2237. [DOI] [PubMed] [Google Scholar]
- 23. Felson DT, Zhang Y, Hannan MT, et al Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 1993; 8: 567–573. [DOI] [PubMed] [Google Scholar]
- 24. Klibanski A, Adams‐Campbell LL, Bassford T, et al (NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy) Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–795.11176917 [Google Scholar]
- 25. Saito M, Fujii K, Mori Y, et al Role of collagen enzymatic and glycation induced cross‐links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 2006; 17: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto M, Yamaguchi T, Yamauchi M, et al Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 27. Burghardt AJ, Issever AS, Schwartz AV, et al High‐resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melton LJ 3rd, Leibson CL, Achenbach SJ, et al Fracture risk in type 2 diabetes: update of a population‐based study. J Bone Miner Res 2008; 23: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwaltz AV, Sellmeyer DE, Ensrud KE, et al Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 2001; 86: 32–38. [DOI] [PubMed] [Google Scholar]
- 30. Fujiwara S, Mizuno S, Ochi Y, et al The incidence of thoracic vertebral fractures in a Japanese population, Hiroshima and Nagasaki, 1958‐86. J Clin Epidemiol 1991; 44: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 31. Ross PD, Fujiwara S, Huang C, et al Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in US. Int J Epidemiol 1995; 24: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 32. Huang C, Ross PD, Fujiwara S, et al Determinants of vertebral fracture prevalence among native Japanese women and women of Japanese descent living in Hawaii. Bone 1996; 18: 437–442. [DOI] [PubMed] [Google Scholar]
- 33. Fujiwara S, Kasagi F, Yamada M, et al Risk factors for hip fracture in a Japanese cohort. J Bone Miner Res 1997; 12: 998–1004. [DOI] [PubMed] [Google Scholar]
- 34. Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fracture in type 2 diabetes: a meta‐analysis. CMAJ 2009; 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanazawa I, Yamaguchi T, Yamamoto M, et al Relationship between treatments with insulin and oral hypoglycemic agents versus the presence of vertebral fractures in type 2 diabetes mellitus. J Bone Miner Metab 2010; 28: 554–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Selection process of study participants for this study.
Figure S2 | C‐reactive protein, white blood cell levels and inflammatory diseases according to sex or generation in all study participants (n = 7,205).
Figure S3 | C‐reactive protein levels and inflammatory diseases according to sex or generation in all eligible participants (n = 6,556).


