Zhang et al. show that hyperphosphorylated STAT1 in patients with STAT1 gain-of-function and STAT3 loss-of-function is caused by impaired SOCS3 expression and leads to upregulation of PD-L1 and defects in Th17 cell differentiation that underlie susceptibility to chronic mucocutaneous candidiasis in these patients.
Abstract
Patients with hypomorphic mutations in STAT3 and patients with hypermorphic mutations in STAT1 share several clinical and cellular phenotypes suggesting overlapping pathophysiologic mechanisms. We, therefore, examined cytokine signaling and CD4+ T cell differentiation in these cohorts to characterize common pathways. As expected, differentiation of Th17 cells was impaired in both cohorts. We found that STAT1 was hyperphosphorylated in response to cytokine stimulation in both cohorts and that STAT1-dependent PD-L1 up-regulation—known to inhibit Th17 differentiation in mouse models—was markedly enhanced as well. Overexpression of SOCS3 strongly inhibited phosphorylation of STAT1 and PD-L1 up-regulation, suggesting that diminished SOCS3 expression may lead to the observed effects. Defects in Th17 differentiation could be partially overcome in vitro via PD-L1 inhibition and in a mouse model of STAT3 loss-of-function by crossing them with PD-1 knockout mice. PD-L1 may be a potential therapeutic target in several genetic diseases of immune deficiency affecting cytokine signaling.
Introduction
Over the past several decades, increasing numbers of patients have been identified with inborn errors of cytokine receptors and downstream JAK-STAT signaling molecules that manifest with highly diverse immunologic and clinical phenotypes (Casanova et al., 2012). Patients with autosomal-dominant, hyper-IgE syndrome have elevated serum IgE levels, eczema, and connective tissue disease, including intracranial aneurysms and other vascular abnormalities (Fathi et al., 2011; Chandesris et al., 2012a). They display a particular susceptibility to skin and lung infection with Staphylococcus aureus and Candida albicans (Casanova et al., 2012) and have impaired antigen-specific antibody responses (Leung et al., 1988; Avery et al., 2010; Meyer-Bahlburg et al., 2012). This syndrome is due to mutations in STAT3 primarily affecting the DNA-binding, Src homology 2 (SH2), linker, or transactivation domains; most of which act in a dominant negative manner (STAT3 loss-of-function [LOF]) by destabilizing the STAT3 protein (Chandesris et al., 2012b; Bocchini et al., 2016). Patients with hypermorphic, gain-of-function (GOF) mutations in STAT1 have autoimmune diseases and, similar to patients with STAT3 LOF, have an increased incidence of vascular malformations, humoral immune defects, and susceptibility to fungal infections (Casanova et al., 2012; Romberg et al., 2013; Toubiana et al., 2016). Mutations leading to STAT1 GOF have been found predominantly in the coiled-coil domain or DNA-binding domain of STAT1 and lead to increased STAT1 phosphorylation in response to cytokine stimulation at least in part by impairing nuclear dephosphorylation (Toubiana et al., 2016).
In addition to their clinical phenotypic similarities, patients with STAT3 LOF and STAT1 GOF also share similar cellular phenotypes. Reduced circulating Th17 (IL-17A+ and IL-17F+) cells and impaired Th17 differentiation are seen in patients with both STAT3 LOF and STAT1 GOF and underlie clinical susceptibility to chronic mucocutaneous candidiasis (CMC; de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008; Liu et al., 2011). These defects are thought to be due to either the direct requirement for STAT3-mediated transcription of retinoic acid receptor–related orphan receptor (ROR)-γt, the master transcription factor for Th17 differentiation (Yang et al., 2008), or inhibition of STAT3 function by enhanced STAT1 (Amadi-Obi et al., 2007). In mouse models, inhibition of STAT3 signaling or IL-17 led to increased vascular aneurysm severity (Chandesris et al., 2012a), raising the question of whether the vascular abnormalities seen in patients with STAT1 GOF and STAT3 LOF could relate to their Th17 defect. Although vascular anomalies have not been described in patients with inherited mutations in IL17F, IL17RA, or IL17RC, the numbers of these patients reported is low and this could account for the lack of observed vascular issues (Puel et al., 2011; Ling et al., 2015). Alternatively, deregulated TGF-β signaling, which has been reported in STAT3 LOF, could underlie these vascular defects (Lyons et al., 2017).
STAT family members have the ability to directly interact and form homo- and heterodimers, and significant evidence exists for competition between STAT proteins for binding sites. For example, STAT3 and STAT5 compete for the Bcl6 and the Il17 loci (Yang et al., 2011; Oestreich et al., 2012). Competition also exists between STAT1 and STAT3, including for binding on the Il21r and the Ifngr1 promoters (Qing and Stark, 2004; Spolski and Leonard, 2008). The delicate balance between signaling of various STATs determines downstream effector fates. Thus, it is plausible that mutations leading to GOF or LOF of one STAT protein may disrupt that balance and have pleiotropic effects because of reciprocal LOF or GOF in other STAT proteins and their downstream targets.
Recently, the oral JAK 1/2 inhibitor ruxolitinib was used to successfully treat CMC and alopecia areata in patients with STAT1 GOF (Higgins et al., 2015; Mössner et al., 2016). In the case of STAT3 LOF, defining a treatment approach is more challenging because direct replacement of the deficient STAT3 protein is limited by the significant potential for oncogenesis (Yu et al., 2009). However, a possible therapeutic opportunity in patients with this and other LOF mutations is in the use of inhibitors of downstream proteins that are dysregulated.
Suppressors of cytokine signaling (SOCS) proteins are induced by cytokines and regulate the cellular response via a negative feedback loop (Yoshimura et al., 2007). STAT3-dependent up-regulation of SOCS3 impairs Th17 cell differentiation via inhibition of IL-6 and IL-23 signaling, whereas STAT1-driven SOCS1 is required for Th17 differentiation (Chen et al., 2006; Tanaka et al., 2008). Although naive T cells from patients with STAT3 LOF have reduced SOCS3 transcripts (Siegel et al., 2011; Chandesris et al., 2012b), those patients paradoxically also have a decrease in Th17 cells (Milner et al., 2008). One possible explanation is that the decrease in SOCS3 leads to disinhibition of other cytokine signaling pathways, such as IL-27 signaling via STAT1. IL-27 priming of naive cluster of differentiation (CD) 4+ T cells leads to up-regulation of programmed cell death protein ligand 1 (PD-L1) in a STAT1-dependent manner (Hirahara et al., 2012). Consistent with this, PD-L1 can be found ex vivo on naive CD4+ T cells in patients with STAT1 GOF (Romberg et al., 2013). PD-L1 delivers an inhibitory signal after T cell activation via its interactions with PD-1 and B7-1, and the PD-L1/PD-1 interaction has been shown in a mouse model to impair Th17 differentiation (Butte et al., 2007; Hirahara et al., 2012).
