SUMMARY
Ménière's disease, a condition first described in the 1800's, has been an advancing area of clinical interest and scientific research in recent decades. Guidelines published by the American Academy of Otolaryngology – Head and Neck Surgery remained nearly static for almost 20 years, although we have certainly expanded our knowledge of the aetiology of the disease since that time. This review of the literature highlights the breadth and detail of the current theories in understanding the pathophysiology of this enigmatic disease. Histopathological specimens providing evidence of many of the aetiologies are presented as well. We aim to provide a centralised and updated resource regarding current and emerging theories for Ménière's disease.
KEY WORDS: Ménière's disease, Pathology, Aetiology, Update
RIASSUNTO
La Sindrome di Ménière, una condizione descritta nel 1800, è stata un'area di grande interesse clinico e di ricerca scientifica negli ultimi decenni. Le linee guida pubblicate dall' American Academy of Otolaryngology-Head and Neck Surgery sono rimaste pressoché invariate per quasi 20 anni, benché la ricerca scientifica sugli aspetti eziopatologici sia indubbiamente molto progredita nel frattempo. La presente revisione della letteratura evidenzia gli importanti progressi compiuti nella comprensione della fisiopatologia di questa malattia enigmatica. Le evidenze discusse sono inoltre accompagnate da una documentazione iconografica istopatologica. L'obiettivo della presente trattazione è fornire al lettore un quadro aggiornato ed accurato sulle teorie inerenti la Sindrome di Ménière.
Introduction
Prosper Ménière brought the attention of vertigo and its possible relationship to the inner ear to light in his controversial paper presented in 1861 to the Imperial Academy of Medicine 1. His series of articles challenged the existing theories of vertigo as a cerebral disease. The eventual development and acceptance of criteria used to describe the disease with Ménière's namesake has increased efforts in understanding the disease. The Committee on Hearing and Equilibrium of the American Academy of Otolaryngology – Head and Neck Surgery established diagnostic criteria and reporting guidelines for treatment. Their guidelines, published in 1995, define Ménière's disease (MD) as "an idiopathic syndrome of endolymphatic hydrops" 2. This inner ear disorder is characterised by episodic vertigo with neurovegetative symptoms, sensorineural hearing loss, and tinnitus or aural fullness. Either tinnitus or aural fullness, or both, must be present in the affected ear. The Classification Committee of the Bárány Society (CCBS) has developed an international consensus for the diagnostic criteria for MD, released for publication in 2015. The new guidelines provide criteria for definite and probable MD, as seen in Table I 3.
Table I.
Definitions of MD
| Definition | Symptoms |
|---|---|
| Definite MD |
|
| Probable MD |
|
As our knowledge regarding the aetiology of the disease expands, the controversy surrounding the pathophysiology of MD deepens. The debate is rooted in the histopathologic finding of endolymphatic hydrops (ELH). This significant step toward understanding the disease was based on the discovery of marked inflammation of the cochlear scala media in the temporal bones of patients with classic symptoms 4 5. Although the certainty of ELH can only currently be identified post-mortem via temporal bone histopathology, our current guidelines provide the means to identify those who may suffer from the disease. The relapsing nature of the disease, and the sampling bias in prior studies, contributes to the varied rates of incidence and prevalence. Recognising these limitations, the incidence likely ranges between 10-150 per 100,000 persons. The most recent estimate of prevalence in the United States is at 190 per 100,000. The prevalence increases markedly with increasing age 6-10.
This review of the literature highlights the breadth and detail of the current theories in understanding the pathophysiology of this enigmatic disease. We aim to provide a centralised and updated resource regarding current and emerging theories behind MD.
Materials and methods
Using the PubMed MEDLINE NCBI database, a total of 291 results were retrieved on December 8, 2013 by utilising the search query "Ménière's Disease." Inclusion criteria include: articles published within the last 5 years, English, full-text, and a journal impact factor of 1 or greater. Two hundred and twenty-three of the 291 total articles which had no direct relation to either disease aetiopathogenesis or histopathology were excluded. Of the remaining 68 articles, there were: 2 case reports, 2 theoretical research articles, 42 original research articles, and 22 review articles. Each publication was meticulously analysed for this comprehensive review. A few choice articles were selected due to further elaboration of the aetiopathogenesis after the original query was performed.
Discussion
Normal physiology of endolymph
Understanding the endolymphatic system physiology will permit a clearer elaboration of the various pathologic alterations described in the literature. The cochlea is a coiled, bony tube approximately 35 mm long and composed of three chambers: scala vestibuli, scala media, and scala tympani. Together, the scala vestibuli and the scala tympani are part of a perilymph-filled periotic labyrinth encased in the bony labyrinth. The scala vestibuli and scala tympani are connected at the apex of the cochlea via the helicotrema. The scala tympani connects to the cerebrospinal fluid containing arachnoid space via the cochlear aqueduct.
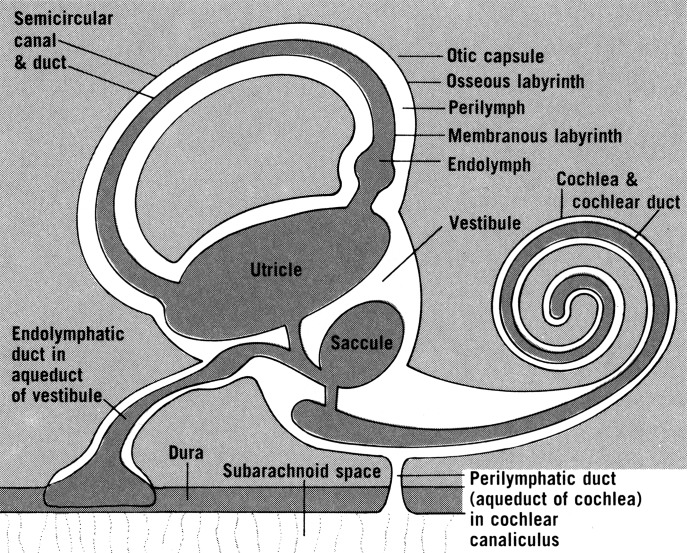
The membranous labyrinth of the cochlea is the scala media, the endolymphatic containing chamber. It is connected to the saccule by the ductus reuniens. The remainder of the membranous labyrinth consists of the semicircular canals, saccule, utricle, endolymphatic duct and sac, and cochlear duct. The utricle is located in the posterosuperior portion of the vestibule. The saccule is located in the anteroinferior portion of the vestibule. The utricle connects posteriorly to the semicircular canals. Anteriorly it connects to the endolymphatic and saccular ducts via the utricular duct. The utricular valve likely serves to protect the superior part of the labyrinth against endolymph loss due to membrane rupture from the inferior part of the labyrinth (Fig. 1) 11.
Fig. 1.
Anatomy of the endolymph system. Used with permission from Rand S. Swenson. O'Rahilly R, Mueller F, Carpenter S, Swenson RS. Basic Human Anatomy: A Regional Study of Human Structure. 2009; http://www.dartmouth.edu/~humananatomy/figures/chapter_44/44-8.HTM. Accessed December, 24, 2014.
The endolymphatic sac is one of the first structures of the membranous labyrinth to appear in embryonic development, and it is the last to stop growing 12. It is closely associated with the layers of dura mater. The sac is connected to the endolymphatic duct, which lies within the bony vestibular aqueduct. The endolymphatic sac can be divided into portions based upon the cellular lining. The proximal, rugose portion is in a bony niche and has cells similar to the endolymphatic duct. The intermediate portion is between bone and dura and has light and dark cylindrical cells. The distal, smooth portion is within layers of dura mater and consists of cuboidal cells 13. In 1997, Gibson and Arenberg described the likely functions of the endolymphatic sac (Table II) 13.
Table II.
Functions of the endolymphatic sac.
|
The endolymphatic sac's role in endolymph homeostasis is well recognised, but the control mechanisms are still being established. The composition of the fluid is maintained by ion transport systems 14. It is unclear as of yet where the sensor might be for maintaining the volume of the scala media, and if it is even in the cochlea. Endolymph is unique to other extracellular fluids in that it has a high potassium, low sodium and low calcium concentrations (Table III) 15 16. The high potassium concentration and low calcium concentration are critical for sensory conduction in the cochlea 15.
Table III.
Composition of cochlear fluids.
| Component | Unit | Endolymph scala media | Intrastrial fluid | Perilymph scala vestibuli | Perilymph scala tympani | Plasma |
|---|---|---|---|---|---|---|
| Na+ | mM | 1.3 | 85 | 141 | 148 | 145 |
| K+ | mM | 157 | 2 | 6.0 | 4.2 | 5.0 |
| Ca2+ | mM | 0.023 | 0.8 | 0.6 | 1.3 | 2.6 |
| Cl– | mM | 132 | 55 | 121 | 119 | 106 |
| HCO3– | mM | 31 | n/a | 18 | 21 | 18 |
| Glucose | mM | 0.6 | n/a | 3.8 | 3.6 | 8.3 |
| pH | pH units | 7.4 | n/a | 7.3 | 7.3 | 7.3 |
| Protein | mg dl | 38 | n/a | 242 | 178 | 4238 |
The ion transport system is mostly well established, with a few remaining concepts under debate. In general, potassium in endolymph is driven by the endocochlear potential into sensory hair cells via apical transduction channels. Basolateral potassium channels carry it out into the perilymph. From there, potassium is taken up by fibrocytes of the spiral ligament where it diffuses into strial intermediate cells. These cells, and potentially other potassium channels, release potassium into the intrastrial space where it is taken up by strial marginal cells and secreted out of the stria vascularis and into endolymph 15.
