SUMMARY
Cocaine abuse occasionally causes extensive destruction of the osteocartilaginous structures of the nose, sinuses and palate, which mimics the clinical picture of other diseases associated with necrotising midfacial lesions. The differentiation of cocaine-induced midline destructive lesions (CIMDL) and limited granulomatosis with polyangiitis (GPA) may be difficult, particularly if patients do not readily admit substance abuse. We studied 10 patients with CIMDL and palate perforation referred to our Unit between 2002 and 2015. All cases underwent nasal endoscopy, sinus CT or MRI and ANCA test. In 8 patients, a nasal biopsy was performed. The PubMed database was searched to review all cases of palate perforation described in patients affected by CIMDL or GPA. All 10 cases presented with septal perforation and inferior turbinate destruction. We found hard palate perforation in 7 patients, soft palate perforation in 2 patients, and perforation of both in one patient. ANCA testing was negative in 8 patients and positive in 2, with C-ANCA and P-ANCA specificity, respectively. A review of the English literature identified palate perforation in 5 patients with GPA and in 73 patients with CIMDL. The presence of palate perforation in patients with MDL may represent a clinical marker that strongly favors CIMDL over GPA.
KEY WORDS: Palatal perforation, Cocaine, Granulomatosis with polyangiitis (GPA)
RIASSUNTO
L' abuso di cocaina può talvolta causare lesioni destruenti della struttura osteocartilaginea del naso, dei seni paranasali, del palato, con caratteristiche cliniche che ricordano altre patologie sistemiche associate a lesioni necrotizzanti centrofacciali. La diagnosi differenziale tra lesioni destruenti della linea mediana indotte da cocaina (CIMDL) e granulomatosi associata a poliangioite (GPA) può essere complessa, in particolare se il paziente non ammette l'abuso di sostanze. 10 pazienti con CIMDL e perforazione palatale sono stati trattati presso la nostra Unità Operativa tra il 2002 ed il 2015. Tutti i casi sono stati sottoposti ad endoscopia nasale, TC o RMN del massiccio facciale ed Anca test. In 8 casi è stata effettuata anche la biopsia nasale. Contestualmente è stata eseguita una revisione della letteratura presente su PubMed riguardante i casi di perforazione palatale in pazienti affetti da CIMDL e GPA. Tutti i 10 pazienti oggetto dello studio presentavano perforazione palatale e distruzione dei turbinati inferiori; inoltre 7 pazienti presentavano perforazione del palato duro , 2 pazienti perforazione del palato molle ed 1 paziente perforazione di entrambi. Gli Anca test erano negativi in 8 pazienti e positivi in 2, sia per C-Anca sia per P-Anca. La revisione della letteratura edita in lingua inglese ha evidenziato perforazioni palatali in 5 pazienti affetti da GPA e in 73 pazienti affetti da CIMDL. La presenza di perforazione palatale in pazienti con lesioni destruenti della linea mediana può rappresentare un nuovo marker clinico a favore delle CIMDL nella diagnosi differenziale con GPA.
Introduction
The United Nations Office on Drugs and Crime reports that cocaine use has remained stable during the last three years, with 14 to 21 million estimated users per year worldwide. In particular, cocaine use has remained high in North America (5 million), South America (4.5 million), Africa (2.8 million), and Western and Central Europe (4 million) 1. In the last 2016 European Drug Report, it was estimated that around 17 million adults (15-64 years) have used cocaine at least once in their lifetime with some countries (such as Spain and UK) showing a prevalence of use among young adults (15-34 years) that matches or even exceeds rates in the USA 2.
