Abstract
Purpose
To compare the safety and efficacy of Baerveldt implantation and trabeculectomy with mitomycin C (MMC) in patients who have not had prior incisional glaucoma surgery.
Design
Retrospective comparative case series
Patients
A total of 125 patients with low risk glaucoma undergoing primary glaucoma surgery, including 55 patients who received a 350-mm2 Baerveldt glaucoma implant and 70 patients who had a trabeculectomy with mitomycin C
Methods
Eligible patients were identified using current procedural terminology codes, and their medical records were retrospectively reviewed.
Main Outcome Measures
The primary outcome measure was surgical success (IOP ≤ 21 mm Hg and reduced ≥ 20% from baseline, IOP > 5 mm Hg, no reoperation for glaucoma, no loss of light perception vision). Secondary outcome measures included visual acuity, intraocular pressure (IOP), number of glaucoma medications, and complications.
Results
The cumulative probability of success at 3 years with or without medical therapy was 87% in the Baerveldt group and 76% in the trabeculectomy group (p = 0.23). Postoperative complications occurred in 11 (20%) patients in the Baerveldt group and 20 (29%) patients in the trabeculectomy group (p = 0.27). Follow-up (mean ± SD) was 27 ± 19 months in the Baerveldt group and 34 ± 20 in the trabeculectomy group (p = 0.053).
Conclusions
Similar rates of surgical success and postoperative complications were observed with trabeculectomy with mitomycin C and Baerveldt implantation during 3 years of follow-up. Both are viable primary glaucoma procedures in patients without prior ocular surgery.
Incisional glaucoma surgery is generally employed when medical and laser therapy do not provide adequate control of intraocular pressure (IOP). Trabeculectomy remains the most commonly performed incisional glaucoma procedure worldwide, but the popularity of this procedure has been declining.1 While tube shunts have traditionally been reserved for refractory glaucoma, their role in the surgical management of glaucoma has been expanding in recent years. In a survey of the American Glaucoma Society membership, 85% preferred trabeculectomy and 11% favored tube shunt placement as the initial incisional glaucoma procedure in a phakic patient with primary open angle glaucoma.2 Results from the Tube Versus Trabeculectomy (TVT) Study have supported the shift in practice patterns toward use of tube shunts as an alternative to trabeculectomy. The Baerveldt implant was found to have a higher success rate compared to trabeculectomy with mitomycin C (MMC) in eyes with prior cataract and/or glaucoma surgery and limited conjunctival scarring.3 Although trabeculectomy is generally used as a primary glaucoma procedure, tube shunts are a viable alternative.
This retrospective comparative case series evaluates the safety and efficacy of Baerveldt shunt implantation and trabeculectomy with mitomycin C (MMC) in patients who have not had prior incisional surgery. The goal of this study is to provide evidence-based information that will assist in surgical decision making for patients requiring a primary procedure to manage their glaucoma.
METHODS
Institutional Review Board approval was obtained from the University of Miami Human Subjects Research Committee. Patients who underwent either primary trabeculectomy with MMC or 350-mm2 Baerveldt shunt placement between 1985 and 2010 were identified using current procedural terminology (CPT) codes. All procedures were performed by one of 6 glaucoma surgeons who were proficient in both procedures under study. Patients were excluded if they had prior incisional ocular surgery, an IOP less than 18 mm Hg immediately prior to surgery, secondary glaucomas at high risk for failure (i.e., neovascular glaucoma, uveitic glaucoma, iridocorneal endothelial syndrome, fibrous/epithelial downgrowth), or less than 6 months of postoperative follow-up. Information obtained from the medical records of each patient included: patient demographic data, preoperative diagnosis, date of surgery, preoperative and postoperative IOPs, visual acuity, number of glaucoma medications used, and postoperative complications.
