Abstract
Purpose
To describe the incidence and outcomes of reoperations for glaucoma in the Tube Versus Trabeculectomy (TVT) Study.
Design
Cohort study of patients in a multicenter randomized clinical trial.
Methods
The TVT Study enrolled 212 patients with medically uncontrolled glaucoma who had previous cataract and/or glaucoma surgery. Randomization assigned 107 patients to surgery with a tube shunt (350-mm2 Baerveldt glaucoma implant) and 105 patients to trabeculectomy with mitomycin C (0.4 mg/ml for 4 minutes). Data were analyzed from patients who failed their assigned treatment and had additional glaucoma surgery. Outcome measures included intraocular pressure (IOP), use of glaucoma medications, visual acuity, surgical complications, and failure (IOP > 21 mm Hg or not reduced by 20%, IOP ≤ 5 mm Hg, additional glaucoma surgery, or loss of light perception vision).
Results
Additional glaucoma surgery was performed in 8 patients in the tube group and 18 patients in the trabeculectomy group in the TVT Study, and the 5-year cumulative reoperation rate was 9% in the tube group and 29% in the trabeculectomy group (p = .025). Follow-up (mean ± SD) after additional glaucoma surgery was 28.0 ± 16.0 months in tube group and 30.5 ± 20.4 months in the trabeculectomy group (p = .76). At 2 years after a glaucoma reoperation, IOP (mean ± SD) was 15.0 ± 5.5 mm Hg in the tube group and 14.4 ± 6.6 mm Hg in the trabeculectomy group (p = .84). The number of glaucoma medications (mean ± SD) after 2 years of follow-up was 1.1 ± 1.3 in the tube group and 1.4 ± 1.4 in the trabeculectomy group (p = .71). The cumulative probability of failure at 1, 2, 3, and 4 years after additional glaucoma surgery was 0%, 43%, 43%, and 43% respectively in the tube group, and 0%, 9%, 20%, and 47% in the trabeculectomy group (p = .28). Reoperations to manage complications were required in 1 (13%) patient in the tube group and 5 (28%) patients in the trabeculectomy group (p = .63).
Conclusions
The rate of reoperation for glaucoma was higher following trabeculectomy with mitomycin C than tube shunt surgery in the TVT Study. Similar surgical outcomes were observed after additional glaucoma surgery, irrespective of initial randomized treatment in the study.
The Tube Versus Trabeculectomy (TVT) Study is a multicenter randomized clinical trial comparing the safety and efficacy of tube shunt surgery and trabeculectomy with mitomycin C (MMC) in eyes with previous ocular surgery. The study found similar intraocular pressure (IOP) and use of glaucoma medical therapy with both surgical procedures after 5 years of follow-up.1 Trabeculectomy with MMC had higher rates of surgical failure and reoperation for glaucoma compared with tube shunt surgery. No difference in the rate of vision loss was observed following the two procedures. Early postoperative complications were more frequent after trabeculectomy with MMC relative to tube shunt placement, but both procedures had similar rates of late postoperative complications and serious complications after 5 years.2
In previous publications of TVT Study data, patients were censored from analysis of IOP, use of glaucoma medications, and complications after a glaucoma reoperation was performed.1–6 Therefore, the outcomes of patients who had additional glaucoma surgery in the study have not been previously described. The purpose of this study is to report the incidence and outcomes of reoperations for glaucoma in the TVT Study.
METHODS
The design and methods of the TVT Study have been previously described in detail.7 The present investigation is a cohort study of patients in a multicenter randomized clinical trial. The study was approved by the Institutional Review Board at each Clinical Center. Written informed consent was obtained from all subjects for both the treatment and participation in the research. The study adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). The TVT Study is registered in http://www.clinicaltrials.gov (NCT00306852).
Enrolled patients were randomly assigned to treatment with a tube shunt or trabeculectomy with MMC. Patients in the tube group underwent placement of a 350-mm2 Baerveldt glaucoma implant in the superotemporal quadrant with a complete restriction of flow at the time of implantation. Patients in the trabeculectomy group had a superior trabeculectomy with a standard dosage of MMC of 0.4 mg/ml for 4 minutes. Follow-up visits were scheduled 1 day, 1 week, 1 month, 3 months, 6 months, 1 year, 18 months, 2 years, 3 years, 4 years, and 5 years postoperatively. Each examination included measurement of Snellen visual acuity (VA), intraocular pressure (IOP), slit lamp biomicroscopy, Seidel testing, and ophthalmoscopy. The examining clinician provided a reason for loss of two or more lines of Snellen VA at follow-up visits after 3 months.
Reoperations for glaucoma or complications were defined as additional procedures that required a return to the operating room. Cyclodestruction, whether performed in the clinic or operating room setting, was also counted a reoperation for glaucoma. Interventions done at the slit lamp, such as needling procedures, were not considered glaucoma reoperations. The decision to perform additional glaucoma surgery was left to the discretion of the investigator. Patients were censored from several analyses at the time of reoperation for glaucoma in previous publications reporting TVT Study outcomes. However, data were still collected at scheduled follow-up visits after a reoperation. The IOP and number of glaucoma medications were determined immediately prior to repeat glaucoma surgery. The criteria used to define surgical failure after initial randomized treatment (IOP > 21 mm Hg or not reduced by 20% below baseline on two consecutive follow-up visits after 3 months, IOP ≤ 5 mm Hg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, or loss of light perception vision) were applied after reoperation for glaucoma in the present study.
Patients were grouped for data analysis based upon their initial randomized treatment in the TVT Study. Univariate comparisons between treatment groups were performed using the two-sided Student t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Snellen VA measurements were converted to logMAR equivalents for the purpose of data analysis, as reported previously.8 The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma, or as the time from surgical treatment to the first of two consecutive follow-up visits after 3 months in which the patient had persistent hypotony (IOP ≤ 5 mm Hg) or inadequately reduced IOP (IOP > 21 mm Hg or not reduced by 20%). A p-value of .05 or less was considered statistically significant in our analyses.
RESULTS
Patient Characteristics
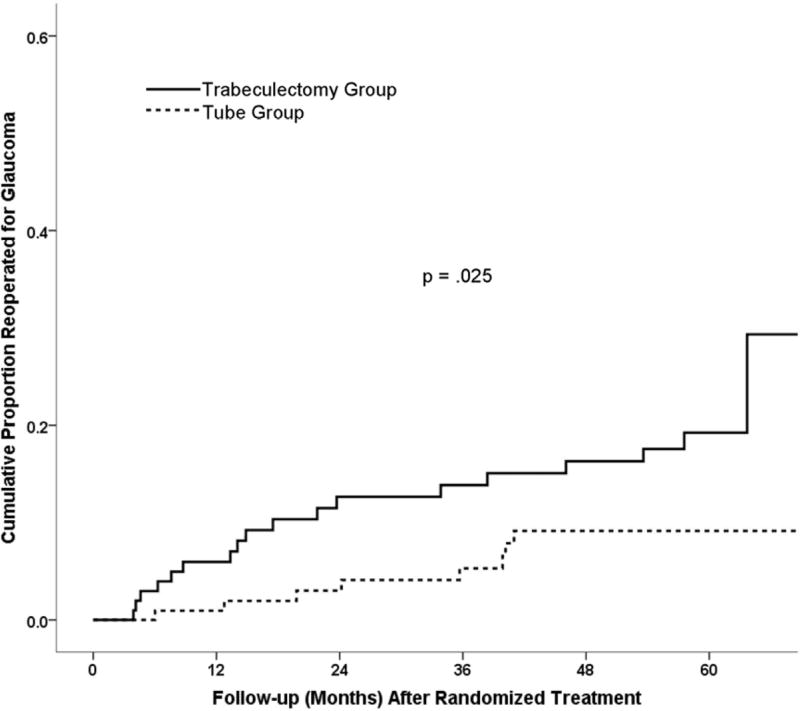
The TVT Study enrolled 212 patients, including 107 in the tube group and 105 in the trabeculectomy group. Figure 1 presents the results from Kaplan-Meier survival analysis comparing the rates of reoperation between the two treatment groups in the TVT Study after initial randomization. The 5-year cumulative reoperation rate was 9% in the tube group and 29% in the trabeculectomy group (p = .025, log-rank test adjusted for stratum). Table 1 shows the patient characteristics at the time of glaucoma reoperation in the TVT Study. No significant differences in any of the demographic or ocular features were observed between the two treatment groups, although there was a tendency for patients in tube group to have a lower IOP at the time of additional glaucoma surgery.
