Abstract
Purpose of review
The Tube Versus Trabeculectomy (TVT) Study is a multicenter randomized clinical trial comparing the safety and efficacy of tube shunt surgery to trabeculectomy with mitomycin (MMC) in eyes with previous cataract and/or unsuccessful glaucoma surgery. This article reviews published results from the TVT Study.
Recent findings
Tube shunt surgery had a higher success rate than trabeculectomy with MMC during the first 3 years of follow-up. Trabeculectomy with MMC produced greater intraocular pressure (IOP) reduction in the early postoperative period compared with tube shunt placement, but similar IOPs were observed after 3 months. Tube shunt surgery was associated with greater use of adjunctive medical therapy than trabeculectomy with MMC during the first 2 years of the study, but no difference in medication use was seen at 3 years. The incidence of postoperative complications was higher after trabeculectomy with MMC compared to tube shunt surgery, but serious complications associated with vision loss and/or reoperation developed with similar frequency after both surgical procedures. No difference in the rate of vision loss was present following trabeculectomy with MMC and tube shunt surgery after 3 years of follow-up. Cataract progression was common, but occurred with similar frequency with both procedures.
Summary
Intermediate-term results of the TVT Study support the expanded use of tube shunts beyond refractory glaucomas. Tube shunt surgery is an appropriate surgical option in patients who have undergone prior cataract and/or unsuccessful filtering surgery.
Keywords: glaucoma surgery, trabeculectomy, tube shunt
INTRODUCTION
Despite the introduction of several new incisional glaucoma procedures in recent years, trabeculectomy and tube shunt surgery remain the most frequently performed glaucoma operations in the United States. Medicare data show a steady reduction in the number of trabeculectomies performed between 1995 and 2004, and a concurrent rise in tube shunt surgery has occurred [1]. Surveys of the American Glaucoma Society membership also demonstrate a growing preference for tube shunts and a decline in the popularity of trabeculectomy in various clinical settings [2,3]. Concern about bleb-related complications, such as bleb leaks and bleb infections, has likely contributed to the expanded use of tube shunt surgery as an alternative to trabeculectomy. When tube shunts were introduced, they were generally reserved for refractory glaucomas at high risk for failure with filtering surgery. However, an increasingly positive experience with these devices has prompted their implantation in glaucomas with better surgical prognoses. No clear consensus exists among glaucoma surgeons regarding the preferred surgical approach for managing glaucoma in eyes with previous ocular surgery [2,3]. In particular, some surgeons favor an antifibrotic augmented trabeculectomy, while others prefer placement of a tube shunt in eyes with prior cataract or failed filtering surgery.
The Tube Versus Trabeculectomy (TVT) Study is a multicenter randomized clinical trial comparing the safety and efficacy of tube shunt surgery to trabeculectomy with mitomycin C (MMC) in eyes with prior ocular surgery. Patients with uncontrolled glaucoma who had previously undergone cataract extraction with intraocular lens implantation and/or unsucessful trabeculectomy were randomized to receive a 350-mm2 Baerveldt glaucoma implant or trabeculectomy with MMC. The goal of this investigator initiated study is to provide valid evidence-based information that will assist surgical decision making in these patient groups.
STUDY DESIGN
The design and methods of the TVT Study were previously described in detail [4]. The study is registered with http://www.clinicaltrials.gov (NCT00306852). The inclusion and exclusion criteria for the study are listed in Table 1. Enrolled patients were randomly assigned to treatment with a tube shunt or trabeculectomy with MMC. Patients in the tube group underwent placement of a 350-mm2 Baerveldt glaucoma implant in the superotemporal quadrant with a complete restriction of flow at the time of implantation. Patients in the trabeculectomy group had a trabeculectomy superiorly with a standard dosage of MMC of 0.4 mg/ml for 4 minutes. All of the investigators were glaucoma surgeons who were experienced in performing both of the surgical procedures under study. Follow-up visits were scheduled 1 day, 1 week, 1 month, 3 months, 6 months, 1 year, 18 months, 2 years, 3 years, 4 years, and 5 years postoperatively. Each examination included measurement of Snellen visual acuity (VA), intraocular pressure (IOP), slit lamp biomicroscopy, Seidel testing, and ophthalmoscopy. Standard automated perimetry, best-corrected VA on Early Treatment Diabetic Retinopathy Study (ETDRS) charts, and quality of life assessment with the National Eye Institute Questionnaire (NEI VFQ-25) were performed at baseline and at the annual follow-up visits. A formal motility evaluation was performed in all patients at baseline and at the 1-year and 5-year follow-up visits, and at any other visits after 3 months if the patient reported diplopia. The examining clinician provided a reason for loss of two or more lines of Snellen VA at follow-up visits after 3 months.