Interestingly, in a euvolemic state the endolymph is maintained without significantly detectable volume flow. However, in abnormal volume states the direction of volume flow may contribute to endolymph homeostasis. Salt has shown that an enlarged endolymphatic space will result in endolymph flow toward the base of the cochlea, contributing to the removal of volume and electrolytes. Contrary to this, in hypovolemic states, the flow is apically oriented in the cochlea, leading to increased volume and electrolytes in the endolymph 17.
Aetiopathologies
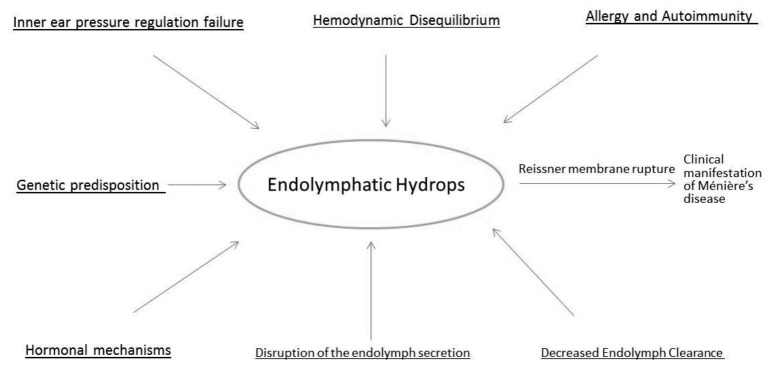
A variety of pathologic findings have been identified during the course of MD. Perhaps the most established is the finding of endolymphatic hydrops. This histologic finding was first reported by Hallpike and Cairns in 1938 as well as Yamakawa who demonstrated this pathologic finding in the same time period 4. Since then, many reports have been described about the histopathologic findings in MD. We present the most updated comprehensive list in at least the past 10 years. Figure 2 provides a concise look at the relationships between the most recent pathologic mechanisms for MD.
Fig. 2.
Aetiologies of endolymphatic hydrops.
Endolymphatic hydrops (ELH)
The mechanism for clinical manifestation of MD secondary to endolymphatic hydrops was led by Schuknecht's theory of Reissner membrane rupture secondary to endolymphatic duct distention. This would have allowed for potassium rich endolymph to bathe the basal surface of hair cells as well as the eighth cranial nerve. Repeated hair cell and nerve exposure to potentially toxic levels of potassium-infused perilymph could cause episodic vertigo as well as long-term decline in auditory and vestibular function (Fig. 3a) 18. The rupture theory eventually stimulated new ideas about endolymphatic flow and blockage along the endolymphatic duct pathway as a potential cause for hydrops. Prevalence of endolymphatic hydrops in MD is reported as 100% in the cochlea, 86.3% in the saccule, 50% in the utricle and 36.4% in the semicircular canals (Fig. 3b) 19. However, one of our histopathologic exams interestingly showed spared cochlear involvement in a patient with MD (Fig. 3c). Earlier studies showed that in the late stages of MD, there might be severe dilatation or collapse of the ampullary walls, which could be restricted with the cupular movement, resulting in a poor caloric response 20; caloric response is reduced in about 65% of patients with MD (Fig. 3d) 21.
Fig. 3.
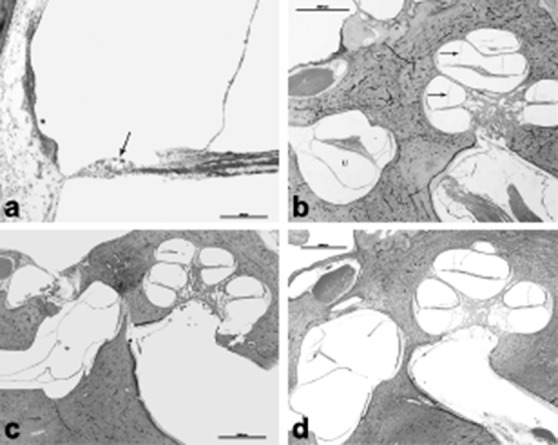
a) HB 655 Lt 130 10x: A higher magnification of the cochlea showed outer hair cell loss (arrow) and stria vascularis atrophy (*), b) HB 655 Lt 100 1x: 72-year-old male patient diagnosed with Ménière's disease. He had a history of bilateral fluctuating hearing loss, tinnitus and vertigo episodes. Histopathologic exam of the left ear showed profound cochlear hydrops (arrow). Utricle (U), c) HB650Lt110. This 79-year-old patient had a history of bilateral profound mixed type hearing loss and left stapedectomy. She was extremely vertiginous right after the surgery for months. Histopathologic exam showed bilateral otosclerosis, which is located anterior to the oval window. This slide shows the surgery site, otosclerosis and hydropic saccular membrane that showed healed perforation. Interestingly, cochlea did not show any hydropic changes, d) HB593Lt 470. This was an 86-year-old patient who had a history of Ménière's disease and 8th nerve resection. Histopathologic exam showed profound hydropic changes in the cochlea, utricle and saccule. Note the outpouching, and hydropic saccular membrane.
Saccular hydrops may be dependent upon the degree of membrane distention toward the stapes footplate; cochlear hydrops is seen as bowing of Reissner's membrane into the scala vestibuli (Fig. 4 a, b) 18. The saccule is the second most common site of hydrops; severe hydrops is most frequently in the saccule.
Fig. 4.
a) HB333Lt 142. This was a 79-year-old patient who had a history of bilateral Ménière's disease. Histopathologic exam shows profound cochlea-saccular hydrops. Note that saccular membrane shows fistulae and touches to the footplate of stapes, b) HB839 Lt 260 1x: This 86-year-old male patient was diagnosed with left Ménière's disease. Histopathologic exam of his left ear showed profound hydrops in every turns of the cochlea as well saccule. Arrow shows that saccular membrane touches the footplate of stapes.
Reports have also been made regarding displacement of the basilar membrane in the apical segments of the cochlea, to the degree that the basilar membrane contacts the bony wall of the scala tympani. This too may further explain the variable degree to which hydrops is seen in different areas of the same temporal bone specimen 22.
Salt applies the theory that abnormal volume states in ELH are detected by small changes in volume when pressure changes are minimal. The endolymphatic sinus, located between the saccule and the utriculoendolymphatic valve at the entrance to the endolymphatic duct, may be able to detect small mechanical pressure changes. For example, as pressure is applied to perilymph, the endolymphatic duct is occluded; this is presumably secondary to displacement of endolymph from the sinus to the endolymphatic sac, thereby allowing the sinus membrane to occlude endolymphatic duct opening 22 23. As of yet, there is no definitive theory for what is causing the pressure fluctuations.
Decreased endolymph clearance
a. Endolymphatic sac (ES)
Just as there are multiple theories regarding the pathogenesis for ELH, many other potential aetiopathologies have been introduced for MD. Observations of changes and dysfunction in the ES may contribute to this disease process. Theories for this dysfunction developed around the ES's role in endolymph regulation, embryologic abnormalities, immune mediator effects on the ES, and hormonal mechanisms.
Hornibrook et al. favour that in states of endolymph excess, such as MD, the ES removes endolymph by longitudinal flow. They note that the ES contains absorptive and secretory cells, suggesting that hydrophilic glycoproteins produced by the secretory cells are stimulated in this state of excess 24. Paparella et al. suggested that patients may have an underlying predisposition to MD in the setting of developmental anomalies of the ES, such as hypoplasia 25. However, the leading theory at that time was the endolymphatic flow concept which newer research has brought into question, as aforementioned. A 2009 study showed that the ES may play a role in endolymph pressure regulation. The use of systemic isoproterenol, a β-adrenergic agonist, increased endolymph pressure and decreased the ES lumen potential. The CSF pressure was not affected by the systemic agent. When ablating the ES, the effects of isoproterenol on endolymph pressure and ES lumen potential were suppressed. This suggests that the ES plays a role in pressure regulation 22. As we identify which components of the ear play a role in endolymph homeostasis, particularly those under hormonal control, we will also need to clarify how these systems are interrelated. The ES is generally accepted to play a role in endolymph volume regulation, although the exact sensor location is not clear. Given the location of the sac, near the pulsations of the sigmoid sinus, Salt proposes that it may not be as well suited to monitor pressure changes 22. He supports this by pointing out that the ES is not bounded by perilymph, the endolymphatic duct restricts access of perilymph to the periphery of the sac, and the detection of hydrops likely requires the detection of small changes in endolymph pressure with respect to perilymph. Instead, the endolymphatic sinus, discussed later, may be the more likely candidate 22.
