Abstract
Matrix metalloproteinases (MMP) have been implicated in multiple stages of cancer metastasis. Tissue inhibitor of metalloproteinase-2 (TIMP-2) plays an important role in regulating MMP-2 activity. By forming a ternary complex with pro-MMP-2 and its activator MMP-14 on the cell surface, TIMP-2 can either initiate or restrain the cleavage and subsequent activation of MMP-2. Our recent work has shown that breast cancer cell adhesion to vascular endothelial cells activates endothelial MMP-2, promoting tumor cell transendothelial migration (TEME). However, the mechanism of MMP-2 regulation during TEME remains unclear. In the current study, we present evidence that MMP-14 is expressed in both invasive breast cancer cells (MDA-MB-231 and MDA-MB-436) and lung microvascular endothelial cells (HBMVEC-L), whereas TIMP-2 is exclusively expressed and released from the cancer cells. The tumor cell–derived TIMP-2 was further identified as a major determinant of endothelial MMP-2 activity during tumor cell transmigration in the presence of MMP-14. This response was associated with endothelial barrier dysfunction because coculture of MDA-MB-231 or MDA-MB-436 with HBMVEC-L caused a significant decrease in transendothelial electrical resistance concomitantly with endothelial cell-cell junction disruption and tumor cell transmigration. Knockdown of TIMP-2 or inhibition of TIMP-2/MMP-14 attenuated MMP-2–dependent transendothelial electrical resistance response and TEME. These findings suggest a novel interactive role of breast cancer cells and vascular endothelial cells in regulating the TIMP-2/MMP-14/MMP-2 pathway during tumor metastasis.
Introduction
Tumor metastasis to vital organs such as the lungs is a leading cause of death in breast cancer patients (1–3). As an essential step in tumor cell invasion and extravasation, transendothelial migration (TEM) across the vascular endothelium is not only dependent on directed tumor cell motility but is also regulated by the local endothelial barrier function. In the past decades, tremendous efforts have been devoted to characterize the molecular biology of invasive tumor cells, and an array of cytokine receptors (e.g., CXCR4) and adhesion molecules (e.g., integrins, E-selectin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, thrombospondin receptors) have been identified as participants in the endothelial response to tumor cell adhesion and transmigration. However, the precise mechanism of tumor-endothelial cell interactions and the specific role of endothelial barrier properties in controlling the TEM process are still far from clear. Further characterization of tumor cell–mediated endothelial responses may lead to the identification of new therapeutic targets for treatment of breast cancer metastasis.
Matrix metalloproteinase-2 (MMP-2) has long been associated with tumor angiogenesis and metastasis (4–8). Compelling evidence has suggested MMP-2 in tumors, as well as the level of its active form in the circulation, as a sensitive indicator of metastasis in breast cancer (9–12). A high level of active MMP-2 in breast cancer tissues has been correlated with decreased overall survival and recurrence-free survival (12–14). As an essential member of the MMP family of zinc-dependent endopeptideases, MMP-2 is secreted in its latent form (pro-MMP-2) and activated on the cell surface by MMP-14, which is also known as membrane type 1 MMP (15–17). A dual role for tissue inhibitor of metalloproteinase-2 (TIMP-2) has been proposed to explain the regulatory mechanism of MMP-14–induced MMP-2 activation (18). On one hand, TIMP-2 acts as a ligand of MMP-14. The NH2-terminal domain of TIMP-2 can bind the catalytic site of MMP-14, resulting in a TIMP-2/MMP-14 binary complex (19). On the other hand, the COOH-terminal domain of TIMP-2 serves as a receptor for the COOH-terminal region of MMP-2, thereby preventing it from further interacting with MMP-14 and subsequent MMP-2 activation (19, 20). It has been reported that the COOH-terminal region of TIMP-2 can form a noncovalent complex with latent MMP-2 through binding to the hemopexin-like domain of the zymogen (21). However, if the concentration of TIMP-2 is relatively low and cell surface MMP-14 is not saturated, non–TIMP-2-bound MMP-14 in close proximity can cleave the Asn37-Leu38 bond of a latent MMP-2 in the ternary complex (20, 22, 23), producing an inactive intermediate form of MMP-2 that subsequently undergoes autocatalytic hydrolysis at the Asn80-Tyr81 peptide bond, rendering a fully active form of MMP-2 (24, 25). In addition to TIMP-2, TIMP-4 is capable of binding to MMP-2 as well as MMP-14, preventing the autocatalytic activation process (26, 27). When TIMP-4 is coexpressed with TIMP-2, it inhibits the activation of latent MMP-2 via MMP-14 (26). These findings suggest that the balance between TIMP-2 and TIMP-4 in the extracellular microenvironment is a critical factor in regulating MMP-2 activity.
It is well documented that active MMP-2 degrades fibrillar collagens in the extracellular matrix (ECM; refs. 28–31), thereby facilitating cell invasion during various physiologic or pathologic processes (32–34). MMP-2 has also recently been found to be able to degrade cell-cell adhesion structures, such as N-cadherin and leukocyte cell adhesion molecules (35, 36). MMP-2 activation may therefore lead to reduced cell-cell and/or cell-ECM adhesions. It has been proposed that increased MMP-2 activity contributes to the initial process of metastasis by promoting tumor cell penetration across the basement membrane and invasion into the blood or lymphatic circulation (37, 38). However, little is known about the effect and mechanism of MMP-2–dependent extravasation of tumor cells from the circulation. In an effort to characterize the effect of MMP-2 in breast cancer cell TEME, we have shown that microvascular endothelial cells serve as an important source of MMP-2 production following breast cancer attachment to the endothelial surface (39).
In the present study, we investigated the underlying mechanism of MMP-2 activation during tumor cell transmigration. Our data revealed that MMP-14, a MMP-2 activator, was expressed in both breast cancer cells (MDA-MB-231 and MDA-MB-436) and lung microvascular endothelial cells (HBMVEC-L), whereas TIMP-2 was detected exclusively in tumor cells. We further showed that it was the tumor cell–derived TIMP-2 that activated endothelial MMP-2. This activation process required the presence of active MMP-14. Subsequent assays using the electric cell-substrate impedance sensing system and fluorescence immunocytochemistry showed that TEME was coupled with endothelial barrier dysfunction and cell-cell junction disruption; the effects were caused by TIMP-2/MMP-14–induced activation of endothelial MMP-2. Taken together, these findings suggest a cooperative mechanism between metastatic breast cancer cells and microvascular endothelial cells in promoting MMP-2 activation and tumor cell transmigration.
Materials and Methods
Antibodies and reagents
Blocking antibodies against the catalytic domain of MMP-14 (MAB3328/LEM-2) and the NH2 terminus of MMP-2 (MAB13405) were from Millipore. Human TIMP-2 and TIMP-4 ELISA kits, as well as the polyclonal TIMP-2 (amino acids 27–220) neutralizing antibody, were from R&D Systems. TIMP-4, β-tubulin, VE-cadherin, and normal IgG antibodies, as well as horseradish peroxidase– and TRITC-conjugated secondary antibodies, were from Santa Cruz Biotechnology. Radioimmunoprecipitation assay lysis buffer was from Upstate Cell Signaling Solutions. Protease inhibitor cocktail was from Thermo Scientific. Triton X-100 and phosphatase inhibitor cocktails were purchased from Sigma-Aldrich. Hoechst 33342, calcein-AM, Tris-glycine SDS sample buffer, sample reducing agent, 4–12% Tris-glycine gradient gel, 10% zymogram gelatin gel, zymogram renaturing and developing buffers, and the Colloidal Blue staining kit were purchased from Invitrogen.