Figure 1.
Kaplan-Meier plots of the cumulative probability of reoperation for glaucoma in the Tube Versus Trabeculectomy Study after randomized treatment. Tube reoperation rate at 1 year = 1% (n = 101), 2 years = 3% (n = 90); 3 years = 5% (n = 79), 4 years = 9% (n = 67), 5 years = 9% (n = 42). Trabeculectomy reoperation rate at 1 year = 6% (n = 90), 2 years = 13% (n = 74); 3 years = 14% (n = 71), 4 years = 16% (n = 66), 5 years = 29% (n = 39).
Table 1.
Patient Characteristics at the Time of Glaucoma Reoperation in the Tube Versus Trabeculectomy Study
| Tube Group (n = 8) |
Trabeculectomy Group (n = 18) |
P-value | |
|---|---|---|---|
|
| |||
| Age (years) | .87b | ||
| Mean ± SD | 69.3 ± 12.6 | 68.4 ± 11.9 | |
| Range | 48–83 | 33–88 | |
|
| |||
| Gender, n (%) | .22c | ||
| Male | 6 (75) | 8 (44) | |
| Female | 2 (25) | 10 (56) | |
|
| |||
| Race, n (%) | 1.00d | ||
| White | 3 (38) | 6 (33) | |
| Black | 5 (63) | 10 (56) | |
| Hispanic | 0 | 1 (6) | |
| Other | 0 | 1 (6) | |
|
| |||
| Diabetes mellitus, n (%) | 2 (25) | 9 (50) | .40c |
|
| |||
| Hypertension, n (%) | 4 (50) | 9 (50) | 1.00c |
|
| |||
| IOP (mm Hg), | .078b | ||
| Mean ± SD | 21.3 ± 5.6 | 27.5 ± 8.6 | |
| Range | 14–33 | 18–47 | |
|
| |||
| Glaucoma medications, mean ± SD | 3.3 ± 1.2 | 2.9 ± 1.1 | .46b |
|
| |||
| Diagnosis, n (%) | .24d | ||
| POAG | 8 (100) | 12 (67) | |
| CACG | 0 | 2 (11) | |
| Other | 0 | 4 (22) | |
|
| |||
| Lens status, n (%) | .85d | ||
| Phakic | 1 (13) | 2 (11) | |
| PCIOL | 7 (88) | 13 (72) | |
| ACIOL | 0 | 2 (11) | |
| Aphakic | 0 | 1 (6) | |
|
| |||
| Stratuma | .39d | ||
| 1 | 2 (25) | 9 (50) | |
| 2 | 4 (50) | 4 (22) | |
| 3 | 0 | 2 (11) | |
| 4 | 2 (25) | 3 (17) | |
|
| |||
| Previous intraocular surgery | .70b | ||
| Mean ± SD | 2.88 ± .99 | 2.72 ± .89 | |
| Range | 2–5 | 2–5 | |
|
| |||
| Time interval since randomized surgical treatment (months), | .67b | ||
| Mean ± SD | 27.4 ± 13.7 | 24.1 ± 19.8 | |
| Range | 6–41 | 4–64 | |
|
| |||
| Snellen VA | .31b | ||
| LogMAR mean ± SD | .94 ± .89 | .60 ± .58 | |
| Median | 20/100 | 20/50 | |
| Range | 20/20-CF | 20/25-CF | |
ACIOL = anterior chamber intraocular lens; CACG = chronic angle-closure glaucoma; CF = count fingers; IOP = intraocular pressure; PCIOL = posterior chamber intraocular lens; POAG = primary openangle glaucoma; SD = standard deviation; VA = visual acuity
Stratum 1 = previous cataract extraction; stratum 2 = previous trabeculectomy or combined procedure without an antifibrotic agent; stratum 3 = previous trabeculectomy with 5-fluorouracil or combinedprocedure with 5-fluorouracil or mitomycin C; stratum 4 = previous trabeculectomy with mitomycin C
Student t-test
Fisher’s exact test
Exact permutation chi-square test
Types of Glaucoma Reoperations
Table 2 shows the reoperations that were performed for glaucoma in the TVT Study after 5 years of follow-up. A total of 18 patients in the trabeculectomy group underwent additional glaucoma surgery, which involved placement of a tube shunt in 15 patients, a bleb revision with tube shunt placement in 2 patients, and a trabeculectomy with 5-fluorouracil in 1 patient. One of these patients underwent a transscleral cyclophotocoagulation 31 months after tube shunt placement as a second reoperation for glaucoma in the study eye. In the tube group, 8 patients had glaucoma reoperations, including placement of a second tube shunt in 4 patients, transscleral cyclophotocoagulation in 3 patients, and endocyclophotocoagulation performed in conjunction with cataract surgery in 1 patient. Transscleral cyclophotocoagulation was performed as a second reoperation for glaucoma 13 months after combined cataract extraction and endocyclophotocoagulation in 1 patient, and 12 months after transcleral cyclophotocoagulation in another patient.
Table 2.
Reoperations for Glaucoma After 5 Years of Follow-up in the Tube Versus Trabeculectomy Study
| Tube Group (n = 107) |
Trabeculectomy Group (n = 105) |
|
|---|---|---|
| Tube shunt | 4 | 15 |
| Transscleral cyclophotocoagulation | 3 | 0 |
| Endocyclophotocoagulation/cataract extraction | 1 | 0 |
| Bleb revision and tube shunt | 0 | 2 |
| Trabeculectomy with 5-FU | 0 | 1 |
| Total number of patients (cumulative percentage) with reoperation for glaucomaa | 8b (9) | 18c (29) |
5-FU = 5-fluorouracil
Data are presented as number of patients.
P = .025 for the difference in 5-year cumulative reoperation rates for glaucoma between treatment groups from Kaplan-Meier analysis (log rank test adjusted for stratum)
2 patients underwent transscleral cyclophotocoagulation as a second reoperation for glaucoma.
1 patient underwent transscleral cyclophotocoagulation as a second reoperation for glaucoma.
