Abstract
Background
Fascioliasis is a zoonotic disease caused by Fasciola species. Patient may be asymptomatic or presents with jaundice and biliary colic or right hypochondriac pain due to bile duct obstruction with gastrointestinal symptoms.
Case presentation
We report a case of human fascioliasis in a 45 years old female presented to Tribhuvan University Teaching Hospital (TUTH), Kathmandu, Nepal on August, 2015 with fever, right hypochondriac pain, jaundice and occasional vomiting with anorexia for 4 months whose alkaline phosphatase was elevated and peripheral blood smear revealed eosinophilia. The patient also gives the history of consumption of water-cress. Endoscopic Retrograde Cholagiopancretography (ERCP) showed the presence of a flat worm resembling Fasciola hepatica and stool routine examination revealed ova of F. hepatica. The patient was treated with nitazoxanide by which she got improved. Repeat stool examination 2 weeks after treatment revealed no ova of F. hepatica.
Conclusions
Patient with fascioliasis can be simply diagnosed with stool routine microscopy and treated with nitazoxanide. So patient with right hypochondriac pain, sign and symptoms of obstructive jaundice, eosinophilia and history of water-cress consumption should be suspected for fascioliasis and investigated and treated accordingly.
Keywords: Fasciola hepatica, Fascioliasis, Right hypochondriac pain, TUTH (Tribhuvan University Teaching Hospital), Nepal
Background
Fascioliasis is caused by infection of trematodes belonging to the genus Fasciola (F. hepatica and F. gigantica). Its infection is known to cause bile duct inflammation and biliary obstruction [1, 2]. Fascioliasis can be presented as various clinical manifestation from mild to severe in nature. Patient may be asymptomatic or presents as gastrointestinal symptoms, chronic cholecystitis, cholangitis and liver abscesses which may be accompanied by biliary colic, epigastric pain, jaundice, pruritus and upper right quadrant pain [3]. Patient of fascioliasis often give the history of consumption of water-cress, a water plant which F. hepatica requires for completion of its life cycle. Many other aquatic plants including like water caltrops, water lettuce, mint and parsley have also been associated with completion of the life cycle. Individuals can also be infected by drinking water containing viable metacercariae [2].
Case presentation
We are reporting a case of 45 years old female from Surkhet presented to the medicine department of TUTH on August, 2015 with the complain of fever for 4 months which was irregular in nature, pain in the right hypochondriac region with jaundice and occasional vomiting with anorexia for same duration. She gave the history of drinking local river water with the habit of eating aquatic plant (water-cress), there was no history of consumption of raw fish. She had visited to different health institute of Nepal as well as India for seeking proper treatment, however no proper diagnosis was done and finally she was admitted in department of medicine (gastroenterology) of TUTH, Kathmandu, Nepal. A complete blood count was performed that revealed eosinophilia (Eosinophil’s: 25%, Neutrophils: 53%, Lymphocytes: 18%, Monocytes: 4% and Basophils: 0%). Total leucocytes count was 13,120/μL of blood and platelet count 325,000/μL of blood. Her hemoglobin was reduced to 7% and packed cell volume (PCV) was 22.3%. Her liver function test revealed an elevated alkaline phosphatase level (679 U/L) and gamma-glutamyl transferase (121 U/L) but normal ALT (17 U/L) and AST (10 U/L) with reduced albumin level (22 g/L). So, ERCP was done to evaluate the cause of her right hypochondriac pain, eosinophilia, an elevated alkaline phosphatase level by which an uncommon morphology of an adult worm was seen in common bile duct (Figs. 1, 2).
Fig. 1.
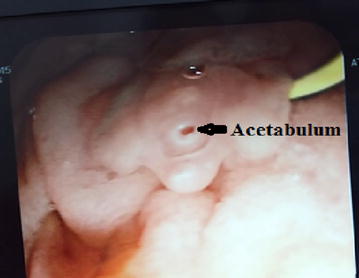
Adult worm of Fasciola hepatica during ERCP
Fig. 2.
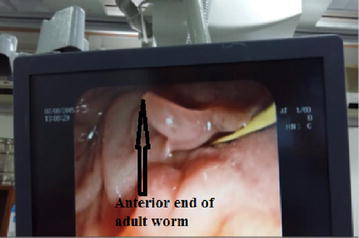
Adult worm of Fasciola hepatica during ERCP
The morphology of the adult worm revealed flat, leaf like measuring approximately 2–2.5 cm in length by 1 cm in width and brown to pale grey in color (Fig. 3). It had a distinct conical projection at the anterior end and broadly pointed posterior end. Patient’s stool sample was collected and processed for routine macroscopic and microscopy examination. For increased yield of ova, stool was concentrated by modified zinc sulphate concentration technique [4] and wet mount was prepared for microscopy. On macroscopic examination of stool, it was yellowish–brown with soft consistency. Microscopic examination of the wet mount of the stool sample showed large, elliptical to oval, operculated, light yellowish brown ova (Fig. 4) measuring 140–142 μm by 70–75 μm (Fig. 5). The size of the detected ova was measured using cell sensation software version 1.12 for DP73 camera installed to the Olympus BX53 microscope used for the microscopy. On the basis of morphological appearance of adult worm and characteristic feature of the detected ova and its measurement, F. hepatica was identified. The photographic evidence of the ova was sent to Centre for Disease Control and prevention (CDC), Atlanta, USA for confirmation and it was confirmed as ova of F. hepatica by CDC, Atlanta. Patient was treated with nitazoxanide 500 mg twice daily for 7 days. On third of treatment she developed high fever due to immunological reaction against the toxic product liberated by the dead worms and was managed with steroids. Follow up stool examination 2 weeks after treatment revealed no ova of F. hepatica.
