Summary
Introduction.
Nosocomial infections are one of the greatest problems in public health. Several studies have highlighted the role played by the hospital environment as a possible source of transmission of nosocomial pathogens.
Methods.
A five-year monitoring of bacterial contamination on healthcare workers hands, surfaces most closely in contact with inpatient wards, operating theatres and "at rest" and "in use" operating theatre air samples. For the samples, we used sterile swabs, contact slides, manual API, and automated VITEK systems for identification.
Results.
In the five-year period, a total of 9396 samples were collected and analysed. In ward patients, 4398 samplings were carried out with 4.7%, 9.4%, 7%, 10.8% and 7.9% positive results respectively from 2010 to 2014. For hands, 648 samplings were carried out, with a positivity of 40.74%. In operating theatres, 4188 samples were taken, with a positivity of 11.9%. Regarding air in empty and full theatres, 1962 samplings were carried out with a positivity rate equal to 31.9%. The monitoring showed a low rate of contamination with a progressive decrease in the fiveyear period on operating theatres surfaces and hands, while there was an increase in the surgical site wards and in the air of operating rooms.
Conclusions.
Our investigation has revealed the presence of pathogens on the assessed surfaces and the need for environmental monitoring, which can be a valuable tool for reducing contamination.
Key words: Microbiological surveillance, HCAI, Operating rooms, Wards
Introduction
Hospital infections are, even today, one of the main problems of public health [1]. Much importance was given, in recent years, to the contamination of the hospital environment in the onset of these infections. One of the most controversial and debated issues is the qualitative and quantitative role of the environment in the process of patient contamination, in particular the role of adjacent surfaces and furniture. It is known that these surfaces act as reservoirs for microorganisms, increasing the risk of cross-contamination through direct and/or indirect contact with the patient [2-5]. Recent studies have focused on the role of hospital environment sanitation processes, establishing a correlation between microbiological contamination of surfaces in direct contact with the patient and Healthcare Associated Infections (HCAI) [6]. The spread of microorganisms is undoubtedly related to the presence of the patients themselves, the latter being the first source of contamination of the environment and especially of all those sites that are closely associated with them, such as the bed, the bedside table, the power supply carriage etc., which are frequently touched ("high-touch surfaces") and easily contaminated [7]. For many infections of the surgical site, in addition to the patient's endogenous flora, the main source of infection is the contamination of the surgical site with desquamative cells [8]. Appropriate clothing and appropriate behaviour on the part of the health workers, along with a controlled ventilation system (CVS) are indispensable measures to reduce microbial air contamination. It was also demonstrated that many important nosocomial pathogens, such as the methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), are frequently found in the areas around the patients infected with these pathogens [9-12]. Several studies have also shown that microorganisms can pass directly from the contaminated surfaces to the hands of healthcare workers, in the absence of direct contact with patients [13, 14]. They also possess an ability to survive for a long time on dry surfaces. MRSA, VRE and Acinetobacter spp, under certain conditions, can survive for 4-5 months [15]. It is also important to underline the fact that, for many pathogens, the infective dose appears to be very low and therefore a slight contamination of the environment is sufficient to cause the onset of infection. For example, it has been shown that less than 15 cells of S. aureus are sufficient to cause infection in experimental lesions [16]. For many infections of the surgical site, in addition to the patient's endogenous flora, the main source of infection is the contamination of the surgical site's air by desquamative cells.
From all this we understand the importance of a continuous monitoring of hospital environments in order to minimize the contamination of the surfaces and, consequently, the possibility of the occurrence of infections. Dancer has proposed the introduction of routine microbiological checks of surfaces in hospitals although this practice is not recommended by the CDC [17, 18].
Materials and methods
The purpose of our research was to detect, through five-year monitoring (January 2010-December 2014) in Messina's University Hospital, the presence of bacterial contamination of the surfaces of operating theatres and hospital wards. The presence of microorganisms on the hands of healthcare workers, which today represent one of the main vehicles of transmission of pathogens, particularly in the hospital setting, was also evaluated [19]. The wards and operating theatres included in the study were part of Medical (Internal Medicine and Paediatrics Operating Unit), Surgical (General Surgery, Orthopaedics, Neurosurgery, Maxillofacial Surgery, Thoracic- Vascular Surgery, Gynaecology and Obstetrics, Paediatric Surgery, Plastic Surgery Operating Units) and Emergency (Intensive Care, Neonatal Intensive Care Unit) areas.