Risk Factor Analysis for Glaucoma Reoperation
Baseline demographic and clinical characteristics of the overall TVT Study population were evaluated as possible predictors for reoperation for glaucoma after 5 years of follow-up, and the results are provided in Table 3. Reoperations were pooled from both treatment groups for this risk factor analysis. Only assigned treatment was significantly associated with reoperation for glaucoma in univariate analysis (p = .025, log-rank test). Stratum, age, gender, ethnicity, diabetes mellitus, hypertension, lens status, number of previous intraocular surgies, glaucoma type, preoperative number of medications, preoperative IOP, preoperative Snellen VA, and clinical centers were not associated with additional glaucoma surgery either univariately or in a multivariate model adjusted for treatment.
Table 3.
Risk Factor Analysis for Reoperation for Glaucoma After 5 Years of Follow-up in the Tube Versus Trabeculectomy Study
| Risk Factor | Number (%) |
Cumulative Probability of Reoperation for Glaucoma (%)b |
P-value | |
|---|---|---|---|---|
|
| ||||
| Univariatec | Multivariated | |||
|
| ||||
| Stratuma | .45 | .54 | ||
| 1 | 94 (44) | 14.1 | ||
| 2 | 49 (23) | 30.3 | ||
| 3 | 35 (17) | 7.6 | ||
| 4 | 34 (16) | 17.6 | ||
|
| ||||
| Age (years) | .11 | .12 | ||
| < 60 years | 31 (15) | 16.4 | ||
| 60–69 | 59 (28) | 47.4 | ||
| 70–79 | 79 (37) | 12.0 | ||
| ≥ 80 | 43 (20) | 6.2 | ||
|
| ||||
| Gender | .40 | .43 | ||
| Male | 100 (47) | 16.5 | ||
| Female | 112 (53) | 20.7 | ||
|
| ||||
| Race | .11 | .12 | ||
| White | 95 (45) | 10.7 | ||
| Black | 82 (39) | 31.6 | ||
| Hispanic | 30 (14) | 3.8 | ||
| Other | 5 (2) | 50.0 | ||
|
| ||||
| Diabetes mellitus | .18 | .18 | ||
| Yes | 67 (32) | 20.5 | ||
| No | 145 (68) | 17.2 | ||
|
| ||||
| Hypertension | .36 | .22 | ||
| Yes | 124 (59) | 19.9 | ||
| No | 88 (42) | 17.0 | ||
|
| ||||
| Lens status | .34 | .35 | ||
| Phakic | 45 (21) | 14.5 | ||
| PCIOL | 160 (76) | 18.3 | ||
| ACIOL | 7 (3) | 31.4 | ||
|
| ||||
| Previous intraocular surgery | .27 | .13 | ||
| 1 | 163 (77) | 18.3 | ||
| 2 | 41 (19) | 19.9 | ||
| 3 or 4 | 8 (4) | 27.1 | ||
|
| ||||
| Time since last intraocular surgery (months) | .76 | .89 | ||
| < 6 months | 15 (7) | 14.4 | ||
| ≥ 6 months | 190 (93) | 18.6 | ||
|
| ||||
| Glaucoma type | .31 | .14 | ||
| Primary | 190 (90) | 18.3 | ||
| Secondary | 22 (10) | 18.2 | ||
|
| ||||
| Preoperative number of glaucoma medications | .86 | .58 | ||
| 0–1 | 21 (10) | 10.0 | ||
| 2–3 | 108 (51) | 21.1 | ||
| 4–6 | 83 (39) | 15.2 | ||
|
| ||||
| Preoperative IOP (mm Hg) | .61 | .67 | ||
| < 23 | 77 (36) | 21.7 | ||
| 23–26 | 66 (31) | 18.7 | ||
| > 26 | 69 (33) | 13.9 | ||
|
| ||||
| Preoperative Snellen VA | .27 | .58 | ||
| ≥ 20/30 | 106 (50) | 12.1 | ||
| 20/40–20/150 | 74 (35) | 20.0 | ||
| ≤ 20/200 | 32 (15) | 38.9 | ||
|
| ||||
| Clinical Centers | .38 | .40 | ||
| Enrolled ≥ 50% patients | 133 (63) | 19.0 | ||
| Enrolled < 50% patients | 79 (37) | 15.9 | ||
|
| ||||
| Treatment | .025 | - | ||
| Tube | 107 (50) | 9.1 | ||
| Trabeculectomy | 105 (50) | 29.3 | ||
ACIOL = anterior chamber intraocular lens; IOP = intraocular pressure; PCIOL = posterior chamber intraocular lens; VA = visual acuity
Stratum 1 = previous cataract extraction; stratum 2 = previous trabeculectomy or combined procedure without an antifibrotic agent; stratum 3 = previous trabeculectomy with 5-fluorouracil or combined procedure with 5-fluorouracil or mitomycin C; stratum 4 = previous trabeculectomy with mitomycin
Kaplan-Meier survival analysis
Log-rank test
Cox proportional hazard regression analysis, p value adjusted for treatment.
Evaluation for Reoperation Bias
Because the surgeon was not masked to the treatment assignment, a potential bias existed in the decision to reoperate for glaucoma. To evaluate for selection bias, the IOP levels were compared between the tube and trabeculectomy groups in patients who had inadequate IOP control after randomized treatment. The IOP (mean ± SD) was 21.1 ± 5.7 mm Hg for the 8 patients in the tube group and 27.0 ± 9.0 mm Hg for the 18 patients in the trabeculectomy group at the time of reoperation for glaucoma (p = .11, Student t-test). The IOP levels were also compared between the 12 patients in the tube group and 11 patients in the trabeculectomy group who failed because of inadequate IOP reduction (i.e. IOP > 21 mm Hg or not reduced by 20% from baseline) but did not undergo additional glaucoma surgery during 5 years of follow-up. In this patient subgroup, the IOP (mean ± SD) was 23.0 ± 5.1 mm Hg in the tube group and 20.1 ± 2.6 in the trabeculectomy group (p = .11, Student t-test). The mean IOP prior to reoperation for glaucoma was similar in the tube and trabeculectomy groups, and no significant difference was seen between treatment groups in mean IOP among patients who failed because of inadequate IOP reduction but did not undergo additional glaucoma surgery.
IOP and Glaucoma Medical Therapy
Table 4 provides data on IOP and use of glaucoma medical therapy after a glaucoma reoperation in the tube and trabeculectomy groups. No significant differences in mean IOP and use of medical therapy were seen between the two treatment groups at any time point.
Table 4.
