SUMMARY
The aim of this study was to elucidate the risk factors for cerebrospinal fluid (CSF) rhinorrhoea following transsphenoidal surgery and discuss its prevention and treatments. We retrospectively reviewed 474 consecutive cases of pituitary adenoma treated with 485 transsphenoidal surgical procedures from January 2008 to December 2011 in our department. We analysed the incidence of intra- and post-operative CSF leakage and outcomes of various repair strategies. Intra-operative CSF leakage was encountered in 85 cases (17.9%), and post-operative CSF rhinorrhoea in 13 cases (2.7%). Seven of the 13 patients with post-operative CSF rhinorrhoea did not experience intra-operative CSF leakage; three of these patients had adrenocorticotropic hormone-secreting adenomas. Of the remaining 6 patients with both intra- and post-operative CSF leakage, 2 were treated for giant invasive prolactinomas, and 2 had previously undergone transsphenoidal surgery. In eight patients, the leak was resolved by lumbar puncture, lumbar external drainage, resting in a semi-reclining position, or other conservative treatment. Two CSF leaks were repaired with gelatine foam and fibrin glue using a transsphenoidal approach, and two with autologous fat graft and sellar floor reconstruction using a transnasal endoscopic approach. After undergoing two transnasal endoscopic repairs, one patient with post-operative CSF rhinorrhoea was successfully treated by further lumbar subarachnoid drainage. In conclusion, procedures using gelatine foam, fibrin glue and autologous fat graft are common and effective techniques for the management of CSF rhinorrhoea after transsphenoidal surgery. When a CSF leak is detected during transsphenoidal surgery, thorough sellar reconstruction and long-term follow-up are necessary.
KEY WORDS: Pituitary adenoma, Cerebrospinal fluid rhinorrhea, Sellar reconstruction
RIASSUNTO
Lo scopo del presente studio è stato quello di chiarire i fattori di rischio della rinoliquorrea a seguito di un approccio transfenoidale e di discuterne la prevenzione e il trattamento. Abbiamo revisionato retrospettivamente 474 casi consecutivi di adenoma ipofisario trattati con 485 procedure chirurgiche per via transfenoidale da Gennaio 2008 a Dicembre 2011 nel nostro dipartimento. Abbiamo analizzato l'incidenza di fuoriuscita di liquor cefalorachidiano intraoperatoriamente e nel postoperatorio, e la riuscita di varie strategie di riparazione. Abbiamo riscontrato fuoriuscita di liquor intraoperatoriamente in 85 casi (17.9%) e postoperatoriamente in 13 casi (2.7%). Sette dei 13 pazienti con rinoliquorrea postoperatoria non avevano mostrato fuoriuscita di liquor intraoperatoriamente; tre di questi pazienti avevano adenomi secernenti ADH. Dei rimanenti 6 pazienti con fuoriuscita di liquor sia intra che postoperatoria, 2 erano stati trattati per prolattinoma gigante e invasivo e 2 erano già stati sottoposti in passato a chirurgia trasnfenoidale. In 8 pazienti la fuoriuscita è stata risolta mediante puntura lombare, drenaggio lombare, riposo in posizione semi-reclinata o altri trattamenti conservativi. Due casi sono stati trattati mediante schiuma di gelatina e colla di fibrina utilizzando un approccio transfenoidale e due con grasso autologo e ricostruzione del pavimento della sella utilizzando un approccio transnasale endoscopico. Dopo essere stato sottoposto a due tentativi di riparazione per via transasale, un paziente è stato trattato con successo mediante un ulteriore drenaggio subaracnoideo. In conclusone le procedure che fanno uso di schiuma di gelatina, colla di fibrina e impianti di grasso autologo sono efficaci ai fini del trattamento della rinoliquorrea postoperatoria in pazienti sottoposti a chirurgia transfenoidale. Quando viene rilevata una perdita di liquido cefalorachidiano in corso di chirurgia transfenoidale, un'appropriata ricostruzione del pavimento della sella e un follow up a lungo termine sono necessari.
Introduction
Persistent cerebrospinal fluid (CSF) leakage is the leading cause of morbidity following transsphenoidal surgery (TSS) for pituitary adenomas 1. CSF leakage can lead to headache and meningitis. Although various repair methods have been described, a national survey of complications following TSS found that the incidence of postoperative CSF leak remains high at 3.9% 2. The economic and psychological burden for patients with CSF rhinorrhoea is enormous. In our study, we aimed to elucidate the risk factors for CSF rhinorrhoea following TSS and discuss its prevention and treatment. Our objective was to analyse the incidence of CSF fistula after TSS to remove tumours in the sellar region, discuss factors associated with CSF leakage and describe a method for sellar closure.