All operating theatres (General Surgery, Otolaryngology, Ophthalmology, Plastic Surgery, Orthopaedics, Neurosurgery, Maxillofacial Surgery, Thoracic-Vascular Surgery, Gynaecology and Obstetrics and Paediatric Surgery Operating Units) were also included in the study. Surfaces considered to be the most immediately in contact with the patients were identified and, along with the hands of healthcare workers, were then sampled: bed bars and header, bedside table, taps, and handles in wards; surgical carts, light and tables in surgical theatre areas.
Random sampling on the hands of healthcare workers, both medics and nurses, who performed diagnostic and/ or therapeutic procedures on the patient were also carried out. Contact slides (manufacturer: PBI) containing various culture media were used for sampling: PCA for the bacterial load, Vogel-Johnson agar for Staphylococcus spp, Cetrimide agar for Pseudomonas spp, Rose Bengal agar + CAF for yeast and mould, VRBG agar for Enterobacteriaceae and Bile-Esculin agar for Enterococcus spp. Each slide was placed in direct contact with the surfaces for 10 seconds. At the same time, sterile cotton swabs swiped in all directions (horizontal, vertical and diagonal) were used within a sterile disposable mask (10x10 cm2) that was placed on the analysed surface. The swabs were immediately placed in a 5 ml tube containing an enrichment broth (brain-heart infusion broth) and, only subsequently, incubated at 37° C for 24 h.
For the evaluation of microbial contamination in operating theatre air, expressed as CFU/m3, the sampling was carried out with a semi-automatic sampler (SAS Super100, Sampler Air System, PBI) containing a Plate Count agar (PCA) that aspired a volume of 180 l/min for 200 seconds. In particular, a sampling was performed in the "at rest" operating theatre and one hour after surgery commencement in the "in use" operating theatre.
All samples were taken immediately to the laboratory. The contact slides were incubated at a temperature of 37°C for 24-72 h and the PCA plates for the measurement of air bacteria were incubated at 37°C for 48h. Samples from the operating theatres were considered positive according to the parameters suggested by the ISPESL "guidelines on standards of security and occupational health in the operating department" [20]. Samples from wards and healthcare workers' hands were considered positive according to the manufacturer's instructions of the contact slides (> 14 colonies on slide corresponding to 117 CFU/100 cm2). From the samples resulted positive, sub-cultures on various agar culture media were set up: mannitol salt agar (Oxoid) for the isolation of Staphylococcus spp, MacConkey agar (bioMérieux) for the Gram-negative, Enterococcosel agar (bioMérieux) for Enterococcus spp and Sabouraud agar (bioMérieux) for yeast and mould. The isolated microorganisms were then identified by manual (API Identification System, bioMérieux) and automatic (VITEK, bioMérieux) biochemical methods.
Results
During the five years in question a total of 9396 microbiological samples from a wide range of surfaces (bed bars and header, bedside table, taps, handles for wards; surgical carts, lights and tables in surgical theatres) were collected and analysed. Below we analyse the results concerning microbiological contamination expressed in percentage of positivity by area, for single microorganism and, for the latter, for each individual year.
Wards
Of a total of 4398 microbiological samples carried out, positivity for wards was 4.7%, 9.4%, 7.0%, 10.8% and 7.9% from 2010 to 2014 respectively.
By evaluating the positivity for each individual organism isolated, a greater presence of Staphylococcus spp and, specifically, mostly for Staphylococcus aureus was shown (Fig. 1).
Fig. 1.
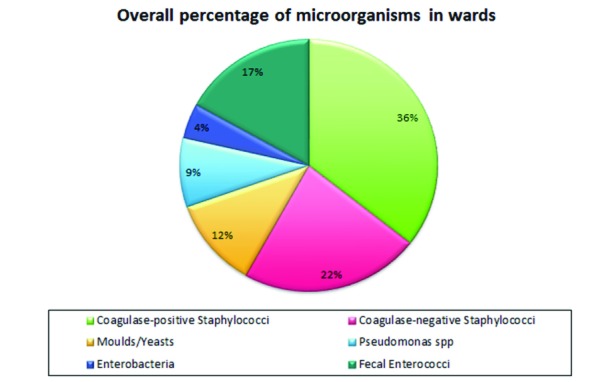
Percentage of microorganisms by type in the 5 years in the wards.