Table 1.
Patient Eligibility Criteria in the Tube Versus Trabeculectomy (TVT) Study
| Inclusion criteria | Age 18 to 85 years |
| Inadequately controlled glaucoma with IOP > 18 mm Hg and < 40 mm Hg on maximum tolerated medical therapy | |
| Previous cataract extraction with intraocular lens implantation, trabeculectomy, or both | |
| Exclusion criteria | Unwilling or unable to give consent, unwilling to accept randomization, or unable to return for scheduled protocol visits |
| Pregnant or nursing women | |
| No light perception vision | |
| Active iris neovascularization or active proliferative retinopathy | |
| Iridocorneal endothelial syndrome | |
| Epithelial of fibrous downgrowth | |
| Aphakia | |
| Vitreous in the anterior chamber for which a vitrectomy is anticipated | |
| Chronic or recurrent uveitis | |
| Severe posterior blepharitis | |
| Unwilling to discontinue contact lens use after surgery | |
| Previous cyclodestructive procedure, scleral buckling procedure, or silicone oil present | |
| Conjunctival scarring precluding a trabeculectomy superiorly | |
| Need for glaucoma surgery combined with other ocular procedures (i.e. cataract surgery, penetrating keratoplasty, or retinal surgery) or anticipated need for additional ocular surgery |
Failure was prospectively defined as IOP > 21 mm Hg or not reduced by 20% below baseline on two consecutive follow-up visits after 3 months, IOP ≤ 5 mm Hg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision. Reoperation for glaucoma or a complication was defined as additional intervention that required a return to the operating room. Cyclodestruction was also counted as a reoperation for glaucoma, and a vitreous biopsy with injection of intravitreal antibiotics was a reoperation for a complication. Serious complications were defined as surgical complications that were associated with loss of two or more lines of Snellen VA and/or reoperation to manage the complication.
BASELINE CHARACTERISTICS
A total of 212 eyes of 212 patients were enrolled at 17 Clinical Centers, including 107 in the tube group and 105 in the trabeculectomy group [4]. The mean age of the study population was 71.0 years, and 53% were women. The baseline IOP was 25.3 ± 5.3 mm Hg (mean ± SD) on 3.1 ± 1.2 glaucoma medications (mean ± SD), and 81% of patients had primary open-angle glaucoma. Among enrolled patients, 44% had undergone cataract surgery, 35% trabeculectomy, and 20% combined cataract and glaucoma surgery as the qualifying previous ocular surgery for the study. The demographic and ocular characteristics were not significantly different between the tube group and the trabeculectomy group, suggesting that randomization was very effective in generating two balanced treatment groups.
OPERATIVE DATA AND POSTOPERATIVE INTERVENTIONS
All enrolled patients received the assigned surgical treatment. A majority of patients had a fornix-based conjunctival flap in the tube group (77%) and a limbus-based conjunctival flap in the trabeculectomy group (74%) [5]. An external polyglactin ligature was the most common method of tube occlusion (75%), and tube fenestration was frequently performed (77%) for IOP reduction in the early postoperative period.