Materials and methods
Patients
We retrospectively reviewed 474 consecutive cases of pituitary adenomas treated by 485 TSS procedures from January 2008 to December 2011 at the Department of Neurosurgery, Shanghai Changzheng Hospital. Written approval for this study was obtained from the ethics committee of Shanghai Second Military Medical University. All patients or their family members provided written consent for study participation in accordance with the ethics committee standards during hospital stay or outpatient follow-up. We obtained information about patient demographics, tumour type, degree of resection (gross total resection or subtotal resection), intra- and post-operative CSF leakage, and repair strategy. Various techniques of sellar closure and indications for each specific condition were retrospectively reviewed. Potential risk factors for post-operative CSF rhinorrhoea were analysed.
Diagnosis of CSF fistula
The diagnostic algorithm for patients with suspected postoperative CSF fistula was pre-operative nasal endoscopy, β-trace protein test (followed by a β-2-transferrin test when necessary to confirm CSF fistula), and 1-mm computed tomography scan slices of the paranasal sinus and anterior cranial base in the axial and coronal planes.
Tumour removal technique
Because endoscopy was unpopular in our department during this period, all patients underwent pituitary adenoma resection by microscopic endonasal TSS. After accessing the sella using a microscopic approach, a fine needle was used to puncture the sellar dura before performing the dual incision to prevent an aneurysm. The tumour was resected using a pseudocapsular technique.
CSF leakage repair techniques
The Valsalva manoeuvre was performed by an anaesthetist to determine whether intra-operative leakage had occurred. If intra-operative CSF leakage was detected, we usually first confirmed the arachnoid leak and then repaired it using one of the following strategies. If the arachnoid laceration was small and the CSF flow rate was low, a piece of Gelfoam covered with Surgicel was placed into the defect and covered with Surgicel and fibrin glue as an overlay graft. The sphenoid sinus was then packed with Gelfoam to support the graft. If the arachnoid laceration was large and the CSF flow rate was high, autologous fat harvested from the lower abdomen was formed into a dumbbell shape and embedded in the defect. The fat graft was then covered immediately with fibrin glue and Gelfoam (Fig. 1). An artificial cerebral dura mater patch and septal cartilage were used to reconstruct the sellar floor. The sphenoid sinus was also packed with fat graft and collagen sponge. External CSF lumbar drainage was kept for 2 to 4 days. Patients were discharged from the clinic 1 to 2 days after surgery with a scheduled date for the next appointment.
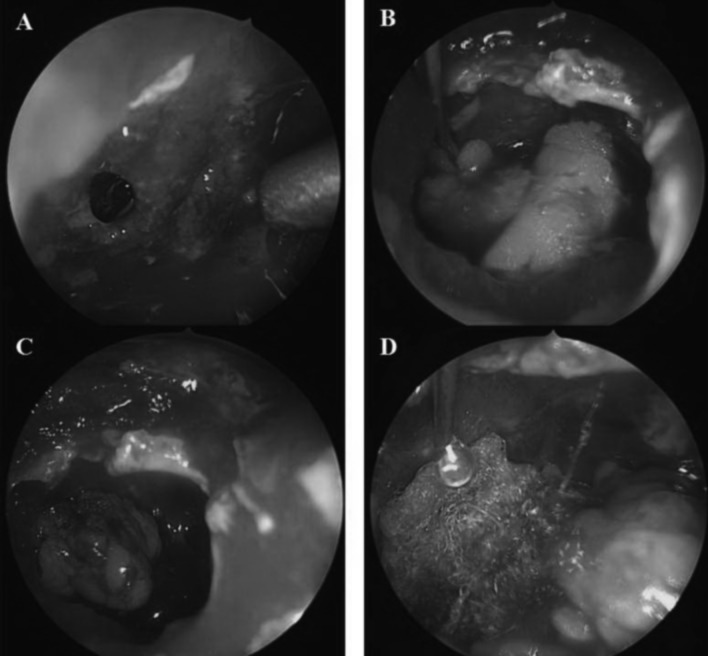
Fig. 1.
A) Identify the arachnoid defect laceration. B, C) Embed the dumbdell- shaped autologous fat in the defect. D) The fat graft was covered immediately with fibrin glue and surgicel.
Statistical analysis
Fisher's exact test was used to compare the odds of CSF leakage. Results are expressed as mean ± SEM. P < 0.05 was considered significant.