Cell culture and siRNA transfection
Breast cancer MDA-MB-231 and MDA-MB-436 cells were purchased from the American Type Culture Collection and were grown in DMEM supplemented with 10% fetal bovine serum. Human lung blood microvascular endothelial cells (HBMVEC-L) were obtained from Lonza and maintained in EGM-2 medium. MDA-MB-231 and MDA-MB-436 cells were grown in six-well tissue culture plates until 80% confluence and then transfected with 1 µg of TIMP-2 siRNA (a pool of three target-specific 20- to 25-nt siRNAs, Santa Cruz Biotechnology) per well using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Protein expression was examined 48 hours after transfection.
Gelatin zymography
HBMVEC-L cells were seeded (105 cells/cm2) on the gelatin-coated 48-well plates (Corning Costar) overnight at 37°C, followed by serum starvation for 12 hours in EBM medium. In coculture experiments, MDA-MB-231 or MDA-MB-436 cells were the added onto the endothelial cells at 105 cells/cm2. Equal amounts of MDA-MB-231 or MDA-MB-436 cells were seeded into a series of empty wells in tumor cell monoculture experiments. In endothelial monoculture assays, HBMVEC-L cells were refreshed only with EBM medium. At various time points (1, 6, 12, and 24 hours), conditioned media were collected and examined by gelatin zymography for MMP-2 activities. The effect of tumor cell–derived TIMP-2 on MMP-2 activation was examined using MDA-MB-231 and MDA-MB-436 cells transfected with TIMP-2 siRNA or blank vehicles (mock transfection). For functional blocking, cells were treated with neutralizing antibodies against TIMP-2 (5 µg/mL) or MMP-14 (20 µg/mL). Normal IgG (20 µg/mL) was used as an isotype control. At each time point, 20 µL of conditioned media were mixed thoroughly with Tris-glycine sample buffer without reducing agent, and then subjected to zymogram gel electrophoresis. After sequential incubations in renaturing and developing buffers, the zymogram gels were incubated with Colloidal Blue stain buffer and then destained in deionized water. The developed bands were imaged with an Alpha Imager (Alpha Innotech) and quantified using the ImageJ software (NIH).
Western blot analysis
HBMVEC-L cells and breast cancer cells (MDA-MB-231 and MDA-MB-436) were harvested and homogenized in radioimmunoprecipitation assay lysis buffer freshly supplemented with 1% protease and phosphatase inhibitor cocktails. After the insoluble components were pelleted at 14,000 × g for 1 minute, the concentration of proteins in the supernatant was determined using a Bio-Rad detergent-compatible protein assay kit. Equal amounts (20 µg) of proteins were then separated by 4–12% Tris-glycine gradient gel, transferred onto Millipore Immobilon-P membranes, and blotted with appropriate primary antibodies. Following washing and incubation with horseradish peroxidase–conjugated secondary antibodies, the proteins of interest were visualized in Kodak BioMax Film using ECL Plus detection reagent (GE Healthcare). The films were then scanned and quantified using ImageJ software for protein levels.
ELISA assays
Conditioned media from either monoculture or coculture were collected as described above. The TIMP-2 and TIMP-4 levels were measured using ELISAs with the R&D Quantikine kits according to the manufacturer's protocol. Briefly, aliquots of samples and standard controls were added in microplate strips and incubated for 2 hours. After aspiration and four washes, horseradish peroxidase–conjugated TIMP-2 or TIMP-4 antibody was added for 2 hours. This was followed by sequential incubation with substrate solution and stop solution. The absorbance of each sample was determined at 450 nm (wavelength correction at 540 nm) using a microplate reader.
Transendothelial migration assay
HBMVEC-L cells were seeded (105 cells/cm2) on gelatin-coated Transwell inserts (Corning Costar, 6.5-mm diameter, 8-µm pore size) overnight to get a confluent monolayer in the upper chamber. The luminal (apical) and abluminal (subendothelial) compartments were therefore generated in the upper and bottom chambers, respectively (40–43). After serum starvation in EBM medium, 500 µL of EBM medium containing 10% fetal bovine serum were added to the bottom chamber and 200 µL of EBM containing calcein-stained breast cancer cells (MDA-MB-231 or MDA-MB-436) with or without TIMP-2 siRNA transfection were added (105 cells/cm2) to the upper chamber, and cells were incubated for 12 hours. The mock-transfected cells were used as controls. In functional blockage experiments, MDA-MB-231 or MDA-MB-436 cells were added to the upper chamber and incubated in the presence of an antibody against TIMP-2 (5 µg/mL), MMP-14 (20 µg/mL), or MMP-2 (5 µg/mL). The cells cocultured in the presence of normal IgG were used as controls. After incubation, the Transwell membranes were fixed with 3.7% formaldehyde for 10 minutes, followed by washing with PBS three times. The cells adhering to the upper side of the membrane were removed with a cotton swab, and transmigrated cells at the bottom side of the membrane were visualized using an Axiovert Zeiss 200M fluorescent microscope and counted from six random fields.
Transendothelial electrical resistance
The transendothelial electrical resistance (TER) was examined using the electric cell-substrate impedance sensing system. Briefly, HBMVEC-L cells were seeded on gelatin-coated gold electrode arrays (Applied Biophysics) and starved in EBM medium as previously described. With 1-V, 4,000-Hz alternating current signal supplied through a 1-MΩ resistor to a constant-current source, and culture media serving as the electrolyte, the electrical impedance was recorded with ECMS 1.0 software (CET). As the electrical impedance of a cell monolayer to a current flow is predominantly determined by the tightness of cell-cell and cell-matrix adhesions, decreased TER represents increased current flow that primarily results from disruption of the endothelial barrier integrity (44–46). After addition of MDA-MB-231 cells, 3.7% formaldehyde-fixed MDA-MB-231 cells, or blank vehicles (EBM only), TER was monitored continuously for 12 hours. In separate groups, the TER was measured using MDA-MB-231 or MDA-MB-436 cells transfected with TIMP-2 siRNA or in the presence of a blocking antibody against TIMP-2 (5 µg/mL), MMP-14 (20 µg/mL), or MMP-2 (5 µg/mL), as well as an isotype control antibody (20 µg/mL). The TER values from multiple wells were normalized to baseline, averaged, and expressed as a fractional change in TER as an indicator of endothelial barrier function.
Immunofluorescence microscopy
HBMVEC-L cells were seeded on gelatin-coated Lab-Tek chamber slides (Nalge Nunc). After serum starvation, endothelial monolayers were cocultured with calcein-AM–stained MDA-MB-231 cells with or without TIMP-2 siRNA transfection in the presence of a blocking antibody against TIMP-2 (5 µg/mL), MMP-14 (20 µg/mL), or MMP-2 (5 µg/mL). Twelve hours after coculture, the cells were fixed with 3.7% formaldehyde for 10 minutes, washed three times with PBS, and then permeabilized with 0.1% Triton X-100 for 5 minutes at room temperature. After blocking with 1% bovine serum albumin in PBS for 30 minutes, the cells were incubated sequentially with VE-cadherin antibody (4 µg/mL, 1 hour), TRITC-conjugated secondary antibody (4 µg/mL, 1 hour), and Hoechst 33342 (1:500 dilution, 10 minutes). Each incubation step was followed by three washings with PBS. The images were then captured using an Axiovert Zeiss 200M fluorescent microscope.