The most commonly performed postoperative intervention was laser suture lysis (49%) in the trabeculectomy group and rip cord removal (18%) in the tube group [5]. Needling procedures were infrequently performed in the trabeculectomy group (8%) and the tube group (2%) during the first year of follow-up, and were not considered glaucoma reoperations. Several patients in the trabeculectomy group (22%) received adjunctive subconjunctival injections of 5-fluorouracil.
INTRAOCULAR PRESSURE AND MEDICAL THERAPY
Data on IOP and glaucoma medical therapy at baseline and at the annual follow-up visits are presented in Table 2. Patients who underwent additional glaucoma surgery were censored from analysis after reoperation. The trabeculectomy group had significantly lower mean IOPs than the tube group at all follow-up visits during the first 3 months, but no significant difference in the degree of IOP reduction persisted between treatment groups after 3 months [6,7••]. Adjunctive medical therapy use was significantly greater in the tube group compared with the trabeculectomy group at all follow-up visits during the first 2 postoperative years. However, no significant difference in the mean number of medications was present between treatment groups at 3 years [7••].
Table 2.
Intraocular Pressure and Medical Therapy in the Tube Versus Trabeculectomy (TVT) Study
| Tube Group | Trabeculectomy Group | P-value* | |
|---|---|---|---|
|
| |||
| Baseline | |||
| IOP (mm Hg) | 25.1 ± 5.3 | 25.6 ± 5.3 | 0.56 |
| Glaucoma medications | 3.2 ± 1.1 | 3.0 ± 1.2 | 0.17 |
|
| |||
| 1 year | |||
| IOP (mm Hg) | 12.5 ± 3.9 | 12.7 ± 5.8 | 0.73 |
| Glaucoma medications | 1.3 ± 1.3 | 0.5 ± 0.9 | < 0.001 |
|
| |||
| 2 years | |||
| IOP (mm Hg) | 13.4 ± 4.8 | 12.1 ± 5.0 | 0.101 |
| Glaucoma medications | 1.3 ± 1.3 | 0.8 ± 1.2 | 0.016 |
|
| |||
| 3 years | |||
| IOP (mm Hg) | 13.0 ± 4.9 | 13.3 ± 6.8 | 0.78 |
| Glaucoma medications | 1.3 ± 1.3 | 1.0 ± 1.5 | 0.3 |
Data are presented as mean ± standard deviation.
IOP = intraocular pressure
Student t-test
Adapted from [7••].
SURGICAL SUCCESS
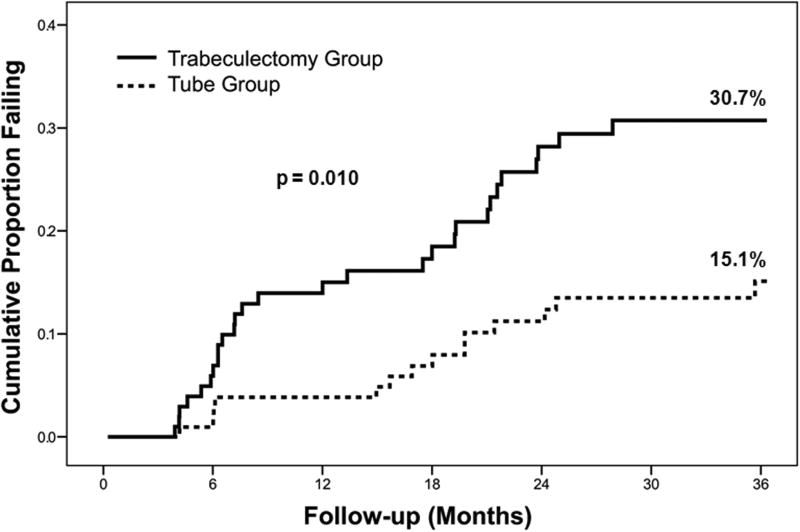
The cumulative probability of failure was 15.1% in the tube group and 30.7% in the trabeculectomy group at 3 years, a difference that was statistically significant (p = 0.010, log rank test adjusted for stratum) [7••]. Kaplan-Meier plots of the probability of failure are shown in Figure 1. The most common cause for failure during the first 3 years of the study was inadequate IOP control (i.e. reoperation for glaucoma or IOP above the failure criteria) in both the tube group (87% of failures) and the trabeculectomy group (68% of failures). Persistent hypotony was a less frequent reason for treatment failure in the tube group (13% of failures) and the trabeculectomy group (29% of failures), and only 1 patient in the trabeculectomy group lost light perception vision. No significant difference in the distribution of reasons for treatment failure was present between treatment groups (p = 0.71, exact permutation chi-square test). A greater proportion of patients in the trabeculectomy group (11%) underwent reoperations for glaucoma compared with the tube group (6%), but this difference did not quite reach the level of statistical significance (p = 0.091, log rank test adjusted for stratum).