The evaluation of the slides carried out on ward surfaces highlighted the presence of microorganisms in 35.4% of cases (1557/4398) on all samples; analysing them for years, there has been a constant presence albeit in a different proportion of enterobacteriaceae, yeasts and moulds and coagulase-negative Staphylococci (CoNS), a substantial increase for Staphylococcus aureus, and less marked, with fluctuating values, for Pseudomonas and Enterococcus faecalis (Tab. I).
Tab. I.
Positive microorganisms per year in hospitalization.
| S. aureus | CoNS | Moulds/yeasts | Pseudomonas spp | Enterobacteria | Enterococcus faecalis | |
|---|---|---|---|---|---|---|
| 2010 | 27.4% | 23.2% | 15.8% | 9.5% | 5.3% | 18.9% |
| 2011 | 33.3% | 6.7% | 6.7% | 10% | 6.7% | 36.7% |
| 2012 | 32.6% | 28.5% | 13.0% | 8.3% | 5.2% | 12.4% |
| 2013 | 32.2% | 31.7% | 9.4% | 8.3% | 3.9% | 14.4% |
| 2014 | 47.3% | 8.3% | 10.7% | 9.5% | 4.1% | 20.1% |
Comparing the trend over time for each individual operating unit (OU) considered, a decrease of positivity only for intensive care (ICU) and the neonatal intensive care unit (NICU) was observed (Fig. 2).
Fig. 2.
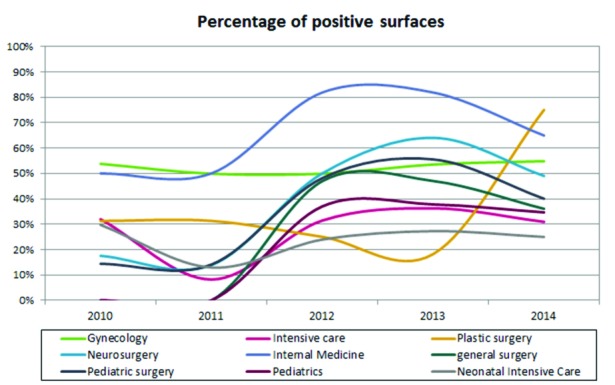
Temporal trend in the five years of positivity for operating units.
Healthcare workers' hands
Microbiological tests on the hands of 648 healthcare workers present in wards were carried out, of which 40.74% (264/648) tested positive. The results obtained highlight a decrease in the percentage of positivity equal to 9.5%, 8.0%, 5.5%, 6.9% and 5.1% from 2010 to 2014 respectively.
On the total sampling conducted from 2010 to 2014, performing a single microorganism evaluation it was also found, as for the wards, a higher positivity in contamination by Staphylococcus spp, although in this case the majority are coagulase-negative staphylococci (Fig. 3).
Fig. 3.
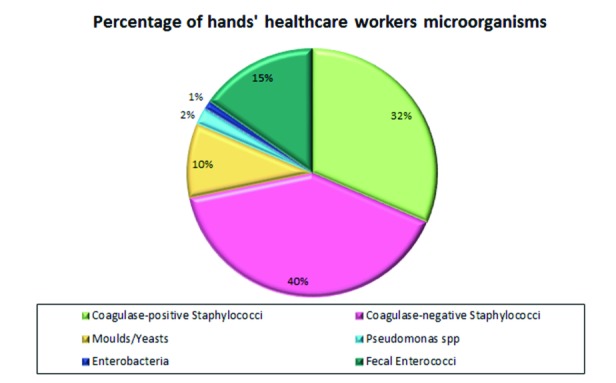
Percentage of microorganisms by type in the period on the hands of health care workers.
Comparing the isolated microorganisms per year there is always a growing trend for microbiological contamination of hands by Staphylococcus aureus, a decrease for coagulase-negative staphylococci, yeasts, and moulds; an up and down trend for Enterococcus faecalis, an initial increase (2011 and 2012) with an overall reduction from 2013 for Pseudomonas while Enterobacteriaceae already appear absent since 2011 (Tab. II).
Tab. II.