Results
Tumor cells activate endothelial MMP-2
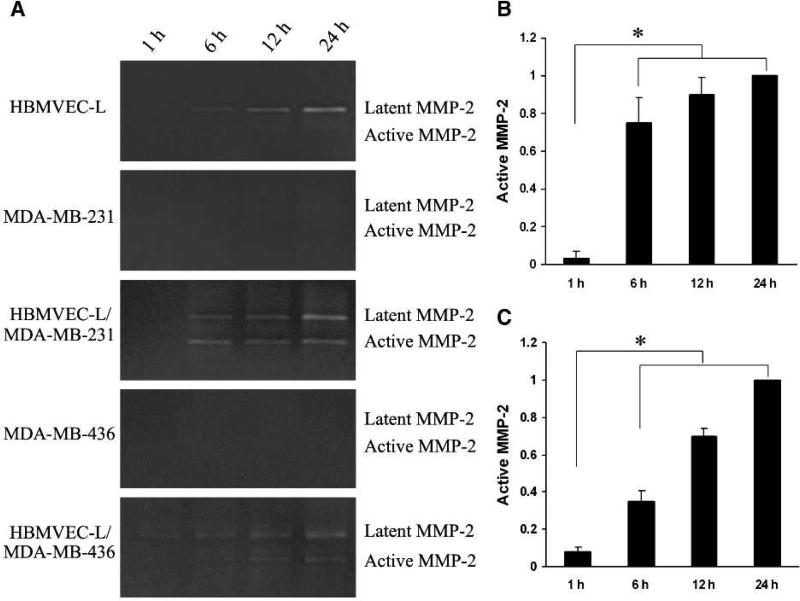
Human lung microvascular endothelial cells (HBMVEC-L) and breast cancer cells (MDA-MB-231 and MDA-MB-436) were grown as monoculture or coculture for various time courses (1, 6, 12, and 24 hours). Serum-free conditioned media were collected and analyzed for the presence and activity of MMP-2 using gelatin zymography. The latent form of MMP-2 (pro-MMP-2) was detected in the conditioned media of HBMVEC-L and HBMVEC-L cocultured with MDA-MB-231 or MDA-MB-436, but not the cancer cells alone (Fig. 1A). Active MMP-2 was not detected in either HBMVEC-L or breast cancer cell monoculture (Fig. 1A). However, when HBMVEC-L cells were cocultured with MDA-MB-231, this latent MMP-2 was cleaved and activated, with a plateau reached at 12 hours (Fig. 1A and B). Similar results were also observed in the conditioned media of HBMVEC-L cocultured with MDA-MB-436 (Fig. 1A and C). These findings are in line with our previous study (39) suggesting that tumor cell–originated factors are responsible for the activation of MMP-2 released primarily from endothelial cells.
FIGURE 1.
Breast cancer cells (MDA-MB-231 and MDA-MB-436) activate human lung microvascular endothelial cells (HBMVEC-L) MMP-2. A, zymographic analysis of conditioned media collected from HBMVEC-L, MDA-MB-231, or MDA-MB-436 cell monoculture and coculture at various time points. HBMVEC-L cells produce latent MMP-2 that can be activated when cocultured with breast cancer MDA-MB-231 or MDA-MB-436 cells. B, quantitative analysis of active MMP-2 in conditioned media of HBMVEC-L/MDA-MB-231 coculture. C, quantitative analysis of active MMP-2 in conditioned media of HBMVEC-L/MDA-MB-436 coculture. Data were normalized by the levels of active MMP-2 at 24 h and presented as mean ± SE (n = 3). *, P < 0.01.
Tumor cell–derived TIMP-2 induces MMP-2 activation
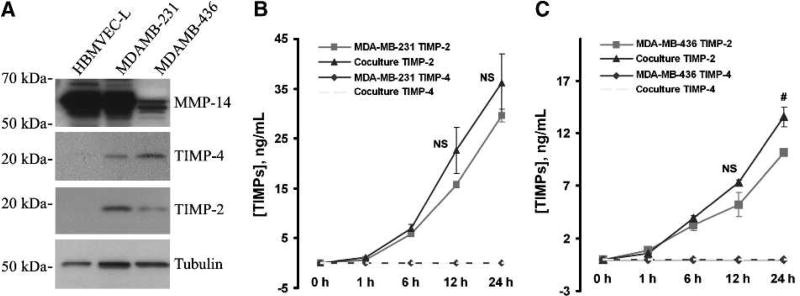
Recent evidence indicates that the activity of MMP-2 is tightly regulated through the actions of TIMP-2, TIMP-4, and membrane-associated MMP-14. As shown in Fig. 2A, MMP-14 is highly expressed in both HBMVEC-L cells and MDA-MB-231 cells. Likewise, a moderate level of MMP-14 is detected in MDA-MB-436 cells. In contrast, TIMP-2 and TIMP-4 were found expressed primarily in MDA-MB-231 and MDA-MB-436 cells (Fig. 2A), and only negligible levels of TIMP-2 and TIMP-4 were detected in HBMVEC-L cells (Fig. 2A). To further investigate if these tissue inhibitors of MMP were actually released by these breast cancer cells during the monoculture or coculture process, aliquots of conditioned media were subjected to ELISA assays. It was found that the level of TIMP-2 was continuously increased in the conditioned media over the culturing time (Fig. 2B and C). There is no statistically significant difference in the TIMP-2 level between the monoculture and the coculture conditions over a 12-hour culturing period. Intriguingly, TIMP-4 was not detected in the conditioned media of either culture condition (Fig. 2B and C), although it was detected in breast cancer cells (Fig. 2A).
FIGURE 2.
Expression of MMP-14, TIMP-4, and TIMP-2 in lung microvascular endothelial cells and breast cancer cells (MDA-MB-231 and MDA-MB-436). A, Western blot analysis showing the protein levels of MMP-14, TIMP-4, and TIMP-2 in HBMVEC-L, MDA-MB-231, and MDA-MB-436 cells. Tubulin is used as a loading control. B, ELISA assays for TIMP-2 and TIMP-4 levels in conditioned media from MDA-MB-231 monoculture or MDA-MB-231/HBMVEC-L coculture. C, TIMP-2 and TIMP-4 levels in conditioned media of MDA-MB-436 monoculture or MDA-MB-436/HBMVEC-L coculture. Data presented as mean ± SE (n = 3). NS, not statistically significant; #, P < 0.05.
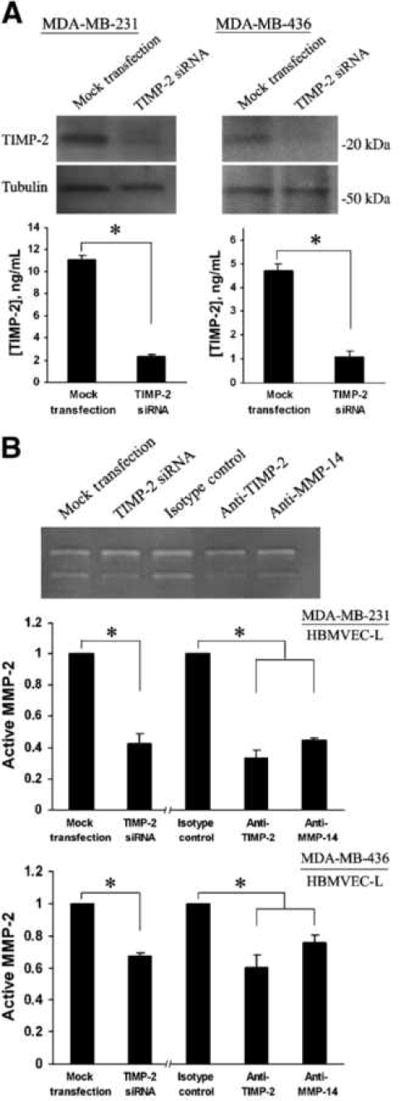
There is some evidence that TIMP-2 plays a crucial role in the regulation of MMP-2 activation. At a relatively low concentration, TIMP-2 binds and presents a latent MMP-2 to its membrane-associated activator MMP-14, which cleaves the MMP-2, thus initiating its activation process. However, at high levels, it blocks latent MMP-2 from interacting with MMP-14, thereby inhibiting MMP-2 activation. In this study, we detected MMP-14 expression in both breast cancer cells and lung microvascular endothelial cells. To characterize the role of tumor cell–derived TIMP-2 in the activation of endothelial cell MMP-2, MDA-MB-231 or MDA-MB-436 cells were transfected with TIMP-2 siRNA followed by coculturing with HBMVEC-L. As shown in Fig. 3A, compared with mock transfection, the levels of TIMP-2 were substantially reduced in both MDA-MB-231 and MDA-MB-436 cells, as well as in the conditioned media at 48 hours post-transfection. In agreement with this, gelatin zymography showed that the level of active MMP-2 was significantly reduced in conditioned media of coculture with TIMP-2-knockdown MDA-MB-231 or MDA-MB-436 cells (Fig. 3B). These findings suggest that tumor cell–derived TIMP-2 is essential for activating MMP-2 from HBMVEC-L cells. To confirm this result and further investigate the role of MMP-14 in this process, coculture assays were conducted in the presence of a blocking antibody against MMP-14 or TIMP-2 (47–51), with normal IgG used as an isotype control. The results from the subsequent gelatin zymography study substantiated that TIMP-2/MMP-14 mediated the activation of latent MMP-2 from HBMVEC-L cells (Fig. 3B).