Intraocular Pressure and Medical Therapy Preoperatively and Postoperatively Following Repeat Glaucoma Surgery in the Tube Versus Trabeculectomy Study
| Tube Group | Trabeculectomy Group | P-valueb | |
|---|---|---|---|
|
| |||
| Preoperative | |||
| IOP (mm Hg) | 21.3 ± 5.6 | 27.5 ± 8.6 | .078 |
| Glaucoma medications | 3.3 ± 1.2 | 2.9 ± 1.1 | .46 |
| N | 8 | 17a | |
|
| |||
| 6 months | |||
| IOP (mm Hg) | 12.2 ± 5.6 | 17.4 ± 8.1 | .35 |
| Glaucoma medications | 0 (0) | .3 ± .8 | .52 |
| N | 3 | 6 | |
|
| |||
| 1 year | |||
| IOP (mm Hg) | 11.6 ± 3.9 | 15.5 ± 5.6 | .15 |
| Glaucoma medications | 1.8 ± 1.3 | 1.1 ± 1.4 | .29 |
| N | 6 | 13 | |
|
| |||
| 2 years | |||
| IOP (mm Hg) | 15.0 ± 5.5 | 14.4 ± 6.6 | .84 |
| Glaucoma medications | 1.1 ± 1.3 | 1.4 ± 1.4 | .71 |
| N | 7 | 10 | |
|
| |||
| 3 years | |||
| IOP (mm Hg) | 8.2 ± 3.2 | 14.1 ± 6.3 | .16 |
| Glaucoma medications | 1.7 ± 1.5 | 1.2 ± 1.0 | .56 |
| N | 3 | 9 | |
|
| |||
| 4 years | |||
| IOP (mm Hg) | 7.8 ± 4.6 | 16.4 ± 4.1 | .078 |
| Glaucoma medications | 1.0 ± 1.4 | 1.8 ± 1.7 | .63 |
| N | 2 | 4 | |
|
| |||
| 5 years | |||
| IOP (mm Hg) | – | 19.6 ± 1.1 | – |
| Glaucoma medications | – | .5 ± 1.0 | – |
| N | – | 4 | |
|
| |||
| Last follow-up | |||
| IOP (mm Hg) | 13.4 ± 6.6 | 16.4 ± 7.0 | .35 |
| Glaucoma medications | 1.7 ± 1.4 | .9 ± 1.3 | .21 |
| N | 7 | 16 | |
Data presented as mean ± standard deviation.
IOP = intraocular pressure
IOP was not available immediately prior to reoperation in 1 patient.
Student t-test
Surgical Success
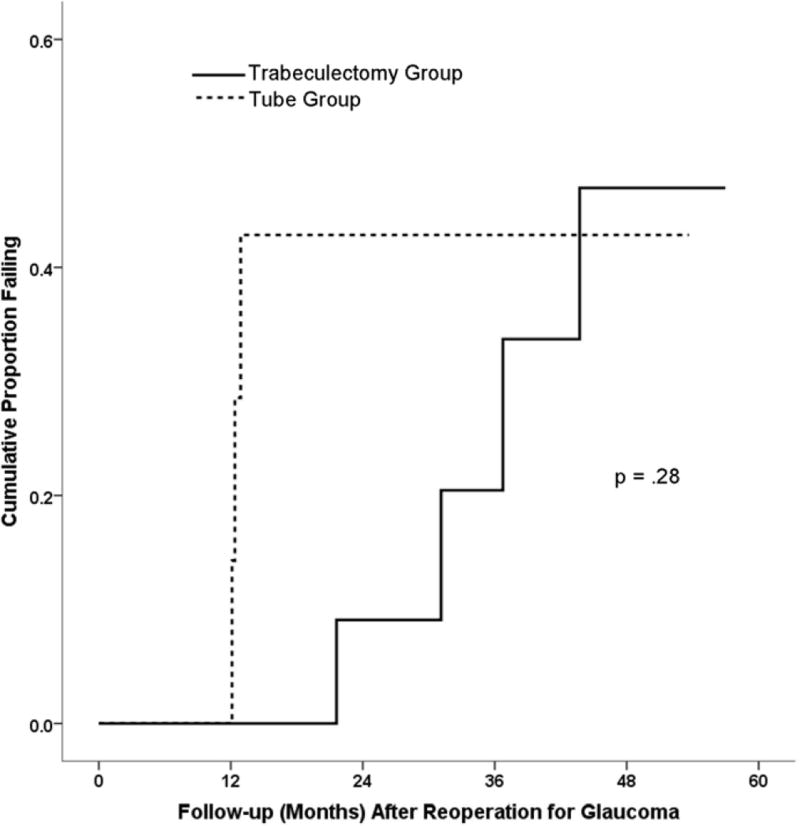
Kaplan-Meier survival analysis was used to compare failure rates in patients who had glaucoma reoperations, and the results are shown in Figure 2. The cumulative probability of failure at 1, 2, 3, and 4 years after additional glaucoma surgery was 0%, 43%, 43%, and 43% respectively in the tube group, and 0%, 9%, 20%, and 47% in the trabeculectomy group (p = .28, log rank test). Earlier failure was observed in the tube group compared with the trabeculectomy group, although this difference was not statistically significant. Treatment failure occurred in 3 (38%) patients in the tube group, including 1 patient who had inadequate IOP reduction and 2 patients who underwent a cyclophotocoagulation as a second reoperation for glaucoma. There were 4 (22%) patients who failed in the trabeculectomy group, including 2 patients who had inadequate IOP reduction, 1 patient who had a cyclophotocoagulation as another glaucoma reoperation, and 1 patient who developed persistent hypotony.
Figure 2.
Kaplan-Meier plots of the cumulative probability of failure in the Tube Versus Trabeculectomy Study after a reoperation for glaucoma. Tube failure rate at 1 year = 0% (n = 7), 2 years = 43% (n = 3), 3 years = 43% (n = 2), 4 years = 43% (n = 1). Trabeculectomy failure rate at 1 year = 0% (n = 14), 2 years = 9% (n = 9), 3 years = 20% (n = 6), 4 years = 47% (n = 2).
Complications
Table 5 lists postoperative complications that developed after reoperation for glaucoma. A total of 5 complications were reported in 2 (25%) patients in the tube group, and 15 complications were reported in 8 (44%) patients in the trabeculectomy group (p = .42, Fisher’s exact test). Among patients who had additional glaucoma surgery, reoperations were required to manage complications in 1 (13%) patient in the tube group and 5 (28%) patients in the trabeculectomy group (p = .63, Fisher’s exact test). A penetrating keratoplasty was performed in 1 patient in the tube group for persistent corneal edema, and this patient subsequently underwent removal of the intraocular lens, anterior vitrectomy, and tube repositioning for tube-cornea touch. There were 3 patients in the trabeculectomy group who had a penetrating keratoplasty for persistent corneal edema, and 1 of these patients subsequently underwent a repeat penetrating keratoplasty. A pars plana vitrectomy with injection of intravitreal antibiotics was performed in 1 patient in the trabeculectomy group for endophthalmitis. Another patient in the trabeculectomy group had a pars plana vitrectomy and scleral buckling procedure for a retinal detachment, and the tube shunt was later removed and a new shunt placed in a different quadrant for a tube erosion.
Table 5.
Postoperative Complications After Reoperation for Glaucoma in the Tube Versus Trabeculectomy Study
| Tube Group (n = 8) |
Trabeculectomy Group (n = 18) |
|
|---|---|---|
| Persistent corneal edema | 1 | 5 |
| Choroidal effusion | 0 | 2 |
| Cystoid macular edema | 0 | 2 |
| Hypotony maculopathy | 0 | 2 |
| Shallow anterior chamber | 2 | 0 |
| Suprachoroidal hemorrhage | 0 | 1 |
| Endophthalmitis | 0 | 1 |
| Retinal detachment | 0 | 1 |
| Tube erosion | 0 | 1 |
| Tube-cornea touch | 1 | 0 |
| Iridocorneal adhesions | 1 | 0 |
| Total number of patients with postoperative complicationsa,b | 2 | 8 |
Data are presented as number of patients.