The most frequently used route of cocaine administration is intranasal inhalation, or "snorting", and thus adverse effects on the nasal tract are very common 3. Habitual nasal insufflations of cocaine may cause mucosal lesions, and if cocaine use becomes chronic and compulsive, progressive damage of the mucosa and perichondrium leads to ischaemic necrosis of septal cartilage and perforation of the nasal septum 4. Occasionally, cocaine-induced lesions cause extensive destruction of the osseocartilaginous structures of nose, sinuses and palate that can mimic other diseases such as tumours, infections and immunological disorders. Several problems have been reported in differentiating cocaine-induced midline destructive lesion (CIMDL) from granulomatosis with polyangiitis (GPA) with limited ear-nose-throat involvement (ENT) 5. An essential element for achieving correct diagnosis is clinical history, although cocaine abusers rarely admit drug dependency. The presence of a positive ANCA test with either proteinase 3 (PR3) or myeloperoxidase (MPO) specificity facilitates differential diagnosis of GPA from CIMDL, although not in all cases.
In the present study, we focus on patients with CIMDL and palatal perforation and review the available literature to discuss the utility of this type of midface destructive lesion as a possible clinical marker that might orient differential diagnosis.
Materials and methods
A series of 10 patients with CIMDL and palate perforation evaluated at the Department of Otorhinolaryngology of San Raffaele Hospital between February 2002 and October 2015 was retrospectively reviewed. This retrospective study adhered to ethical standards according to the Declaration of Helsinki. The patients ranged in age from 28 to 60 years; there were 8 males and 2 females. Follow-up lasted from 8 to 86 months. The duration of cocaine abuse was available in 7 patients, ranging from 2 to 30 years, at doses varying from 1 to 10 g/week. Demographics and past medical history were collected at the initial visit. It is important to underline that data concerning duration and daily dose of cocaine abuse are difficult to estimate because of poor collaboration between cocaine abusers and physicians. All patients underwent physical examination at the outpatient clinic of our Institute that included inspection of the face, oral cavity and oropharynx, as well as inspection of the nasal cavities and nasopharynx using 30° rigid telescopes (4 mm in diameter). During endoscopy, biopsies and samples for bacterial and fungal cultures were taken. A total of 8 mucosal biopsy specimens were evaluated. Sections were stained with haematoxylineosin. Orcein staining was used to evaluate elastic fibres, and periodic-acid Schiff and Ziehl-Nielsen stains were used to identify fungi and mycobacteria, respectively. Ten patients underwent imaging studies; in particular, 7 patients were evaluated using computed tomography (CT) scan, and 3 patients with magnetic resonance imaging (MRI). Sera from 10 patients were tested for ANCA in the laboratory at our Institute. We used indirect immunofluorescence (IIF) microscopy on ethanol-fixed blood donor neutrophils following the standard procedure outlined at the first ANCA workshop 6. The PubMed database was searched for the available English literature (original papers, case series, and single reports) describing palate perforation in cocaine abusers and in patients suffering from GPA (available at: http://www.actaitalica.it/issues/2017/4-2017/06_TRIMARCHI).
Results
The 10 patients in our study cohort presented with a variable combination of common symptoms, such as epistaxis, oro-nasal regurgitation of solids and liquids, dysphagia, oropharyngeal pain, nasal speech and halitosis. At rhinoscopy, all patients showed necrotising ulcerative lesions, extensive crusting, intranasal destruction of the vomer and perforation of the nasal septum cartilage as well as of the hard and/or soft palate. Eight patients presented destruction of inferior turbinates, 7 of middle turbinates, and one of the right superior turbinate. The medial wall of maxillary sinus was completely reabsorbed in one patient. Oro-nasal communication affected the hard palate in 7 cases, the soft palate in 2 cases, and both the soft and hard palate in one patient. The diameter of the oro-nasal fistula ranged from 1 to 4 cm (Figs. 1, 2).