Surgical success was defined as an IOP ≤21 mm Hg and reduced ≥ 20% from baseline on two consecutive follow up visits (conducted at least one month apart) after 3 months, IOP > 5 mm Hg, no reoperation for glaucoma, and no loss of light perception vision. Secondary outcome measures included visual acuity (VA), IOP, number of glaucoma medications, and complications. Patient who were on no supplemental medical therapy were categorized as complete successes. Patients were considered qualified successes if they had a successful surgical outcome with or without glaucoma medical therapy. Reoperation for glaucoma or a complication was defined as additional surgery necessitating a return to the operating room. A vitreous tap with injection of antibiotics was considered a reoperation for a complication. Interventions that the surgeon performed at the slit-lamp, such as reformation of the anterior chamber or transconjunctival needling procedures, were not considered reoperations. Corneal edema, dysesthesia, and diplopia were defined as the postoperative development of these complications with their continued presence after 6-months of follow-up. Eyes that were noted to be Seidel positive within the first month of follow-up were labeled as wound leaks, and those occurring after 1 month were classified as bleb leaks. The time to failure was defined as the time from surgical treatment to reoperation for glaucoma, or as the time from surgical treatment to the first of two consecutive study visits after 3 months in which the patient had persistent low IOP (IOP < 5 mm Hg) or inadequately reduced IOP (IOP > 21 mm Hg or < 20% reduction from baseline). Kaplan Meier survival analysis was used to evaluate success rates.
Surgical Technique
Baerveldt Implantation
A fornix-based conjunctival flap was made and Tenon’s capsule was dissected from the sclera to create space for the implant in the desired quadrant. The lateral wings of a 350-mm2 Baerveldt glaucoma implant (Abbott Medical Optics, Santa Ana, California) were placed beneath the adjacent rectus muscles. The end plate was attached to the sclera approximately 8–10 mm posterior to the limbus with nonabsorbable sutures (nylon or polypropylene). The tube was ligated with a 7-0 polyglactin suture near the tube-plate junction. After occluding the tube, fenestrations were made anterior to the tube ligature with a 7-0 polyglactin suture needle (TG-140) at the discretion of the surgeon. The tube was trimmed to an appropriate length. A 23-gauge needle was used to make an entry incision, and the tube was then inserted into the anterior chamber through the needle track. The limbal portion of the tube was covered with a patch graft, and the conjunctiva was reapproximated to the limbus with polyglactin sutures.
Trabeculectomy with MMC
A fornix- or limbus-based conjunctival flap was dissected. A partial thickness scleral flap was created. Mitomycin-C (0.2–0.4 mg/ml) was applied to the scleral surface using a saturated Weck-Cel sponge for 2–4 minutes. A copious amount of balanced salt solution was used to irrigate the area after the sponge was removed. A temporal paracentesis was made. The anterior chamber was entered under the scleral flap, and a sclerostomy was created using a Kelly punch. A peripheral iridectomy was then made, and the scleral flap was closed with interrupted 10-0 nylon sutures. The conjunctiva was then closed with polyglactin sutures.
Statistical Analysis
Univariate comparisons between treatment groups were performed using the two-sided Student t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Snellen VA measurements were converted to logMAR equivalents for the purpose of data analysis, as reported previously.4 A p-value of 0.05 or less was considered statistically significant in our analyses.
RESULTS
Demographic Features
The baseline characteristics of study patients are presented in Table 1. A total of 125 patients met the eligibility criteria for the study, including 70 patients who underwent trabeculectomy and 55 patients who received a Baerveldt implant. No significant differences were observed in the baseline characteristics between the Baerveldt and trabeculectomy groups, with the exception of glaucoma type. The Baerveldt group had a greater proportion of patients with primary open angle glaucoma, and the trabeculectomy group had more patients with pseudoexfoliative glaucoma and chronic angle closure glaucoma.
Table 1.