We describe here a common cellular phenotype of STAT1 hyperphosphorylation in response to cytokine stimulation in patients with STAT1 GOF and STAT3 LOF. We show that STAT1-dependent PD-L1 up-regulation is markedly enhanced in STAT3 LOF and STAT1 GOF lymphocytes compared with healthy donor cells. Both STAT1 hyperphosphorylation and PD-L1 up-regulation are associated with significantly reduced SOCS3 protein expression, and overexpression of SOCS3 strongly inhibited phosphorylation of STAT1. Furthermore, defects in Th17 differentiation could be partially overcome via PD-L1 inhibition.
Results and discussion
Enhanced cytokine-induced phosphorylation of STAT1 in patients with STAT3 LOF is similar to that seen in STAT1 GOF and is, in part, caused by decreased SOCS3 expression
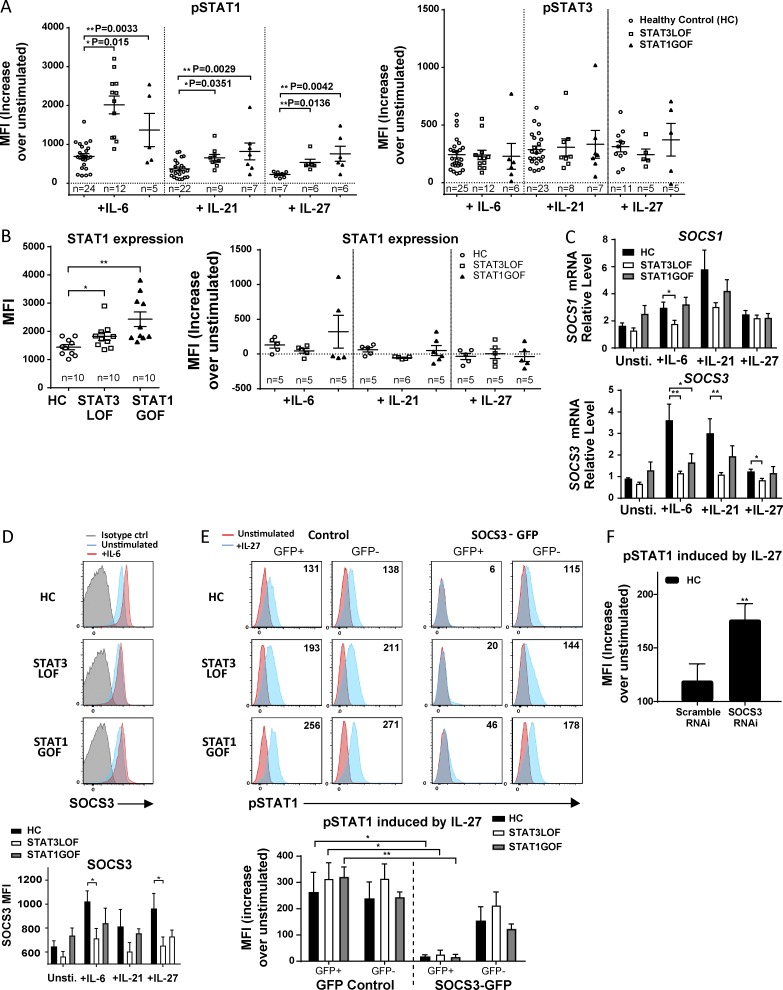
After stimulation for 15 min with IL-6, IL-21, IFN-γ (not depicted), or IL-27 (cytokines known to signal via STAT1 and/or STAT3), the level of cytokine-induced STAT1 phosphorylation (pSTAT1) among CD3+ T cells was significantly increased in patients with STAT1 GOF and STAT3 LOF compared with healthy controls (HCs; Fig. 1 A, left). Consistent with recently reported data (Tabellini et al., 2017), total STAT1 protein levels were also increased at baseline and after cytokine stimulation in some patients with STAT1 GOF compared with HCs (Fig. 1 B) potentially explaining some of the increased pSTAT1 in patients with STAT1 GOF. In contrast, only one patient with STAT3 LOF was identified with increased total STAT1. No difference was seen in the levels of cytokine-induced pSTAT3 (Fig. 1 A, right), consistent with previous studies examining pSTAT3 in patients with STAT3 LOF and STAT1 GOF (Renner et al., 2008; Higgins et al., 2015; Zheng et al., 2015), suggesting regulation of pSTAT1 by a STAT3-dependent mechanism, but not necessarily the reciprocal.
Figure 1.
STAT3 LOF and STAT1 GOF cells have enhanced pSTAT1 that is partially dependent on impaired induction of SOCS3. (A) pSTAT1 (left) and pSTAT3 (right) in PBMCs after stimulation with IL-6, IL-21, or IL-27 for 15 min. (B) Total STAT1 in PBMCS before stimulation (left) and after cytokine stimulation (right) for 15 min. (C and D) SOCS3 and SOCS1 mRNA (C) and SOCS3 protein (D) levels in naive CD4 T cells after stimulation with IL-6, IL-21, or IL-27 for 1 h. (E and F) Naive (CD45RO−) CD4+ T cells were transfected with SOCS3–GFP or GFP–control or SOCS3–RNAi or scramble RNAi–control. IL-27 was added 5 h after transfection, and pSTAT1 levels were analyzed 18–24 h later. One-way ANOVA (A–D) and paired and Student’s t tests (E and F) were performed. *, P < 0.05; **, P < 0.01. Data are representative of three independent experiments, unless otherwise indicated on the graph; graphs show means ± SEM. MFI, mean fluorescence intensity.
