Watanabe et al. report that, contrary to the prevailing paradigm, there are unique cellular requirements for B7 and CD40 expression in primary GC responses. B7 is required on DCs but not on B cells, whereas CD40 is required on B cells but not on DCs for generation of Tfh cells, GC B cells, and high-affinity class-switched antibody production.
Abstract
T cell–dependent germinal center (GC) responses require coordinated interactions of T cells with two antigen-presenting cell (APC) populations, B cells and dendritic cells (DCs), in the presence of B7- and CD40-dependent co-stimulatory pathways. Contrary to the prevailing paradigm, we found unique cellular requirements for B7 and CD40 expression in primary GC responses to vaccine immunization with protein antigen and adjuvant: B7 was required on DCs but was not required on B cells, whereas CD40 was required on B cells but not on DCs in the generation of antigen-specific follicular helper T cells, antigen-specific GC B cells, and high-affinity class-switched antibody production. There was, in fact, no requirement for coexpression of B7 and CD40 on the same cell in these responses. Our findings support a substantially revised model for co-stimulatory function in the primary GC response, with crucial and distinct contributions of B7- and CD40-dependent pathways expressed by different APC populations and with important implications for understanding how to optimize vaccine responses or limit autoimmunity.
Introduction
T helper cell (Th)–dependent (TD) antibody production is a critical aspect of the adaptive immune response to pathogens and other foreign antigens (Victora and Nussenzweig, 2012). In vivo TD antibody responses and the critical events of Ig class switching and somatic hypermutation (SHM) are dependent on the formation of germinal centers (GCs), which provide a highly specialized microenvironment for the interaction of T and B cells (Victora and Nussenzweig, 2012; Crotty, 2014; Vinuesa et al., 2016). Recent studies of GC biology have led to elegant models for the cross talk between follicular helper T cells (Tfh cells) and APCs in the formation of GCs; in the regulated cell trafficking that allows iterative Tfh cell–GC B cell interactions; and ultimately in functional outcomes including affinity maturation, T and B cell memory, negative selection of autoreactive B cells, and Ig class switch recombination (Victora and Nussenzweig, 2012; Crotty, 2014; Vinuesa et al., 2016). Several studies have visualized the dynamics of T cell–APC interactions in GC responses. Antigen-activated T and B cells first interact at the border of T and B cell zones (Pape et al., 2003; Kerfoot et al., 2011; Kitano et al., 2011). However, expression by antigen-activated T cells of Bcl6, an essential transcription factor for Tfh cell differentiation (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), precedes this T–B cell interaction (Kerfoot et al., 2011; Kitano et al., 2011), suggesting that APCs other than B cells, possibly DCs (Qi et al., 2008; Deenick et al., 2010; Choi et al., 2011; Goenka et al., 2011), are responsible for initiation of the Tfh cell differentiation program. Given the evidence for sequential interaction of T cells with DCs and B cells during the GC response (Pape et al., 2003; Qi et al., 2008; Deenick et al., 2010; Kerfoot et al., 2011; Kitano et al., 2011), it was of interest to compare the requirements for DC and B cell functions in these responses.
In addition to T cell recognition of peptide-MHCII (pMHCII) ligands shown to be critical in TD antibody responses (Singer and Hodes, 1983; Steinman et al., 1988; Cosgrove et al., 1991; Grusby et al., 1991; Shimoda et al., 2006; Deenick et al., 2010), GC formation and function are dependent on CD80/CD86 ligands (B7.1/B7.2)–CD28 receptor and CD154 ligand (CD40L)–CD40 receptor interactions. Disruption of either of these co-stimulatory pathways results in severe defects in GC formation and antigen-specific class-switched antibody production (Armitage et al., 1992; Kawabe et al., 1994; Han et al., 1995; Ferguson et al., 1996; Borriello et al., 1997). Whereas CD28 and CD40L are expressed on T cells, B7 and CD40 are expressed on multiple cell types, including DCs and B cells. Thus, the requirement for B7–CD28 and CD40L–CD40 interactions could reflect requirements for both pathways in T–DC and T–B cell interactions, as presented in currently proposed models of the GC response (Nutt and Tarlinton, 2011; Victora and Nussenzweig, 2012; Zotos and Tarlinton, 2012; Crotty, 2014; Vinuesa et al., 2016). It has in fact been posited that signaling interactions between B7 and CD40 expressed by the same B cell or DC are important for the function of these populations (Kapsenberg, 2003; Nutt and Tarlinton, 2011; Zotos and Tarlinton, 2012; Bakdash et al., 2013). Alternatively, these co-stimulatory pathways might have distinct roles restricted to either T–DC or T–B cell interactions, analogous to the SAP–SLAM pathway that is specifically required in stable T–B cell conjugation but dispensable for T–DC conjugation for GC responses (Qi et al., 2008; Cannons et al., 2010). However, elucidation of the cellular and molecular interactions involved in the co-stimulatory signaling supporting GC responses, including Tfh cell and GC B cell development, has been limited, in part because of the lack of models for conditional expression of the critical B7 and CD40 molecules.