The role of the ES as an immune mediator for the middle ear presents another fashion by which MD could be provoked. Details of the immunologic factors involving the ES will be discussed later, but a brief background is warranted. Immunoglobulins (Ig) G, IgM, and IgA, as well as secretory components of the immune system have been found in the ES. Additionally, macrophages and plasma cells reside in the perisaccular connective tissue 26 27. The ES can process antigen and produce its own antibody response, but the overall role of this in inner ear immune response is not fully elucidated. Surgical destruction of the ES has been shown to result in locally decreased antigen and antibody responses 26 27. Currently, one proposed mechanism is access of immune complexes, mast cells, and viral antigens into the highly vascular subepithelial space via the fenestrated vasculature 26 27. Immune complex access to this otherwise barrier protected region is thought to be via the many fenestrated branches of the posterior meningeal artery which help supply the ES and the endolymphatic duct 26 27. This will be discussed later along with a review of mast cell degranulation in the perisaccular connective tissue and viral antigen-allergic reaction with T-cell homing to the ES.
Investigations of immune-mediated dysfunction of the ES started in 1979 when McCabe described patients with sensorineural hearing loss (SNHL) who benefited from corticosteroids and cyclophosphamide. By the 1990's, Bloch et al. had purified an antibody seen in 35% of patients with progressive SNHL; this antibody targeted heat shock protein 70 (HSP70) 24. The question arose as to whether there was any overlap in subjects with progressive SNHL and MD. After multiple follow-up studies, there has been no correlation between HSP70 antibodies and MD. The question remains about an autoimmune component for the disease, but certainly HSP70 antibodies are not causative agents in MD.
In the setting of ELH, models studied by surgical ablation of the endolymphatic duct and sac were performed in guinea pigs. The most obvious limitation to this study is that the ES is completely non-functional, which does not seem to be reflective of the ES in MD 22. One study circumvented this limitation while examining the effect of aldosterone on hydrops. The distal portion of the ES was detached from the sigmoid sinus while leaving the intraosseous portion alone. This study was able to demonstrate that even partial sac ablation can cause hydrops. Aldosterone and its effect on sodium-potassium adenosine triphosphatase (Na-K ATPase) levels in stria vascularis as well as the potential enhanced secretion of potassium into endolymph are discussed in the Hormone section 22.
b. Endolymphatic duct (ED)
The ED lies within the bony vestibular aqueduct. A recent histologic study of the otic capsule evaluated the cellular appearances of the normal vestibular arch, and with that, a theory of how the canals in the otic capsule may contribute to the homeostasis of the ED. It appears that lamellar bone contributes to the foundation of the otic capsule. Many Volkmann's canals and micro-Volkmann's canals are present as channels throughout the bone in the vestibular arch. Osteoblasts likely line the mature vestibular arches, as the arch is derived from osteogenic cells of the external layer of the otic capsule 28. The development of the otic capsule skeleton is activated by signalling molecules from the nearby epithelium, including that of the ED 28. Michaels et al. suggest this close relationship may result in osteoblast proliferation and consequent microcanalisation throughout life. They propose that the slow breakdown of proliferated osteoblasts, via apoptotic mechanisms, may nourish the nearby endolymph with potassium ions 28.
The ED has also been implicated in theories of endolymph obstruction as aforementioned, and in particular, Schuknecht's theory of Reissner membrane rupture secondary to ED distention, allowing for potassium rich endolymph to bathe the basal surface of hair cells as well as the eighth cranial nerve (Fig. 5) 18.
Fig. 5.
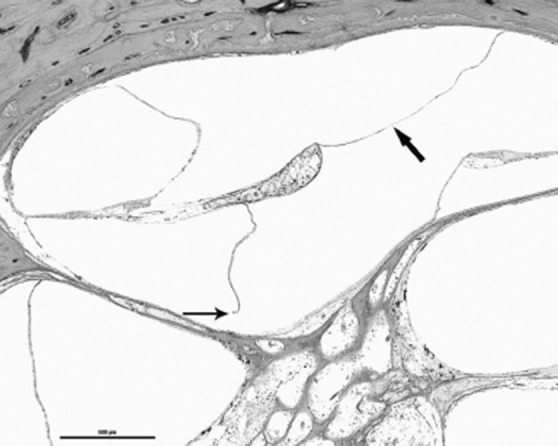
HB839 Lt 260 4x: A higher magnification of the cochlear hydrops is seen. Thick arrow shows profound hydrops; thin arrow indicates the rupture of Reissner's membrane.
Additionally, the ED may play a role in the effect of spiral ligament fibrocytes on ELH. ED obstruction is thought to alter the cytochemistry of the perilymph, by a yet unknown mechanism, leading to cellular stress of the spiral ligament fibrocytes. These fibrocytes, in turn, disrupt the potassium recycling mechanism within the scala media, resulting in an osmotic imbalance and expansion of endolymphatic compartment 29.
c. Endolymphatic sinus
The potential role of the endolymphatic sinus was briefly discussed during our review of ELH. The endolymphatic sinus may assist in endolymph volume regulation by detecting minute changes in endolymph pressure with respect to perilymph. The walls of the sinus are distensible, and given the position of the sinus at the entrance to the ED, it would be ideally placed to monitor pressure changes. Prior studies showing ED occlusion with increased pressure in the perilymph suggests the endolymph is displacing the sinus walls into the ES, thereby occluding the duct opening 22. Additionally, the amount of endolymph being displaced into the ES may depend upon the degree of distention of the sinus, adding yet another means of volume control for the endolymphatic sinus 22.
The projected role of the sinus may be compatible within the controversial theories of longitudinal flow and ion transport regulation of endolymph. Further research will need to be done to elucidate such details. One prominent theory is that under exceptional circumstances, in situations with large volume increase, endolymph will move longitudinally toward to the ES to be resorbed. In such situations, the rate of longitudinal flow would be limited by the narrow isthmus of the ED, and the endolymphatic sinus may act as a reservoir 30. In situations where the sinus has an overflow state, or blocks the entrance to the ED, the utriculoendolymphatic valve may be affected.
d. Utriculoendolymphatic valve (UEV)
The exact purpose of the UEV, or Bast's valve, remains unclear. One theory is that it anatomically acts as a shutter, allowing for excessive volumes of endolymph to be processed in the ES, in a fashion regulated by unknown means as of yet. It would simultaneously prevent an excessive loss of endolymph, consequently leading to membrane distortions and interference of vestibular sense organs 30 31. It is uncertain if the UEV is open or closed in normal situations, or situations without volume excess of endolymph. Additionally, the mechanisms which open and close the valve are not yet elucidated 31. The UEV has been observed to be open for a few days once ELH develops, then closes due to compression from increasing hydrops 32. A more recent evaluation of the UEV in a temporal bone series did not show significance between closure of the valve in MD patients and normal ears 33. Shimizu et al. suggested that the position of the UEV in temporal bones does not directly reflect the pathologic condition of MD 33.
e. Ductus reuniens (DR)
The DR (reuniting duct) is a membranous path between the saccule and cochlea. Blockage of this usually patent pathway can be caused by ELH. The longitudinal flow of endolymph was shown to be blocked most frequently, between 59-67%, at the DR as determined by two case series 34 35. These series evaluated the bony saccular orifice to the bony groove of the DR utilising computed tomography. The mechanism of obstruction at the DR needs further elucidation, but one report from Gussen et al. shows that otoconia traveled from the saccule, fell into the DR, and then migrated further to the cochlea 36. If the cochlear endolymphatic pressure builds to a critical point, the material blocking the DR can be washed into the sacculus, resulting in a classic vertiginous attack characteristic of MD. Given the relative reported frequency of DR obstruction, pathology at the level of the DR likely contributes to the aetiology of MD.
f. Vestibular arch
Within the inner layer of the bony vestibular aqueduct lies the vestibular arch, a cylindrical lining of thin, osseous cells extending into the posteromedial aspect of the vestibule. It closely envelops the ED in an arch-like fashion and surrounds it throughout most of its length. A striking loss of these cells was previously observed in 20 cases of MD, together with the apparent new formation of large intraskeletal channels 37. Additional studies have revealed associated degenerative changes with apoptotic bodies and denudation of osteoblasts throughout both the microcanals and Volkmann's canals in the vestibular arch. Dislocation of dead microcanals into blood vessels of Volkmann's canals has also been described in some MD patients. Collectively, these findings suggest that the origin of ELH could potentially be localised to a pathologic lesion located at the vestibular arch 28.
g. Semicircular canals
Recently, a proposed theory by Phillips and Prinsley described the presence of free floating particle (FFP) deposits distal to the semicircular canals at five different sites within the labyrinth as a potential source of aberrations in vestibular function seen in Ménière's patients. These changes would result in disturbances of balance and hearing, depending on the quantity and size of the floating particulate matter. Progressive damage would initially be reversible but would ultimately result in irreversible harm to the auditory and vestibular apparatus. The role of mineral intake may have an effect on the composition of these FFPs; it may be that the mineral composition of endolymphatic fluid is critical with respect to thresholds for crystallisation. Becvarovski coined the term "ductolithiasis" to describe these phenomena, and the associated histopathological findings of ELH in the temporal bones of Ménière's patients may simply be the consequence of FFP matter 38. This theory is certainly reasonable, but unable to be verified at this time.