Patients can have more than one complication.
P = .42 for the difference in total number of patients with postoperative complications between treatment groups (Fisher’s exact test)
Visual Acuity
LogMAR Snellen VA (mean ± SD) was .94 ± .89 in the tube group and .60 ± .58 at the time of glaucoma reoperation (p = .31, Student t-test), which represented a .52 ± .91 and .25 ± .49 decrease from the time of entry into the TVT Study in the tube and trabeculectomy groups, respectively (p = .41, Student t-test). At last follow-up, logMAR Snellen VA (mean ± SD) was reduced from its preoperative level an additional .20 ± .58 in the tube group and .36 ± .71 in the trabeculectomy group (p = .64, Student t-test) following glaucoma reoperation. Loss of 2 or more Snellen lines occurred in 2 (33%) patients in the tube group and 7 (58%) patients in the trabeculectomy group (p = .62, Fisher’s exact test). Snellen VA immediately prior to glaucoma reoperation was not available in 1 patient in the tube group and 5 patients in the trabeculectomy group, and patients were excluded from the calculation of rates of vision loss of 2 Snellen lines if they had less than 6 months of follow-up. The length of follow-up (mean ± SD) was 28.0 ± 16.0 months in the tube group and 30.5 ± 20.4 months in the trabeculectomy group (p = .62, Student t-test).
DISCUSSION
The TVT Study is a multicenter randomized clinical trial that enrolled patients with medically uncontrolled glaucoma who had prior cataract and/or glaucoma surgery and randomized them to surgical treatment with a 350-mm2 Baerveldt glaucoma implant or trabeculectomy with MMC. In prior publications describing treatment outcomes and surgical complications in the TVT Study, patients were censored from analysis after a reoperation for glaucoma. Therefore, the outcomes of patients who had additional glaucoma surgery in the study have not been previously reported.
The rate of reoperation for glaucoma was higher in the trabeculectomy group relative to the tube group. Following randomization to the study procedure, the decision to reoperate for glaucoma and the surgical approach were left to the discretion of the surgeon. Nearly all patients in the trabeculectomy group underwent placement of a tube shunt superotemporally when filtration failure occurred. One patient had a repeat trabeculectomy with 5-fluorouracil by an outside ophthalmologist who was not an investigator in the study. In the tube group, implantation of a second tube shunt and cyclodestruction were selected with equal frequency when initial tube shunt surgery failed.
Additional glaucoma surgery in patients with unsuccessful tube shunt surgery is generally considered to be more complex than in those who have failed trabeculectomy. Because investigators were not masked to the treatment assignment and the decision to reoperate was left to the surgeon’s judgment, a potential bias existed in the decision to perform additional surgery for glaucoma. We explored for this potential reoperation bias by comparing the IOPs between the two treatment groups among patients who failed because of inadequate IOP control. If surgeons had a higher threshold for reoperating on patients who underwent tube shunt surgery, one would expect that the IOP level at the time of additional glaucoma surgery would have been higher in the tube group than the trabeculectomy group. The opposite was actually observed. There was a tendency for the tube group to have a lower IOP immediately prior to reoperation, although this difference did not quite reach the level of statistical significance. Additionally, mean IOP was similar between treatment groups at the time of failure in patients with inadequate IOP reduction (i.e. IOP > 21 mm Hg or not reduced by 20% from baseline) who did not undergo a glaucoma reoperation. These observations strongly suggest that there was not a bias against reoperating for glaucoma in patients in the tube group.
IOP reduction was generally achieved in patients who underwent additional glaucoma surgery in the TVT Study with an associated reduction in the use of glaucoma medical therapy. Similar mean IOP and use of glaucoma medications were observed in the tube and trabeculectomy groups after glaucoma reoperations. The cumulative probability of success was 53% at 4 years among patients who failed a trabeculectomy with MMC and had a subsequent glaucoma reoperation. This success rate is consistent with other studies investigating the results of glaucoma surgery in eyes with failed filtering surgery (Table 6), which range from 32% to 92% for repeat trabeculectomy with an adjunctive antifibrotic agent8–17 and 44% to 88% for placement of a tube shunt.18–23 Among patients who failed initial tube shunt surgery in the TVT Study, the cumulative probability of success was 57% at 4 years with repeat glaucoma surgery. This result is similar to the rates reported in previous studies (Table 7), including success rates of 37% to 86% for second tube shunts24–29 and 67% to 71% for cyclodestruction29–31 in patients who failed tube shunt implantation.
Table 6.
Surgical Results in Eyes with Failed Filters in the Tube Versus Trabeculectomy Study and Other Studies
| Authors | Procedure | Number of Eyes |
IOP Reduction | Success Rate | IOP Success Criteria (mm Hg) |
Follow-up (months) | |
|---|---|---|---|---|---|---|---|
| Mean | Range | ||||||
|
| |||||||
| Heuer, et al | Trab with 5-FU | 16 | – | 81% at last follow-up | ≤ 21 with meds ≤ 25 without meds | 18.5 | 9-27 |
|
| |||||||
| Weinreb | Trab with 5-FU | 12 | 51% | 92% at 1 year | ≤ 21 with meds ≤ 25 without meds | 12 | – |
|
| |||||||
| FFSS | Trab with 5-FU | 24 | – | 47% at 5 years | ≤ 21 | 60 | – |
|
| |||||||
| Chen, et al | Trab with MMC | 45 | – | 77.8% at last follow-up | < 21 | 36 | 12–96 |
|
| |||||||
| Singh, et al | Trab with MMC | 12 | 33% at last follow-up | 83% at last follow-up | ≤ 21 | 11.6 | 4–24 |
|
| |||||||
| Andreanos, et al | Trab with MMC | 24 | – | 83.3% at last follow-up | ≤ 20 without meds | 18 | 11–34 |
|
| |||||||
| You, et al | Trab with MMC | 44 | 56% at last follow-up | 88.6% at last follow-up | ≤ 21 | 38.2 | 6–53 |
|
| |||||||
| Law, et al | Trab with MMC | 75 | 39% at 3 years | 54.6% at 3 years | ≤ 18 and ≥ 20% reduction | 62.4 | 34–133 |
| 41.3% at 3 years | ≤ 15 and ≥ 25% reduction | ||||||
| 32.0% at 3 years | ≤ 12 and ≥ 30% reduction | ||||||
|
| |||||||
| Olali, et al | Trab with MMC | 50 | 54% at 1 year | 88% at 1 year | ≤ 21 with ≥ 20% reduction and ≥ 6 | 36.7 | 12–91 |
| 78% at 1 year | ≤ 16 with ≥ 20% reduction and ≥ 6 | ||||||
|
| |||||||
| Cankaya, et al | Trab with MMC | 28 | 29% at last follow-up | 82.1% at last follow-up | ≤ 21 | 19.4 | 12–30 |
| 57.1% at last follow-up | ≤ 18 | ||||||
| 50.0% at last follow-up | 30% reduction | ||||||
|
| |||||||
| Minckler, et al | Tube shunt | 10 | – | 70% at last follow-up | ≤ 21 | 12.3 | 6–25 |
|
| |||||||
| Lloyd, et al | Tube shunt | 12 | – | 83% at 4 years | ≤ 21 and > 5 | 41.4 | 15–64 |
|
| |||||||
| Hodkin, et al | Tube shunt | 12 | – | 75% at last follow-up | ≤ 21 | 16.1 | 7.1–26.1 |
|
| |||||||
| Mills, et al | Tube shunt | 9 | – | 46% at last follow-up | ≤ 22 | 42 | 8–78 |
|
| |||||||
| Broadway, et al | Tube shunt | 59 | 47% at last follow-up | 57.6% at last follow-up | < 22 | 43 | 6–120 |
|
| |||||||
| Roy, et al | Tube shunt | 17 | – | 88% at last follow-up | ≤ 21 and > 6 | 37.6 | 12–68 |
|
| |||||||
| Present study | Tube shunt | 18 | 48% at 2 years | 100% at 1 year | ≤ 21 with ≥ 20% reduction and > 5 | 30.5 | 1–58 |
| Tube shunt/bleb revision | 91% at 2 years | ||||||
| 80% at 3 years | |||||||
| Trab with 5-FU | 53% at 4 years | ||||||
FFSS = Fluorouracil Filtering Surgery Study; 5-FU = 5-fluorouracil; trab = trabeculectomy; MMC = mitomycin C
Table 7.