Baseline Characteristics of Baerveldt and Trabeculectomy Patients
| Baerveldt Group (n = 55) |
Trabeculectomy Group (n = 70) |
P-value | |
|---|---|---|---|
| Age (years), mean ± SD | 62 ± 16 | 66 ± 12 | 0.10 |
| Gender, n (%) | 0.70 | ||
| Male | 29 (54) | 40 (57) | |
| Female | 25 (46) | 30 (43) | |
| Race, n (%) | |||
| White | 13 (26) | 17 (25) | 0.19 |
| Black | 19 (37) | 19 (28) | |
| Hispanic | 17 (33) | 32 (47) | |
| Other | 2 (4) | 0 | |
| Diabetes mellitus, n (%) | 7 (13) | 14 (20) | 0.28 |
| Hypertension, n (%) | 29 (53) | 38 (54) | 0.86 |
| IOP (mm Hg), mean ± SD | 28.0 ± 9.8 | 28.5 ± 8.6 | 0.75 |
| Glaucoma medications, mean ± SD | 3.4 ± 1.1 | 3.0 ± 1.3 | 0.11 |
| Diagnosis, n (%) | |||
| POAG | 45 (85) | 40 (57) | 0.025 |
| CACG | 4 (8) | 14 (20) | |
| PXFG | 2 (4) | 9 (13) | |
| PG | 1 (2) | 2 (3) | |
| Other | 1 (2) | 5 (7) | |
| Snellen VA, LogMAR mean ± SD | 0.59 ± 0.70 | 0.47 ± 0.52 | 0.31 |
Intraocular Pressure Reduction
Baseline and follow-up IOP measurements for the Baerveldt and trabeculectomy groups are reported in Table 2. There was no significant difference in baseline IOP between the two study groups. Patients were censored from analysis at the time of failure. The number of patients who were censored at 6 months, 1 year, 18 months, 2 years, 3 years, 4 years, and 5 years were 5, 8, 10, 11, 8, 7, and 3 respectively in the trabeculectomy group, and 1, 1, 2, 1, 3, 5, and 5 in the Baerveldt group. Additional reductions in the number of patients at follow-up time points in Table 2 were the result of missed visits. Both surgical procedures produced a significant and sustained reduction in IOP. Postoperative IOPs were lower at all time points in the trabeculectomy group compared with the Baerveldt group, and these differences were statistically significant at 6 months and 1 year.
Table 2.
Intraocular Pressures and Medical Therapy at Baseline and Follow-up for Patients
| Baerveldt Group (n = 55) |
Trabeculectomy Group (n = 70) |
P-value | |
|---|---|---|---|
| Baseline | |||
| IOP (mm Hg) | 28.0 ± 9.8 | 28.5 ± 8.6 | 0.75 |
| Glaucoma medications | 3.4 ± 1.1 | 3.0 ± 1.3 | 0.11 |
| N | 55 | 70 | |
| 6 months | |||
| IOP (mm Hg) | 14.0 ± 4.1 | 11.0 ± 4.8 | 0.001 |
| Glaucoma medications | 1.8 ± 1.5 | 0.7 ± 1.5 | 0.001 |
| N | 44 | 50 | |
| 1 year | |||
| IOP (mm Hg) | 13.1 ± 3.8 | 11.2 ± 3.3 | 0.016 |
| Glaucoma medications | 1.9 ± 1.5 | 0.7 ± 1.4 | <0.001 |
| N | 41 | 44 | |
| 18 months | |||
| IOP (mm Hg) | 14.0 ± 3.6 | 11.9 ± 4.6 | 0.06 |
| Glaucoma medications | 2.0 ± 1.4 | 0.6 ± 1.3 | <0.001 |
| N | 25 | 38 | |
| 2 years | |||
| IOP (mm Hg) | 13.2 ± 2.9 | 12.2 ± 4.2 | 0.30 |
| Glaucoma medications | 2.2 ± 1.5 | 1.0 ± 1.6 | 0.004 |
| N | 27 | 45 | |
| 3 years | |||
| IOP (mm Hg) | 14.9 ± 7.0 | 11.6 ± 3.6 | 0.070 |
| Glaucoma medications | 2.0 ± 1.4 | 0.7 ± 1.2 | 0.002 |
| N | 18 | 28 | |
| 4 years | |||
| IOP (mm Hg) | 13.2 ± 4.0 | 11.6 ± 3.0 | 0.21 |
| Glaucoma medications | 1.9 ± 1.6 | 1.1 ± 1.3 | 0.16 |
| N | 9 | 24 | |
| 5 years | |||
| IOP (mm Hg) | 13.6 ± 2.8 | 11.2 ± 4.2 | 0.16 |
| Glaucoma medications | 2.5 ± 1.9 | 1.8 ± 1.7 | 0.37 |
| N | 8 | 14 |
Patients were censored from analysis at the time of failure
Data are presented as mean ± standard deviation
Fenestration of the tube was performed in 26 (47%) patients at the time of Baerveldt implantation. Baseline IOP was 30.6 ± 12.1 mm Hg in the fenestration group and 24.9 ± 6.2 mm Hg in the group without fenestrations (p = 0.040). No significant differences in mean IOPs were observed at follow-up time points between patients who did and did not undergo tube fenestration.