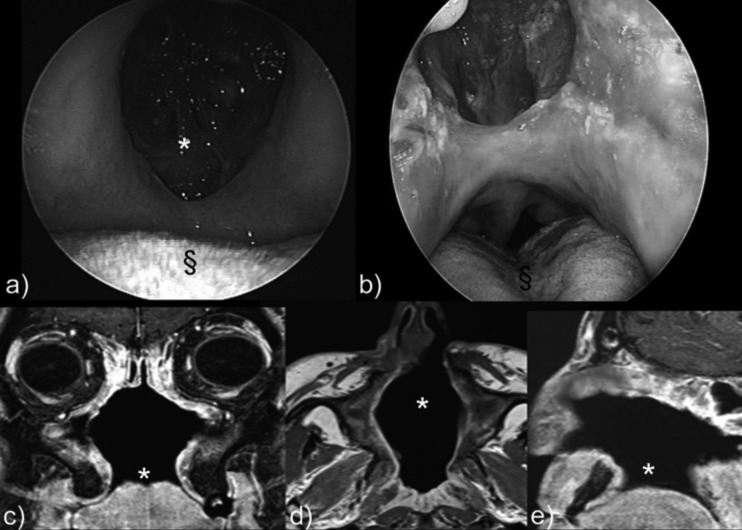
Fig. 1.
a) Oral endoscopy (0° rigid fiberscope): large 4 cm palate perforation. b) Nasal endoscopy (0° rigid fiberscope): 4 cm hard palate perforation (§). c) MRI in the coronal plane shows destruction of the inferior, middle, and superior turbinates on both sides, partial reabsorption of the left medial wall, and palate perforation. d) Destruction of the central nasal septum and lateral nasal structures (middle turbinate) is shown by MRI axial study. e) Palate perforation (*) is shown in the sagittal plane.
Fig. 2.
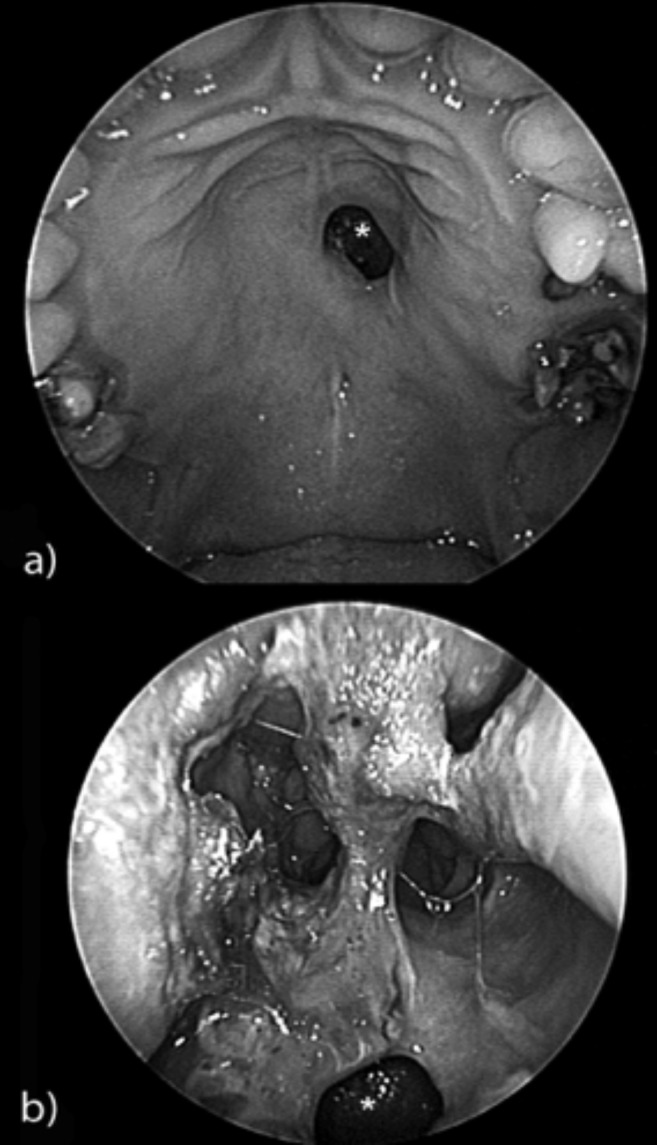
a) Oral endoscopy (0° rigid fiberscope): a 1 cm hard palate perforation with regular margins (*). b) Nasal endoscopy (0° rigid fiberscope): typical nasal crusting in a cocaine abuser with 1 cm hard palate perforation (*).