Positive microorganisms per year on the hands of healthcare workers.
| S. aureus | CoNS | Moulds/yeasts | Pseudomonas spp | Enterobacteria | Enterococcus faecalis | |
|---|---|---|---|---|---|---|
| 2010 | 31% | 42% | 11% | 0% | 3% | 14% |
| 2011 | 18% | 42% | 9% | 3% | 0% | 27% |
| 2012 | 38% | 44% | 13% | 6% | 0% | 0% |
| 2013 | 50% | 17% | 0% | 0% | 0% | 33% |
| 2014 | 86% | 14% | 0% | 0% | 0% | 0% |
Operating theatres
The microbiological assessment carried out on operating theatre surfaces has shown the presence of microorganisms in 11.9% of cases (498/4188) of the total number of samples taken in the five-year period, equal to 4188.
The percentage of total microbial positivity has been decreasing steadily over the years, going from 2.8% to 2.5%, 2.3%, 2.3% and 1.9% respectively from 2010 to 2014.
A higher positivity for Staphylococcus spp was noted (Fig. 4).
Fig. 4.
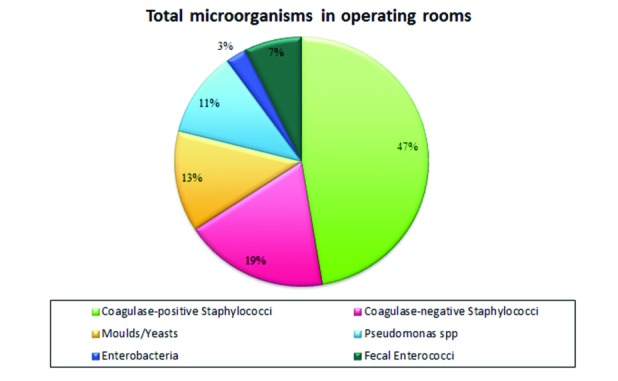
Percentage of microorganisms by type in the five years in operating rooms.
If we observe the trend by year for an isolated microorganism, as done previously for wards, there is a definite correspondence as regards the behaviour of Staphylococcus aureus; in fact, in both cases, there is a substantial increase of positivity percentages. A different behaviour of CONS, yeasts, moulds, and Pseudomonas is also observed in operating theatres; as regards enterobacteria there is a net improvement with absence of contamination in the year 2014 (Tab. III).
Tab. III.
Positivity of microorganisms per year in the operating rooms.
| S. aureus | CoNS | Moulds/yeasts | Pseudomonas spp | Enterobacteria | Enterococcus faecalis | |
|---|---|---|---|---|---|---|
| 2010 | 26.3% | 31.6% | 15.8% | 18.4% | 2.6% | 5.3% |
| 2011 | 44.4% | 0.0% | 33.3% | 0.0% | 0.0% | 22.2% |
| 2012 | 48.3% | 16.7% | 15.0% | 11.7% | 3.3% | 5.0% |
| 2013 | 50.0% | 15.5% | 13.8% | 12.1% | 3.4% | 5.2% |
| 2014 | 65.5% | 13.8% | 0.0% | 3.4% | 0.0% | 17.2% |
Evaluating the trend over time, for the operating theatres taken into account, a decrease of positivity in a majority of theatres was found, except for one surgical complex (maxillofacial surgery, plastic surgery and orthopaedics) (Fig. 5).
Fig. 5.
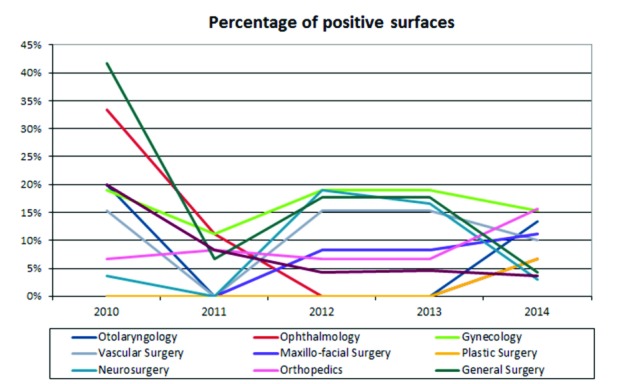
Time course in the five years of positive operating rooms.
Air
Air samples were taken for each operating empty "at rest" and full "in use" theatre for a total, over five years, of 1962 samples with a positivity rate of 31.9% (627/1962).