The clinical setting of MD can be clouded when patients have associated disorders such as benign paroxysmal positional vertigo (BPPV). Recent literature suggests a possible correlation between the two disorders, and it has been hypothesised that the pathophysiology of one disease may lead to the other. Although many authors support the idea that BPPV can be secondary to MD, some suggest that MD secondary to BPPV is also a possibility. The mechanism by which this may occur is thought to be due to loose otoconia decreasing endolymphatic absorption, resulting in endolymphatic hydrops 39.
Disruption of the endolymph secretion
Another model which attempted to explain ELH by means of disruption of the endolymph secretion in the cochlea and resorption of endolymph in the ES has been disproven. This theory was introduced as a result of iatrogenically introducing the effect of endolymphatic flow when a large volume of marker was injected into endolymphatic space. The homeostasis of endolymph seems more dependent upon ion transport and osmotic gradients than volume secretion. These ion transport systems have been shown to be under the regulation of hormonal mechanisms, which will be discussed later 22.
Hormonal mechanisms
Various hormones have been associated with effects on endolymph homeostasis. Based upon our recent literature review, a few key hormonal influences have associations with the pathophysiology of MD. Specifically, we review antidiuretic hormone, the aquaporin system, ion channels, epinephrine and aldosterone.
A correlation between antidiuretic hormone (ADH, vasopressin) and the exacerbation of MD has often been proposed. ADH works on the vasopressin-2 receptors (V2R) to activate aquaporin-2 channels, which in turn moves water from the tubular lumen of the renal collecting duct into the cell, regulating body fluid homeostasis and maintaining systemic osmotic pressure. Endolymphatic homeostasis appears to be related to ADH although the exact mechanism remains unclear; when there is no change in plasma osmolality, the predominant theory is that inner ear pressure plays a role in controlling plasma ADH release. ADH may suppress water reabsorption in the ES, leading to ELH 40 41. However, recent follow-up studies investigating serum concentrations of ADH in MD patients have been inconclusive. Lim and colleagues found no significant correlation of serum ADH levels with unilateral disease 42. One proposed reason for this contrasting evidence is the variable accuracy of measuring plasma versus serum ADH. Aoki and colleagues showed that during a Ménière's attack there was a significant increase in ADH concentration 40. Interestingly, both nausea and emesis are stimuli for ADH and often accompany an attack 40.
In conjunction with ADH, the aquaporin system presents a feasible means for the development and manifestation of MD from the vantage point of a hormonal fluid balance disorder. These ubiquitous transmembrane channels that actively move water and other solutes through cell membranes within the kidneys, brain and lungs could be involved in the ES as well. Within the inner ear, aquaporin-2 receptors, the only humorally controlled aquaporin, have been found with high concentrations in human ESs 43. Kitahara and colleagues have also shown that V2R mRNA expression in the ES is up-regulated in MD and clearly distributed together with aquaporin-2 in the luminal epithelium of the human ES. This V2R overexpression in the ES could attenuate the membrane turnover and cause the endolymphatic fluid overflow into the endolymphatic space after even a small increase in plasma vasopressin. V2R and subsequent cyclic AMP-linked signalling could suppress the endolymphatic fluid absorption in the ES, resulting in the inner ear hydrops 44. Other aquaporin channels have been identified in the ear, including aquaporin-1 in most of the inner ear, aquaporin-3 in the epithelium of the ELS, aquaporin-4 in the supporting cells of the cochlea and vestibular end organs, aquaporin-5 in the organ of Corti and Reissner membrane and aquaporin-6 in the epithelium of the ELS 45.
In addition to the ADH-aquaporin system, voltage and non-voltage dependent ion channels play a crucial role in the regulation of the endolymphatic fluid composition, and more importantly, in the regulation of the endocochlear potential. Potassium plays an integral role in the generation and maintenance of the endocochlear potential. Its recirculation through the supporting cells of the organ of corti, the fibrocytes of the spiral ligament and the marginal cells of the stria vascularis, known as the potassium cycle, maintains the endocochlear potential 46. This cycle involves an active and passive transport of potassium via ionic channels, gap junction (connexins) and tight junctions.
Animal model studies using systemic dosing of aldosterone have shown an elevation of Na-K ATPase levels in the stria vascularis 47. Speculation suggested an enhanced secretion of potassium into endolymph would increase the rate of endolymph production, thereby creating volume excess in the partially impaired endolymphatic sac. Additional investigations into the genes KCNE1 and KCNE3, two voltage-gated potassium channels expressed in the inner ear, have been studied in MD patients. Doi et al. found that single nucleotide polymorphisms (SNPs) in KCNE1 and KCNE3 are associated with MD in the Japanese population, whereas Campbell et al. found no association in the Caucasian population and could not duplicate the Doi et al. results 48 49. A subsequent study evaluating the influence of epinephrine, a known modulator of potassium secretion by marginal cells, on endolymph in the cochlea and sac revealed an increase in the dilation of the intraosseous portion of the sac in the hydrops model 50 51. A disturbance in the function of any of these channels or associated electrolytes may result in alterations of the endocochlear potential and subsequent auditory dysfunction. Calcium channel blockers have been explored in the treatment for MD by Teggi et al. Use of cinnarizine improved cochlear and vestibular symptoms, likely due to its inhibition of potassium currents rather than blocking voltage-gated calcium currents 52.
Treatment with intratympanic lipopolysaccharide (LPS) combined with systemic aldosterone in mice has been shown to induce ELH. This study left the ES undisturbed, unlike prior models. LPS causes a non-infectious immune response in the ear. The degree of hydrops in the cochlea was shown to be similar for an LPS alone group and an LPS plus systemic aldosterone group. However, the ES was more dilated in the LPS plus aldosterone group and the sac lumen was partially collapsed 53. Using the same model, the effect of epinephrine on endolymph in the cochlea and sac has been evaluated. Epinephrine increased the dilation of the intraosseous portion of the sac in the hydropic model 22 50. Since potassium secretion in strial marginal cells can be modulated by epinephrine, ongoing investigation with this mouse model is being used to evaluate the influence of different transport processes 22 51.
Hemodynamic disequilibrium
a. Dysfunctional cochlear blood flow
Evidence from research on animal models suggests the pathophysiology in MD is closely associated with dysfunctional cochlear blood flow (CoBF). Miller demonstrated that the magnitude of an evoked CoBF response was reduced by approximately one-third in hydropic ears compared to normal ears 54. Brechtelsbauer reported reduced autoregulation of CoBF in guinea pigs with ELH 55. Andrews revealed that that increased blood viscosity can result in inner ear dysfunction with symptoms of hearing loss, tinnitus and vertigo 56. Radiological MR imaging has also confirmed the presence of an intense contrast effect in ears with hydrops, indicating that the blood–labyrinth barrier is impaired in ears with MD 57. However, the evidence is not consistent. For example, Larsen found no change in regional or total cochlear blood flow in hydropic ears 58. CoBF measurement in patients with MD and control groups has shown no statistically significant difference with respect to CoBF amplitudes 59. Resolving the issue of whether microcirculation and ear hydrops are correlated rests on the development and advancement of means to measure blood flow in the cochlea.
An emerging area of research for endolymphatic hydrops is at the level of the venous drainage of the inner ear. Filipo et al. evaluated patients to determine if cerebrospinal venous abnormalities were associated with MD patients. If there is an increase in arterial pressure at the inner ear microcirculation, theory suggests an increase in endolymph pressure would occur if the venous outflow were impaired 60. Filipo's study assumes that radial resorption of endolymph drains into cochlear and vestibular aqueduct veins and that impaired outflow would not permit physiologic endolymphatic drainage. This recent study suggests MD patients have a significantly higher rate of chronic venous insufficiency in the head and neck compared to controls 60.
Allergy and autoimmunity
a. Allergy mechanisms
Since 1923, inhalant and food allergies have been linked with MD symptoms, and many studies have addressed the difficulties in confirming a relationship between allergy and MD 27. Studies by Derebery and Berliner have shown that 59.2 and 40.3% of patients with MD, respectively, have confirmed or suspected airborne and food allergies 61. Three mechanisms have been described which explore the role of allergic reactions in MD. First, the ES itself could be a target organ of the allergic reaction. The sac's peripheral and fenestrated blood vessels could allow antigen entry, stimulating mast cell degranulation in the perisaccular connective tissue. The resulting inflammatory mediator release could affect the sac's filtering capability, resulting in a toxic accumulation of metabolic products, and interfering with hair cell function. Also, the fenestrated blood vessels to the ES could be pharmacologically vulnerable to the effects of vasoactive mediators such as histamine, which are released in a distal allergic reaction 26. A second possible mechanism involves the production of a circulating immune complex, such as a food antigen, which is then deposited through the fenestrated blood vessels of the ES, producing inflammation. A third possible mechanism is a viral antigen-allergic interaction. A predisposing viral upper respiratory infection in childhood (e.g., mumps, herpes) antigenically stimulates Waldeyer's ring, with subsequent T-cell homing to the ES, resulting in a chronic low-grade inflammation 61.