Surgical Results in Eyes with Failed Tube Shunts in the Tube Versus Trabeculectomy Study and Other Studies
| Authors | Procedure | Number of Eyes |
IOP Reduction | Success Rate | IOP Success Criteria (mm Hg) |
Follow-up (months) |
|
|---|---|---|---|---|---|---|---|
| Mean | Range | ||||||
|
| |||||||
| Shah, et al | Shunt revision | 12 | 7% at last follow-up | 56% at 2 years | ≥ 25% reduction | 25.2 | 3–108 |
| Second tube shunt | 21 | 40% at last follow-up | 84% at 2 years | 34.8 | 6–84 | ||
|
| |||||||
| Burgoyne, et al | Second tube shunt | 22 | 33% at last follow-up | 86% at last follow-up | ≤ 21 and ≥ 20% reduction | 35 | 2–89 |
|
| |||||||
| Godfrey, et al | Second tube shunt | 18 | 34% at last follow-up | 37% at 3 years | ≤ 21 and ≥ 20% reduction | 19.6 | 6–47 |
|
| |||||||
| Smith, et al | Second tube shunt | 19 | 43% at 1 year | 74% at 1 year | ≤ 21 with ≥ 20% reduction and ≥ 5 | 38.8 | 12–80 |
|
| |||||||
| Anand, et al | Second tube shunt | 43 | 44% at last follow-up | 83% at 3 years | < 21 and ≥ 25% reduction | 32.6 | 12–76 |
| 75% at 3 years | < 17 and ≥ 25% reduction | ||||||
|
| |||||||
| Sood, et al | Second tube shunt | 8 | 39% at last follow-up | 63% at 2 years | ≤ 22 | 26.3 | 13–42 |
| TSCPC | 9 | 38% at last follow-up | 67% at 2 years | 19.8 | 5–53 | ||
|
| |||||||
| Semchyshya, et al | TSCPC | 21 | 62% at last follow-up | 71% at last follow-up | ≤ 21 and > 5 | 26.9 | 7–58 |
|
| |||||||
| Ness, et al | TSCPC | 32 | 43% at 1 year | – | – | 17.1 | 2–68 |
|
| |||||||
| Present study | Second tube shunt | 8 | 28% at 2 years | 100% at 1 year | ≤ 21 with ≥ 20% reduction and > 5 | 28.0 | 2–54 |
| TSCP | 57% at 2 years | ||||||
| ECP/CE | 57% at 3 years | ||||||
| 57% at 4 years | |||||||
ECP/CE = endocyclophotocoagulation/cataract extraction; TSCPC = transscleral cyclophotocoagulation
Surgical complications occurred in a large proportion of patients who had glaucoma reoperations in the TVT Study. Many of these complications were transient and self-limited, such as choroidal effusions and shallowing of the anterior chamber. However, several complications required additional surgery to manage the complication. Corneal edema was the most common postoperative complication, and the most frequent indication for reoperation for a complication. The mean age at the time of glaucoma reoperation was approximately 69 years, and all patients had multiple ocular procedures. Advancing age and ocular surgery are known causes of reduced corneal endothelial cell density and increase the risk of corneal decompensation.32–41 Almost all of the patients in this study had a tube shunt present, either as the initial randomized procedure and/or the reoperation for glaucoma. Tube shunts are known to be associated with progressive endothelial cell loss.34,39–41 It is interesting to note that none of the patients who underwent placement of a second tube shunt developed corneal edema in the TVT Study. The rates of postoperative complications and reoperations for complications were similar between the tube and trabeculectomy groups.
Even though the TVT Study showed a higher success rate with tube shunt surgery compared with trabeculectomy with MMC during 5 years of follow-up,1 some surgeons may still advocate trabeculectomy over tube shunt placement in patients with similar characteristics as those enrolled in the TVT Study. One reason for such an approach may be the perception that trabeculectomy offers more surgical options, with a presumed higher probability of success should repeat glaucoma surgery be required. Patients who fail trabeculectomy may undergo repeat trabeculectomy or tube shunt placement. However, patients with unsuccessful tube shunt surgery are generally not candidates for trabeculectomy and undergo either placement of a second tube shunt or cyclodestruction. No significant differences in mean IOP, use of glaucoma medications, and rates of surgical success, vision loss, and complications were observed between patients who were randomized to initial treatment with trabeculectomy or tube shunt surgery and subsequently required additional glaucoma surgery. The results of this study indicate that patients who underwent initial treatment with a tube shunt or trabeculectomy with MMC in the TVT Study had similar outcomes when a reoperation for glaucoma was performed.
There are several limitations to the present study. While patients were randomized to their initial treatment in the TVT Study, the decision to reoperate for glaucoma and the surgical approach were decided by the surgeon. There was a tendency for the tube group to have lower IOP immediately prior to reoperation, and this may have affected the likelihood of subsequent surgical success. The small number of patients requiring additional glaucoma surgery in the TVT Study limits the power to detect significant differences between the two treatment groups. The length of follow-up was variable depending upon when the reoperation occurred during the course of the TVT Study. Neither the surgeon nor the patient was masked to the original treatment assignment and subsequent procedures.
In summary, IOP reduction was usually achieved in patients who had glaucoma reoperations in the TVT Study. Tube shunt placement was generally the preferred surgical approach in patients who failed trabeculectomy with MMC, while implantation of a second tube shunt and cyclophotocoagulation were selected with equal frequency in patients who failed initial tube shunt surgery. No significant differences in mean IOP, number of glaucoma medications, and rates of surgical success, vision loss, and complications were observed between the tube and trabeculectomy groups. These results suggest that the outcomes of reoperations for glaucoma were similar, irrespective of whether the patient was initially treated with placement of a Baerveldt glaucoma implant or trabeculectomy with MMC in the TVT Study.
Acknowledgments
Funding/Support: The study was supported by research grants from Pfizer, Inc., New York, New York, Abbott Medical Optics, Santa Ana, California, the National Eye Institute (grant EY014801), National Institutes of Health, Bethesda, Maryland, and Research to Prevent Blindness, Inc., New York, New York. The TVT Study is registered in http://www.clinicaltrials.gov (NCT00306852).