Figure 1.
Kaplan-Meier plots of the probability of failure in the Tube Versus Trabeculectomy (TVT) Study. Adapted from [7••].
VISUAL ACUITY
Reduction in VA occurred in both the tube group and the trabeculectomy group during the first 3 years of follow-up, but Snellen and ETDRS VA were similar between treatment groups at 3 years [7••]. The rate of loss of two or more lines of Snellen VA was not significantly different between the tube group (31%) and the trabeculectomy group (34%) after 3 years (p = 0.65, log rank test). Many of the causes of vision loss were not directly attributable to the surgical procedures under study, such as macular degeneration and diabetic retinopathy. However, the proportion of patients who lost two or more Snellen lines was significantly higher for patients who developed postoperative complications (45%) than patients without complications (20%) after 1 year of follow-up (p < 0.001, chi-square test) [5].
SURGICAL COMPLICATIONS
Although a substantial number of surgical complications developed in the TVT Study, most were transient and self-limited. A similar rate of intraoperative complications was observed in the tube group (7%) and trabeculectomy (10%) (p = 0.59, chi-square test) [5]. Significantly more patients in the trabeculectomy group (60%) experienced postoperative complications compared with the tube group (39%) during the first 3 years of follow-up (p = 0.004, chi-square test) [7••]. However, all complications are not equal in severity, and serious complications associated with reoperation to manage the complication and/or loss of two Snellen lines of vision occurred with similar frequency in the trabeculectomy group (27%) and tube group (22%) at 3 years (p = 0.58, chi-square test). Wound leaks (p = 0.004, chi-square test), dysesthesia (p = 0.018, Fischer’s exact test), and bleb leaks (p = 0.028, Fisher’s exact test) were significantly more common in the trabeculectomy group compared with the tube group. New postoperative motility disturbances developed more frequently in the tube group (9.9%) than the trabeculectomy group (0%) during the first year of follow-up (p = 0.005, Fisher’s exact test) [8•], and there was a tendency for diplopia to occur more commonly in the tube group (5%) compared with the trabeculectomy group (0%) (p = 0.06, Fisher’s exact test) [5,7••,8•].
In multivariate risk factor analyses after 1 year of follow-up, persistent corneal edema (p < 0.001, exact permutation logistic regression) and choridal effusion (p = 0.001, exact permutation logistic regression) were significant independent predictors of vision loss [5]. Wound leaks (p = 0.026, exact permutation logistic regression) and hypotony maculopathy (p = 0.049, exact permutation logistic regression) were significantly associated with treatment failure. Shallowing of the anterior chamber significantly increased the risk of cataract progression (p = 0.008, exact permutation logistic regression).
CATARACT PROGRESSION
Cataract progression was common in the TVT Study, but occurred with similar frequency in the tube group (58%) and the trabeculectomy group (57%) during the first 3 years of follow-up (p = 1.00, chi-square test) [7••]. The rate of cataract surgery was not significantly different between the tube group (46%) and the trabeculectomy group (29%) after 3 years (p = 0.38, chi-square test).