In clinical practice, it is well known that MD patients frequently complain of gastrointestinal tract symptoms such as diarrhoea, abdominal pain, dyspepsia, and weight fluctuation; however, this observation is hidden by the importance of otological manifestations (hypacusis and vertigo). Multiple studies have reported that wheat is the most common food allergen found in patients with MD, up to 68.2% of patients, and the presence of late skin reactions to gliadin in MD suggests a potential relationship between the immune response to wheat proteins and MD symptoms 62.
b. Autoimmune mechanisms
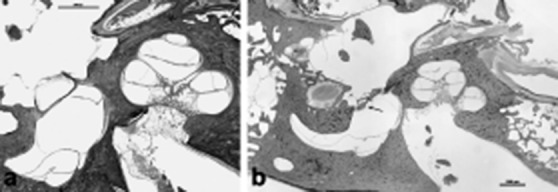
The concept of an autoimmune aetiology of inner ear disease was first introduced more than 30 years ago, and it still remains an active area of research today. Many of MD's clinical characteristics suggest an underlying inflammatory or autoimmune aetiology, such as its propensity to wax and wane 26. The finding of lymphocytes, macrophages, and a rich network of lymphatics and capillaries, venules and fenestrated blood vessels in the guinea pig and human ES raised the possibility that the sac may have an immune function. Subsequent experiments demonstrated antibody production in the inner ear and showed that the ES can generate an immune response 24. It is assumed that autoimmunity occurs via one of three basic pathways to cause tissue damage in MD, which may or may not be organ-specific: (1) autoantibodies directed against antigens found upon or within tissue cells, (2) circulation and deposition of antigen-antibody complexes with activation of the complement system and resultant inflammatory tissue destruction, or (3) an inflammatory reaction mediated by sensitised T lymphocytes. This hypothesis is supported by the fact that hydrops can be induced experimentally by injection of antigens or monoclonal antibodies and the deposition of circulating immune complexes may produce inflammation and interfere with the ES's filtering capability. Several studies demonstrated raised levels of circulating immune complexes (CIC) in a percentage varying from 21 to 96% of MD patients 63. These abnormalities include immune responses to homogenous or heterogeneous inner ear proteins (28–30 kDa, 40 kDa, 42 kDa, 50 kDa, 68–70 kDa, 220 kDa), S-100 beta protein and type II or type IV collagen antigens 64. Yoo et al. suggested a possible role for type II collagen autoimmunity in the aetiology of otosclerosis as well 65. Our experience and histopathological studies suggest that certain combinations of otologic diseases, such as otosclerosis and MD, can occur and may have causative links, although other associations (e.g. otitis media) are commonly only coincidental (Fig. 6 a, b) 66 67. Otosclerosis may result in endolymphatic hydrops by abutting the spiral ligament, resulting in a chemical disruption of ion-fluid recycling, obstruction of the ED and sac, and biochemical changes 68-70.
Fig. 6.
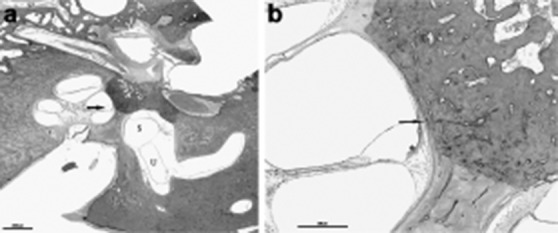
a) HB 856 Rt 560 1x: This is an 80-year-old man who had bilateral mixed hearing loss and vertigo episodes. Histopathologic exam of his right ear showed otosclerosis that involves the otic capsule and endolymhatic hydrops in the basal turn of the cochlea (arrow), saccule (S) and utricle (U), b) HB 856 Rt 560 4x: A higher magnification of the basal turn of the cochlea showed clearly otosclerotic involvement of the otic capsule (arrow) and stria vascularis atrophy (*).
Certain D-related loci may be associated with MD. The elevated CIC induces endothelial injury and leads to an increase in the permeability of the 'leaky capillaries' that surrounds the ES. This sudden efflux of fluid results in acute ELH and rupture of Reissner's membrane. The mixture of potassiumrich endolymph with perilymph hyperpolarises the neural afferents and causes the acute Ménière's attack 71.
Finally, an association of thyroid dysfunction in patients with MD has also been suggested. Brenner and colleagues showed that 32% of patients with MD were being concomitantly treated with supplemental thyroid hormone, and those older than 60 years of age were taking a supplement at a higher rate than the general population 72. Of the Ménière's patients Fattori et al. studied, 38% showed the presence of thyroid autoantibodies, which was significantly higher than the two control groups 73. These studies suggest a predisposition of MD patients for autoimmune disease, given the significant association MD patients have with thyroid dysfunction.
c. Viral infection
As previously mentioned, ELH is a well-known and clinically appreciable finding in many patients with MD. Recently, a temporal bone study has suggested that ELH is a marker for disordered homeostasis of the labyrinth in which an unknown factor produces both the clinical symptoms of MD and ELH 29. A possible chemical injury to the labyrinth could be the release of infectious nucleic acids from vestibular nerve terminals following virus reactivation in the vestibular ganglion (VG) 74. The presence of Herpes simplex virus (HSV-1) antibodies in the perilymph and HSV-1 DNA in the VG excised from MD patients have shown promise, although the ubiquitous nature of these viruses in the worldwide population raise questions with respect to the direct correlation between these findings 75 76. However, electron microscopy by Gacek has shown that virion particles were noted to be present in transport vesicles of vestibular ganglion cells excised from a patient with MD. The virions reportedly led to focal vestibular nerve axonal degeneration and subsequent inflammatory changes associated with ELH 77. Since reactivation of latent neurotropic viruses like herpesviridae is dependent on viral load in a sensory ganglion, VG loss in the contralateral nerve may also represent the development of bilateral MD, which has been reported to occur in 15-50% of patients with MD 78.
Genetic predisposition
Multiple hereditary mechanisms have been proposed over the years as means to explain the events that occur in MD. Genetic predisposition has been reported in 2.6–12% of patients with MD 79. Familial MD appears to follow an autosomal dominant inheritance pattern with 60% penetrance and evidence of anticipation, or a more severe phenotype, in offspring 80. Early investigations into the causes of MD focused on autoimmunity and sought to identify human leukocyte antigen (HLA) associations; although many have been proposed, none have been proven 81 82. Linkage analysis in a large Swedish family defined an interval on chromosome 12p12.3 and screened two candidate genes (RERGL and PIK3C2G), although a precise MD gene on chromosome 12 has yet to be found 83 84. Furthermore, two studies have shown associations with MD and SNPs: one variation in heat shock protein HSP70-1, which is thought to be involved in the cellular stress response 85; the other being a variation in adducin (Gly460Trp), previously known for its association in hypertension, where heterozygous individuals manifest changes in sodium excretion and blood pressure. The adducin variation mechanism is theorised to act via increased Na-K ATPase activity, inducing hyperosmolarity in endolymph which may lead to pathologic hydrops 86.
Additionally, the genetics of immune signalling pathways has been studied in MD. Many protein tyrosine phosphatases (PTPs) play a negative role in T cell receptor signalling. In particular, PTPN22 1858C/T genotype, primarily expressed in T cells, B cells, monocytes, neutrophils, dendritic cells, and natural killer cells, has been associated with autoimmune disease and may confer differential susceptibility to bilateral MD 87. Conversely, longer alleles of (CA)17-20 poly (ADP-ribose)-polymerase 1, a nuclear enzyme that contributes to both neuronal death and survival under stress conditions, has been shown to be protective against bilateral MD 87. Host cell factor C1on the X chromosome haplotype block, known for its role in herpes virus replication within neurons, has been shown to be protective against MD, suggesting a potential trigger between an external source and activation of a molecular pathway that leads to the development of cochleovestibular symptoms.
Inner ear pressure regulation failure
a. Cochlear aqueduct
The cochlear aqueduct plays a key role in the regulation and transmission of pressure equalisation between the middle and inner ear. In particular, the flow resistance of the cochlear aqueduct is crucial for pressure release. The anatomic complex of the round window, connected to the cochlear aqueduct over a pouch-like extension of the round window, allows its position to influence the patency of the cochlear aqueduct and inner ear pressure 88. Multiple studies have reported pathological middle ear pressures and alterations of cochlear aqueduct patency in patients with MD. In 1966, Tumarkin postulated that middle ear ventilation changes influenced vertigo 89. Lall reported that 30.9% of patients with MD had pathological middle ear pressure changes 89. Furthermore, Morinaka and Nakamura found an increased difference of middle ear pressure between bilateral ears in MD patients 90. Finally, patients with MD showed on average a significant negative middle ear pressure, - 43 decapascals, compared with healthy subjects and patients with sudden hearing loss 91. Given these findings, due to the previously described importance of the cochlear aqueduct and its functional anatomic proximity to the round window, inner ear disorders such as ELH present conditions in which abnormal middle ear pressures might trigger or worsen vertigo attacks.
b. Vestibular aqueduct
The vestibular aqueduct is also involved in inner ear pressure regulation and plays a role in MD by causing changes in hydrostatic pressure equilibration during states of dysfunction. Hypoplasia of the vestibular aqueduct already appears in the development of the labyrinth before childhood and might have an impact on the aetiology of MD 92. Numerous radiographic studies by Sennaroglu et al. have demonstrated that the vestibular aqueduct is significantly narrower in the affected ear in patients with MD than the unaffected ear 93. Any condition that causes narrowing of the vestibular aqueduct and the production of excess endolymph could result in the same symptom complex as patients with MD 30. In Figure 7, we present a histopathologic slide of a 78-year-old woman diagnosed with Ménière's disease. Histopathologic evaluation of her right ear indicated endolymphatic hydrops associated with extensive otosclerosis that involves the otic capsule and blocked the vestibular aqueduct (Fig. 7, arrow). Please note cochlear, saccular and utricular hydrops. Secondary causes, such as treponemal disease blocking the aqueduct and viral labyrinthitis narrowing the aqueduct, have added further support to pathological changes in the vestibular aqueduct and subsequent ELH.