Biography

Hady Saheb, M.D., M.P.H. is Assistant Professor of Ophthalmology and Director of Resident Research at McGill University. He completed his ophthalmology residency and medical school at McGill University. He then pursued glaucoma fellowships at the Bascom Palmer Eye Institute and University of Toronto. He also completed a Master's in Public Health at the Johns Hopkins University Bloomberg School of Public Health. His research interests include surgical glaucoma, micro-invasive glaucoma surgery and angle closure glaucoma.
APPENDIX
Participating Centers and Committees in the Tube Versus Trabeculectomy Study
Clinical Centers:
Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami (Miami, Florida): Principal Investigator: Steven Gedde, M.D.; Coinvestigators: Douglas Anderson, M.D., Donald Budenz, M.D., M.P.H., Madeline Del Calvo, Peter Chang, M.D., Francisco Fantes, M.D., Fouad El Sayyad, M.D., David Greenfield, M.D., Jessica Hochberg, Elizabeth Hodapp, M.D., Richard Lee, M.D., Ph.D., Alexia Marcellino, Paul Palmberg, M.D. Ph.D., Richard Parrish II, M.D.
Duke University (Durham, North Carolina): Principal Investigator: Leon Herndon, M.D.; Coinvestigators: Pratap Challa, M.D., Cecile Santiago-Turla, M.D.
Indiana University (Indianapolis, Indiana): Principal Investigator: Darrell WuDunn, M.D., Ph.D.
Loyola University (Maywood, Illinois): Principal Investigator: Geoffrey Emerick, M.D.
Medical College of Wisconsin (Milwaukee, Wisconsin): Principal Investigator: Dale Heuer, M.D.
Medical University of South Carolina (Charleston, South Carolina): Principal Investigator: Alexander Kent, M.D.; Coinvestigators: Carol Bradham, Lisa Langdale.
Moorfields Eye Hospital (London, England): Principal Investigator: Keith Barton, M.D.; Coinvestigator: Francesca Amalfitano, Poornima Rai, M.D.
New York Eye and Ear Infirmary (New York, New York): Principal Investigator: Paul Sidoti, M.D.; Coinvestigators: Amy Gedal, James Luayon, Roma Ovase, Katy Tai
Scripps Clinic (La Jolla, California): Principal Investigator: Quang Nguyen, M.D.; Coinvestigator: Neva Miller
St. Louis University (St. Louis Missouri): Principal Investigator: Steven Shields, M.D.; Coinvestigators: Kevin Anderson, Frank Moya, M.D.
University of California, Davis (Sacramento, California): Principal Investigator: James Brandt, M.D.; Coinvestigator: Michele Lim, M.D., Marilyn Sponzo.
University of Florida (Gainesville, Florida): Principal Investigator: Mark Sherwood, M.D.; Coinvestigator: Revonda Burke
University of Oklahoma (Oklahoma City, Oklahoma): Principal Investigator: Gregory Skuta, M.D.; Coinvestigators: Jason Jobson, Lisa Ogilbee, Adam Reynolds, M.D., Steven Sarkisian, M.D.
University of Southern California (Los Angeles, California): Principal Investigator: Rohit Varma, M.D., M.P.H.; Coinvestigators: Brian Francis, M.D., Frances Walonker
University of Texas Houston (Houston, Texas): Principal Investigator: Robert Feldman, M.D.; Coinvestigators: Laura Baker, Nicholas Bell, JoLene Carranza, Athena Espinoza
University of Virginia (Charlottesville, Virginia): Principal Investigator: Bruce Prum, M.D.; Coinvestigator: Janis Beall
University of Wisconsin (Madison, Wisconsin): Principal Investigator: Todd Perkins, M.D.; Coinvestigators: Paul Kaufman, M.D., Tracy Perkins, Barbara Soderling.
Statistical Coordinating Center, Bascom Palmer Eye Institute, Miller School of Medicine, University of Miami (Miami, Florida): William Feuer, M.S., Luz Londono, Joyce Schiffman, M.S., Wei Shi, M.S.
Safety and Data Monitoring Committee: Philip Chen, M.D., William Feuer, M.S., Joyce Schiffman, M.S., Kuldev Singh, M.D., M.P.H., George Spaeth, M.D., Martha Wright, M.D.
Steering Committee: Keith Barton, M.D., James Brandt, M.D., Geoffrey Emerick, M.D., Robert Feldman, M.D., Steven Gedde, M.D., Leon Herndon, M.D., Dale Heuer, M.D., Alexander Kent, M.D., Quang Nguyen, M.D., Richard Parrish II, M.D., Todd Perkins, M.D., Bruce Prum, M.D., Mark Sherwood, M.D., Steven Shields, M.D., Paul Sidoti, M.D., Gregory Skuta, M.D., Rohit Varma, M.D., M.P.H., Darrell WuDunn, M.D., Ph.D.
Study Chairmen: Steven Gedde, M.D., Dale Heuer M.D., Richard Parrish II, M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. The following disclosures were reported: H.S.: consultancy (Alcon, Allergan), payment for lectures (Alcon, Allergan, Bausch and Lomb), travel/accommodations/meeting expenses (Ivantis); S.J.G.: grants (National Eye Institute, Research to Prevent Blindness, Abbott Medical Optics), consultancy (Alcon, Allergan); J.C.S.: grants (National Eye Institute, Research to Prevent Blindness, Abbott Medical Optics), support for travel to meetings for the study (Abbott Medical Optics); W.J.F.: grants (National Eye Institute, Abbott Medical Optics), support for travel to meetings for the study (Abbott Medical Optics).
Contributions of Authors: Involved in design and conduct of study (H.S., S.J.G., J.C.S., W.J.F.); collection, management, analysis, and interpretation of data (H.S., S.J.G., J.C.S., W.J.F.); and preparation, review, and approval of the manuscript (H.S., S.J.G., J.C.S., W.J.F.)
Other Acknowledgements: None.