CONCLUSIONS
The TVT Study enrolled patients with medically uncontrolled glaucoma who had previous cataract extraction with intraocular lens implantation and/or failed filtering surgery and randomly assigned treatment to trabeculectomy with MMC or tube shunt placement. Patients who were treated with tube shunt surgery were more likely to maintain IOP control and avoid persistent hypotony, loss of light perception vision, or reoperation for glaucoma than trabeculectomy with MMC during the first 3 years of follow-up [7••]. Both surgical procedures were associated with similar IOP reduction and use of supplemental medical therapy at 3 years. The incidence of postoperative complications was higher after trabeculectomy with MMC compared with tube shunt surgery, but serious complications associated with reoperation and/or vision loss occurred with similar frequency with both surgical procedures. The rate of vision loss after trabeculectomy with MMC and tube shunt surgery was not significantly different after 3 years of follow-up. Cataract progression was common, but occurred with similar frequency with both surgical procedures.
The TVT Study challenges several current concepts and recent publications about the safety and efficacy of incisional glaucoma surgery. Based upon a systematic review of the medical literature on tube shunts, a panel of glaucoma specialists concluded that low IOP levels usually cannot be attained with these devices and the IOP typically settles in the high teens postoperatively [9••]. The TVT Study contradicts these opinions, as evidenced by a mean IOP of 13.0 mm Hg and IOP of 14 mm Hg or less in 62% of patient in the tube group at 3 years [7••]. Wilson and associates conducted a randomized clinical trial in Sri Lanka comparing the Ahmed glaucoma valve implant (New World Medical, Inc., Rancho Cucamonga, California) to trabeculectomy with or without an adjunctive antifibrotic agent in patients with primary open angle glaucoma or angle-closure glaucoma and no previous ocular surgery [10]. With an average follow-up of 31 months, success rates were comparable between the trabeculectomy group and the Ahmed group. In contrast, the TVT Study found that tube shunt surgery had a higher success rate compared with trabeculectomy with MMC after 3 years of follow-up [7••]. In a retrospective review of data derived from Medicare claims, Stein and colleagues found higher rates of adverse outcomes after tube shunt surgery than primary trabeculectomy or trabeculectomy with scarring [11•]. The opposite observation was made in the TVT Study, with a higher rate of postoperative complications after trabeculectomy with MMC relative to tube shunt surgery [5,7••]. The differences in results between the TVT Study and other studies likely relates to differences in study populations, implant types, surgical techniques, outcome measures, and study designs. Importantly, the TVT Study excluded several types of refractory glaucomas that have historically undergone tube shunt surgery (e.g., neovascular glaucoma) and included patients at lower risk for failure after trabeculectomy than have traditionally had placement of a tube shunt (e.g., only prior clear cornea cataract extraction).
There are several limitations to the TVT Study. The study enrolled patients who met specific inclusion and exclusion criteria, and the results should not be generalized to different patient groups. An attempt was made to standardize the two glaucoma procedures under investigation, but variations in surgical technique are expected with different surgeons. The low incidence of certain complications and the small size of many patient subgroups limits the power of the study to detect significant differences. There were no standardized definitions or quantification of surgical complications. A limbus-based conjunctival flap was used in most patients randomized to the trabeculectomy group, and MMC (0.4 mg/ml) was applied intraoperatively for 4 minutes. A trend toward use of fornix-based conjunctival flaps with a more diffuse application of MMC at a lower dosage has developed since the TVT Study was initiated [12]. This modification in surgical technique may result in lower rates of hypotony and bleb-related complications after trabeculectomy [13]. A subgroup of patients enrolled in the TVT Study (i.e., those with a history of prior trabeculectomy with MMC) had already failed one treatment arm of the study, and potentially could have introduced bias in favor of the tube group. We felt that the study question of whether one surgical procedure was superior to the other was clinically relevant in eyes that had failed a MMC trabeculectomy, and a separate stratum (stratum 4) was created for these eyes to facilitate data analysis and address concerns about possible bias. No significant differences in treatment efficacy were observed between strata.