All patients had positive nasal cultures for Staphylococcus aureus. No positive fungal cultures were observed. Eight biopsies were negative for inflammatory, oncological and immunological diseases (2 patients admitted cocaine use, but did not consent to biopsy). One patient showed strong P-ANCA positivity on immunofluorescence with negative ELISA for both anti-PR3 and anti-MPO antibodies. One patient showed C-ANCA positivity on immunofluorescence with positive ELISA for anti-MPO antibodies.
CT scan confirmed the endoscopic features, showing perforation of nasal septum and palate, and destruction of the inferior middle and superior turbinate, whereas MRI revealed areas of abnormal signal in the septal and palate perforation, such as hypointensity of mucosal and submucosal tissue and nonhomogeneous enhancement.
Discussion
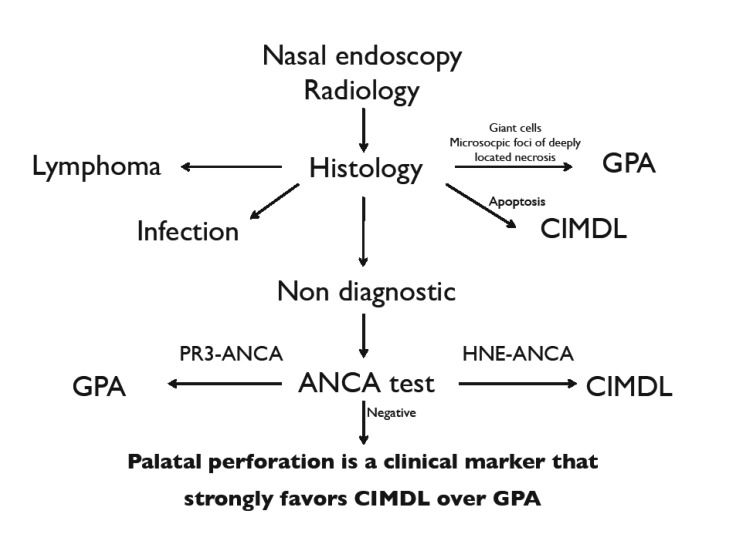
CIMDL represents an uncommon complication of habitual intranasal cocaine insufflations 7. Patients with CIMDL develop extensive destruction of midfacial osseocartilaginous structures, which can resemble different pathological conditions such as oncologic, infectious and immunological diseases 5. If differential diagnosis between oncologic or infectious disorders and CIMDL is facilitated by histopathological features and microbiological studies, respectively, differentiation between CIMDL and limited ENT GPA may pose several problems. Considering the histological features of CIMDL reported in the literature 5 and in our experience, we frequently found the presence of vascular changes including microabscesses in the venule wall and leukocytoclastic vasculitis, which have been proposed as characteristic features of GPA 8-10. Consequently, the histologic changes observed in a large proportion of biopsies from patients with CIMDL might be misinterpreted as "consistent with GPA" 8. Only extravascular changes consisting in giant cells and microscopic foci of deeply located necrosis may be diagnostic of GPA 5-11.
However, as described in a previous study 12, in situ TUNEL assay on biopsy specimens can be useful to demonstrate apoptotic cells because they are highly characteristic features of CIMDL. If histological analysis is non-diagnostic, the next step is ANCA determination, although ANCA test positivity is not always discriminatory between CIMDL and GPA. Anti-neutrophilic cytoplasmic antibodies (ANCAs) directed against proteinase 3 (PR3) or myeloperoxidase (MPO) are sensitive and specific markers for GPA. However, instances of positive ANCA test results have been reported in an unexpectedly large proportion of patients with CIMDL 5. In order to discriminate between CIMDL and GPA, Wiesner et al. made a detailed analysis of the ANCAs and found a high frequency (84%) of anti-human neutophil elastase (HNE) ANCAs in patients presenting with CIMDL. This finding allowed the authors to conclude that HNE ANCAs are discriminatory, whereas the presence of PR3 ANCAs is not. Consequently, in a clinical setting of necrotising inflammation of the upper respiratory tract, additional testing for HNE ANCAs may be useful in differentiating CIMDL from limited ENT GPA 7. Unfortunately, this diagnostic method in not commonly available in routine practice. Differential diagnosis is difficult to reach in the case of HNE ANCA negativity, and in this case there are no discriminating elements.