Medical Therapy
Table 2 shows the number of glaucoma medications in the both groups at baseline and follow-up. Patients were censored from analysis at the time of failure. Throughout the first 3 years of follow up, patients in the trabeculectomy group required significantly fewer medications compared to the tube shunt group.
Treatment Outcomes
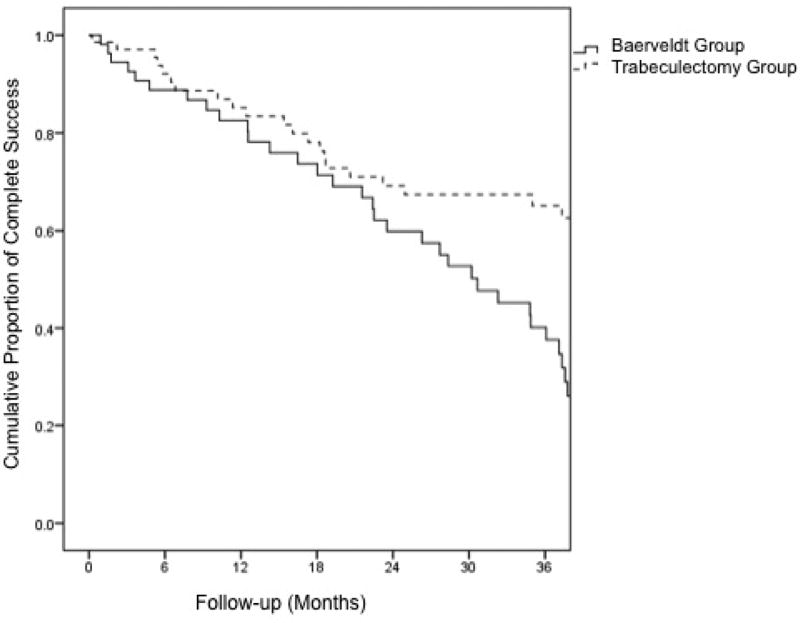
Kaplan-Meier survival curves are shown in Figures 1 and 2. The cumulative probability of qualified success at 1 year, 2 years, and 3 years was 95%, 92%, and 87% respectively in the Baerveldt group, and 87%, 76%, and 76% in the trabeculectomy group (p = 0.23). The cumulative rate of complete success at 1 year, 2 years, and 3 years was 83%, 60%, and 40% respectively in the Baerveldt group, and 85%, 69%, and 65% in the trabeculectomy group (p = 0.002).
Figure 1.
Kaplan-Meier plots of the probability of qualified success after primary Baerveldt implantation and trabeculectomy with mitomycin C.
Figure 2.
Kaplan-Meier plots of the probability of complete success after primary Baerveldt implantation and trabeculectomy with mitomycin C.
The most common cause for failure in both treatment groups was inadequate IOP reduction (IOP > 21 mm Hg or < 20% reduction below baseline on two consecutive follow-up visits after 3 months). There were 5 patients in the Baerveldt group and 6 in the trabeculectomy group who failed because of inadequate IOP reduction. Treatment failure due to persistent hypotony was not observed in the Baerveldt group, but was responsible for treatment failure for 4 patients in the trabeculectomy group. All patients who failed because of persistent hypotony had a decrease in VA from baseline. Follow-up (mean ± SD) was 27 ± 19 months in the Baerveldt group and 34 ± 20 months in the trabeculectomy group (p = 0.053).