FIGURE 3.
Tumor cell–derived TIMP-2 and MMP-14 are required for MMP-2 activation. A, top, TIMP-2 levels in MDA-MB-231 and MDA-MB-436 cells 48 h after transfection of TIMP-2 siRNA or mock transfection. Bottom, TIMP-2 levels in conditioned media (12 h) of MDA-MB-231 and MDA-MB-436 cells transfected with TIMP-2 siRNA. Mock transfectants were used as controls. B, top, representative zymography showing the levels of latent and active MMP-2 in conditioned media of HBMVEC-L cocultured with MDA-MB-231 cells that were transfected with TIMP-2 siRNA or in the presence of a blocking antibody against TIMP-2 or MMP-14. Mock transfectants were used as controls for TIMP-2-knockdown cells. Normal IgG is used as an isotype control for antibody blockage assays. Middle, quantitative analysis of active MMP-2 in HBMVEC-L/MDA-MB-231 coculture conditioned media. For TIMP-2-knockdown MDA-MB-231 cells, data were normalized to the levels of active MMP-2 in coculture with mock transfectants. For the antibody blockage assays, data were normalized to the levels of active MMP-2 in the presence of control antibodies. Bottom, normalized levels of active MMP-2 in HBMVEC-L/MDA-MB-436 coculture conditioned media. Data presented as mean ± SE (n = 3). *, P < 0.01.
TIMP-2 in MMP-14/MMP-2–mediated breast cancer cell TEME
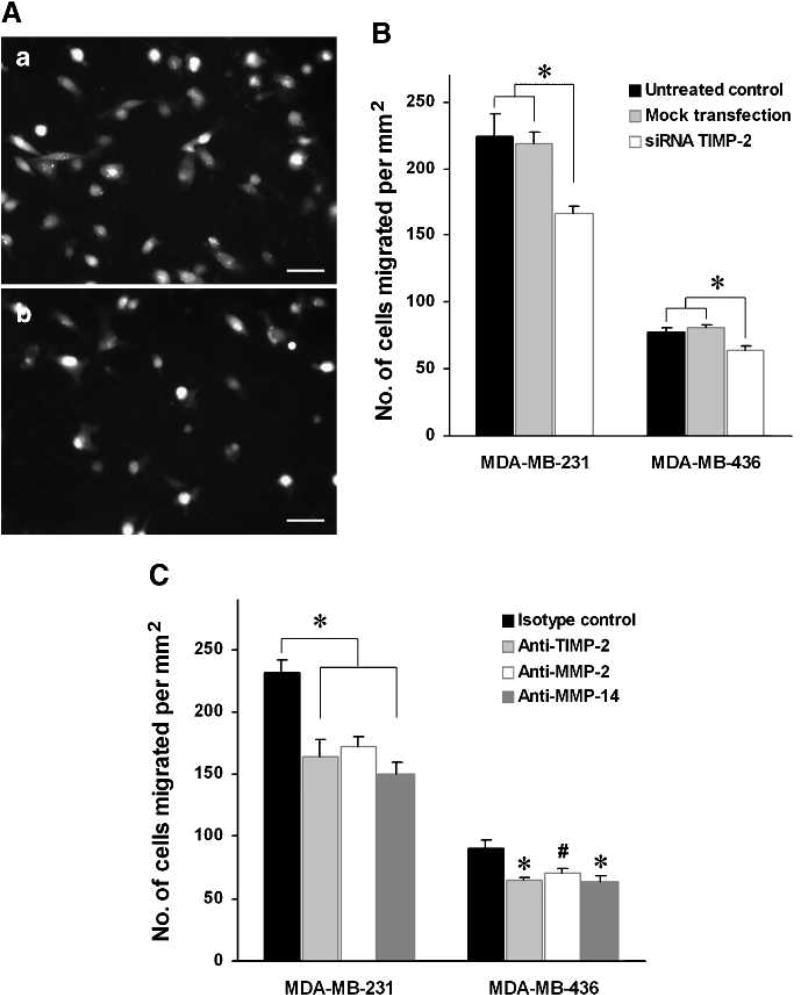
Our previous work has shown a critical role for MMP-2 in breast cancer cell transmigration across the endothelial barrier (39). The current study further shows that tumor cell–derived TIMP-2 is required for activating MMP-2 in the presence of functional MMP-14. To clarify the underlying mechanisms of MMP-2–mediated tumor cell TEME, we investigated the potential role of TIMP-2 and MMP-14 in this process. As shown in Fig. 4A and B, in comparison with the control tumor cells, TIMP-2-knockdown MDA-MB-231 and MDA-MB-436 cells exhibited significantly lower transmigrating activity through the HBMVEC-L monolayers. Moreover, in agreement with previous studies that MMP-2 activation is blocked by TIMP-2 at significantly high concentrations (250–1,250 ng/mL; ref. 52), our data showed that TIMP-2 at 500 ng/mL inhibited transmigration (Supplementary Fig. S1). The crucial role of tumor-derived TIMP-2 in TEME was further supported by the data from the subsequent experiments with TIMP-2, MMP-14, or MMP-2 blocking antibodies (Fig. 4C), which showed that the presence of functionally active MMP-14 was required for the TIMP-2–induced tumor cell TEME. These findings suggest for the first time that the tumor-derived TIMP-2 and MMP-14 mediate MMP-2 activation and tumor cell transmigration across the endothelial barrier.
FIGURE 4.
TIMP-2 promotes transmigration of breast cancer cells (MDA-MB-231 and MDA-MB-436) through HBMVEC-L monolayers. A, representative images of transmigrated MDA-MB-231 cells: a, mock-transfected MDA-MB-231 cells; b, TIMP-2-knockdown MDA-MB-231 cells. MDA-MB-231 cells were identified by the fluorescence of cell-loaded calcein-AM. B, quantitative analysis of transmigrated MDA-MB-231 and MDA-MB-436 cells (number/mm2) that are transfected with mock or TIMP-2 siRNA. Untreated MDA-MB-231 and MDA-MB-436 cells were used as controls. C, quantitative analysis of transmigrated MDA-MB-231 and MDA-MB-436 cells (number/mm2) in the presence of isotype control antibody or anti–TIMP-2, anti–MMP-2, or anti–MMP-14 antibody. Data presented as mean ± SE (n = 6). #, P < 0.05; *, P < 0.01. Bar, 50 µm.