In the work reported here, we have identified spatially and temporally distinct patterns of T cell–APC interactions and have characterized the MHC dependency and co-stimulatory requirements for the primary GC response to vaccine challenge. We have generated conditional KOs (cKOs) for both B7 and CD40 and have used these, together with conditional MHCII KOs and BM chimeric strategies, to analyze the pathways involved in GC and antibody responses to antigen challenge. These experiments confirmed the expected requirement for MHCII-dependent interactions of T cells with both DCs and B cells in the generation of antigen-specific Tfh cells, antigen-specific GC B cells, and class-switched antibody responses to primary immunization with protein antigen and adjuvant. Our findings reveal, however, that the requirements for B7 and CD40 expression on DCs and B cells are distinct. B7 expression is needed on DCs but not on B cells, whereas cell-autonomous expression of CD40 is critical on B cells but not DCs, and there is in fact no requirement for coexpression of B7 and CD40 on the same cell in primary GC responses after vaccination. We have thus identified crucial and distinct contributions from two co-stimulatory pathways operating during T cell–APC interactions in the GC response.
Results
MHCII expression on both B cells and DCs is essential for primary GC responses
Given the evidence for interactions of T cells with DCs and B cells during the GC response (Pape et al., 2003; Qi et al., 2008; Deenick et al., 2010; Kerfoot et al., 2011; Kitano et al., 2011), we examined the location and association of antigen-specific T cells with both of these APCs using histocytometry, a powerful quantitative imaging technique (Gerner et al., 2012, 2015; Liu et al., 2015; Radtke et al., 2015). At multiple time points after OVA-specific OT-II T cell transfer and 4-hydroxy-3-nitrophenylacetyl (NP)–OVA/Alum immunization, histological sections of spleens and LNs were stained with antibodies specific for OT-II T cells, CD4+ T cells, B cells, DCs, and the Tfh cell/GC B cell marker Bcl6 (Fig. S1). We quantified T cell localization and T–APC interactions in the spleen and LN. At day 2, after immunization, antigen-specific OT-II T cells were found predominantly in T cell zones; by day 4, a significant proportion of OT-II T cells were also present in B cell follicles; and by days 7–8, the number of OT-II T cells in B cell follicles and GCs had increased (Fig. S1, A, C, and E). Intimate contacts between T cells and DCs were observed at relatively early time points in the GC response, whereas T–B cell interactions were maximal at later time points (Fig. S1, B and F).