Results
Patient and tumour characteristics
A total of 474 patients (214 males and 260 females) who underwent 485 TSS procedures for resection of pituitary adenomas met the inclusion criteria for this study. Mean age at the time of surgery was 49 ± 15 years (range 14- 87 years). Non-functional adenomas accounted for 62.2% of cases, and invasive adenomas for 26% of cases (Table I). Eleven patients (2.3%) underwent repeat operations because of tumour recurrence.
Table I.
Summary of clinical characteristics and CSF leakage in 474 cases treated with resection of pituitary adenomas.
| CSF Leak | ||||
|---|---|---|---|---|
| Features | N = 474 | Intraop | Postop | P-value |
| Gender | 0.181 | |||
| Male | 214 | 36 | 3 | |
| Female | 260 | 49 | 10 | |
| Age | 0.245 | |||
| < 50 years | 182 | 38 | 7 | |
| ≥ 50 years | 292 | 47 | 6 | |
| Tumour type | 0.157 | |||
| Non-functioning | 295 | 40 | 6 | |
| GH | 50 | 15 | 0 | |
| PRL | 91 | 18 | 3 | |
| ACTH | 29 | 12 | 4 | |
| TSH | 5 | 0 | 0 | |
| GH-PRL | 4 | 0 | 0 | |
| Knosp grade | ||||
| 0 | 115 | 17 | 2 | |
| I | 214 | 25 | 1 | |
| II | 102 | 16 | 4 | |
| IV | ||||
| Repeated surgery | ||||
| Yes | 11 | 5 | 2 | |
| No | 0 | 6 | 0 | |
| Extent of resection | 0.916 | |||
| GTR | 424 | 51 | 8 | |
| STR | 50 | 34 | 5 | |
CSF leakage
Intra-operative CSF leakage was detected in 85 cases (17.9%), and post-operative CSF rhinorrhoea in 13 cases (2.7%) (Table I). Our results showed that none of the clinicopathological features, such as gender, age, tumour type, Knosp grade, repeated surgery, or extent of resection correlated with intra- or post-operative CSF leakage.
Seven of the 13 patients with post-operative CSF rhinorrhoea did not experience intra-operative CSF leakage (Table II); three of these patients had adrenocorticotropic hormone (ACTH)-secreting adenomas. Of the remaining 6 patients who experienced both intra- and post-operative CSF leakage, 2 had giant invasive prolactinomas and 2 had previously undergone TSS. Eight patients were successfully treated by lumbar puncture, lumbar external drainage, resting in a semi-reclining position, or other conservative treatment. In two patients the leak was repaired with gelatine foam and fibrin glue through a transsphenoidal approach, and in two patients the leak was repaired with autologous fat graft and sellar floor reconstruction using a transnasal endoscopic approach. One patient with postoperative CSF underwent two transnasal endoscopic repairs, but the leak was resolved only after further lumbar subarachnoid drainage.
Table II.
The clinicopathological features of 13 patients with post-op CSF leakage.
| Case no | Age | Gender | Subtype | Tumour size (mm) |
Knosp grade | Surgical technique of intraop CSF leakage |
Treatment of postop
leakage |
|---|---|---|---|---|---|---|---|
| 1 | 40 | F | NF | 38.5 | IV | Gelatin foam | Semireclining + LP |
| 2 | 56 | F | ACTH | 60 | IV | No CSF leakage | Semireclining |
| 3 | 44 | F | ACTH | 7 | 0 | Gelatin foam + Fibrin Glue | Semireclining |
| 4 | 56 | F | ACTH | 25 | II | No CSF leakage | Semireclining |
| 5 | 22 | F | PRL | 31 | II | Gelatin foam + Fibrin Glue | TSS repair |
| 6 | 68 | M | NF | 41 | II | Fibrin Glue | TSS repair |
| 7 | 43 | M | PRL | 28 | III | No CSF leakage | Semireclining |
| 8 | 46 | F | NF | 14 | II | Gelatin foam | Semireclining |
| 9 | 48 | F | PRL | 23 | III | Gelatin foam + Fibrin Glue | Endoscopy |
| 10 | 48 | F | ACTH | 30 | I | No CSF leakage | Semireclining + LP |
| 11 | 53 | F | NF | 25 | III | No CSF leakage | Semireclining |
| 12 | 53 | F | NF | 24 | III | No CSF leakage | Semireclining |
| 13 | 70 | M | NF | 9 | 0 | No CSF leakage | Endoscopy + LP |
β-trace protein test for CSF fistula
Laboratory assessment of CSF leakage by quantifying β-trace protein in nasal secretions offers a great diagnostic advantage. This test is highly sensitive and is more specific and much less expensive than β-2 transferrin testing 3. In this study, the β-trace protein test was used for all patients. In cases in which there was a doubt about interpretation, the result was confirmed with the β-2 transferrin test.