Tumor cell–derived TIMP-2 induces endothelial barrier dysfunction
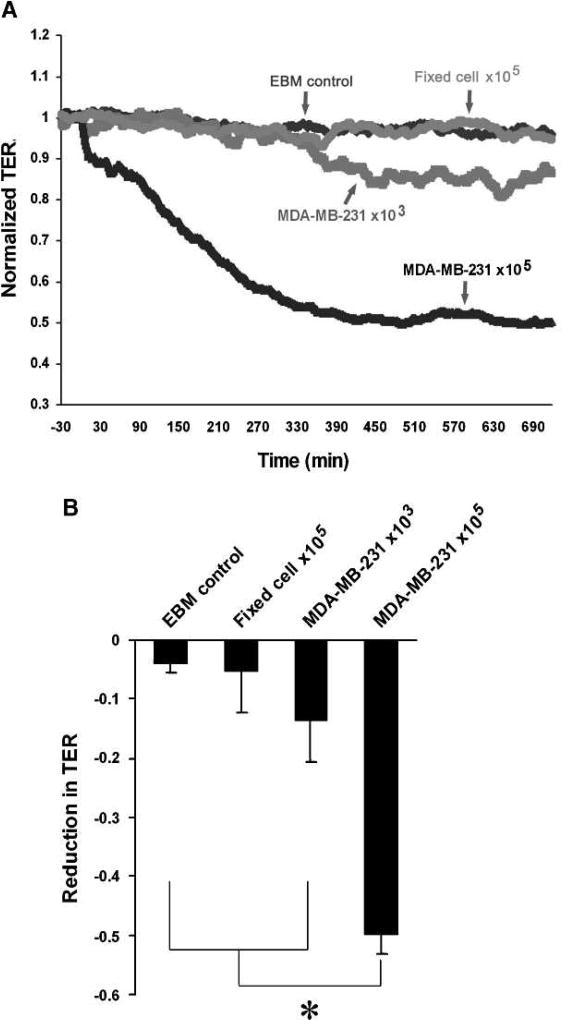
The process of transendothelial migration is determined by directed tumor cell motility and microvascular endothelial barrier function. To test the role of tumor cell–derived TIMP-2 as well as MMP-4 and MMP-2 in microvascular endothelial barrier function, we first examined the TER dynamics of HBMVEC-L monolayers in response to MDA-MB-231 cell stimulation. As shown in Fig. 5A and B, MDA-MB-231 cells reduce the TER of HBMVEC-L monolayers in a concentration (cell number)- and time-dependent manner, which is reversely correlated with the level of active MMP-2 detected in coculture using gelatin zymography assays. Moreover, in contrast to live MDA-MB-231 cells, formaldehyde-fixed MDA-MB-231 cells did not cause significant decreases in TER (Fig. 5A and B), indicating that the tumor cell–released factors, rather than the physical effects of cell-cell contact, are the primary mediators of endothelial barrier dysfunction.
FIGURE 5.
TER indicative of barrier function is reduced in HBMVEC-L monolayers during coculture with MDA-MB-231. A, TER dynamics in the HBMVEC-L monolayers cultured with serum-free EBM medium, MDA-MB-231 cells (103 or 105 cells/cm2), and formaldehyde-fixed MDA-MB-231 cells (105 cells/cm2). B, concentration-dependent effects of MDA-MB-231 cells on the TER of HBMVEC-L monolayer after 12 h of coculture. The data are presented as mean ± SE (n = 6). *, P < 0.01.
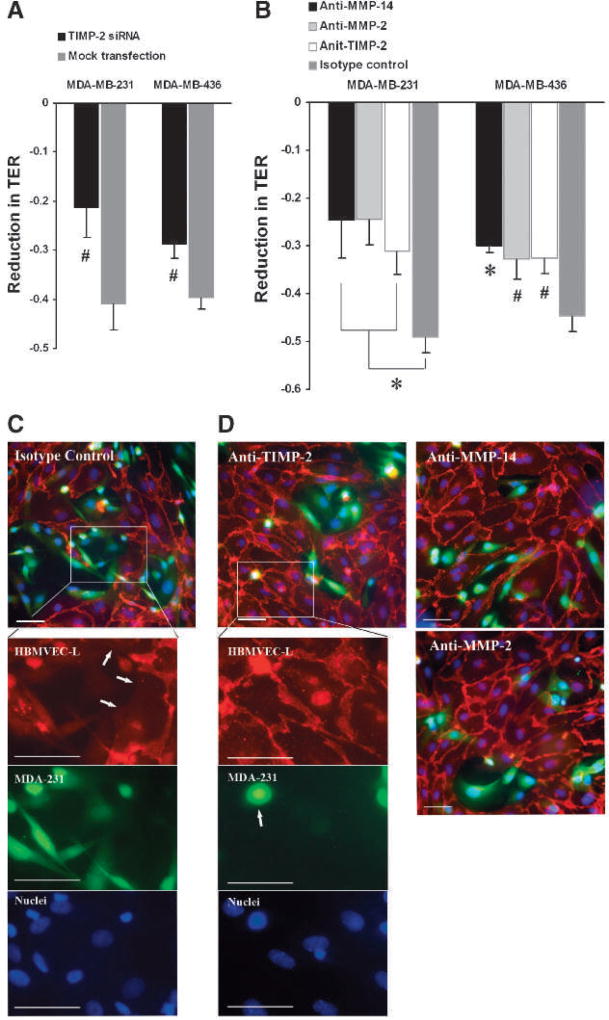
In the following TER assays with TIMP-2-knockdown MDA-MB-231 and MDA-MB-436 cells, we found that TIMP-2 was required for tumor cell–induced endothelial barrier dysfunction (Fig. 6A). Antibody blockage assays additionally showed the critical role of MMP-14 and MMP-2 in tumor cell–induced endothelial barrier dysfunction (Fig. 6B). Supporting these findings, immunofluorescence microscopy showed that the HBMVEC-L monolayer was extensively disrupted following a 12-hour coculture with MDA-MB-231 cells (Fig. 6C). Large intercellular gaps were frequently observed in the HBMVEC-L monolayer, with tumor cells occupying the wide-open interendothelial space (Fig. 6C). In addition, VE-cadherin was sequestrated from the periphery of HBMVEC-L cells at the areas losing intercellular contact (Fig. 6C). In the presence of a blocking antibody against TIMP-2, MMP-14, or MMP-2, the endothelial barrier damage was considerably reduced, although some small interendothelial gaps were still observed (Fig. 6D). Collectively, the data show the critical roles of TIMP-2, MMP-14, and MMP-2 in breast cancer cell–induced endothelial barrier dysfunction.
FIGURE 6.
TIMP-2 induces endothelial barrier dysfunction in HBMVEC-L cell monolayers. A, effects of knocking down TIMP-2 in MDA-MB-231 and MDA-MB-436 cells on the TER of HBMVEC-L monolayers after 12 h of coculture. B, effects of neutralizing TIMP-2, MMP-2, and MMP-4 on MDA-MB-231– and MDA-MB-436–induced TER decreases after 12 h of coculture. Normal IgG was used as isotype control. C, representative image of HBMVEC-L monolayer (red) after a 12-h coincubation with MDA-MB-231 cells (green) in the presence of normal IgG. Insets show higher magnifications of the designated area under individual channel, where HBMVEC-L cells were identified by VE-cadherin (red), MDA-MB-231 cells were identified by cell-loaded calcein-AM (green), and nuclei by Hoechst 33342 (blue). Note the MDA-MB-231–occupied intercellular gaps, where VE-cadherin was sequestrated from the periphery of HBMVEC-L (white arrows). D, representative images of HBMVEC-L monolayers (red) after a 12-h coincubation with MDA-MB-231 cells (green) in the presence of a blocking antibody against TIMP-2, MMP-14, or MMP-2. Insets show higher magnifications of the designated areas in anti–TIMP-2. Compared with the images in C, interendothelial gaps were less frequent and more VE-cadherin (red) was localized to the cell periphery; there were fewer MDA-MB-231 cells (green cells indicated by white arrow) transmigrating to endothelial tricellular corner. Data presented as mean ± SE (n = 6). #, P < 0.05; *, P < 0.01. Bar, 50 µm.