Fig. 7.
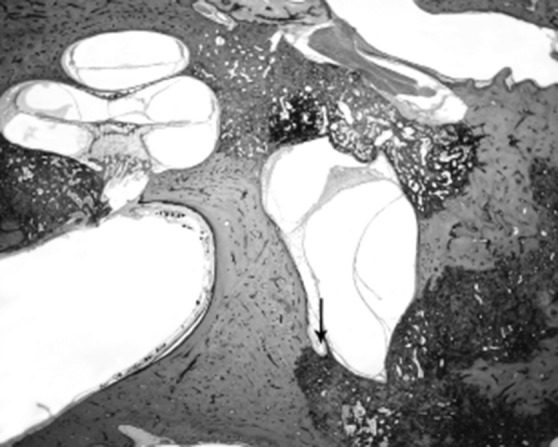
HB 693 Rt 570 1x: 78-year-old woman diagnosed with Ménière's disease. Histopathologic evaluation of her right ear indicated endolymphatic hydrops associated with extensive otosclerosis that involves the otic capsule and block the vestibular aqueduct (arrow). Note cochlear, saccular and utricular hydrops.
c. Middle ear muscles (MEM)
The MEM have a long history of being implicated in many inner ear disorders such as tinnitus, otalgia, MD, and SNHL. In particular, spasm of the tensor tympani (TT) has been implicated in a range of conditions including tinnitus, myofascial pain-dysfunction syndrome, and MD. Sectioning of the TT has been a suggested treatment for MD. Embryologically, the TT, derived from the 1st pharyngeal arch mesenchyme, develops into a mixed muscle containing slow and fast muscle fibres. Innervation is subsequently supplied by the tensor tympani nerve, a purely motor branch of the mandibular division of the trigeminal nerve. It has been speculated that the TT can medialise the stapes into the oval window, resulting in changes in inner ear perilymphatic pressures, which in turn may lead to various inner ear disorders like MD 94. This seems reasonable, considering that a rise in inner ear pressure has three main routes of escape: the vestibular aqueduct, the round window and the oval window. Most importantly, this pressure change and alteration in ossicular function seems to primarily affect lower frequencies, which aligns with the hearing loss spectrum in MD 95.
Klockhoff described a tensor tympani syndrome characterised by fluctuation in the middle ear impedance and complaints of fullness, tinnitus, and dysacusis, which echoes some of the common findings in MD. Although the role of the TT in otologic disease still remains largely elusive, the best expected markers for TT contraction reported thus far include: a decrease in peak static compliance measured with acoustic tympanometry and a low-frequency conductive hearing loss, with a possible smaller low-frequency SNHL component 96.
d. Spontaneous intracranial hypotension (SIH)
The original discussion of SIH is attributed to Schaltenbrand in 1938. A rare pathology, the annual incidence is estimated at 5 per 100,000 persons 97. Typically, it results from a cerebrospinal fluid (CSF) leakage associated with an orthostatic headache, the cause of which is unknown but may be associated with spontaneous trauma in context of fragile spinal meninges 98. Physiologically, there is a balance of pressures between the endolymphatic and perilymphatic compartments, mediated by the CSF. Each compartment is in continuity with the CSF: the perilymph, via the cochlear aqueduct, and the endolymph via the ES. However, if this equilibrium is disrupted in some way, the CSF pressure falls. This in turn is transmitted to the perilymph via the cochlear aqueduct, producing a transitory perilymphatic hypotonia and endolymphatic hydrops, which is clinically comparable to the findings present in MD 99. Additionally, diffuse intracranial venous engorgement has been described in patients with SIH, which causes irritation of the vestibular and cochlear nerves at the internal acoustic meatus, leading to the triad of hearing loss, tinnitus, and vertigo 100.
There are reports in humans and animals that a CSF leak could lead to compensatory expansion of the endolymphatic space. It is proposed that this expansion is the cause of the associated hearing loss with a CSF leak. Reports in MD patients have shown decreased hearing with shifts of intracranial pressure, but no definitive proof is available 22.
e. Low frequency pressure changes
The effect of MD patients' sensitivity to low frequency pressure changes, such as atmospheric pressure changes, has been attributed to prolonged ELH. Prolonged hydrops, experimentally sustained up to 40 minutes, causes displacement of the organ of Corti toward the scala tympani. This was shown to increase endocochlear potential and increase sensitivity to infrasonic frequencies 22.
Conclusions
Our understanding of MD continues to evolve with scientific improvements in animal model studies, imaging modalities and histologic techniques, as well as further comprehension in the fields of allergy, immunology, endocrinology, and genetics. There are an abundant number of theories relating the clinical signs and symptoms of MD to various predispositions and inflammatory states. Our goal is to provide a comprehensive update regarding the aetiopathologies of MD, which unfortunately remains an idiopathic disease despite medical advancements. It is the authors' impression at this time that endolymphatic hydrops seems to be the most appropriate core principle for the ultimate development of MD. Research about MD is broadly focused, which may provide multiple approaches for exploring therapeutic options for disease sufferers in the future.
References
- 1.Baloh RW. Prosper Meniere and his disease. Arch Neurol. 2001;58:1151–1156. doi: 10.1001/archneur.58.7.1151. [DOI] [PubMed] [Google Scholar]
- 2. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc , author. Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere's disease. J Vestib Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 4.Hallpike CS, Cairns H. Observations on the pathology of Meniere's syndrome: (Section of Otology) Proc R Soc Med. 1938;31:1317–1336. doi: 10.1177/003591573803101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamakawa K. Uber die pathologische Veranderung beieinem Ménière-Kranken. Paper presented at: Annual Meeting Oto- Rhino-Laryngol Society. 1938 Japan. [Google Scholar]
- 6.Alexander TH, Harris JP. Current epidemiology of Meniere's syndrome. Otolaryngol Clin North Am. 2010;43:965–970. doi: 10.1016/j.otc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Costa SS, Sousa LC, Piza MR. Meniere's disease: overview, epidemiology, and natural history. Otolaryngol Clin North Am. 2002;35:455–495. doi: 10.1016/s0030-6665(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 8.Dinces EA. Ménière disease. 2013; http://www.uptodate.com/contents/meniere-disease. Accessed December 10, 2014.
- 9.Kotimaki J, Sorri M, Aantaa E, et al. Prevalence of Meniere disease in Finland. Laryngoscope. 1999;109:748–753. doi: 10.1097/00005537-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Vassiliou A, Vlastarakos PV, Maragoudakis P, et al. Meniere's disease: still a mystery disease with difficult differential diagnosis. Ann Indian Acad Neurol. 2011;14:12–18. doi: 10.4103/0972-2327.78043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman R, Segenhout JM, Wit HP. A Bast-like valve in the pigeon? Eur Arch Otorhinolaryngol. 2009;266:1397–1401. doi: 10.1007/s00405-009-0934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KJ. Anatomy of the ear. Essential otolaryngology: head and neck surgery. 7th ed. East Norwalk, CT: McGraw-hill/ appleton & Lange; 1999. [Google Scholar]
- 13.Gibson WP, Arenberg IK. Pathophysiologic theories in the etiology of Meniere's disease. Otolaryngol Clin North Am. 1997;30:961–967. [PubMed] [Google Scholar]
- 14. Salt AN. Measurement of endolymph flow rate. n.d.; http://oto2.wustl.edu/cochlea/res1.htm. Accessed December 5, 2014.