The results of the TVT Study provide further support for the expanded use of tube shunts beyond refractory glaucomas. Although these devices have historically been reserved for surgically managing glaucomas at high risk of trabeculectomy failure, this study enrolled eyes at lower risk for failure. The TVT Study does not demonstrate clear superiority of one glaucoma operation over the other. Other factors that must be considered when selecting a surgical procedure include the surgeon’s skill and experience with both operations, the patient’s willingness to undergo repeat glaucoma surgery, and the planned surgical approach should failure occur. Additional follow-up data will be forthcoming, and is needed to fully assess the risks and benefits of tube shunt surgery and trabeculectomy with MMC in managing medically uncontrolled glaucoma in similar patient groups.
Acknowledgments
The study was supported by research grants from Pfizer, Inc., New York, New York, Advanced Medical Optics, Irvine, California, the National Eye Institute (grant EY014801), National Institutes of Health, Bethesda, Maryland, and Research to Prevent Blindness, Inc., New York, New York.
Appendix
The following are participating centers and committees in the TVT Study.
Clinical Centers:
Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami (Miami, Florida): Principal Investigator: Steven Gedde, M.D.; Coinvestigators: Douglas Anderson, M.D., Donald Budenz, M.D., M.P.H., Madeline Del Calvo, Ivette DePool, Francisco Fantes, M.D., David Greenfield, M.D., Jessica Hochberg, Elizabeth Hodapp, M.D., Richard Lee, M.D., Ph.D., Alexia Marcellino, Paul Palmberg, M.D. Ph.D., Richard Parrish II, M.D.
Duke University (Durham, North Carolina): Principal Investigator: Leon Herndon, M.D.; Coinvestigators: Pratap Challa, M.D., Cecile Santiago-Turla, M.D.
Indiana University (Indianapolis, Indiana): Principal Investigator: Darrell WuDunn, M.D., Ph.D.
Loyola University (Maywood, Illinois): Principal Investigator: Geoffrey Emerick, M.D.
Medical College of Wisconsin (Milwaukee, Wisconsin): Principal Investigator: Dale Heuer, M.D.
Medical University of South Carolina (Charleston, South Carolina): Principal Investigator: Alexander Kent, M.D.; Coinvestigators: Carol Bradham, Lisa Langdale.
Moorfields Eye Hospital (London, England): Principal Investigator: Keith Barton, M.D.; Coinvestigator: Francesca Amalfitano, Poornima Rai, M.D.
New York Eye and Ear Infirmary (New York, New York): Principal Investigator: Paul Sidoti, M.D.; Coinvestigators: Amy Gedal, James Luayon, Roma Ovase, Katy Tai
Scripps Clinic (La Jolla, California): Principal Investigator: Quang Nguyen, M.D.; Coinvestigator: Neva Miller
St. Louis University (St. Louis Missouri): Principal Investigator: Steven Shields, M.D.; Coinvestigators: Kevin Anderson, Frank Moya, M.D.
University of California, Davis (Sacramento, California): Principal Investigator: James Brandt, M.D.; Coinvestigator: Michele Lim, M.D., Marilyn Sponzo.
University of Florida (Gainesville, Florida): Principal Investigator: Mark Sherwood, M.D.; Coinvestigator: Revonda Burke
University of Oklahoma (Oklahoma City, Oklahoma): Principal Investigator: Gregory Skuta, M.D.; Coinvestigators: Jason Jobson, Lisa Ogilbee, Adam Reynolds, M.D., Steven Sarkisian, M.D.
University of Southern California (Los Angeles, California): Principal Investigator: Rohit Varma, M.D., M.P.H.; Coinvestigators: Brian Francis, M.D., Frances Walonker
University of Texas Houston (Houston, Texas): Principal Investigator: Robert Feldman, M.D.; Coinvestigators: Laura Baker, Nicholas Bell, JoLene Carranza, Athena Espinoza
University of Virginia (Charlottesville, Virginia): Principal Investigator: Bruce Prum, M.D.; Coinvestigator: Janis Beall
University of Wisconsin (Madison, Wisconsin): Principal Investigator: Todd Perkins, M.D.; Coinvestigators: Paul Kaufman, M.D., Tracy Perkins, Barbara Soderling.