Discussion
Tumor cell extravasation is a critical step in metastasis. It was originally proposed as a passive process driven primarily by mechanical factors (53). However, numerous in vivo and ex vivo studies show that tumor cell TEME is an active process involving the interaction between tumor cells and local microvascular endothelium (54–56). It is characterized by orchestrated signaling events involving adhesion molecules and cytokines, such as sLe/E-selectin, integrins, mucins, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, stromal cell–derived factor-1/CXC chemokine receptor-4, thrombospondin and its receptors, etc. (57–63).Moreover, increased activity of extracellular proteolytic enzymes has been found to correlate with poor prognosis of breast cancer in patients. Our previous work has shown a critical role of endothelial-originated MMP-2 in promoting breast cancer cell TEME (39). Knockdown of constitutive MMP-2 in endothelial cells diminished MMP-2 production and activation in cocultured tumor/endothelial cells (39). Selective inhibition of MMP-2 activity in endothelial cells, but not MDA-MB-231 cells, significantly attenuated tumor cell transendothelial migration (39). In this study, we provide further mechanistic evidence that TIMP-2 expressed in breast cancer cells activates endothelial MMP-2 in the presence of active MMP-14; this response causes endothelial barrier dysfunction and promotes tumor cell transmigration across endothelial monolayers on tumor cell adhesion. These findings suggest a novel mechanism underlying the interaction between cancer cells and microvascular endothelium during tumor cell extravasation. Malignant breast cancer cells exhibit a preferential pattern of metastases and tend to metastasize to specific organs, such as the lungs, liver, and brain (1, 2, 64, 65). Given that both MDA-MB-231 and MDA-MB-436 cells are highly invasive breast cancer cells originated from lung metastases (66, 67), our study focusing on the interaction between these cells and lung microvascular endothelial cells is of clinical relevance to organ-specific breast cancer metastasis.
Accumulating evidence indicates that TIMP-2 levels in tumors correlate with progress in breast cancer. Consistent with the generally accepted role as a tumor suppressor, overexpression of TIMP-2 was shown to inhibit tumor invasion and metastasis (68–70). On the contrary, Ree et al. found that TIMP-2 mRNA levels were considerably higher in breast cancer patients who developed remote metastasis than in those who were free of metastasis (71). It was further found that a high protein level of TIMP-2 in breast cancer tissues correlated with decreased overall survival and recurrence-free survival (72). These findings suggest that TIMP-2 levels may be tightly regulated at different stages of breast carcinogenesis and its effects vary depending on the microenvironment of the tumor. For prognosis evaluation, TIMP-2 should be considered together with other factors, such as local levels of TIMP-4, MMP-14, and latent MMP-2. Supporting this is the current data that both MDA-MB-231 and MDA-MB-436 produced a low level of TIMP-2; a similar concentration of TIMP-2 has been shown to activateMMP-2 in other studies (52). Furthermore, although both TIMP-2 and TIMP-4 were detected in the tumor cells, TIMP-4 was not detected in culture media of the tumor cells when they were monocultured or cocultured with endothelial cells, suggesting that TIMP-4 was not released from tumor cells. Given that TIMP-4 inhibits TIMP-2/MMP-14–mediated MMP-2 activation (26), selective release of TIMP-2 (but not TIMP-4) may represent a critical mechanism by which tumor cells facilitate their TEME. Decreased production of TIMP-2 to around 2 ng/mL, a level comparable to that in the serum of healthy subjects (data not shown), substantially reduced the level of active MMP-2. On the other hand, consistent with the negligible level of active MMP-2 found in human blood (data not shown), active MMP-2 was not detected in conditioned media of HBMVEC-L monoculture regardless of the presence of MMP-14. Therefore, complex mechanisms involving multiple factors, such as TIMP-2, TIMP-4, andMMP-14, seem to contribute to MMP-2 activation and function.
Tumor cell TEME is an orchestrated process of numerous signaling events in both cancer cells and endothelial cells, characterized by tumor cell invasive motility and endothelial barrier dysfunction. Previous work showed that the heterotypic interaction between breast cancer cells and endothelial cells induces promigratory signaling in tumor cells, resulting in increased cell invasive motility (73–76). Currently, many theories tend to consider tumor cell TEME as a process similar to leukocyte extravasation during inflammation; the sequence of events includes leukocyte adhesion to the endothelium, endothelial cell retraction and junction opening, followed by leukocyte transmigration. Therefore, the barrier function of the vascular endothelium is an important determinant of transmigration and extravasation of cells from the circulation. The integrity of the endothelial barrier is maintained by an equilibrium between actomyosinmediated centripetal tension and cell-cell/ECM adhesive forces (77, 78). An array of inflammatory cells and substances have been identified as mediators of protein leakage and leukocyte diapedesis by altering endothelial cell-cell and cell-matrix adhesive barrier structures (77, 79–82). This study suggests that similar mechanisms may be involved in cancer cell invasion and extravasation across the vascular endothelium. Using TER as an indicator of cell-cell/substrate adhesive barrier properties (44, 45, 83–86), we show that TIMP-2/MMP-14–mediated activation of MMP-2 causes lung microvascular endothelial barrier dysfunction coupled with enhanced tumor cell TEME. Notably, although the TER response and TEME were significantly attenuated with TIMP-2 siRNA transfection and by neutralizing the activity of the TIMP-2/MMP-14/MMP-2 complex, they were not completely blocked. This indicates that tumor cell–derived TIMP-2 is only partially responsible for endothelial barrier dysfunction and tumor cell TEME. As other factors, such as adhesion receptors and cytokines, have been implicated in tumor cell adhesion and transendothelial migration (40, 57, 59, 60), a more dramatic reduction in TEME is expected by targeting multiple related pathways.
Based on the current data and our previous study (39), we hypothesize that MMP-2 functions as a potent metalloproteinase causing structural or conformational changes in endothelial cell-cell junctions and/or endothelial cell-matrix focal adhesions; both are critical components of the endothelial barrier that restricts tumor cell transmigration. In particular, activated MMP-2 may induce ectodomain shedding or degradation of transmembrane molecules that form cell-cell adherens junctions (e.g., VE-cadherin), leading to opening of intercellular gaps and facilitating tumor cell transmigration. In support of this theory, previous studies have shown that MMP-2 is capable of degrading cell-cell adhesion molecules, such as N-cadherin (35, 36). Consistently, our immunofluorescence microscopic study showed TIMP-2/MMP-2–induced junction disruption in human lung microvascular endothelial monolayers during coculture with breast cancer cells; this was accompanied by sequestration of VE-cadherin from the endothelial cells. Alternatively, MMP-2 may cause endothelial barrier dysfunction by affecting endothelial focal adhesion to the basement membrane. In this study, the barrier functional assays were done in endothelial monolayers grown on matrix protein–coated surfaces that resemble the basement membrane. Further, endothelial cells in culture can synthesize and deposit type IV collagen, a major constituent of the matrix underlying the vascular endothelium (87–90).
Using formaldehyde-fixed MDA-MB-231 cells as a control, we found that cell contact–induced mechanical stress did not significantly alter endothelial barrier function. Similar to the result of the TEME assay, selective inhibition of MMP-2 and MMP-14 also exhibited a protective effect on endothelial barrier function, indicating the TIMP-2/MMP-14/MMP-2 pathway as a potential target for preventing breast cancer extravasation during metastasis. With regard to the therapeutic potential of manipulating this pathway, several MMP-2 inhibitors have been developed and tested in clinical trials. For example, batimastat (BB-94), one of the early generations of peptidomimetic inhibitors, was shown to halt breast cancer metastasis in various animal models (18, 91–93). Its phase III trail was, however, terminated due to severe adverse reactions in local tissues. Subsequently, marimastat (BB-2516), a batimastat derivate that can be delivered orally, has been developed and tested (94). Although there is clinical data supporting the use of marimastat in advanced cancer (95), a randomized phase III study in patients with metastatic breast cancer who had responding or stable disease after chemotherapy failed to show evidence of clinical benefit (96, 97). Other metalloproteinase inhibitors that have been tested as anticancer therapies include BMS-275291, BAY 12-9566, CGS27023A, and FYK-1388, etc. (97–99). Nevertheless, the ineffectiveness or serious side effects limit their usage in further clinical application. The results of this study suggest that targeting the TIMP-2/MMP-14 pathway specific to the microvascular endothelial barrier of metastatic organs may serve as a potential alternative means for blockage of TEME signaling from tumor cells.