We next sought to explore the mechanism of STAT3-dependent regulation of pSTAT1. Naive CD4+ T cells from patients with STAT3 LOF and STAT1 GOF were found to have decreased SOCS3 transcripts compared with HCs after stimulation for 1 h with IL-6 and, to a lesser extent, IL-21 and IL-27, consistent with previous studies (Fig. 1 C; Siegel et al., 2011; Zheng et al., 2015). No significant difference was found in SOCS1 mRNA levels at rest or after cytokine stimulation (Fig. 1 C), suggesting a targeted effect of STAT3 signaling on SOCS3. This finding of decreased cytokine-induced SOCS3 was also confirmed at the protein level (Fig. 1 D). To determine whether SOCS3 overexpression could correct the observed hyperphosphorylation of STAT1, naive CD4+ T cells were transfected with a SOCS3–GFP construct or an empty GFP control vector. Overexpression of SOCS3 significantly decreased IL-27–induced pSTAT1 in HCs and in patients with STAT3 LOF and STAT1 GOF (Fig. 1 E), with no change in total STAT1 expression (not depicted). To further confirm that decreased SOCS3 results in dysregulated, cytokine-induced pSTAT1, we used SOCS3 RNAi to knock down SOCS3 expression in HC cells. As expected, IL-27-induced pSTAT1 was significantly increased (Fig. 1 F).
IL-27–driven PD-L1 is up-regulated on naive T cells in patients with STAT3 LOF and STAT1 GOF
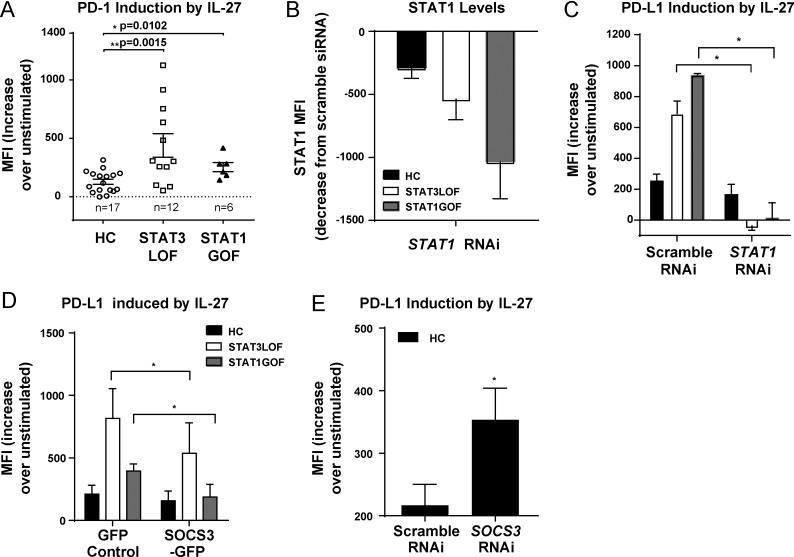
To determine the effects of STAT1 hyperphosphorylation on downstream target genes, we examined the expression of PD-L1 in response to IL-27, which has previously been shown to up-regulate PD-L1 in a STAT1-dependent manner (Hirahara et al., 2012). Basal PD-L1 expression was increased in naive and memory CD4+ T cells from patients with STAT1 GOF (not depicted), consistent with previous studies (Romberg et al., 2013). Levels of PD-L1 were also significantly increased after IL-27 stimulation of naive CD4+ T cells from STAT3 LOF or STAT1 GOF, relative to that of HCs (Fig. 2 A). Treatment with a siRNA molecule targeting STAT1 effectively reduced STAT1 expression levels (Fig. 2 B) and corrected that phenotype (Fig. 2 C). SOCS3 overexpression resulted in significantly lower IL-27–induced PD-L1 in cells from patients with STAT3 LOF and STAT1 GOF (Fig. 2 D). Conversely, SOCS3 silencing in HCs using RNAi resulted in increased PD-L1 levels (Fig. 2 E). Collectively, these results demonstrate enhanced activity of the IL-27/STAT1 signaling pathway, at least in part, because of SOCS3 disinhibition, resulting in PD-L1 overexpression in STAT3 LOF and STAT1 GOF.
Figure 2.
High pSTAT1 drives increased PD-L1 expression in STAT3 LOF and STAT1 GOF cells. (A) PD-L1 expression on naive (CD45RO−) T cells from HCs and patients after 24 h of stimulation with IL-27. Naive (CD45RO−) CD4+ T cells were transfected with STAT1 RNAi or scramble RNAi (B and C), SOCS3-GFP or GFP-control (D), or SOCS3 RNAi or scramble RNAi (E). (B) STAT1 expression after transfection with STAT1 RNAi. (C–E) PD-L1 expression 41 h after IL-27 stimulation. One-way ANOVA (A) and paired Student’s t tests (C–E) were performed. *, P < 0.05; **, P < 0.01. Data are representative of three independent experiments unless otherwise indicated on the graph; graphs show means ± SEM. MFI, mean fluorescence intensity.