In 2010, empty theatre compliance was 64.0%, while when full it was 80.0%. In 2011, there was an increase in empty theatre compliance of 69.0%, while in full theatres the samples showed a compliance of 85.0%. There has been, on the other hand, a decrease in air compliance in operating theatres in the following three years: in particular, in 2012, 59.3% of empty theatres and 79.0% of the theatres in use were compliant. In 2013, however, 57.7% of empty theatres and 58.2% of theatres in use were compliant. In 2014 empty theatre compliance was 58.2% and full theatre 41.8%.
Discussions and conclusions
This work has highlighted the presence of pathogens that are potential cause of nosocomial infections on the surfaces we assessed with a percentage of positivity varying between 1.9% to 10.8%.
For inpatient care contamination levels increased progressively over the five years, especially for some of the surgical sites: in Plastic Surgery, positivity increased from 31.0% to 75.0%, in Neurosurgery from 18.0% to 49.0%, in Pediatric Surgery from 14.0% to 40.0%. Instead, in the Department of Internal Medicine, positivity remained high although with mixed values (50.0% in 2010-2011, an 82.0% spike in 2012-2013 biennium and 65.0% in 2014). There are various risk factors that are evident. The first to consider is that wards are often overcrowded with a high turn-over of both hospitalized patients and visitors, and this promotes the continuous re-contamination of the environments [21]. Another determining element is lesser attention to the sanitation of hospital stays compared to other areas. Indeed, we found a decrease of contamination in intensive care units where the sanitization procedures are more frequent and effective, probably because of the special attention given to these departments where patients are hospitalized in critical conditions and where the influx of visitors is regulated. In all departments, however, the most common microorganism is S. aureus, known agent of nosocomial infections.
As for the hands, finally, the monitoring has highlighted a low rate of contamination with a progressive decrease in the five-year period. This could be the result of greater awareness and attention in the cleaning and disinfection of the hands of healthcare workers, thanks also to the audit campaigns carried out by us constantly, and the presence of hydro-alcoholic gel dispensers in the various departments.
A good result was also obtained for the surfaces of operating theatres where, in five years, there has been a decrease of positivity.
The same does not apply, however, to the air, because contamination has increased in "at rest" operating theatres, especially in the last three years (2012-2014) and increased significantly in the "in use" ones in the 2013- 2014 biennium. This result may be due to various factors that coexist with each other such as poor adherence by healthcare workers to ministerial recommendations [22].
We did in fact observe an inconstant application of these rules, which provide for the closing of doors throughout the duration of surgery, using the lowest possible number of operators in attendance, and proper surgical attire. Another key element to consider is the correct operation and powering of the ventilation system (VCCC) with proper maintenance of the operating theatre filters.
During the final study period we, regularly, conducted audits and educational meetings to inform the healthcare workers about the importance of adopting correct behaviours to the aim of avoiding microbiological contamination of hospital environment and so the transmission to patients of nosocomial pathogens potentially cause of nosocomial infections.
The strength of our research is to show the importance of a constant environmental microbiological monitoring that appears as one of the main tools for reducing environmental microbial load as a whole, through the evaluation and assessment of the following parameters: proper operation of VCCC systems and effectiveness of sanitation procedures put into place. Indeed, as a result of our control, many important policies were adopted in the hospital, especially the increased control and cleaning of air filters in the operating rooms, the greater attention to the sanitation of the hospital surfaces and the increased number of sanitizer dispenser for hands in hospital.
However, microbiological monitoring alone is not sufficient to improve the fight to the nosocomial infections. It is, also, necessary conducting periodic informative meetings to healthcare workers, ensuring correct application of the rules of conduct to be adopted by healthcare staff, performing periodic audits to regulate the influx of visitors and informing them to follow a few simple hygienic-health rules is also necessary, in order to decrease environmental recontamination in the hospital. Moreover, it would be desirable improve the cleaning of the hospital environment combining the classic cleaning methods with those recent such as ultraviolet devices, hydrogen peroxide systems, self-disinfecting surfaces and use of an innovative sanitization procedure using probiotic bacteria based on the principle of biological competition [23-25].
ACKNOWLEDGMENTS
Funding: the funds had been provided by the Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy.
Conflicts of interest: the authors declare that there are no conflicts of interest.
Ethical approval: not required. (This study is not a clinical trial and not involved human subjects).