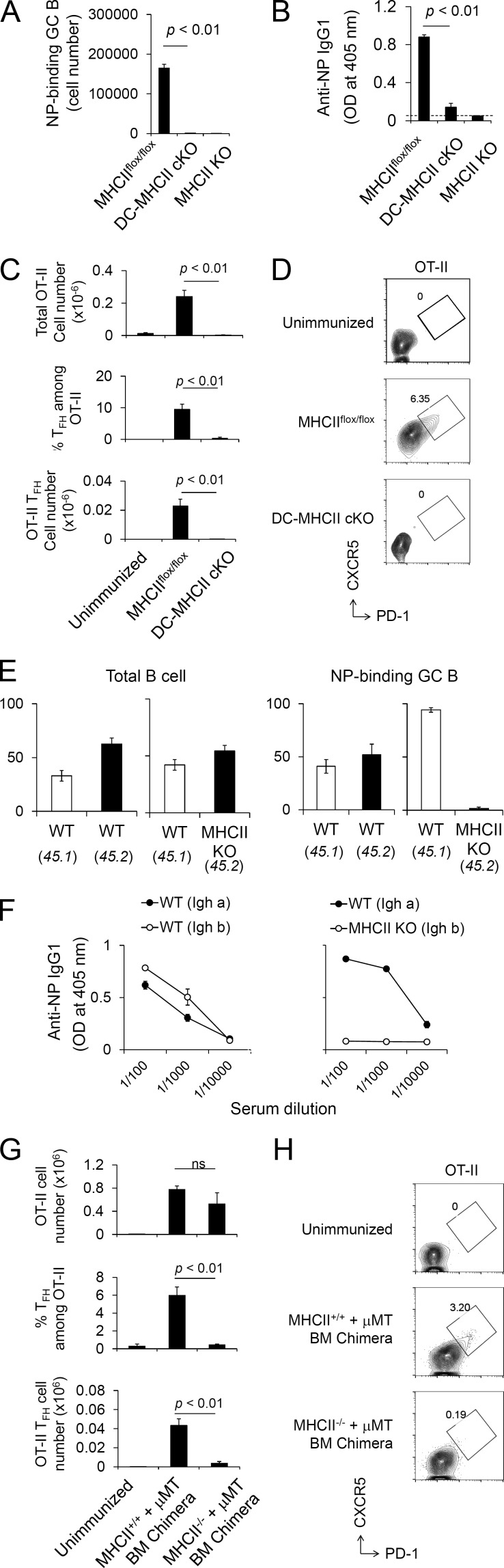
We further assessed the requirements for these T–APC interactions in GC responses by analyzing requirements for MHCII expression in DCs or B cells. MHCII deletion from DCs, achieved by crossing MHCII (I-Ab)flox/flox and CD11c-Cre transgenic (Tg) mice, resulted in an absence of antigen-specific GC B cell development and IgG1 production (Fig. 1, A and B; and Fig. S2 A). We also assessed the cell type–specific requirement for MHCII in the generation of CXCR5high PD-1high GC Tfh cells (Haynes et al., 2007; Choi et al., 2011; Pepper et al., 2011; Tubo et al., 2013) by transfer of OVA-specific OT-II T cells to recipient mice lacking MHCII on either DCs or B cells (Fig. S2 B). MHCII on DCs in recipient mice was critical for antigen-specific GC Tfh cell development as well as clonal expansion (Fig. 1, C and D; and Fig. S2 C). Deletion of MHCII on B cells in MHCII (I-Ab)flox/flox mice by crossing to CD19-Cre mice was previously reported to leave a residual small number of MHCII+ B cells that escape MHCII deletion and selectively expand to generate GC B cells (Shimoda et al., 2006). We therefore used an alternative approach to analyze the role of MHCII on B cells for the GC response. Mixed BM chimeras that were reconstituted with MHCII WT BM + MHCII KO BM (Fig. S2 D) generated antigen-specific GC B cells and anti–NP IgG1 antibody derived exclusively from WT donors, demonstrating a cell-autonomous requirement for MHCII on B cells in these responses (Fig. 1, E and F). BM chimeras were also constructed by a mixture of B cell KO (μ mutation [µMT]) + MHCII KO BM (Fig. S2 E), resulting in a selective absence of MHCII on all B cells (MHCII−/− + µMT). Antigen-specific GC Tfh cell development was essentially absent after OT-II T cell transfer to these chimeras despite intact expansion of overall OT-II numbers (Fig. 1, G and H). Together with the histocytometry findings depicted, these results demonstrated that MHCII-dependent T–DC and T–B cell interactions are spatially and temporally regulated and essential for the GC response.
Figure 1.