To facilitate diagnosis of CIMDL and reduce the rate of misdiagnosis with other pathological conditions, we propose a diagnostic algorithm that takes into account the presence of palate perforation. This can help diagnostic choice when clinical doubt is focused on CIMDL and GPA (Fig. 3).
Fig. 3.
Diagnostic algorithm for CIMDL.
For this reason, we consider palate perforation to be an additional diagnostic element. In our experience, palate perforation is frequently associated with cocaine abuse 5, but we have never observed cases of palate perforation in patients with GPA 13.
From a review of the literature, palate perforation has been described in 73 CIMDL patients, while there are only 5 reports of subjects affected by GPA with palate perforation, for which a few comments are warranted.
Kasifoglu et al. 14 described the case of a 26-year-old woman admitted with nasal septum and palatal destruction. The authors reported indeterminate cANCA positivity and a histopathological diagnosis of GPA, but these elements were not exhaustive for definitive diagnosis because CIMDL could not be excluded. Moreover, the same authors defined palatal destruction as a rare condition in the course of GPA, citing three papers from literature 15-17 in which palatal perforation was not mentioned. Molloy et al. 18 did not present a published case, but indirectly describe their personal GPA experience with one case of palatal perforation in a letter. Manganaro et al. 19 imputed their case of soft palatal loss to GPA without ANCA test positivity or biopsy, and therefore with a debatable diagnosis. Aries et al. 20 presented a case of palatal perforation without biopsy because it was refused by the patient: the final diagnosis relied on non-histological criteria for GPA. In contrast, Sciascia et al. 21 presented the case of a 49-yearold woman with perforation of the midline and no other signs of systemic disease. A biopsy revealed a chronic inflammatory process with giant cells, pathognomonic of GPA.
Recently, a proportion of idiopathic cases of midline destructive lesion have been attributed to IgG4-related disease (IgG4-RD), a novel systemic fibro-inflammatory condition characterised by tumourous swelling of affected organs and serum IgG4 elevation 22 23. IgG4-RD was shown to involve midline structures both in the form of mass-forming lesions and nasal or palatal erosions, thereby introducing an additional differential diagnosis to CIMDL. However, in contrast to GPA and CIMDL, the incidence of IgG4-RD is largely unknown and palatal perforation remains an anecdotal observation from a single centre case series that requires further confirmation. In addition, as we recently reported, IgG4-RD might be accurately differentiated from CIMDL thanks to specific histological hallmarks, such as storiform fibrosis, IgG4+ positive plasma cells, and obliterative phlebitis 24.
In conclusion, our experience and the present review of the literature demonstrate that palatal perforation is strongly suggestive of CIMDL and represents an important diagnostic element that can enrich diagnostic workup between GPA and CIMDL.
Conclusions
In conclusion, the presence of palate perforation in patients with MDL and negative biopsy and negative ANCA test may represent a clinical marker that strongly favours CIMDL over GPA.
References
- 1. United Nations Office on Drugs and Crime. World Drug Report 2014. (United Nations publication, Sales No. E.14. XI.7).
- 2. European Drug Report: Trend and developements 2016.