Fig. 3.
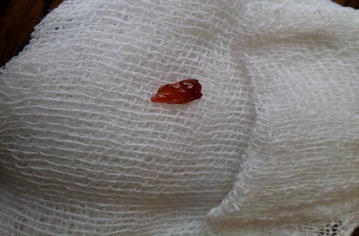
Dead adult worm of Fasciola hepatica
Fig. 4.
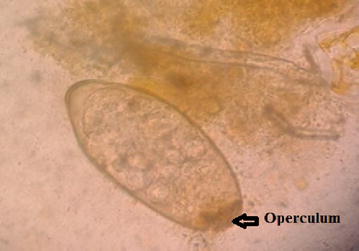
Ova of Fasciola hepatica
Fig. 5.

Measurement of the ova (142 μm by 70 μm) using cell sensation software version 1.12 for DP73 camera installed to the Olympus BX53 microscope used for the microscopy
Discussion and conclusions
To our knowledge, this is the first report of a F. hepatica infection in a human in Nepal, although fascioliasis is regarded as one of the most important platyhelminthic infection of Asia and Africa [5]. Its infection is known to cause biliary tract inflammation and obstruction. Symptoms may include fever, malaise, fatigue, anorexia, weight loss and peripheral eosinophilia. Symptoms may be absent in case of light infection. Infection is more common in indigenous people and farmers who share same water sources with their animals such as sheep, goat and cattle which are the definitive host including humans and also commonly consume fresh-water aquatic plants such as water cresses [5] locally called seem-saag in Nepal that may harbor the encysted cercariae released from the snails [2].
Fasciola hepatica passes its life cycle in two different hosts. Sheep, goat, cattle or man are the definitive host. The adult worms reside in the biliary passage of the definitive host. The eggs are passed out in the faeces of the definitive host which in water develop into a ciliated miracidium that finds its way into the intermediate host, the Lymnaea snails. The miracidium passes through the stages of sporocyst. Two generations of rediae and finally to the stage of cercariae; the whole cycle taking a period of 30–60 days. The mature cercariae escape from the snail into the water and encyst in the blades of grasses or water cress. The encysted cerceriae are swallowed along with the grass and water cress by definitive host including humans. On reaching the duodenum, excystation occurs, migrate through the intestinal wall into the peritoneal cavity, penetrate the capsule of the liver, traverse its parenchyma and settle in the biliary passage. The eggs are passed in the faeces through the bile in 3–4 months of infection.
In fascioliasis, the causative agent could be F. hepatica or F. gigantica. In countries where both species co-exist, size and shape of the eggs passed in the feces are crucial diagnostic feature [5]. Eggs are identified more easily if the specimens are concentrated. Multiple specimens may need to be examined because egg production is relatively low and egg excretion may be intermittent. Negative fecal specimens do not exclude the diagnosis [6]. Serology usually becomes positive during the early phase of migration through the liver, and thus is useful in diagnosing early symptoms prior to the appearance of eggs in the feces. However, although successful treatment often correlates with a decline in antibody titers, antibodies may be detectable for years after infection [7]. So, serodiagnosis may be inconclusive. The differentiation between F. hepatica and F. gigantica infection in humans is very important because of their different transmission and epidemiological characteristics [8]. Species of the freshwater snails from the family Lymnaeidae are well known for their role as intermediate hosts in the life cycle of Fasciola species. The most important intermediate host for F. gigantica is Radix auricularia. However, other species are also known to harbour the fluke including Lymnaea rufescens and L. acuminata in the Indian Subcontinent; Radix rubiginosa and R. natalensis in Malaysia and in Africa respectively. The most important and widespread (Europe, Asia, Africa and North America) intermediate host of F. hepatica is L. truncatula [9]. Fascioliasis has a patchy distribution, with foci being related to the local distribution of intermediate snail host populations in freshwater bodies [10]. The recommended anti-parasitic agent for F. hepatica is triclabendazole 10 mg/kg body weight as a single dose [1]. However, we have treated our patient with nitazoxanide 500 mg twice daily for 7 days as triclabendazole is not available in Nepal and nitazoxanide is an alternative choice [1]. The patient was improved with the treatment and follow up stool routine examination 2 weeks after treatment revealed no ova of F. hepatica. According to Favennec et al., nitazoxanide is well-tolerated and has cure rate of 40% in children and 60 percent in adults [11]. So, treatment should be repeated if radiologic findings or eosinophilia fail to resolve or titers of serologic tests do not decrease.