Enhanced IL-27/STAT1/PD-L1 pathway activation inhibits Th17 differentiation in STAT3 LOF and STAT1 GOF
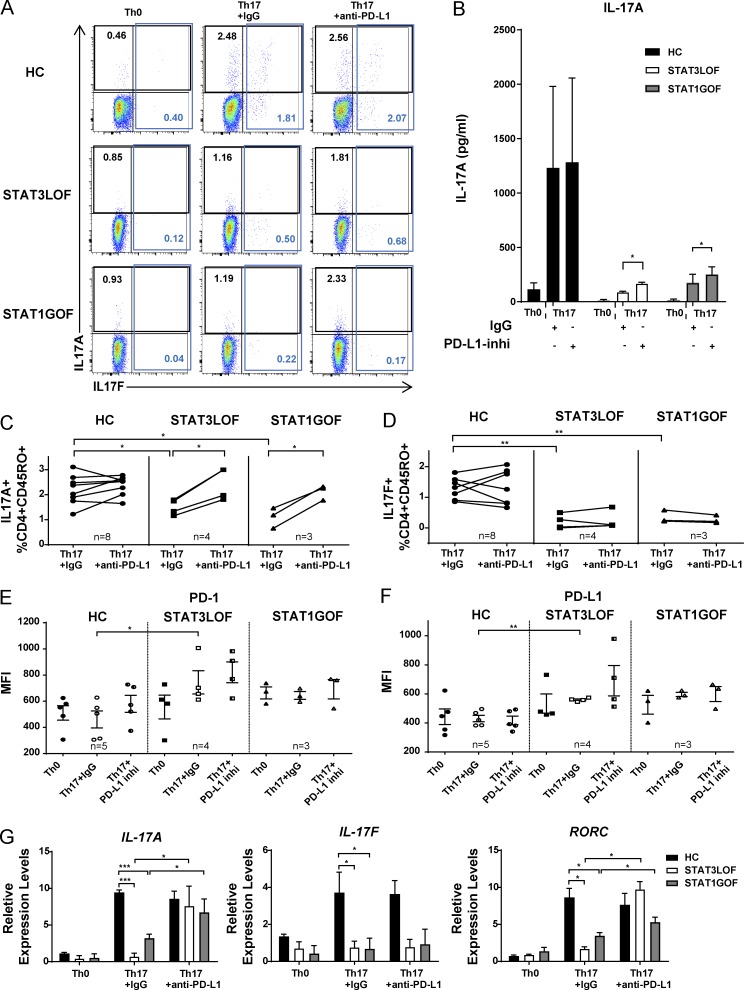
We next sought to determine whether the observed impairment of Th17 differentiation in these patients could be explained by increased signaling via the IL-27/STAT1/PD-L1 pathway. Naive CD4+ T cells from HCs and from patients with STAT3 LOF and STAT1 GOF were cultured with anti-CD3/CD28 under Th17- or non(Th0)-skewing conditions. Decreased frequencies of CD45RO+IL-17A+ and CD45RO+IL-17F+ T cells and decreased IL-17A protein levels were found under Th17-skewing conditions in STAT3 LOF and STAT1 GOF compared with HCs (Fig. 3, A–D). Both PD-1 and PD-L1 levels were increased in STAT3 LOF cells cultured under Th17 conditions, despite being unchanged or decreased in HC cells (Fig. 3, E and F). The addition of an anti-human PD-L1 inhibitory mAb partially restored IL-17A protein and transcript levels in STAT3 LOF and STAT1 GOF (Fig. 3, A–D and G). A modest increase above baseline IL-17A+ cells was also observed in most HCs under PD-L1 blockade (Fig. 3 C), suggesting a greater negative effect of increased PD-L1 in patients’ cells where there are also other pathway disruptions as compared with HCs. IL-17F was not rescued by PD-L1 blockade (Fig. 3, A, D, and G). Additionally, PD-L1 blockade restored transcription of RORC, which encodes for the Th17 master transcription factor ROR-γt (Fig. 3 G).
Figure 3.
Th17 cytokine production is impaired in STAT3 LOF and STAT1 GOF T cells, and IL-17A can be partially restored by blocking PD-L1. Naive CD4+ T cells from HCs and patients were cultured for 6 d under Th0 or Th17 conditions in the presence of PD-L1 blocking antibody (anti-PD-L1) or control IgG. Expression of IL-17A and IL-17F (A–D), PD-1 (E), and PD-L1 (F) were analyzed. (G) IL17A, IL17F, and RORC mRNA levels in cells are shown. One-way ANOVA between genotypes (C–G) and paired Student’s t tests (B) were performed. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data are representative of three independent experiments. Graphs show means ± SEM.
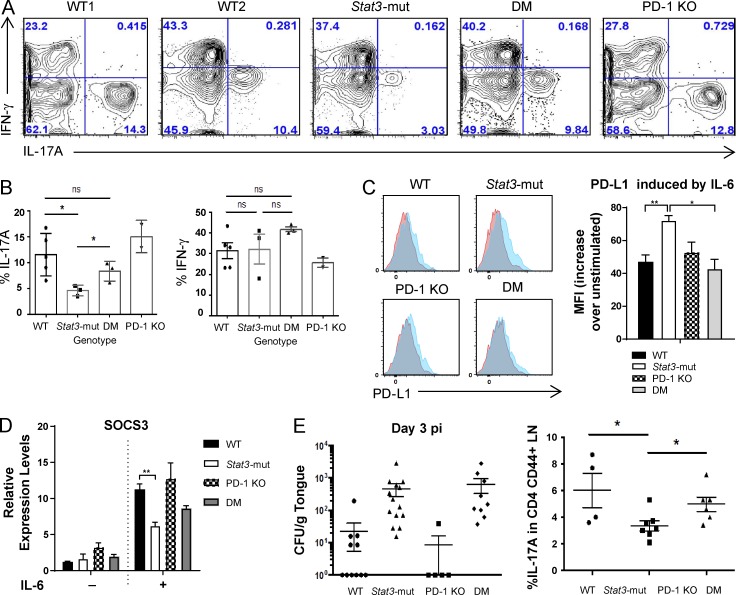
Finally, to confirm that finding in vivo, Stat3-mut mice (Steward-Tharp et al., 2014) were immunized with OVA/CFA, and Th17 cytokine production was measured in cells isolated from LNs 1 wk later. As expected, there was a decreased frequency of CD4+CD44+IL-17A+ memory T cells from Stat3-mut mice compared with WT. Crossing with PD-1 KO mice to generate double-mutant (DM) mice led to restoration of IL-17A frequencies in WT mice (Fig. 4, A and B). Increased PD-L1 protein (Fig. 4 C) and decreased Socs3 mRNA levels (Fig. 4 D) induced by IL-6 were seen in Stat3-mut mice, with normalization of both PD-L1 and Socs3 levels in DM mice. To see whether that correlated with in vivo suppression of C. albicans, we used a model of oropharyngeal candidiasis (OPC) with measurement of tongue fungal load (in CFU/g) as a marker of fungal burden. As expected, the Stat3-mut mouse had a significantly greater fungal burden than the WT mouse had, with a lower frequency of CD4+CD44+IL-17A+ draining LN cells (Fig. 4 E), indicating susceptibility to OPC. Although a partial rescue in the frequency of IL-17A+ cells was observed in DM mice, it was insufficient to confer protection from OPC (Fig. 4 E). This may be because CD4+ T cells are not the sole source of protective IL-17 required for mucosal anti-Candida immunity in mice (Gladiator et al., 2013; Conti et al., 2014). As such, rescue of CD4+ IL-17 production would not necessarily be expected to lead to alteration in the fungal burden.
Figure 4.