- 3.Seyer BA, Grist W, Muller S. Aggressive destructive midfacial lesion from cocaine abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:465–470. doi: 10.1067/moe.2002.126020. [DOI] [PubMed] [Google Scholar]
- 4.Trimarchi M, Bertazzoni G, Bussi M. Cocaine induced midline destructive lesions. Rhinology. 2014;52:104–111. doi: 10.4193/Rhino13.112. [DOI] [PubMed] [Google Scholar]
- 5.Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic and serologic features and their differentiation from Wegener granulomatosis. Medicine. 2001;80:391–404. doi: 10.1097/00005792-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wilk A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS. 2001;97:12–13. [PubMed] [Google Scholar]
- 7.Wiesner O, Russel KA, Lee AS, et al. Antineutrophil cytoplasmatic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004;50:2954–2965. doi: 10.1002/art.20479. [DOI] [PubMed] [Google Scholar]
- 8.Colby TV, Tazelaar H, Specks U, et al. Nasal biopsy in Wegener's granulomatosis. Hum Path. 1991;22:101–104. doi: 10.1016/0046-8177(91)90028-n. [DOI] [PubMed] [Google Scholar]
- 9.Del Buono EA, Flint A. Diagnostic usefulness of nasal biopsy in Wegener's granulomatosis. Hum Pathol. 1991;22:107–110. doi: 10.1016/0046-8177(91)90030-s. [DOI] [PubMed] [Google Scholar]
- 10.Devaney KO, Travis WD, Hoffman G, et al. Interpretation of head and neck biopsies in Wegener's granulomatosis. Am J Surg Pathol. 1990;14:555–564. doi: 10.1097/00000478-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 12.Trimarchi M, Miluzio A, Nicolai P, et al. Massive apoptosis erodes nasal mucosa of cocaine abusers. Am J Rhynol. 2006;20:160–164. [PubMed] [Google Scholar]
- 13.Comite G, Bonavida G, Bozzolo E, et al. Wegener's granulomatosis: an analysis of 50 patients. Reumatismo. 2005;57:187–192. doi: 10.4081/reumatismo.2005.187. [DOI] [PubMed] [Google Scholar]
- 14.Kasifoglu T, Cansu D, Korkmaz C, et al. Clinical images: perforation of the nasal septum and palate due to Wegener's granulomatosis. Arthritis Rheum. 2008;58:2564–2564. doi: 10.1002/art.23502. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 16.Loke YK, Tan MH. An unusual case of Wegener's granulomatosis. Med J Malaysia. 1998;53:107–109. [PubMed] [Google Scholar]
- 17.Matsumura T, Sato-Matsumura KC, Ota M, et al. Two cases of pyoderma gangrenosum complicated with nasal septal perforation. Br J Dermatol. 1999;141:1133–1135. doi: 10.1046/j.1365-2133.1999.03220.x. [DOI] [PubMed] [Google Scholar]
- 18.Molloy E, Hoffman GS, Langford CA. Determining the cause of isolated midline destructive lesions: comment on the clinical images presented by Kasifoglu et al. Arthritis Rheum. 2009;60:632–632. doi: 10.1002/art.24286. [DOI] [PubMed] [Google Scholar]
- 19.Manganaro AM, Bryant AW. Speech aid prosthesis for a patient with Wegener's granulomatosis: a clinical report. J Prosthet Dent. 1997;77:346–347. doi: 10.1016/s0022-3913(97)70156-2. [DOI] [PubMed] [Google Scholar]
- 20.Aries PM, Ullrich S, Gross WL. A case of destructive Wegener's granulomatosis complicated by cytomegalovirus infection. Nat Clin Pract Rheumatol. 2006;2:511–515. doi: 10.1038/ncprheum0269. [DOI] [PubMed] [Google Scholar]
- 21.Sciascia S, Kuzenko A, Bertero MT. Medical image. Palatal perforation. Wegener granulomatosis. N Z Med J. 2009;122:122–123. [PubMed] [Google Scholar]
- 22.Della-Torre E, Mattoo H, Mahajan VS, et al. IgG4-related midline destructive lesion. Ann Rheum Dis. 2014;73:1434–1436. doi: 10.1136/annrheumdis-2014-205187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzillotta M, Campochiaro C, Trimarchi M, et al. Deconstructing IgG4-related disease involvement of midline structures: comparison to common mimickers. Mod Rheumatol. 2016;13:1–8. doi: 10.1080/14397595.2016.1227026. [DOI] [PubMed] [Google Scholar]
- 24.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]