Exemplary case
In a 70-year-old male patient with a microadenoma, we mistook the sellar floor and punctured the dura in the clival direction during the transsphenoidal tumour resection. Although we repaired the dura puncture thoroughly with gelatine foam and fibrin glue, CSF rhinorrhoea was detected 4 days after the operation. The situation did not improve with conservative treatment, and the patient developed tension pneumocephalus. Two neuroendoscopy procedures failed to repair the defect, but the CSF rhinorrhoea resolved with further lumbar puncture drainage. During hospitalisation, the patient was diagnosed with a refractory intracranial infection. When a combination of vancomycin and ceftriaxone did not control the infection, we treated the patient with linezolid and meropenem along with daily intrathecal injections of vancomycin (20 mg). The man was discharged without any neurological deficits.
Follow-up
Follow-up of asymptomatic patients consisted of nasal endoscopy and imaging evaluations once a month for the first 3 months, then twice yearly for the first year and once yearly for the following 2 years. Median follow-up time was 5.2 years (range 3.5-7 years). In two patients, CSF rhinorrhoea recurred but was subsequently resolved by resting in a semi-reclining position.
Discussion
Post-operative CSF fistula rates after microscopic or endoscopic TSS procedures range from 0.5% to 15% 4, which is consistent with the rate of 2.7% in the present study. Intra-operative CSF fistula rates are higher, ranging from 18.1% to 53.2% 4. Similarly, the rate of 17.9% in the present study is consistent with these previous reports. According to Shiley et al., the incidence of post-operative CSF fistula is 6 times greater in patients who experience intra-operative CSF fistula 4. For this reason, it is important to identify dural defects through meticulous haemostasis and use of the Valsalva manoeuvre and Trendelenburg position 5 6.
Regarding the risk of CSF fistula associated with specific tumour types, Tamasauskas et al. reported higher rates of post-operative CSF fistula in patients with growth hormone-producing adenomas 7, whereas Shiley et al 4 reported higher CSF fistula rates in patients with non-adenomatous disease (e.g., craniopharyngioma). However, of the 13 patients with post-operative CSF rhinorrhoea in our case series, 4 had ACTH-secreting adenomas, 3 had prolactinomas and 6 had non-functional macroadenomas. Two patients had undergone TSS previously. Our findings are similar to those of other studies. Nishioka et al. retrospectively reviewed 200 consecutive cases of TSS for sellar lesions and observed intra-operative CSF leakage in 19.0% of cases 8. The risk of post-operative CSF rhinorrhoea was significantly increased in patients who underwent prior TSS, radiotherapy, or both. Macroadenomas (particularly those with suprasellar extension), repeat TSS, intra-operative leaks and even elevated body mass index have previously been reported as predictors of postoperative CSF rhinorrhoea 9 10.
The primary reconstruction technique uses autologous grafts (e.g., fascia lata) or a pedicled nasoseptal flap to reconstruct the skull base when a CSF leak occurs during or after surgery 11 12. However, due to the unpopularity of endoscopy and unfamiliarity with this reconstruction method in our department during the study period, we used alternative repair methods and also obtained excellent results. Most patients in this study chose conservative methods for CSF rhinorrhoea repair, with surgical repair used only if conservative treatment failed. Our strategies often eliminated the need for additional surgery; however, conservative treatment may increase the risk of infection, duration of hospitalisation and economic and psychological burden on the patient. Presutti et al. 13 suggested that surgical repair should be performed as soon as general clinical conditions allow if diagnostic assessments have detected CSF rhinorrhoea and identified the exact site of the leak. They concluded that clinical presentation and office-based endoscopic nasal exam were of primary importance to evaluate suspected CSF leaks. Prospective randomised controlled studies are needed to clarify the optimal approach and time window for surgical repair of CSF rhinorrhoea.
Couldwell et al. reported no incidence of postoperative CSF rhinorrhea if no intraoperative leak was encountered during transphenoidal surgery 14. Nevertheless, post-operative CSF rhinorrhoea without intra-operative leakage although rare, does occur. In seven of the 13 patients (53.8%) with post-operative CSF rhinorrhoea in our study, an intra-operative leak was not detected. One possible reason for this finding is that the enlarged sella from a macroadenoma leads to expansion and possible incompetence of the diaphragma sellae and exposed arachnoid membrane. Insertion of an autologous fat graft in the sella turcica may be a feasible and effective surgical method in this scenario 15.