- 15.Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576:11–21. doi: 10.1113/jphysiol.2006.112888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wangemann P, Schacht J. Homeostasic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay R, editors. Springer Handbook of Auditory Research: The Cochlea. New York, NY: Springer; 1996. pp. 130–185. [Google Scholar]
- 17.Salt AN. Regulation of endolymphatic fluid volume. Ann N Y Acad Sci. 2001;942:306–312. doi: 10.1111/j.1749-6632.2001.tb03755.x. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal Y, Minor LB. Physiologic effects on the vestibular system in Meniere's disease. Otolaryngol Clin North Am. 2010;43:985–993. doi: 10.1016/j.otc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Schuknecht HF, Gulya AJ. Endolymphatic hydrops. An overview and classification. Ann Otol Rhinol Laryngol Suppl. 1983;106:1–20. doi: 10.1177/00034894830920s501. [DOI] [PubMed] [Google Scholar]
- 20.Rizvi SS. Investigations into the cause of canal paresis in Meniere's disease. Laryngoscope. 1986;96:1258–1271. doi: 10.1002/lary.1986.96.11.1258. [DOI] [PubMed] [Google Scholar]
- 21.Stahle J, Bergman B. The caloric reaction in Meniere's disease. An electronystagmographical study in 300 patients. Laryngoscope. 1967;77:1629–1643. doi: 10.1288/00005537-196709000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am. 2010;43:971–983. doi: 10.1016/j.otc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191:90–100. doi: 10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Hornibrook J, George P, Spellerberg M, et al. HSP70 antibodies in 80 patients with "clinically certain" Meniere's disease. Ann Otol Rhinol Laryngol. 2011;120:651–655. doi: 10.1177/000348941112001004. [DOI] [PubMed] [Google Scholar]
- 25.Paparella MM, Djalilian HR. Etiology, pathophysiology of symptoms, and pathogenesis of Meniere's disease. Otolaryngol Clin North Am. 2002;35:529–545. doi: 10.1016/s0030-6665(02)00019-1. vi. [DOI] [PubMed] [Google Scholar]
- 26.Derebery MJ. Allergic and immunologic features of Meniere's disease. Otolaryngol Clin North Am. 2011;44:655–666. doi: 10.1016/j.otc.2011.03.004. ix. [DOI] [PubMed] [Google Scholar]
- 27.Derebery MJ, Berliner KI. Allergy and its relation to Meniere's disease. Otolaryngol Clin North Am. 2010;43:1047–1058. doi: 10.1016/j.otc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Michaels L, Soucek S, Linthicum F. The intravestibular source of the vestibular aqueduct. II: its structure and function clarified by a developmental study of the intraskeletal channels of the otic capsule. Acta Otolaryngol. 2010;130:420–428. doi: 10.3109/00016480903253561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant SN, Adams JC, Nadol JB., Jr. Pathophysiology of Meniere's syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Gibson WP. Hypothetical mechanism for vertigo in Meniere's disease. Otolaryngol Clin North Am. 2010;43:1019–1027. doi: 10.1016/j.otc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Hofman R, Segenhout JM, Buytaert JA, et al. Morphology and function of Bast's valve: additional insight in its functioning using 3D-reconstruction. Eur Arch Otorhinolaryngol. 2008;265:153–157. doi: 10.1007/s00405-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konishi S. The ductus reuniens and utriculo-endolymphatic valve in the presence of endolymphatic hydrops in guineapigs. J Laryngol Otol. 1977;91:1033–1045. doi: 10.1017/s0022215100084747. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu S, Cureoglu S, Yoda S, et al. Blockage of longitudinal flow in Meniere's disease: A human temporal bone study. Acta Otolaryngol. 2011;131:263–268. doi: 10.3109/00016489.2010.532155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitamura K, Schuknecht HF, Kimura RS. Cochlear hydrops in association with collapsed saccule an ductus reuniens. Ann Otol Rhinol Laryngol. 1982;91:5–13. doi: 10.1177/000348948209100104. [DOI] [PubMed] [Google Scholar]
- 35.Yamane H, Takayama M, Sunami K, et al. Blockage of reuniting duct in Meniere's disease. Acta Otolaryngol. 2010;130:233–239. doi: 10.3109/00016480903096648. [DOI] [PubMed] [Google Scholar]
- 36.Yamane H, Imoto T, Nakai Y, et al. Otoconia degradation. Acta Otolaryngol Suppl. 1984;406:263–270. doi: 10.3109/00016488309123047. [DOI] [PubMed] [Google Scholar]
- 37.Michaels L, Soucek S, Linthicum F. The intravestibular source of the vestibular aqueduct: Its structure and pathology in Meniere's disease. Acta Otolaryngol. 2009;129:592–601. doi: 10.1080/00016480802342416. [DOI] [PubMed] [Google Scholar]
- 38.Phillips JS, Prinsley PR. A unified hypothesis for vestibular dysfunction? Otolaryngol Head Neck Surg. 2009;140:477–479. doi: 10.1016/j.otohns.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Balatsouras DG, Ganelis P, Aspris A, et al. Benign paroxysmal positional vertigo associated with Meniere's disease: epidemiological, pathophysiologic, clinical, and therapeutic aspects. Ann Otol Rhinol Laryngol. 2012;121:682–688. doi: 10.1177/000348941212101011. [DOI] [PubMed] [Google Scholar]
- 40.Aoki M, Ando K, Kuze B, et al. The association of antidiuretic hormone levels with an attack of Meniere's disease. Clin Otolaryngol. 2005;30:521–525. doi: 10.1111/j.1749-4486.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 41.Takeda T, Kakigi A, Saito H. Antidiuretic hormone (ADH) and endolymphatic hydrops. Acta Otolaryngol Suppl. 1995;519:219–222. doi: 10.3109/00016489509121909. [DOI] [PubMed] [Google Scholar]
- 42.Lim JS, Lange ME, Megerian CA. Serum antidiuretic hormone levels in patients with unilateral Meniere's disease. Laryngoscope. 2003;113:1321–1326. doi: 10.1097/00005537-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Couloigner V, Berrebi D, Teixeira M, et al. Aquaporin-2 in the human endolymphatic sac. Acta Otolaryngol. 2004;124:449–453. doi: 10.1080/00016480310000700a. [DOI] [PubMed] [Google Scholar]
- 44.Kitahara T, Doi K, Maekawa C, et al. Meniere's attacks occur in the inner ear with excessive vasopressin type-2 receptors. J Neuroendocrinol. 2008;20:1295–1300. doi: 10.1111/j.1365-2826.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 45.Beitz E, Zenner HP, Schultz JE. Aquaporin-mediated fluid regulation in the inner ear. Cell Mol Neurobiol. 2003;23:315–329. doi: 10.1023/A:1023636620721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 47.Cate WJ, Curtis LM, Rarey KE. Effects of low-sodium, highpotassium dietary intake on cochlear lateral wall Na+,K(+)- ATPase. Eur Arch Otorhinolaryngol. 1994;251:6–11. doi: 10.1007/BF00175950. [DOI] [PubMed] [Google Scholar]
- 48.Campbell CA, Della Santina CC, Meyer NC, et al. Polymorphisms in KCNE1 or KCNE3 are not associated with Meniere disease in the Caucasian population. Am J Med Genet A. 2010;152A:67–74. doi: 10.1002/ajmg.a.33114. [DOI] [PubMed] [Google Scholar]
- 49.Doi K, Sato T, Kuramasu T, et al. Meniere's disease is associated with single nucleotide polymorphisms in the human potassium channel genes, KCNE1 and KCNE3. ORL J Otorhinolaryngol Relat Spec. 2005;67:289–293. doi: 10.1159/000089410. [DOI] [PubMed] [Google Scholar]
- 50.Takumida M, Akagi N, Anniko M. Effect of inner ear blood flow changes in Meniere's model mice. Acta Otolaryngol. 2009;129:244–253. doi: 10.1080/00016480802241980. [DOI] [PubMed] [Google Scholar]
- 51.Wangemann P, Liu J, Shimozono M, et al. K+ secretion in strial marginal cells is stimulated via beta 1-adrenergic receptors but not via beta 2-adrenergic or vasopressin receptors. J Membr Biol. 2000;175:191–202. doi: 10.1007/s00232001067. [DOI] [PubMed] [Google Scholar]
- 52.Teggi R, Gatti O, Sykopetrites V, et al. Association of cinnarizine and betahistine in prophylactic therapy for Meniere's disease with and without migraine. Acta Otorhinolaryngol Ital. 2014;34:349–353. [PMC free article] [PubMed] [Google Scholar]
- 53.Takumida M, Akagi N, Anniko M. A new animal model for Meniere's disease. Acta Otolaryngol. 2008;128:263–271. doi: 10.1080/00016480701497436. [DOI] [PubMed] [Google Scholar]
- 54.Miller JM, Ren TY, Laurikainen E, et al. Hydrops-induced changes in cochlear blood flow. Ann Otol Rhinol Laryngol. 1995;104:476–483. doi: 10.1177/000348949510400611. [DOI] [PubMed] [Google Scholar]
- 55.Brechtelsbauer PB, Ren TY, Miller JM, et al. Autoregulation of cochlear blood flow in the hydropic guinea pig. Hear Res. 1995;89:130–136. doi: 10.1016/0378-5955(95)00130-4. [DOI] [PubMed] [Google Scholar]
- 56.Andrews JC, Hoover LA, Lee RS, et al. Vertigo in the hyperviscosity syndrome. Otolaryngol Head Neck Surg. 1988;98:144–149. doi: 10.1177/019459988809800208. [DOI] [PubMed] [Google Scholar]