Stat3-mut mice have impaired IL-17A production that can be restored in Stat3-mut/PD-1 KO (DM) mice. (A and B) IFN-γ and IL-17A expression by CD4+CD44+ draining LN cells from WT, Stat3-mut, PD-1 KO, and DM mice was analyzed 1 wk after OVA/CFA immunization. (C) PD-L1 expression on CD4+CD44− T cells after 24 h of stimulation with IL-6. (D) Socs3 mRNA levels in CD4+CD44− T cells before and after stimulation with IL-6 for 1 h. (E) Fungal load in the tongues and the percentage of CD4+CD44+IL-17A+ cells in cervical LN in Candida-infected mice was determined at d 3 postinfection (pi). One-way ANOVA was performed (B–E). *, P < 0.05; **, P < 0.01. Graphs show means ± SEM. (A-D) WT, n = 5; hyper-IgE syndrome, n = 3; DM, n = 3; PD-1 KO, n = 2. (E) Data were combined from three independent experiments, n = 5–14 mice/group.
Collectively, these data demonstrate a STAT1 GOF phenotype in patients with either STAT1 GOF or STAT3 LOF and illustrate how seemingly disparate molecular defects, each of which results in an alteration in the balance between JAK-STAT signaling molecules, can lead to similar downstream defects. Reciprocal effects exist: enhanced STAT1 activity interferes with STAT3 function, but mutations in STAT3 seem to interfere with appropriate control of STAT1. The dysregulated STAT1 signal enables enhanced IL-27–mediated up-regulation of PD-LI, which is associated with impaired Th17 differentiation and may contribute to the shared phenotype of susceptibility to CMC observed in both cohorts. Additional mechanisms in STAT1 GOF, including reduced transcription of STAT3-dependent Th17 gene transcription because of reduced histone acetylation, may also contribute to the CMC phenotype (Zheng et al., 2015).
Intriguingly, the impairment in Th17 differentiation could be partially overcome by treatment with a PD-L1 inhibitor, with restoration of RORC transcription and increase in IL-17A, but not IL-17F, expression. Differential regulation of IL-17A and IL-17F has been noted previously, with a greater dependence of IL-17F on prostaglandin E2/STAT3 and IL-17A on PI3K/mTOR/NFAT (Gomez-Rodriguez et al., 2009; Melton et al., 2013). In addition, enhanced chromatin accessibility of the IL-17A compared with the IL-17F locus has been noted in a primary human Th17 cell line, which could explain enhanced restoration of IL-17A (Adamik et al., 2013). In mice, IL-17A deficiency leads to susceptibility to both CMC and invasive candidiasis and S. aureus, whereas, in contrast, IL-17F has been found to be redundant (Ishigame et al., 2009; Saijo et al., 2010). The relative importance of IL-17A compared with IL-17F in humans (as reviewed in Cypowyj et al., 2012) has not been clearly established. In humans, patients with IL-17RA deficiency (Puel et al., 2011), IL-17RC deficiency (Ling et al., 2015), and IL-17F deficiency (Puel et al., 2011) have all been identified as having CMC. However, each of these defects impairs the cellular response to both IL-17A and IL-17F homo- and heterodimers. Intriguingly, in the kindred with autosomal-dominant IL17F mutations, two mutation-carrying family members were asymptomatic, raising the possibility that IL17F in humans, similar to that found in mice, may be dispensable (Puel et al., 2011). Because humans with IL17A mutations have not yet been identified, the relative importance of each individual cytokine in host defense is not yet clearly established.
Importantly, despite the in vitro restoration in primary human CD4+ T cells of RORC and IL17A in the setting of PD-L1 blockade, PD-1 blockade was not sufficient in our model to protect Stat3-mut mice against OPC. Important differences exist between mouse and human host defenses to Candida, including an important role for innate immune cells, which may explain the result. Certainly, the in vitro findings in humans provide the basis for further studies into the use of immune checkpoint inhibitors targeting PD-1/PD-L1 for the treatment of infectious phenotypes associated with IL17 defects (Webster, 2014). Additionally, there is the potential to restore other lineages that are restrained by PD-1/PD-L1 and are found to be defective in these diseases. The broader concept of inhibiting a molecule that is increased because of the inherited LOF of another protein (or the reciprocal), holds promise as a novel therapeutic approach to be explored in patients with mutations in cytokine signaling pathways.
Materials and methods
Human subjects and clinical studies
All patients or their guardians provided informed consent under approved protocols of the National Institute of Allergy and Infectious Diseases Institutional Review Board (10-I-0148 and 00-I-0159), as well as the guidelines in the Declaration of Helsinki. Subjects with STAT3 LOF had mutations in the SH2 domain (n = 6), DNA binding domain (n = 8), and transactivation domain (n = 1) and ranged from 11 to 47 yr old; five were male. Subjects with STAT1 GOF had mutations in the coiled-coil domain (n = 5), DNA-binding domain (n = 4), and SH2 domain (n = 1) and ranged from 4 to 60 yr old; 3 were male. Additional genotype and phenotype information is included in Table S1. Blood from HCs was obtained under Institutional Review Board–approved protocols.
Flow cytometry
PBMCs were isolated from whole blood by Ficoll-Hypaque gradient centrifugation and washed with FACS buffer. Events were collected on an LSRFortessa with Diva 6.1.3 software (BD Biosciences) and analyzed with FlowJo 10 software (Tree Star). All plots are gated on live, singlet, CD3+ lymphocytes. All values used for analyzing proportionate responses are background subtracted.
STAT phosphorylation
To assay STAT1 and STAT3 phosphorylation, PBMCs were incubated at 37°C for 1 h in serum-free RPMI 1640 and then stimulated for 15 min with media alone, IL-6 (100 ng/ml; PeproTech), IFN-γ (2,000 U/ml; PeproTech), IL-21 (100 ng/ml; PeproTech), or IL-27 (50 ng/ml; R&D Systems) before being fixed with paraformaldehyde and permeabilized in methanol. Cells were stained with CD3 Alexa Fluor 700, pSTAT1 (pY701) AF488, pSTAT3 (pY705) PE, and STAT1 FITC (all BD Biosciences).
Real-time PCR
To assay SOCS1 and SOCS3/Socs3 up-regulation in response to cytokine stimulation, naive CD4 T cells were stimulated with or without IL-6 (100 ng/ml; PeproTech), IL-21 (100 ng/ml; PeproTech), or IL-27 (50 ng/ml, R&D Systems) for 1 h, as indicated in the text and figures. Cells were then washed and pelleted. Total RNA was extracted from PBMCs with the RNeasy Mini kit (QIAGEN). For real-time PCR, 1 µg of total RNA was reverse-transcribed (Thermo Fisher Scientific), and the resulting cDNA was amplified by PCR with the ABI 7500 Sequencer and TaqMan expression assays (Thermo Fisher Scientific). 18S was used as a normalization control. The data were analyzed with the 2−▵▵Ct method, and results are expressed as mean fold induction.