Postoperative Interventions
Laser suture lysis was performed in 38 (54%) patients in the trabeculectomy group. There were 4 (6%) patients in the trabeculectomy group who underwent bleb needling. Subconjunctival injections of 5-fluorouracil were administered in 3 (4%) patients in the trabeculectomy group. No postoperative interventions were performed in the Baerveldt group.
Reoperation for Glaucoma
A total of 7 patients in the trabeculectomy group had reoperations for glaucoma, including 2 trabeculectomy revisions and 6 tube shunt implantations (1 patient had both procedures). In the Baerveldt group, 2 patients underwent additional glaucoma surgery involving placement of a second Baerveldt implant in both cases. No significant difference in the rate of reoperation for glaucoma was observed between the two treatment groups (p = 0.30).
Visual Acuity
Snellen VA was decreased by 2 or more lines from baseline in 18 (33%) patients in the Baerveldt group and 28 (40%) patients in the trabeculectomy group at last follow-up (p = 0.40). Snellen VA (logMAR mean ± SD) was 0.66 ± 0.76 in the Baerveldt group and 0.72 ± 0.88 in the trabeculectomy group (p = 0.69) after 3 years of follow-up.
Postoperative Complications
Table 3 lists postoperative complications. A total of 11 complications in 55 (20%) patients were reported in the Baerveldt group, and 20 complications in 70 (29%) patients were noted in the trabeculectomy group (p = 0.27). Complications presenting at least 1 month following surgery included persistent corneal edema, bleb leak, persistent diplopia, and endophthalmitis/blebitis. Choroidal effusion, wound leak, and malignant glaucoma were observed in the early postoperative period. Wound leak occurred with significantly greater frequency in the trabeculectomy group than the Baerveldt group (p = 0.006).
Table 3.
Postoperative Complications
| Baerveldt Group (n = 55) |
Trabeculectomy Group (n = 70) |
|
|---|---|---|
| Early postoperative complications* | ||
| Choroidal effusion | 4 | 3 |
| Wound leak | 0 | 9 |
| Malignant glaucoma | 0 | 1 |
| Late postoperative complications# | ||
| Persistent corneal edema | 2 | 0 |
| Persistent diplopia | 4 | 1 |
| Bleb leak | 1 | 4 |
| Endophthalmitis/blebitis | 0 | 2 |
|
| ||
| Total number of patients with postoperative complications^ | 11 | 20 |
Complications ≤ 1 month after surgery
Complications > 1 month after surgery
P = 0.27 for the difference in the total number of patients with postoperative complications between treatment groups
Reoperations for complications are shown in Table 4. There were 3 (5.5%) patients in the Baerveldt group and 7 (10%) patients in the trabeculectomy group who had surgical complications that required a reoperation to manage the complication (p = 0.24).
Table 4.
Reoperations for Complications
| Baerveldt Group (n = 55) |
Trabeculectomy Group (n = 70) |
|
|---|---|---|
| Pars plana vitrectomy | 0 | 1 |
| Bleb revision | 1 | 4 |
| Drainage of choroidal effusion | 1 | 1 |
| Vitreous tap with injection of intravitreal antibiotics | 0 | 1 |
| Removal of tube shunt | 1 | - |
|
| ||
| Total number of patients with reoperations for complications* | 3 | 7 |
P = 0.35 for the difference in the total number of patients with reoperations for complications between treatment groups
Cataract Surgery
No significant difference in the rate of cataract extraction between the 2 treatment groups was observed. There were 16 (29%) patients in the tube group and 23 (33%) patients in the trabeculectomy group who underwent cataract extraction with intraocular lens implantation (p = 0.65).
DISCUSSION
Trabeculectomy with MMC and Baerveldt implantation were both effective in lowering IOP in patients without prior incisional surgery. Among patients who completed 3-years of follow-up, placement of a Baerveldt implant produced a 47% decrease in IOP while trabeculectomy with MMC achieved a 59% reduction in IOP. The results from the present study are similar to earlier reports in the literature, which found a reduction of IOP ranging from 46.4% to 58.3% in patients who underwent tube shunt surgery and 38.6% to 61.4% in those treated with trabeculectomy with an antifibrotic agent.5–12 Mean IOPs were lower at all postoperative time points following trabeculectomy than Baerveldt placement, and these differences were statistically significant at 6 months and 1 year.