- 57.Tagaya M, Yamazaki M, Teranishi M, et al. Endolymphatic hydrops and blood-labyrinth barrier in Meniere's disease. Acta Otolaryngol. 2011;131:474–479. doi: 10.3109/00016489.2010.534114. [DOI] [PubMed] [Google Scholar]
- 58.Larsen HC, Albers F, Jansson B, et al. Cochlear blood flow in endolymphatic hydrops. Acta Otolaryngol. 1988;106:404–408. doi: 10.3109/00016488809122263. [DOI] [PubMed] [Google Scholar]
- 59.Munoz DJ, Kendrick IS, Rassam M, et al. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 60.Filipo R, Ciciarello F, Attanasio G, et al. Chronic cerebrospinal venous insufficiency in patients with Meniere's disease. Eur Arch Otorhinolaryngol. 2015;272:77–82. doi: 10.1007/s00405-013-2841-1. [DOI] [PubMed] [Google Scholar]
- 61.Derebery MJ, Berliner KI. Prevalence of allergy in Meniere's disease. Otolaryngol Head Neck Surg. 2000;123:69–75. doi: 10.1067/mhn.2000.105715. [DOI] [PubMed] [Google Scholar]
- 62.Berardino F, Cesarani A. Gluten sensitivity in Meniere's disease. Laryngoscope. 2012;122:700–702. doi: 10.1002/lary.22492. [DOI] [PubMed] [Google Scholar]
- 63.Bovo R, Ciorba A, Martini A. Vertigo and autoimmunity. Eur Arch Otorhinolaryngol. 2010;267:13–19. doi: 10.1007/s00405-009-1122-5. [DOI] [PubMed] [Google Scholar]
- 64.Tan CQ, Dong WD, Guo L, et al. Auditory function in women with autoimmune inner ear diseases and their offspring. Int J Pediatr Otorhinolaryngol. 2009;73:1702–1711. doi: 10.1016/j.ijporl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Yoo TJ, Stuart JM, Kang AH, et al. Type II collagen autoimmunity in otosclerosis and Meniere's disease. Science. 1982;217:1153–1155. doi: 10.1126/science.7112122. [DOI] [PubMed] [Google Scholar]
- 66.Cawthorne T. Otosclerosis. J Laryngol Otol. 1955;69:437–456. doi: 10.1017/s0022215100050933. [DOI] [PubMed] [Google Scholar]
- 67.Paparella MM, Mancini F, Liston SL. Otosclerosis and Meniere's syndrome: diagnosis and treatment. Laryngoscope. 1984;94:1414–1417. [PubMed] [Google Scholar]
- 68.Lawrence M. Possible influence of cochlear otosclerosis on inner ear fluids. Ann Otol. 1966;75:553–558. [Google Scholar]
- 69.Liston SL, Paparella MM, Mancini F, et al. Otosclerosis and endolymphatic hydrops. Laryngoscope. 1984;94:1003–1007. [PubMed] [Google Scholar]
- 70.Yoon TH, Paparella MM, Schachern PA. Otosclerosis involving the vestibular aqueduct and Meniere's disease. Otolaryngol Head Neck Surg. 1990;103:107–112. doi: 10.1177/019459989010300116. [DOI] [PubMed] [Google Scholar]
- 71.Derebery MJ, Rao VS, Siglock TJ, et al. Meniere's disease: an immune complex-mediated illness? Laryngoscope. 1991;101:225–229. doi: 10.1288/00005537-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Brenner M, Hoistad DL, Hain TC. Prevalence of thyroid dysfunction in patients with Meniere's disease. Arch Otolaryngol Head Neck Surg. 2004;130:226–228. doi: 10.1001/archotol.130.2.226. [DOI] [PubMed] [Google Scholar]
- 73.Fattori B, Nacci A, Dardano A, et al. Possible association between thyroid autoimmunity and Meniere's disease. Clin Exp Immunol. 2008;152:28–32. doi: 10.1111/j.1365-2249.2008.03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herriott RM. Infectious nucleic acids, a new dimension in virology. Science. 1961;134:256–260. doi: 10.1126/science.134.3474.256. [DOI] [PubMed] [Google Scholar]
- 75.Arnold W, Niedermeyer HP. Herpes simplex virus antibodies in the perilymph of patients with Meniere disease. Arch Otolaryngol Head Neck Surg. 1997;123:53–56. doi: 10.1001/archotol.1997.01900010061008. [DOI] [PubMed] [Google Scholar]
- 76.Vrabec JT. Herpes simplex virus and Meniere's disease. Laryngoscope. 2003;113:1431–1438. doi: 10.1097/00005537-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Gacek RR. Meniere's disease is a viral neuropathy. ORL J Otorhinolaryngol Relat Spec. 2009;71:78–86. doi: 10.1159/000189783. [DOI] [PubMed] [Google Scholar]
- 78.Paparella MM, Griebie MS. Bilaterality of Meniere's disease. Acta Otolaryngol. 1984;97:233–237. doi: 10.3109/00016488409130984. [DOI] [PubMed] [Google Scholar]
- 79.Birgerson L, Gustavson KH, Stahle J. Familial Meniere's disease: a genetic investigation. Am J Otol. 1987;8:323–326. [PubMed] [Google Scholar]
- 80.Morrison AW, Bailey ME, Morrison GA. Familial Meniere's disease: clinical and genetic aspects. J Laryngol Otol. 2009;123:29–37. doi: 10.1017/S0022215108002788. [DOI] [PubMed] [Google Scholar]
- 81.Fung K, Xie Y, Hall SF, et al. Genetic basis of familial Meniere's disease. J Otolaryngol. 2002;31:1–4. doi: 10.2310/7070.2002.19261. [DOI] [PubMed] [Google Scholar]
- 82.Rawal SG, Thakkar KH, Ziai K, et al. HLA-B27-associated bilateral Meniere disease. Ear Nose Throat J. 2010;89:122–127. [PubMed] [Google Scholar]
- 83.Gabrikova D, Frykholm C, Friberg U, et al. Familiar Meniere's disease restricted to 1.48 Mb on chromosome 12p12.3 by allelic and haplotype association. J Hum Genet. 2010;55:834–837. doi: 10.1038/jhg.2010.122. [DOI] [PubMed] [Google Scholar]
- 84.Klar J, Frykholm C, Friberg U, et al. A Meniere's disease gene linked to chromosome 12p12.3. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:463–467. doi: 10.1002/ajmg.b.30347. [DOI] [PubMed] [Google Scholar]
- 85.Kawaguchi S, Hagiwara A, Suzuki M. Polymorphic analysis of the heat-shock protein 70 gene (HSPA1A) in Meniere's disease. Acta Otolaryngol. 2008;128:1173–1177. doi: 10.1080/00016480801901675. [DOI] [PubMed] [Google Scholar]
- 86.Teggi R, Lanzani C, Zagato L, et al. Gly460Trp alpha-adducin mutation as a possible mechanism leading to endolymphatic hydrops in Meniere's syndrome. Otol Neurotol. 2008;29:824–828. doi: 10.1097/MAO.0b013e318180a4b1. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Escamez JA, Saenz-Lopez P, Acosta L, et al. Association of a functional polymorphism of PTPN22 encoding a lymphoid protein phosphatase in bilateral Meniere's disease. Laryngoscope. 2010;120:103–107. doi: 10.1002/lary.20650. [DOI] [PubMed] [Google Scholar]
- 88.Feijen RA, Segenhout JM, Albers FW, et al. Cochlear aqueduct flow resistance depends on round window membrane position in guinea pigs. J Assoc Res Otolaryngol. 2004;5:404–410. doi: 10.1007/s10162-004-5001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lall M. Meniere's disease and the grommet (a survey of its therapeutic effects) J Laryngol Otol. 1969;83:787–791. doi: 10.1017/s002221510007095x. [DOI] [PubMed] [Google Scholar]
- 90.Morinaka S, Nakamura H. Middle ear pressure in patients with dizziness. Ann Otol Rhinol Laryngol. 2004;113:906–913. doi: 10.1177/000348940411301110. [DOI] [PubMed] [Google Scholar]
- 91.Park JJ, Luecke K, Luedeke I, et al. Long-term middle ear pressure measurements in inner ear disorders. Acta Otolaryngol. 2012;132:266–270. doi: 10.3109/00016489.2011.636378. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto E, Mizukami C. Development of the vestibular aqueduct in Meniere's disease. Acta Otolaryngol Suppl. 1993;504:46–50. doi: 10.3109/00016489309128121. [DOI] [PubMed] [Google Scholar]
- 93.Sennaroglu L, Yilmazer C, Basaran F, et al. Relationship of vestibular aqueduct and inner ear pressure in Meniere's disease and the normal population. Laryngoscope. 2001;111:1625–1630. doi: 10.1097/00005537-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 94.Rock EH. Objective tinnitus and the tensor tympani muscle. Int Tinnitus J. 1995;1:30–37. [PubMed] [Google Scholar]
- 95.Jang CH, Park H, Choi CH, et al. The effect of increased inner ear pressure on tympanic membrane vibration. Int J Pediatr Otorhinolaryngol. 2009;73:371–375. doi: 10.1016/j.ijporl.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Bance M, Makki FM, Garland P, et al. Effects of tensor tympani muscle contraction on the middle ear and markers of a contracted muscle. Laryngoscope. 2013;123:1021–1027. doi: 10.1002/lary.23711. [DOI] [PubMed] [Google Scholar]
- 97.Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8:325–328. doi: 10.1007/s10194-007-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia. 2008;28:1345–1356. doi: 10.1111/j.1468-2982.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 99.Fontaine N, Charpiot A, Debry C, et al. A case of spontaneous intracranial hypotension: from Meniere-like syndrome to cerebral involvement. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:153–156. doi: 10.1016/j.anorl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Mokri B. Low cerebrospinal fluid pressure syndromes. Neurol Clin. 2004;22:55–74. doi: 10.1016/S0733-8619(03)00089-6. vi. [DOI] [PubMed] [Google Scholar]