SOCS3 flow cytometry
Human, naïve, CD4+ T cells isolated from PBMC were stimulated with IL-6 (100 ng/ml; PeproTech), IL-21 (100 ng/ml; PeproTech), or IL-27 (50 ng/ml; R&D Systems) for 90 min in RPMI 1640 plus 10% FBS. Cells were then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), per the manufacturer’s protocol, then stained with anti-SOCS3 antibodies and DyLight 488 Anti-Mouse IgG (Abcam).
PD-L1 induction
PBMCs were incubated at 150,000 cells/well in 96-well, round-bottom plates for 24 h at 37°C and 5% CO2 in the presence or absence of IL-27 (50 ng/ml; R&D Systems) or IFN-γ (100 ng/ml; R&D Systems). Cells were harvested and stained with Live/Dead Fixable Aqua viability dye (Thermo Fisher Scientific), CD3 AF700, CD4 PE-Cy7, CD27 PE-Cy5, CD45RO PE-Texas Red (BD Biosciences), and PD-L1 PE (BioLegend). Median fluorescence intensity of PD-L1 expression was assessed in the naive lymphocyte gate (CD3+CD4+CD27+CD45RO−).
SOCS3 transfection
PBMCs were transfected with either GFP control (Lonza) or SOCS3–GFP construct (OriGene) using Amaxa Human T cell Nucleofection kit (Lonza) per the manufacturer’s protocol, with the Amaxa nucleofection program U-014. Cells were stimulated with IL-27 (20 ng/ml; R&D Systems) after allowing cells to rest for 5–7 h after transfection. STAT1 phosphorylation (24 h stimulation) and PD-L1 expression (41 h stimulation) were assessed in live CD3+CD4+ lymphocytes as described above.
siRNA transfection
PBMCs were transfected with 30 pM STAT1 or SOCS3 siRNA (Thermo Fisher Scientific) using Amaxa Human T cell Nucleofection kit (Lonza) per the manufacturers’ protocols. Control cells were transfected with 30 pM nonspecific, scrambled siRNA. Cells were stimulated with IL-27 (50 ng/ml; R&D Systems) after allowing cells to rest for 5–7 h after transfection. Cells were harvested and stained for pSTAT1 and PD-L1 as per above.
Th17 cell differentiation and cytokine analyses
Naive CD4+ T cells were isolated from PBMCs using negative-selection, magnetic-activated, cell-sorting microbeads (Miltenyi Biotec) per manufacturer’s protocol. Purity was >95% in all samples run. For T cell differentiation, cells were stimulated at 100,000 cells/well in 96-well, flat-bottom plates that had been coated with anti-CD3 (10 µg/ml, OKT3; eBioscience) antibodies. The cells were cultured in X-VIVO 15 medium (Lonza) at 37°C and 5% CO2 for 6 d in the presence of soluble anti-CD28 (0.5 μg/ml). For Th17, anti–IFN-γ (10 μg/ml; BD Biosciences), IL-23 (100 ng/ml; R&D Systems), IL-1β (10 ng/ml; PeproTech), and IL-6 (20 ng/ml; PeproTech) were added in the culture. For Th0, 100 U/ml IL-2 (R&D Systems) was added. In certain experiments, cells were pretreated with 10 μg/ml of anti-human PD-L1 mAb (AMP-714; kindly supplied by Amplimmune, Inc., AstraZeneca) or UltraLEAF-purified human-isotype control IgG4 antibody (BioLegend). ELISA analysis was performed for IL-17A (R&D Systems) secretion on d 6 for expanded T cells in culture. On d 6, the cells were stimulated with 20 ng/ml PMA and 1 µM ionomycin for 5 h. 10 µg/ml brefeldin A was added after 2.5 h. Cells were then fixed and permeabilized with Cytofix/Cytoperm per the manufacturer’s protocol and stained with Live/Dead Fixable Aqua dye, CD3 AF700, CD4 PE-Cy7, IL-17F AF488, IFN-γ fluorescein v450 (BD Biosciences), CD45RO ECD (Beckman Coulter), and IL-17A APC (eBioscience).
Stat3-mut and PD-1 KO mouse
All animal work was performed under an Animal Care and Use Committee–approved protocol. All mice were derived from a C57BL/6 background. Littermates are obtained by breeding from the original sources, and no outside WT mice were used as controls or for breeding. The Stat3-mut mouse was a gift from John O’Shea (NIAMS, NIH, Bethesda, MD). The mouse has a single aa deletion (ΔV463) in the STAT3 DNA-binding domain to mimic the heterozygous mutation seen in patients (Steward-Tharp et al., 2014). PD-1 KO mouse is a gift from Daniel Barber (NIAID, NIH, Bethesda, MD). For OVA/CFA-induced Th17, mice were injected with 25 µg OVA plus 1:1 CFA per site s.c. on the back (four sites per mouse). After 1 wk, cells were harvested from draining LNs and were cultured in 1 ml RPMI 1640 with 10% FBS, 2-ME, 20 ng/ml PMA, and 1 µM ionomycin in a 48-well plate. After 1 h of incubation, 5 µg/ml brefeldin A was added to the culture, and incubation was continued for another 5–6 h. Cells were fixed, permeabilized, and stained with antibodies using the Cytofix/Cytoperm kit (BD Biosciences), according to the manufacturer’s protocol, and flow cytometry was performed.
Mouse model of OPC and quantification of fungal load
C. albicans strain SC5314 was grown in yeast, peptone, and dextrose medium (containing penicillin and streptomycin) by incubation in a shaking incubator at 30°C. To induce OPC in mice, mice were sublingually infected, as previously described (Solis and Filler, 2012; Break et al., 2015). In brief, mice were sedated with a ketamine/xylazine mixture and cotton swabs were saturated in 107/ml C. albicans. A swab was placed securely under the tongues of mice for 90 min, with repeated administrations of ketamine/xylazine as needed. To determine the fungal load in the tongues of mice on d 3 after infection, tongues were weighed, homogenized with an Omni tissue homogenizer, and the entire tongue was plated on yeast, peptone, and dextrose agar plates containing penicillin and streptomycin. The plates were incubated at 37°C for 24–48 h and the CFUs were counted. If no colonies were counted, then a value of zero was assigned. PBMCs from cervical LN were isolated on d 3 after infection and were treated with PMA/ionomycin in the presence of brefeldin A for 5 h. Intracellular IL-17A in CD4+CD44+ cells was measured by flow cytometry.