Parasitic infestations are common in developing countries. However, they are wrongly diagnosed as other medical or surgical conditions and remain untreated for long. Infections like fascioliasis can be diagnosed by simple stool routine microscopy examination and their treatment is simply a short course of anti-helminthic therapy. So, patient with supporting clinical history and clinical findings should be suspected and searched for the evidence of such parasitic infections. Family members of the patient should also be screened for the infection as they may harbor the parasite with or without symptoms since they share the common food and water.
Authors’ contributions
RS and SK made the diagnosis, designed the manuscript, reviewed the literature and prepared the article for submission. MK, DG, JBS, KP, NPS, HPK, BMP and BR helped for literature review, gave concept of research paper and critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thanks Assistant Professor Dr. Sangita Sharma, Assistant Professor Shyam Kumar Mishra, Dr. Mahesh Adhikari, Dr. Neha Shrestha, Shiva Khadka, Ratna Kumari Gurung, Sarmila Tandukar and Dinesh Bhandari for their constant support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data generated or analyzed during this study are included in this published article and remaining are available from the corresponding author on reasonable request.
Consent to publish
Written informed consent was taken from the patient to publish this case report and related photographic evidence.
Ethical approval and consent to participate
There is no need for ethical approval for a case report according to the local ethical guidelines.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- F. hepatica
Fasciola hepatica
- F. gigantica
Fasciola gigantica
- TUTH
Tribhuvan University Teaching Hospital
- ERCP
Endoscopic Retrograde Cholangiopancreatography
- CDC
Centre of Disease Control and Prevention
- USA
United States of America
- µL
microlitre
- mg
milligram
- g/L
gram per litre
- U/L
unit per litre
- PCV
packed cell volume
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
Contributor Information
Ranjit Sah, Email: ranjitsah57@gmail.com, Email: ranjitsah@iom.edu.np.
Shusila Khadka, Email: shushila.mbbs9@gmail.com.
Mohan Khadka, Email: khamohan620@gmail.com.
Dipesh Gurubacharya, Email: dipeshgurubacharya@gmail.com.
Jeevan Bahadur Sherchand, Email: jeevanbsherchand@gmail.com.
Keshab Parajuli, Email: drkparajuli@iom.edu.np.
Niranjan Prasad Shah, Email: niranjshah@yahoo.com.
Hari Prasad Kattel, Email: kattelhari@hotmail.com.
Bharat Mani Pokharel, Email: bmp268@hotmail.com.
Basista Rijal, Email: basistarijal@gmail.com.
References
- 1.Goral V, Senturk S, Mete O, Cicek M, Ebik B, Kaya B. A case of biliary fascioliasis by Fasciola gigantica in Turkey. Korean J Parasitol. 2011;49(1):65–68. doi: 10.3347/kjp.2011.49.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee KD. Parasitology (Protozoology and helminthology) 13th edition. Kolkata. 2014. p. 186–8.
- 3.Arora DR, Arora BB. Medical parasitology, 3rd edition. 2010. p. 158–60.
- 4.Mackie TJ, McCartney JE. Practical medical microbiology, 14th edition. 2006. p. 769.
- 5.Marcilla A, Barques MD, Mas-Coma S. A PCR-RFLP assay for the distinction between Fasciola hepatica and Fasciola gigantica. Mol Cell Probes. 2002;16(5):327–333. doi: 10.1006/mcpr.2002.0429. [DOI] [PubMed] [Google Scholar]
- 6.Jones EA, Kay JM, Milligan HP, Owens D. Massive infection with Fasciola hepatica in man. Am J Med. 1977;63:836. doi: 10.1016/0002-9343(77)90171-1. [DOI] [PubMed] [Google Scholar]
- 7.Santiago N, Hillyer GV. Antibody profiles by EITB and ELISA of cattle and sheep infected with Fasciola hepatica. J Parasitol. 1988;74:810. doi: 10.2307/3282259. [DOI] [PubMed] [Google Scholar]
- 8.McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology. 2006;133:S43–S61. doi: 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- 9.Soliman MFM. Epidemiological review of human and animal fascioliasis in Egypt. J Infect Dev ctries. 2008;2(3):182–189. doi: 10.3855/jidc.260. [DOI] [PubMed] [Google Scholar]
- 10.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ. 1999;77(4):340–346. [PMC free article] [PubMed] [Google Scholar]
- 11.Favennec L, Jave Ortiz J, Gargala G, Lopez Cheqne N, Ayoub A, Rossignol JF. Doule-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adutls and children from northern Peru. Aliment Pharmacol Ther. 2003;17:265. doi: 10.1046/j.1365-2036.2003.01419.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analyzed during this study are included in this published article and remaining are available from the corresponding author on reasonable request.