Supplementary Material
Acknowledgments
We thank Olesya P. Litovka, Bert J. Frederich, Dr. Jing Tang, and Dr. Nathalie Gaudreault for technical support.
Grant Support
NIH grants HL61507, HL70753, HL73324, and HL84852.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kamby C, Ejlertsen B, Andersen J, et al. The pattern of metastases in human breast cancer. Influence of systemic adjuvant therapy and impact on survival. Acta Oncol. 1988;27:715–9. doi: 10.3109/02841868809091774. [DOI] [PubMed] [Google Scholar]
- 2.Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–12. [PubMed] [Google Scholar]
- 3.Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy G, Stanton H, Cowell S, et al. Mechanisms for pro matrix metalloproteinase activation. Apmis. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 5.Ellerbroek SM, Stack MS. Membrane associated matrix metalloproteinases in metastasis. Bioessays. 1999;21:940–9. doi: 10.1002/(SICI)1521-1878(199911)21:11<940::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–32. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 7.Masson V, de la Ballina LR, Munaut C, et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–6. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fromigue O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNKc-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–56. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu SC, Yang SF, Yeh KT, et al. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clin Chim Acta. 2006;371:92–6. doi: 10.1016/j.cca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–42. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppa S, Saarto T, Vehmanen L, Blomqvist C, Elomaa I. A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin Cancer Res. 2004;10:1057–63. doi: 10.1158/1078-0432.ccr-03-0047. [DOI] [PubMed] [Google Scholar]
- 12.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004;90:1414–21. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer. 2003;89:1270–5. doi: 10.1038/sj.bjc.6601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah FD, Shukla SN, Shah PM, Shukla HK, Patel PS. Clinical significance of matrix metalloproteinase 2 and 9 in breast cancer. Indian J Cancer. 2009;46:194–202. doi: 10.4103/0019-509X.52953. [DOI] [PubMed] [Google Scholar]
- 15.Seiki M. Membrane-type matrix metalloproteinases. Apmis. 1999;107:137–43. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 16.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type matrix metalloproteinases. Semin Cancer Biol. 2002;12:131–8. doi: 10.1006/scbi.2001.0421. [DOI] [PubMed] [Google Scholar]
- 18.Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009;15:RA32–40. [PubMed] [Google Scholar]
- 19.Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- 20.Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993;268:14033–9. [PubMed] [Google Scholar]
- 21.Overall CM, King AE, Sam DK, et al. Identification of the tissue inhibitor of metalloproteinases-2 (TIMP-2) binding site on the hemopexin carboxyl domain of human gelatinase A by site-directed mutagenesis. The hierarchical role in binding TIMP-2 of the unique cationic clusters of hemopexin modules III and IV. J Biol Chem. 1999;274:4421–9. doi: 10.1074/jbc.274.7.4421. [DOI] [PubMed] [Google Scholar]
- 22.Butler GS, Butler MJ, Atkinson SJ, et al. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–80. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 23.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–8. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson SJ, Crabbe T, Cowell S, et al. Intermolecular autolytic cleavage can contribute to the activation of progelatinase A by cell membranes. J Biol Chem. 1995;270:30479–85. doi: 10.1074/jbc.270.51.30479. [DOI] [PubMed] [Google Scholar]
- 25.Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–23. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Barrantes S, Shimura Y, Soloway PD, Sang QA, Fridman R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem Biophys Res Commun. 2001;281:126–30. doi: 10.1006/bbrc.2001.4323. [DOI] [PubMed] [Google Scholar]
- 27.Liu YE, Wang M, Greene J, et al. Preparation and characterization of recombinant tissue inhibitor of metalloproteinase 4 (TIMP-4) J Biol Chem. 1997;272:20479–83. doi: 10.1074/jbc.272.33.20479. [DOI] [PubMed] [Google Scholar]
- 28.Huhtala P, Chow LT, Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990;265:11077–82. [PubMed] [Google Scholar]
- 29.Collier IE, Wilhelm SM, Eisen AZ, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988;263:6579–87. [PubMed] [Google Scholar]
- 30.Karagiannis ED, Popel AS. A theoretical model of type I collagen proteolysis by matrix metalloproteinase (MMP) 2 and membrane type 1 MMP in the presence of tissue inhibitor of metalloproteinase 2. J Biol Chem. 2004;279:39105–14. doi: 10.1074/jbc.M403627200. [DOI] [PubMed] [Google Scholar]
- 31.Monaco S, Sparano V, Gioia M, et al. Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006;15:2805–15. doi: 10.1110/ps.062430706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets. 2005;5:203–20. doi: 10.2174/1568009053765799. [DOI] [PubMed] [Google Scholar]
- 33.Luo J. Role of matrix metalloproteinase-2 in ethanol-induced invasion by breast cancer cells. J Gastroenterol Hepatol. 2006;21(Suppl 3):S65–8. doi: 10.1111/j.1440-1746.2006.04578.x. [DOI] [PubMed] [Google Scholar]
- 34.Riches K, Morley ME, Turner NA, et al. Chronic hypoxia inhibits MMP-2 activation and cellular invasion in human cardiac myofibroblasts. J Mol Cell Cardiol. 2009;47:391–9. doi: 10.1016/j.yjmcc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartland SN, Murphy F, Aucott RL, et al. Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int. 2009;29:966–78. doi: 10.1111/j.1478-3231.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 36.Jezierska A, Matysiak W, Motyl T. ALCAM/CD166 protects breast cancer cells against apoptosis and autophagy. Med Sci Monit. 2006;12:BR263–73. [PubMed] [Google Scholar]
- 37.Cockett MI, Murphy G, Birch ML, et al. Matrix metalloproteinases and metastatic cancer. Biochem Soc Symp. 1998;63:295–313. [PubMed] [Google Scholar]
- 38.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–7. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kargozaran H, Yuan SY, Breslin JW, et al. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis. 2007;24:495–502. doi: 10.1007/s10585-007-9086-6. [DOI] [PubMed] [Google Scholar]
- 40.Rahn JJ, Chow JW, Horne GJ, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22:475–83. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 41.Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem. 2003;278:5277–84. doi: 10.1074/jbc.M210063200. [DOI] [PubMed] [Google Scholar]
- 42.Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol. 2001;280:C814–22. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 43.Moreland JG, Bailey G. Neutrophil transendothelial migration in vitro to Streptococcus pneumoniae is pneumolysin dependent. Am J Physiol Lung Cell Mol Physiol. 2006;290:L833–40. doi: 10.1152/ajplung.00333.2005. [DOI] [PubMed] [Google Scholar]
- 44.Giaever I, Keese CR. A morphological biosensor for mammalian cells. Nature. 1993;366:591–2. doi: 10.1038/366591a0. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka N, Iwaki K, Hashimoto K, et al. Measurements of endothelial cell-to-cell and cell-to-substrate gaps and micromechanical properties of endothelial cells during monocyte adhesion. Proc Natl Acad Sci U S A. 2002;99:15638–43. doi: 10.1073/pnas.242590799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giaever I, Keese CR. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci U S A. 1991;88:7896–900. doi: 10.1073/pnas.88.17.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–92. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 48.Matias-Roman S, Galvez BG, Genis L, et al. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–64. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- 49.Simeone AM, McMurtry V, Nieves-Alicea R, et al. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–21. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491–500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- 52.Lafleur MA, Tester AM, Thompson EW. Selective involvement of TIMP-2 in the second activational cleavage of pro-MMP-2: refinement of the pro-MMP-2 activation mechanism. FEBS Lett. 2003;553:457–63. doi: 10.1016/s0014-5793(03)01094-9. [DOI] [PubMed] [Google Scholar]