Statistical Coordinating Center, Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami (Miami, Florida): William Feuer, M.S., Luz Londono, Joyce Schiffman, M.S., Wei Shi, M.S.
Safety and Data Monitoring Committee: Philip Chen, M.D., William Feuer, M.S., Joyce Schiffman, M.S., Kuldev Singh, M.D., M.P.H., George Spaeth, M.D., Martha Wright, M.D.
Steering Committee: Keith Barton, M.D., James Brandt, M.D., Geoffrey Emerick, M.D., Robert Feldman, M.D., Steven Gedde, M.D., Leon Herndon, M.D., Dale Heuer, M.D., Alexander Kent, M.D., Quang Nguyen, M.D., Richard Parrish II, M.D., Todd Perkins, M.D., Bruce Prum, M.D., Mark Sherwood, M.D., Steven Shields, M.D., Paul Sidoti, M.D., Gregory Skuta, M.D., Rohit Varma, M.D., M.P.H., Darrell WuDunn, M.D., Ph.D.
Study Chairmen: Steven Gedde, M.D., Dale Heuer M.D., Richard Parrish II, M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Gedde has no financial interest in any of the products discussed in this article. Drs. Heuer and Parrish are consultants and members of the speakers bureau for Pfizer, Inc.
References
- 1.Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–2270. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen PP, Yamamoto T, Sawada A, et al. Use of antifibrosis agents and glaucoma drainage devices in the American and Japanese Glaucoma Societies. J Glaucoma. 1997;6:192–196. [PubMed] [Google Scholar]
- 3.Joshi AB, Parrish RK, Feuer WF. 2002 Survey of the American Glaucoma Society. Practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172–174. doi: 10.1097/01.ijg.0000151684.12033.4d. [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Schiffman JC, Feuer WJ, et al. The Tube Versus Trabeculectomy Study: Design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140:275–287. doi: 10.1016/j.ajo.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2007;143:23–31. doi: 10.1016/j.ajo.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy Study after one year of follow-up. Am J Ophthalmol. 2007;143:9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 7••.Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol. 2009 Aug 10; [Epub ahead of print]. This article reports the 3-year results of the TVT Study. Tube shunt surgery had a higher success rate than trabeculectomy with MMC during the first 3 years of follow-up. Both surgical procedures were associated with similar IOP reduction, use of adjunctive medical therapy, and rates of serious complications at 3 years. [Google Scholar]
- 8•.Rauscher FM, Gedde SJ, Schiffman JC, et al. Motility disturbances in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2009;147:458–466. doi: 10.1016/j.ajo.2008.09.019. This article describes preoperative and postoperative motility disturbances encountered in the TVT Study during the first year of follow-up. New motility disturbances were more common after tube shunt surgery compared with trabeculectomy with MMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1089–1098. doi: 10.1016/j.ophtha.2008.03.031. A panel of glaucoma specialists use an evidence-based approach to address questions related to commercially available aqueous shunts. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136:464–470. doi: 10.1016/s0002-9394(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 11•.Stein JD, Ruiz D, Belsky D, et al. Longitudinal rates of postoperative adverse outcomes after glaucoma surgery among Medicare beneficiaries 1994 to 2005. Ophthalmology. 2008;115:1109–1116. doi: 10.1016/j.ophtha.2008.03.033. This retrospective study of Medicare data from 1994 to 2005 found a higher rate of severe adverse outcomes in glaucoma drainage device implantation than primary trabeculectomy or trabeculectomy with scarring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones E, Clarke J, Khaw PT. Recent advances in trabeculectomy technique. Curr Opin Ophthalmol. 2005;16:107–113. doi: 10.1097/01.icu.0000156138.05323.6f. [DOI] [PubMed] [Google Scholar]
- 13.Wells AP, Cordeiro MF, Bunce C, Khaw PT. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology. 2003;110:2192–2197. doi: 10.1016/S0161-6420(03)00800-5. [DOI] [PubMed] [Google Scholar]