The trend toward greater IOP reduction following trabeculectomy with MMC relative to Baerveldt implantation was observed with the use of less adjunctive medical therapy, which was statistically significant throughout 3 years of follow-up. A higher rate of surgical success without glaucoma medications (i.e., complete success) was seen after trabeculectomy compared with Baerveldt placement. Although more medications were prescribed after Baerveldt implantation, this does not ensure that they were being used. Nonadherence to glaucoma medical therapy is well described.13–17
Numerous studies have reported outcomes of primary trabeculectomy in eyes at low risk of filtration failure (e.g., primary open angle glaucoma, chronic angle closure, pseudoexfoliative glaucoma, pigmentary glaucoma).5–9 Although many studies have evaluated tube shunts in refractory glaucoma (e.g., neovascular glaucoma, uveitic glaucoma, conjunctival scarring),18–25 limited information exists about the use of these devices in eyes with non-refractory glaucoma and no prior ocular surgery. Valimaki and colleagues reported IOP reduction to 15.4 mm Hg with the Molteno3 implant (Molteno Ophthalmic Limited, Dunedin, New Zealand) in 38 patients with primary open angle glaucoma and pseudoexfoliative glaucoma.26 Forty-seven percent of patients had no previous incisional ocular surgery, and 57% had only cataract extraction with intraocular lens implantation before Molteno implantation. Molteno and associates conducted a prospective comparative case series of trabeculectomy and single- and double-plate Molteno implantation as primary surgery.27 Using the definition of failure of an IOP greater than 21 mm Hg, phthisis, reoperation for glaucoma, or total vision loss due to complications from surgery, there were 96 failures (13%) in the trabeculectomy group and 8 failures (3%) in the Molteno implant group. Complication rates as well as rate of visual field loss were noted to be similar between both treatment groups. The authors concluded that Molteno shunt implantation may be superior to trabeculectomy when utilized as a primary procedure.
Despite lower mean IOPs and mean number of glaucoma medications after trabeculectomy compared with Baerveldt shunt implantation, similar rates of surgical failure were observed with both surgical procedures. This counterintuitive observation relates to the fact that treatment failures represented only a small subset of the overall group used to calculate mean IOP and number of glaucoma medications during follow-up. Mean IOP values can be misleading because high and low IOP measurements can counterbalance each other resulting in a false impression of good IOP control in some cases. The rate of surgical success is a better indicator of the effectiveness of a glaucoma procedure, and it was selected as the primary outcome measure for this reason.
Wilson and colleagues conducted a randomized clinical trial comparing the Ahmed glaucoma valve and trabeculectomy in eyes with primary glaucoma. As with the present study, significantly greater IOP reduction was seen at several study visits following trabeculectomy compared with Ahmed implantation, and similar rates of surgical success were observed with both glaucoma procedures.28,29 The TVT Study was another randomized clinical trial comparing trabeculectomy with MMC to Baerveldt implantation in patients with prior cataract and/or glaucoma surgery. In contrast with the current study and Wilson’s trial, no significant differences were observed in long-term IOP control between the two study groups and higher rates of surgical success were found with tube shunt surgery relative to trabeculectomy (cumulative probability of success was 70.2% in the tube group and 53.1% in the trabeculectomy group at 5 years).3 The varying study results may relate to differences in patient characteristics, success/failure criteria, retention in the study, and length of follow-up. In addition, implants with larger surface end plates have been reported to offer greater pressure reduction.30
The benefits of tube shunt implantation or trabeculectomy with MMC in reducing IOP must be weighed against the risks of surgical complications. Similar rates of vision loss were observed after trabeculectomy with MMC and Baerveldt implantation. No significant differences were observed in the rates of postoperative complications, reoperation for complications, and cataract extraction following both surgical procedures. Wound leaks were more common following trabeculectomy with MMC, occurring in 13% of patients. It is our practice to perform a Seidel test at all postoperative visits after glaucoma surgery. The rate of wound leaks following trabeculectomy with an adjunctive antifibrotic agent was 32% in the Fluorouracil Filtering Surgery Study and 11% in the TVT Study with protocols that required Seidel testing at all study visits.4,31 A trend toward a higher incidence of diplopia and persistent corneal edema were noted after Baerveldt implantation. The power of this study to detect differences in complications with low rates of occurrence was limited by the sample size.