Footnotes
the authors declare that there are no conflicts of interest.
the funds had been provided by the Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team, author. Multistate pointprevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26(4):338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 3.Otter J, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32(7):687–699. doi: 10.1086/660363. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 4.Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73:378–385. doi: 10.1016/j.jhin.2009.03.030. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65:50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 6.Dancer SJ, White LF, Lamb J, Girvan EK, Robertson C. Measuring the effect of enhanced cleaning in a UK hospital: a prospective cross-over study. BMC Med. 2009;7:28–28. doi: 10.1186/1741-7015-7-28. doi: 10.1186/1741-7015-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining "high-touch" surfaces in hospitals. Infect Control Hosp Epidemiol. 2013;31:850–853. doi: 10.1086/655016. [DOI] [PubMed] [Google Scholar]
- 8.Pasquarella C, Sansebastiano GE, Ferretti S, Saccani E, Fanti M, Moscato U, Giannetti G, Fornia S, Cortellini P, Vitali P, et al. A mobile laminar airflow unit to reduce air bacterial contamination at surgical area in a conventionally ventilated operating theatre. J Hosp Infect. 2007;66:313–319. doi: 10.1016/j.jhin.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Squeri R, Grillo OC, La Fauci V. Surveillance and evidence of contamination in hospital environment from methicillin and vancomycin-resistant microbial agents. J Prev Med Hyg. 2012;53:143–145. [PubMed] [Google Scholar]
- 10.Sexton T, Clarke P, O'Neill E, Dillane T, Humphreys H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implications for hospital hygiene. J Hosp Infect. 2006;62:187–194. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein BC, Adams DA, Eckstein EC, et al. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis. 2007;7:61–61. doi: 10.1186/1471-2334-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French GL, Otter JA, Shannon KP, Adams NM, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Hayden MK, Blom DW, Lyle EA, Moore CG, Weinstein RA. Risk of hand or glove contamination after contact with patients colonized with vancomycin-resistant Enterococcus or the colonized patients' environment. Infect Control Hosp Epidemiol. 2008;29:149–154. doi: 10.1086/524331. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25:164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 15.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130–130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster WD, Hutt MS. Experimental staphylococcal infections in man. Lancet. 1960;2:1373–1376. doi: 10.1016/s0140-6736(60)92612-x. [DOI] [PubMed] [Google Scholar]
- 17.Dancer SJ. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect. 2004;56:10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities: recommendation of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–42. [PubMed] [Google Scholar]
- 19.Squeri R, Genovese C, Palamara MA, Trimarchi G, La Fauci V. "Clean care is safer care": correct handwashing in the prevention of healthcare associated infections. Ann Ig. 2016;28(6):409–415. doi: 10.7416/ai.2016.2123. doi: 10.7416/ai.2016.2123. [DOI] [PubMed] [Google Scholar]
- 20. Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (ISPESL). Linee guida sugli standard di sicurezza e di igiene del lavoro nel reparto operatorio. 2009.
- 21.Hardya KJ, Gossaina S, Hendersonb N, Druganb C, Oppenheima BA, Gaob F, Hawkeya PM. Rapid recontamination with MRSA of the environment of an intensive care unit after decontamination with hydrogen peroxide vapour. J Hosp Infect. 2007;66(4):360–368. doi: 10.1016/j.jhin.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 22. Ministero del Lavoro, della Salute e delle Politiche Sociali. Manuale per la Sicurezza in sala operatoria: Raccomandazioni e Checklist. Roma, 2009.
- 23.Weber DJ, Kanamori H, Rutala WA. 'No touch' technologies for environmental decontamination: focus on ultraviolet devices and hydrogen peroxide systems. Curr Opin Infect Dis. 2016;29(4):424–431. doi: 10.1097/QCO.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 24.Weber DJ, Rutala WA, Anderson DJ, Chen LF, Sickbert-Bennett EE, Boyce JM. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am J Infect Control. 2016;44(5 Suppl):e77–e84. doi: 10.1016/j.ajic.2015.11.015. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Fauci V, Costa GB, Anastasi F, Facciolà A, Grillo OC, Squeri R. An innovative approach to hospital sanitization using probiotics: in vitro and field trials. J Microb Biochem Technol. 2015;7:160–164. doi: 10.4172/1948-5948.1000198. [Google Scholar]