Statistics
Two-group comparisons of normal data were made using the Student’s t test, and comparisons of nonnormal data were made with the Mann–Whitney U test. Multiple-group comparisons used ANOVA (Prism; GraphPad Software). The statistical significance level adopted was P < 0.05.
Online supplemental material
Table S1 provides genotype and phenotype information on the patients in the study.
Supplementary Material
Acknowledgments
This work was supported by the intramural research program of the National Institutes of Health 1ZIAAI001098-02.
D.L. Barber has patents and has received royalties related to the therapeutic targeting of the PD-1 pathway. The other authors declare no competing financial interests.
Author contributions: Y. Zhang, C.A. Ma, M.G. Lawrence, T.J. Break, M.P. O’Connell, J.J. Lyons, D.B. Lopez, J.S. Barber, Y. Zhao, and D.L. Barber designed and performed the experiments. M.S. Lionakis and J.D. Milner supervised the experimental design and the data analysis. A.F. Freeman and S.M. Holland recruited human subjects. Y. Zhang, C.A. Ma, M.G. Lawrence, and J.D. Milner wrote the original draft of the manuscript, and all authors reviewed and edited the manuscript.
Footnotes
Abbreviations used:
- CMC
- chronic mucocutaneous candidiasis
- DM
- double-mutant
- GOF
- gain-of-function
- HC
- healthy control
- LOF
- loss-of-function
- OPC
- oropharyngeal candidiasis
- p
- phosphorylated
- ROR
- retinoic acid receptor–related orphan receptor
- SOCS
- suppressors of cytokine signaling
References
- Adamik J., Henkel M., Ray A., Auron P.E., Duerr R., and Barrie A.. 2013. The IL17A and IL17F loci have divergent histone modifications and are differentially regulated by prostaglandin E2 in Th17 cells. Cytokine. 64:404–412. 10.1016/j.cyto.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi-Obi A., Yu C.R., Liu X., Mahdi R.M., Clarke G.L., Nussenblatt R.B., Gery I., Lee Y.S., and Egwuagu C.E.. 2007. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 13:711–718. 10.1038/nm1585 [DOI] [PubMed] [Google Scholar]
- Avery D.T., Deenick E.K., Ma C.S., Suryani S., Simpson N., Chew G.Y., Chan T.D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. . 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207:155–171. 10.1084/jem.20091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini C.E., Nahmod K., Katsonis P., Kim S., Kasembeli M.M., Freeman A., Lichtarge O., Makedonas G., and Tweardy D.J.. 2016. Protein stabilization improves STAT3 function in autosomal dominant hyper-IgE syndrome. Blood. 128:3061–3072. 10.1182/blood-2016-02-702373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Break T.J., Jaeger M., Solis N.V., Filler S.G., Rodriguez C.A., Lim J.K., Lee C.C., Sobel J.D., Netea M.G., and Lionakis M.S.. 2015. CX3CR1 is dispensable for control of mucosal Candida albicans infections in mice and humans. Infect. Immun. 83:958–965. 10.1128/IAI.02604-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., and Freeman G.J.. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 27:111–122. 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J.L., Holland S.M., and Notarangelo L.D.. 2012. Inborn errors of human JAKs and STATs. Immunity. 36:515–528. 10.1016/j.immuni.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandesris M.O., Azarine A., Ong K.T., Taleb S., Boutouyrie P., Mousseaux E., Romain M., Bozec E., Laurent S., Boddaert N., et al. . 2012a Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ Cardiovasc Genet. 5:25–34. 10.1161/CIRCGENETICS.111.961235 [DOI] [PubMed] [Google Scholar]
- Chandesris M.O., Melki I., Natividad A., Puel A., Fieschi C., Yun L., Thumerelle C., Oksenhendler E., Boutboul D., Thomas C., et al. . 2012b Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: Molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore). 91:e1–e19. 10.1097/MD.0b013e31825f95b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., and O’Shea J.J.. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 103:8137–8142. 10.1073/pnas.0600666103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H.R., Peterson A.C., Brane L., Huppler A.R., Hernández-Santos N., Whibley N., Garg A.V., Simpson-Abelson M.R., Gibson G.A., Mamo A.J., et al. . 2014. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J. Exp. Med. 211:2075–2084. 10.1084/jem.20130877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypowyj S., Picard C., Maródi L., Casanova J.L., and Puel A.. 2012. Immunity to infection in IL-17-deficient mice and humans. Eur. J. Immunol. 42:2246–2254. 10.1002/eji.201242605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Jannière L., et al. . 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205:1543–1550. 10.1084/jem.20080321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi A.R., Vortmeyer A., Holland S.M., and Pluta R.M.. 2011. Intracranial aneurysms associated with hyperimmunoglobulinaemia E (Job) syndrome: Report of two cases. J. Neurol. Neurosurg. Psychiatry. 82:704–706. 10.1136/jnnp.2009.198283 [DOI] [PubMed] [Google Scholar]
- Gladiator A., Wangler N., Trautwein-Weidner K., and LeibundGut-Landmann S.. 2013. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J. Immunol. 190:521–525. 10.4049/jimmunol.1202924 [DOI] [PubMed] [Google Scholar]
- Gomez-Rodriguez J., Sahu N., Handon R., Davidson T.S., Anderson S.M., Kirby M.R., August A., and Schwartzberg P.L.. 2009. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 31:587–597. 10.1016/j.immuni.2009.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins E., Al Shehri T., McAleer M.A., Conlon N., Feighery C., Lilic D., and Irvine A.D.. 2015. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J. Allergy Clin. Immunol. 135:551–553. 10.1016/j.jaci.2014.12.1867 [DOI] [PubMed] [Google Scholar]
- Hirahara K., Ghoreschi K., Yang X.P., Takahashi H., Laurence A., Vahedi G., Sciumè G., Hall A.O., Dupont C.D., Francisco L.M., et al. . 2012. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 36:1017–1030. 10.1016/j.immuni.2012.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. . 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 30:108–119. 10.1016/j.immuni.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Leung D.Y., Ambrosino D.M., Arbeit R.D., Newton J.L., and Geha R.S.. 1988. Impaired antibody responses in the hyperimmunoglobulin E syndrome. J. Allergy Clin. Immunol. 81:1082–1087. 10.1016/0091-6749(88)90873-1 [DOI] [PubMed] [Google Scholar]