Endoscopic endonasal pituitary surgery differs from the transsphenoidal microsurgery in the following aspects: plane vision, close-up view, no nasal speculum, endonasal approach and ample vision field 16. Microscopy features a three-dimensional visualisation, wider view and use of a transnasal speculum. Use of the endoscope during TSS is important in that it allows maximum tumoural excision and better visualisation of a small CSF fistula. Because of the enhanced illumination and visualisation of lesions, endoscopic surgery for CSF rhinorrhoea is more reliable and convenient than traditional TSS. In addition, we found that the endoscopic approach enables precise confirmation of the leakage site, sufficient exposure, minimal invasiveness and high rate of success. Although endoscopy was underutilised initially in our department, we subsequently used endoscopy to repair CSF leakage with excellent results. We therefore strongly recommend endoscopy for surgical repair as well as tumour removal.
Limitations of this study include its small sample size and retrospective design. Prospective multicentre studies with larger cohorts of patients are needed to confirm our results regarding risk factors for CSF rhinorrhoea and optimal repair strategies following TSS.
Conclusions
In conclusion, repair strategies using gelatin foam, fibrin glue and autologous fat are common and effective techniques for the management of CSF rhinorrhoea after TSS. The repair strategy should be individualised to each patient. Endoscopic repair of CSF leak is superior to the traditional TSS approach.
Acknowledgments
This work was generously supported by the National Natural Sciences Fund Project of China (NSFC Nos. 81500601), Shanghai Municipal Natural Science Foundation (14ZR1413800) and Shanghai Municipal Health Bureau Project (201440383 and 20154Y0036).
References
- 1.Black PM, Zervas NT, Candia GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987;20:920–924. doi: 10.1227/00006123-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ciric I, Ragin A, Baumgartner C, et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–237. doi: 10.1097/00006123-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Meco C, Oberascher G, Arrer E, et al. β-Trace protein test: new guidelines for the reliable diagnosis of cerebrospinal fluid fistula. Otolaryngol Head Neck Surg. 2003;129:508–517. doi: 10.1016/S0194-59980301448-7. [DOI] [PubMed] [Google Scholar]
- 4.Shiley SG, Limonadi F, Delashaw JB, et al. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. 2003;113:1283–1288. doi: 10.1097/00005537-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Nishioka H, Haraoka J, Ikeda Y. Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir. 2005;147:1163–1166. doi: 10.1007/s00701-005-0586-3. [DOI] [PubMed] [Google Scholar]
- 6.Basu D, Haughey BH, Hartman JM. Determinants of success in endoscopic cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg. 2006;135:769–773. doi: 10.1016/j.otohns.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Tamasauskas A, Sinkūnas K, Draf W, et al. Management of cerebrospinal fluid leak after surgical removal of pituitary adenomas. Medicina. 2008;44:302–307. [PubMed] [Google Scholar]
- 8.Nishioka H, Haraoka J, Ikeda Y. Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien) 2005;147:1163–1166. doi: 10.1007/s00701-005-0586-3. [DOI] [PubMed] [Google Scholar]
- 9.Dlouhy BJ, Madhavan K, Clinger JD, et al. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116:1311–1317. doi: 10.3171/2012.2.JNS111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta GU, Oldfield EH. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg. 2012;116:1299–1303. doi: 10.3171/2012.3.JNS112160. [DOI] [PubMed] [Google Scholar]
- 11.Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 12.Pagella F, Pusateri A, Matti E, et al. Endoscopic management of spontaneous clival cerebrospinal fluid leaks: case series and literature review. World Neurosurg. 2016;86:470–477. doi: 10.1016/j.wneu.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Presutti L, Mattioli F, Villari D, et al. Transnasal endoscopic treatment of cerebrospinal fluid leak: 17 years' experience. Acta Otorhinolaryngol Ital. 2009;29:191–196. [PMC free article] [PubMed] [Google Scholar]
- 14.Couldwell WT, Kan P, Weiss MH. Simple closure following transphenoidal surgery. Technical note. Neurosurg Focus. 2006;20:E11–E11. doi: 10.3171/foc.2006.20.3.12. [DOI] [PubMed] [Google Scholar]
- 15.Gkekas N, Primikiris P, Georgakoulias N. Postoperative rhinorrhea without intraoperative cerebrospinal fluid leak after endoscopic transnasaltransphenoidal surgery for pituitary macroadenomas. World Neurosurg. 2014;82:e658–e659. doi: 10.1016/j.wneu.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Romero Adel C, Nora JE, Topczewski TE, et al. Cerebrospinal fluid fistula after endoscopic transsphenoidal surgery: experience in a spanish center. Arq Neuropsiquiatr. 2010;68:414–417. doi: 10.1590/s0004-282x2010000300017. [DOI] [PubMed] [Google Scholar]