- 53.Chambers AF, MacDonald IC, Schmidt EE, et al. Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 1995;14:279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 54.Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–7. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glinskii OV, Huxley VH, Turk JR, et al. Continuous real time ex vivo epifluorescent video microscopy for the study of metastatic cancer cell interactions with microvascular endothelium. Clin Exp Metastasis. 2003;20:451–8. doi: 10.1023/a:1025449031136. [DOI] [PubMed] [Google Scholar]
- 56.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 57.Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170:1781–92. doi: 10.2353/ajpath.2007.060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kam JL, Regimbald LH, Hilgers JH, et al. MUC1 synthetic peptide inhibition of intercellular adhesion molecule-1 and MUC1 binding requires six tandem repeats. Cancer Res. 1998;58:5577–81. [PubMed] [Google Scholar]
- 59.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310–29. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 60.Song JW, Cavnar SP, Walker AC, et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One. 2009;4:e5756. doi: 10.1371/journal.pone.0005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monterrubio M, Mellado M, Carrera AC, Rodriguez-Frade JM. PI3Kγ activation by CXCL12 regulates tumor cell adhesion and invasion. Biochem Biophys Res Commun. 2009;388:199–204. doi: 10.1016/j.bbrc.2009.07.153. [DOI] [PubMed] [Google Scholar]
- 62.Haddad O, Chotard-Ghodsnia R, Verdier C, Duperray A. Tumor cell/endothelial cell tight contact upregulates endothelial adhesion molecule expression mediated by NFκB: differential role of the shear stress. Exp Cell Res. 2010;316:615–26. doi: 10.1016/j.yexcr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Earley S, Plopper GE. Phosphorylation of focal adhesion kinase promotes extravasation of breast cancer cells. Biochem Biophys Res Commun. 2008;366:476–82. doi: 10.1016/j.bbrc.2007.11.181. [DOI] [PubMed] [Google Scholar]
- 64.Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G H.A. Clowes Memorial Award Lecture. Cancer Res. 1990;50:6130–8. [PubMed] [Google Scholar]
- 65.Zhang Y, Ma B, Fan Q. Mechanisms of breast cancer bone metastasis. Cancer Lett. 2010;292:1–7. doi: 10.1016/j.canlet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Jardines L, Callans LS, Torosian MH. Recurrent breast cancer: presentation, diagnosis, and treatment. Semin Oncol. 1993;20:538–47. [PubMed] [Google Scholar]
- 67.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeClerck YA, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992;52:701–8. [PubMed] [Google Scholar]
- 69.Imren S, Kohn DB, Shimada H, Blavier L, DeClerck YA. Overexpression of tissue inhibitor of metalloproteinases-2 retroviral-mediated gene transfer in vivo inhibits tumor growth and invasion. Cancer Res. 1996;56:2891–5. [PubMed] [Google Scholar]
- 70.Noel A, Hajitou A, L'Hoir C, et al. Inhibition of stromal matrix metalloproteases: effects on breast-tumor promotion by fibroblasts. Int J Cancer. 1998;76:267–73. doi: 10.1002/(sici)1097-0215(19980413)76:2<267::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 71.Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O. High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res. 1997;3:1623–8. [PubMed] [Google Scholar]
- 72.Remacle A, McCarthy K, Noel A, et al. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer. 2000;89:118–21. doi: 10.1002/(sici)1097-0215(20000320)89:2<118::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 73.Shen Q, Rahn JJ, Zhang J, et al. MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol Cancer Res. 2008;6:555–67. doi: 10.1158/1541-7786.MCR-07-2033. [DOI] [PubMed] [Google Scholar]
- 74.Rahn JJ, Shen Q, Mah BK, Hugh JC. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–90. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- 75.Bauer K, Mierke C, Behrens J. Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for αvβ3 integrin. Int J Cancer. 2007;121:1910–8. doi: 10.1002/ijc.22879. [DOI] [PubMed] [Google Scholar]
- 76.Lewalle JM, Cataldo D, Bajou K, Lambert CA, Foidart JM. Endothelial cell intracellular Ca2+ concentration is increased upon breast tumor cell contact and mediates tumor cell transendothelial migration. Clin Exp Metastasis. 1998;16:21–9. doi: 10.1023/a:1006555800862. [DOI] [PubMed] [Google Scholar]
- 77.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Q, Wu MH, Yuan SY. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskeleton. 2009;1:43–50. doi: 10.2147/chc.s5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–45. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu MH, Yuan SY, Granger HJ. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. J Physiol. 2005;563:95–104. doi: 10.1113/jphysiol.2004.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breslin JW, Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol. 2004;286:H1057–62. doi: 10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 82.Wu MH, Guo M, Yuan SY, Granger HJ. Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J Physiol. 2003;552:691–9. doi: 10.1113/jphysiol.2003.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo M, Daines D, Tang J, et al. Fibrinogen-γ C-terminal fragments induce endothelial barrier dysfunction and microvascular leak via integrin-mediated and RhoA-dependent mechanism. Arterioscler Thromb Vasc Biol. 2009;29:394–400. doi: 10.1161/ATVBAHA.108.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Umapathy NS, Zemskov EA, Gonzales J, et al. Extracellular β-nicotinamide adenine dinucleotide (β-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J Cell Physiol. 2010;223:215–23. doi: 10.1002/jcp.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun C, Wu MH, Guo M, Day ML, Lee ES, Yuan SY. ADAM15 regulates endothelial permeability and neutrophil migration via Src/ERK1/2 signalling. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq060. Epub 2010 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kramer RH, Fuh GM, Karasek MA. Type IV collagen synthesis by cultured human microvascular endothelial cells and its deposition into the subendothelial basement membrane. Biochemistry. 1985;24:7423–30. doi: 10.1021/bi00346a059. [DOI] [PubMed] [Google Scholar]
- 88.Jaffe EA, Minick CR, Adelman B, Becker CG, Nachman R. Synthesis of basement membrane collagen by cultured human endothelial cells. J Exp Med. 1976;144:209–25. doi: 10.1084/jem.144.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhuri V, Karasek MA. Mechanisms of microvascular wound repair II. Injury induces transformation of endothelial cells into myofibroblasts and the synthesis of matrix proteins. In Vitro Cell Dev Biol Anim. 2006;42:314–9. doi: 10.1290/0607044.1. [DOI] [PubMed] [Google Scholar]
- 90.Brevig T, Rohrmann JH, Riemann H. Oxygen reduces accumulation of type IV collagen in endothelial cell subcellular matrix via oxidative stress. Artif Organs. 2006;30:915–21. doi: 10.1111/j.1525-1594.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 91.Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65:130–6. [PubMed] [Google Scholar]
- 92.Eccles SA, Box GM, Court WJ, Bone EA, Thomas W, Brown PD. Control of lymphatic and hematogenous metastasis of a rat mammary carcinoma by the matrix metalloproteinase inhibitor batimastat (BB-94) Cancer Res. 1996;56:2815–22. [PubMed] [Google Scholar]
- 93.Sledge GW, Jr, Qulali M, Goulet R, Bone EA, Fife R. Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst. 1995;87:1546–50. doi: 10.1093/jnci/87.20.1546. [DOI] [PubMed] [Google Scholar]
- 94.Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–93. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 95.Fielding J, Scholefield J, Stuart J, et al. A randomized double-blind placebo-controlled study of marimastat in patients with inoperable gastric adenocarcinoma. Proc Am Soc Clin Oncol. 2000;19:240a. [Google Scholar]
- 96.Sparano J, Bernardo P, Gradishar W, Ingle J, Zucker S, Davidson N. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: an Eastern Cooperative Oncology Group Trail (E2196). (presentation) Am Soc Clin Oncol. 2002;21:44. doi: 10.1200/JCO.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 97.Pavlaki M, Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22:177–203. doi: 10.1023/a:1023047431869. [DOI] [PubMed] [Google Scholar]
- 98.Naglich JG, Jure-Kunkel M, Gupta E, et al. Inhibition of angiogenesis and metastasis in two murine models by the matrix metalloproteinase inhibitor, BMS-275291. Cancer Res. 2001;61:8480–5. [PubMed] [Google Scholar]
- 99.Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–76. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.