The current study has several limitations. Patients were not randomly assigned to surgical treatment, and there were likely individual patient factors that directed the surgeon in the selection of a glaucoma procedure. As with all retrospective studies, a standard protocol for data collection at the time of follow-up visits was not used. This may have resulted in an underestimation of adverse events, because surgical complications may be overlooked unless specific attention is directed toward their detection. Postoperative interventions and the addition of medical therapy were left to the discretion of the surgeon, and no standardized protocols were used to guide postoperative management. Limited follow-up data was available, particularly after 3 years. Additionally, the study was conducted at one academic center, and this may affect the generalizability of the results.
In summary, similar rates of surgical success were observed after trabeculectomy with MMC and Baerveldt implantation in patients undergoing primary incisional glaucoma surgery. Trabeculectomy with MMC tended to produce lower IOP with less use of adjunctive glaucoma therapy compared with Baerveldt implantation. Vision loss, postoperative complications, reoperation for complications and cataract extraction occurred at similar rates following both surgical procedures. Multiple factors must be considered when selecting a glaucoma procedure, including the patient’s tolerance of glaucoma medical therapy, target level of IOP reduction, risk of postoperative complications, and the surgeon’s experience and comfort with each procedure. A review of the ophthalmic literature indicates this is the first study comparing the Baerveldt glaucoma implant to trabeculectomy as a primary procedure. A randomized clinical trial comparing these two surgical procedures is needed to provide higher level of evidence to guide surgical decision making.
Acknowledgments
The Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD-Grant#W81XWH-09-1-0675).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no proprietary or financial interest in any of the work discussed in this manuscript. No conflicting relationship exists for any author.
Trabeculectomy with mitomycin C and Baerveldt implantation had similar rates of surgical success as a primary glaucoma procedure in patients without prior ocular surgery.
References
- 1.Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–70. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Desai MA, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42:202–8. doi: 10.3928/15428877-20110224-04. [DOI] [PubMed] [Google Scholar]
- 3.Gedde SJ, Schiffman JC, Feuer WF, et al. Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up. Am J Ophthalmol. 2012;153:789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Five-year follow-up of the Fluorouracil Filtering Surgery Study. The Fluorouracil Filtering Surgery Study Group. Am J Ophthalmol. 1996;121:349–66. doi: 10.1016/s0002-9394(14)70431-3. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb RN. Adjusting the dose of 5-fluorouracil after filtration surgery to minimize side effects. Ophthalmology. 1987;94:564–70. doi: 10.1016/s0161-6420(87)33430-x. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SS. Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology. 1991;98:317–321. doi: 10.1016/s0161-6420(91)32293-0. [DOI] [PubMed] [Google Scholar]
- 7.Prata Junior JA, Minckler DS, Baerveldt G, et al. Trabeculectomy in pseudophakic patients: postoperative 5-fluorouracil versus intraoperative mitomycin C antiproliferative therapy. Ophthalmic Surg. 1995;26:73–7. [PubMed] [Google Scholar]
- 8.Singh J, O'Brien C, Chawla HB. Success rate and complications of intraoperative 0.2 mg/ml mitomycin C in trabeculectomy surgery. Eye. 1995;9:460–6. doi: 10.1038/eye.1995.107. [DOI] [PubMed] [Google Scholar]