References
- 1.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy Study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy Study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy Study after one year of follow-up. Am J Ophthalmol. 2007;143(1):9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2007;143(1):23–31. doi: 10.1016/j.ajo.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Rauscher FM, Gedde SJ, Schiffman JC, et al. Motility disturbances in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2009;147(3):458–466. doi: 10.1016/j.ajo.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol. 2009;148(5):670–84. doi: 10.1016/j.ajo.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Gedde SJ, Schiffman JC, Feuer WJ, et al. The Tube Versus Trabeculectomy Study: Design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140(2):275–287. doi: 10.1016/j.ajo.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 8.The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the fluorouracil filtering surgery study. Am J Ophthalmol. 1996;121(4):349–366. doi: 10.1016/s0002-9394(14)70431-3. [DOI] [PubMed] [Google Scholar]
- 9.Heuer DK, Parrish RK, Gressel MG, et al. 5-fluorouracil and glaucoma filtering surgery: III. Intermediate follow-up of a pilot study. Ophthalmology. 1986;93(12):1537–1546. doi: 10.1016/s0161-6420(86)33542-5. [DOI] [PubMed] [Google Scholar]
- 10.Weinreb RN. Adjusting the dose of 5-fluorouracil after filtration surgery to minimize side effects. Ophthalmology. 1987;94(5):564–570. doi: 10.1016/s0161-6420(87)33430-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen CW, Huang HT, Bair JS, Lee CC. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocular Pharmacol. 1990;6(3):175–182. doi: 10.1089/jop.1990.6.175. [DOI] [PubMed] [Google Scholar]
- 12.Singh J, O’Brien C, Chawla HB. Success rate and complications of intraoperative 0.2 mg/ml mitomycin C in trabeculectomy surgery. Eye. 1995;9(4):460–466. doi: 10.1038/eye.1995.107. [DOI] [PubMed] [Google Scholar]
- 13.Andreanos D, Georgopoulos GT, Vergados J, et al. Clinical evaluation of the effect of mitomycin-C in re-operation for primary open angle glaucoma. Eur J Ophthalmol. 1997;7(1):49–54. doi: 10.1177/112067219700700109. [DOI] [PubMed] [Google Scholar]
- 14.You YA, Gu YS, Fang CT, Ma XQ. Long-term effects of simultaneous and subscleral mitomycin C application in repeat trabeculectomy. J Glaucoma. 2002;11(2):110–118. doi: 10.1097/00061198-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Law SK, Shih K, Tran DH, et al. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol. 2009;148(5):685–695. doi: 10.1016/j.ajo.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Olali C, Rotchford AP, King AJ. Outcome of repeat trabeculectomies. Clin Experiment Ophthalmol. 2011;39(7):658–664. doi: 10.1111/j.1442-9071.2011.02519.x. [DOI] [PubMed] [Google Scholar]
- 17.Cankaya AB, Elgin U. Comparison of the outcome of repeat trabeculectomy with adjunctive mitomycin C and initial trabeculectomy. Korean J Ophthalmol. 2011;25(6):401–408. doi: 10.3341/kjo.2011.25.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minckler DS, Heuer DK, Hasty B, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Ophthalmology. 1988;95(9):1181–88. doi: 10.1016/s0161-6420(88)33029-0. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd MA, Sedlak T, Heuer DK, et al. Clinical experience with the single plate Molteno implant in complicated glaucomas. Update of a pilot study. Ophthalmology. 1992;99(5):679–87. doi: 10.1016/s0161-6420(92)31910-4. [DOI] [PubMed] [Google Scholar]
- 20.Hodkin MJ, Goldblatt WS, Burgoyne CF, et al. Early clinical experience with the Baerveldt implant in complicated glaucomas. Am J Ophthalmol. 1995;120(1):32–40. doi: 10.1016/s0002-9394(14)73756-0. [DOI] [PubMed] [Google Scholar]
- 21.Mills RP, Reynolds A, Emond MJ, et al. Long-term survival of Molteno glaucoma drainage devices. Ophthalmology. 1996;103(2):299–305. doi: 10.1016/s0161-6420(96)30700-8. [DOI] [PubMed] [Google Scholar]
- 22.Broadway DC, Iester M, Schulzer M, Douglas GR. Survival analysis for success for Molteno tube implants. Br J Ophthalmol. 2001;85(6):689–95. doi: 10.1136/bjo.85.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Ravinet E, Mermoud A. Baerveldt implant in refractory glaucoma: Long-term results and factors influencing outcomes. Int Ophthalmol. 2001;24(2):93–100. doi: 10.1023/a:1016335313035. [DOI] [PubMed] [Google Scholar]
- 24.Shah AA, WuDunn D, Cantor LB. Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol. 2000;129(4):455–460. doi: 10.1016/s0002-9394(99)00410-9. [DOI] [PubMed] [Google Scholar]
- 25.Burgoyne JK, WuDunn D, Lakhani V, Cantor LB. Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology. 2000;107(2):309–314. doi: 10.1016/s0161-6420(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 26.Godfrey DG, Krishna R, Greenfield DS, et al. Implantation of second glaucoma drainage devices after failure of primary devices. Ophthalmic Surg Lasers. 2002;33(1):37–43. [PubMed] [Google Scholar]
- 27.Smith M, Buys YM, Trope GE. Second Ahmed valve insertion in the same eye. J Glaucoma. 2009;18(4):336–340. doi: 10.1097/IJG.0b013e318182edfb. [DOI] [PubMed] [Google Scholar]
- 28.Anand A, Tello C, Sidoti PA, et al. Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol. 2010;149(1):95–101. doi: 10.1016/j.ajo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009;13(4):379–383. doi: 10.1016/j.jaapos.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser cyclophotocoagulation after aqueous shunt placement in refractory glaucoma. Ophthalmology. 2002;109(6):1078–1084. doi: 10.1016/s0161-6420(02)01019-9. [DOI] [PubMed] [Google Scholar]
- 31.Ness PJ, Khaimi MA, Feldman RM, et al. Intermediate term safety and efficacy of transscleral cyclophotocoagulation after tube shunt failure. J Glaucoma. 2012;21(2):83–88. doi: 10.1097/IJG.0b013e31820bd1ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laule A, Cable MK, Hoffman CE, Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol. 1978;96(11):2031–2035. doi: 10.1001/archopht.1978.03910060419003. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H, Jacobs PM, McPherson K, Noble MJ. Precision of cell density estimates and endothelial cell loss with age. Arch Ophthalmol. 1985;103(10):1478–1481. doi: 10.1001/archopht.1985.01050100054017. [DOI] [PubMed] [Google Scholar]
- 34.McDermott ML, Swendris RP, Shin DH, Juzych MS, Cowden JW. Corneal endothelial cell counts after Molteno implantation. Am J Ophthalmol. 1993;115(1):93–96. doi: 10.1016/s0002-9394(14)73530-5. [DOI] [PubMed] [Google Scholar]
- 35.Menucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: Microincision versus standard technique. J Cataract Refract Surg. 2006;32(8):1351–1354. doi: 10.1016/j.jcrs.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 36.Sheng H, Bullimore MA. Factors affecting corneal endothelial morphology. Cornea. 2007;26(5):520–525. doi: 10.1097/ICO.0b013e318033a6da. [DOI] [PubMed] [Google Scholar]
- 37.Arnavielle S, Lafontaine PO, Bidot S, Creuzot-Garcher C, D’Athis P, Bron AM. Corneal endothelial changes after trabeculectomy and deep sclerectomy. J Glaucoma. 2007;16(3):324–328. doi: 10.1097/IJG.0b013e3180391a04. [DOI] [PubMed] [Google Scholar]
- 38.Buys YM, Chipman ML, Zack B, Rootman DS, Slomovic AR, Trope GE. Prospective randomized comparion of one- versus two-site phacotrabeculectomy two-year results. Ophthalmology. 2008;115(7):1130–1133. doi: 10.1016/j.ophtha.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Kim CS, Yim JH, Lee EK, Lee NH. Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Experiment Ophthalmol. 2008;36(2):142–147. doi: 10.1111/j.1442-9071.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 40.Mendrinos E, Dosso A, Sommerhalder J, Shaarawy T. Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye. 2009;23(9):1836–1844. doi: 10.1038/eye.2008.321. [DOI] [PubMed] [Google Scholar]
- 41.Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol. 2009;148(3):361–367. doi: 10.1016/j.ajo.2009.04.016. [DOI] [PubMed] [Google Scholar]