- Ling Y., Cypowyj S., Aytekin C., Galicchio M., Camcioglu Y., Nepesov S., Ikinciogullari A., Dogu F., Belkadi A., Levy R., et al. . 2015. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J. Exp. Med. 212:619–631. 10.1084/jem.20141065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Okada S., Kong X.F., Kreins A.Y., Cypowyj S., Abhyankar A., Toubiana J., Itan Y., Audry M., Nitschke P., et al. . 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208:1635–1648. 10.1084/jem.20110958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J.J., Liu Y., Ma C.A., Yu X., O’Connell M.P., Lawrence M.G., Zhang Y., Karpe K., Zhao M., Siegel A.M., et al. . 2017. ERBIN deficiency links STAT3 and TGF-β pathway defects with atopy in humans. J. Exp. Med. 214:669–680. (published erratum appears in J. Exp. Med. 2017. http://dx.doi.org/10.1084/jem.2016143503082017c) 10.1084/jem.2016143503082017c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., and Cook M.C.. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557. 10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton A.C., Melrose J., Alajoki L., Privat S., Cho H., Brown N., Plavec A.M., Nguyen D., Johnston E.D., Yang J., et al. . 2013. Regulation of IL-17A production is distinct from IL-17F in a primary human cell co-culture model of T cell-mediated B cell activation. PLoS One. 8:e58966 10.1371/journal.pone.0058966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg A., Renner E.D., Rylaarsdam S., Reichenbach J., Schimke L.F., Marks A., Tcheurekdjian H., Hostoffer R., Brahmandam A., Torgerson T.R., et al. . 2012. Heterozygous signal transducer and activator of transcription 3 mutations in hyper-IgE syndrome result in altered B-cell maturation. J. Allergy Clin. Immunol. 129:559–562. 10.1016/j.jaci.2011.09.017 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. . 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 452:773–776. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mössner R., Diering N., Bader O., Forkel S., Overbeck T., Gross U., Grimbacher B., Schön M.P., and Buhl T.. 2016. Ruxolitinib induces interleukin 17 and ameliorates chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation. Clin. Infect. Dis. 62:951–953. 10.1093/cid/ciw020 [DOI] [PubMed] [Google Scholar]
- Oestreich K.J., Mohn S.E., and Weinmann A.S.. 2012. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 13:405–411. 10.1038/ni.2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Cypowyj S., Bustamante J., Wright J.F., Liu L., Lim H.K., Migaud M., Israel L., Chrabieh M., Audry M., et al. . 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 332:65–68. 10.1126/science.1200439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y., and Stark G.R.. 2004. Alternative activation of STAT1 and STAT3 in response to interferon-γ. J. Biol. Chem. 279:41679–41685. 10.1074/jbc.M406413200 [DOI] [PubMed] [Google Scholar]
- Renner E.D., Rylaarsdam S., Anover-Sombke S., Rack A.L., Reichenbach J., Carey J.C., Zhu Q., Jansson A.F., Barboza J., Schimke L.F., et al. . 2008. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced TH17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J. Allergy Clin. Immunol. 122:181–187. 10.1016/j.jaci.2008.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg N., Morbach H., Lawrence M.G., Kim S., Kang I., Holland S.M., Milner J.D., and Meffre E.. 2013. Gain-of-function STAT1 mutations are associated with PD-L1 overexpression and a defect in B-cell survival. J. Allergy Clin. Immunol. 131:1691–1693. 10.1016/j.jaci.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S.H., et al. . 2010. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 32:681–691. 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Siegel A.M., Heimall J., Freeman A.F., Hsu A.P., Brittain E., Brenchley J.M., Douek D.C., Fahle G.H., Cohen J.I., Holland S.M., and Milner J.D.. 2011. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 35:806–818. 10.1016/j.immuni.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis N.V., and Filler S.G.. 2012. Mouse model of oropharyngeal candidiasis. Nat. Protoc. 7:637–642. 10.1038/nprot.2012.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R., and Leonard W.J.. 2008. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26:57–79. 10.1146/annurev.immunol.26.021607.090316 [DOI] [PubMed] [Google Scholar]
- Steward-Tharp S.M., Laurence A., Kanno Y., Kotlyar A., Villarino A.V., Sciume G., Kuchen S., Resch W., Wohlfert E.A., Jiang K., et al. . 2014. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood. 123:2978–2987. 10.1182/blood-2013-09-523167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabellini G., Vairo D., Scomodon O., Tamassia N., Ferraro R.M., Patrizi O., Gasperini S., Soresina A., Giardino G., Pignata C., et al. . 2017. Impaired natural killer cell functions in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J. Allergy Clin. Immunol. 10.1016/j.jaci.2016.10.051 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichiyama K., Hashimoto M., Yoshida H., Takimoto T., Takaesu G., Torisu T., Hanada T., Yasukawa H., Fukuyama S., et al. . 2008. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-γ on STAT3 and Smads. J. Immunol. 180:3746–3756. 10.4049/jimmunol.180.6.3746 [DOI] [PubMed] [Google Scholar]
- Toubiana J., Okada S., Hiller J., Oleastro M., Lagos Gomez M., Aldave Becerra J.C., Ouachee-Chardin M., Fouyssac F., Girisha K.M., Etzioni A., et al. International STAT1 Gain-of-Function Study Group . 2016. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 127:3154–3164. 10.1182/blood-2015-11-679902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.M. 2014. The immune checkpoint inhibitors: Where are we now? Nat. Rev. Drug Discov. 13:883–884. 10.1038/nrd4476 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., et al. . 2008. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 28:29–39. 10.1016/j.immuni.2007.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G., et al. . 2011. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 12:247–254. 10.1038/ni.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., and Kubo M.. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7:454–465. 10.1038/nri2093 [DOI] [PubMed] [Google Scholar]
- Yu H., Pardoll D., and Jove R.. 2009. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer. 9:798–809. 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., van de Veerdonk F.L., Crossland K.L., Smeekens S.P., Chan C.M., Al Shehri T., Abinun M., Gennery A.R., Mann J., Lendrem D.W., et al. . 2015. Gain-of-function STAT1 mutations impair STAT3 activity in patients with chronic mucocutaneous candidiasis (CMC). Eur. J. Immunol. 45:2834–2846. 10.1002/eji.201445344 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.