- 9.Andreanos D, Georgopoulos GT, Vergados J, et al. Clinical evaluation of the effect of mitomycin-C in re-operation for primary open angle glaucoma. Eur J Ophthalmol. 1997;7:49–54. doi: 10.1177/112067219700700109. [DOI] [PubMed] [Google Scholar]
- 10.You YA, Gu YS, Fang CT, Ma XQ. Long-term effects of simultaneous subconjunctival and subscleral mitomycin C application in repeat trabeculectomy. J Glaucoma. 2002;11:110–8. doi: 10.1097/00061198-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Freedman J, Rubin B. Molteno implants as a treatment for refractory glaucoma in black patients. Arch Ophthalmol. 1991;109:1417–20. doi: 10.1001/archopht.1991.01080100097051. [DOI] [PubMed] [Google Scholar]
- 12.Broadway DC, Lester M, Schulzer M, Douglas GR. Survival analysis for success of Molteno tube implants. Br J Ophthalmol. 2001;85:689–95. doi: 10.1136/bjo.85.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 14.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–7. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:1320–7. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116:191–9. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106:2312–8. doi: 10.1016/S0161-6420(99)90532-8. [DOI] [PubMed] [Google Scholar]
- 19.Freedman J, Bhandari R. Supra-tenon capsule placement of original Molteno vs Molteno 3 tube implants in black patients with refractory glaucoma: a single-surgeon experience. Arch Ophthalmol. 2011;129:993–7. doi: 10.1001/archophthalmol.2011.183. [DOI] [PubMed] [Google Scholar]
- 20.Hodkin M, Goldblatt W, Burgoyne C, et al. Early clinical experience with the Baerveldt implant in complicated glaucomas. Am J Ophthalmol. 1995;120:32–40. doi: 10.1016/s0002-9394(14)73756-0. [DOI] [PubMed] [Google Scholar]
- 21.Huang M, Netland P, Coleman A, et al. Intermediate-term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999;127:27–33. doi: 10.1016/s0002-9394(98)00394-8. [DOI] [PubMed] [Google Scholar]
- 22.Ishida K, Netland PA. Ahmed Glaucoma Valve implantation in African American and white patients. Arch Ophthalmol. 2006;124:800–6. doi: 10.1001/archopht.124.6.800. [DOI] [PubMed] [Google Scholar]
- 23.Roy S, Ravinet E, Mermoud A. Baerveldt implant in refractory glaucoma: Long-term results and factors influencing outcomes. Int Ophthalmol. 2001;24:93–100. doi: 10.1023/a:1016335313035. [DOI] [PubMed] [Google Scholar]
- 24.Syed HM, Law SK, Nam SH, et al. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004;13:38–45. doi: 10.1097/00061198-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Yalvac IS, Eksioglu U, Satana B, Duman S. Long-term results of Ahmed glaucoma valve and Molteno implant in neovascular glaucoma. Eye. 2007;21:65–70. doi: 10.1038/sj.eye.6702125. [DOI] [PubMed] [Google Scholar]
- 26.Valimaki J, A YA-P. Molteno3 Implantation as Primary Glaucoma Surgery. Journal of Ophthalmology. 2014;2014 doi: 10.1155/2014/167564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molteno AC, Bevin TH, Herbison P, Husni MA. Long-term results of primary trabeculectomies and Molteno implants for primary open-angle glaucoma. Arch Ophthalmol. 2011;129:1444–50. doi: 10.1001/archophthalmol.2011.221. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Mendis U, Smith S, Paliwal A. Ahmed glaucoma valve implant vs. trabeculectomy in the surgical treatment of glaucoma: A randomized clinical trial. Am J Ophthalmol. 2000;130:267–73. doi: 10.1016/s0002-9394(00)00473-6. [DOI] [PubMed] [Google Scholar]
- 29.Wilson M, Mendis U, Paliwal A, et al. Long-term follow-up of primary glaucoma surgery with Ahmed glau- coma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136:464–70. doi: 10.1016/s0002-9394(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 30.Gedde S, Panarelli J, Banitt M, Lee R. Evidenced-based comparison of aqueous shunts. Curr Opin Ophthalmol. 2013;24:87–95. doi: 10.1097/ICU.0b013e32835cf0f5. [DOI] [PubMed] [Google Scholar]
- 31.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]