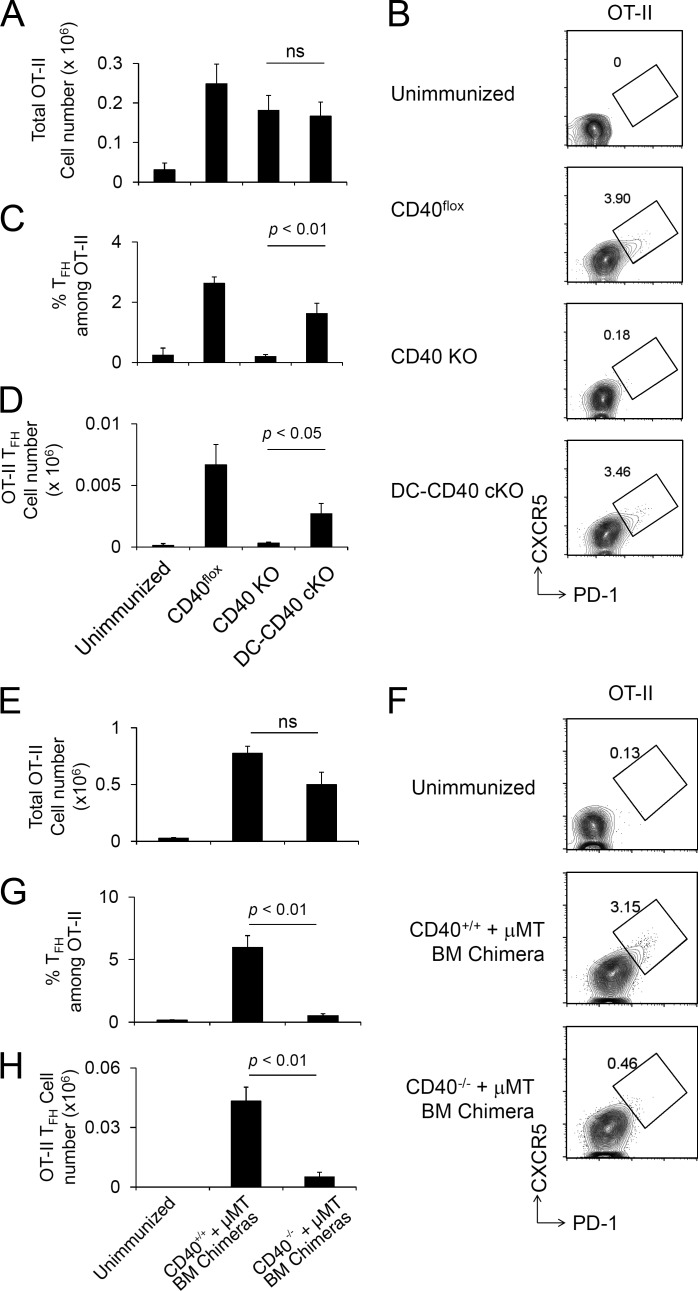
Nonredundant requirements for MHCII on DCs and B cells in GC response. (A) DC-specific MHCII cKO (MHCIIflox/flox × CD11c-Cre, termed DC-MHCII cKO) mice were immunized with NP-KLH/Alum. At day 7 after immunization, NP-specific GC B cell numbers were analyzed in the spleen. Each strain, n = 3. Data are representative of three independent experiments. (B) DC-MHCII cKO mice were immunized with NP-KLH/Alum. 3 wk after immunization, anti-NP IgG1 titers were analyzed by ELISA. Dashed line indicates background OD value in empty wells. MHCIIflox/flox, n = 4; DC-MHCII cKO, n = 4; and MHCII KO, n = 2. Data are representative of two independent experiments. (C and D) OT-II T cells (CD45.1) were transferred to the indicated recipient mice (CD45.2) followed by NP-OVA/Alum immunization, and transferred OT-II cells were analyzed at day 7. (C) Recovered total OT-II cell number (top), frequency of Tfh cells among OT-II (middle), and total OT-II Tfh cell number (bottom) from the spleen are shown. (D) Representative flow cytometry profile of splenic OT-II cells (CD4+ B220− CD45.1+ Vα2+) analyzed for the Tfh CXCR5high PD-1high phenotype. Unimmunized, n = 4; MHCIIflox/flox, n = 5; and DC-MHCII cKO, n = 6. Data shown are the combined result of two independent experiments. (E) Cell-intrinsic requirement of MHCII on B cells for antigen-specific GC development. MHCII WT (CD45.1) + MHCII KO (CD45.2) mixed BM chimeric mice and control MHCII WT (CD45.1) + MHCII WT (CD45.2) BM chimeric mice were prepared and immunized with NP-KLH. At day 7 after immunization, chimerism of total B cells (B220+; left) and NP-binding GC B cells from the spleen (B220+ GL7+ Fas+; right) was analyzed. Each group, n = 4. Data are representative of two independent experiments. (F) Cell-intrinsic requirement of MHCII on B cells for antigen-specific IgG1 production. MHCII WT (Igha) and MHCII KO (Ighb) mixed BM chimera mice and control MHCII WT (Igha) and MHCII WT (Ighb) BM chimera mice were prepared and immunized with NP-KLH/Alum. At 3 wk after immunization, anti–NP-IgG1 titers were analyzed by ELISA. Each group, n = 3. Data are representative of two independent experiments. (G and H) BM chimeras were reconstituted by a mixture of B cell KO (μMT) + MHCII KO BM (MHCII−/−+ μMT; Fig. S2 E), resulting in the selective absence of MHCII on all B cells. Control chimeras were reconstituted by a mixture of B cell KO (μMT) + MHCII WT BM (MHCII+/+ + µMT) BM. OT-II T cells (CD45.1) were transferred to recipient BM chimera mice (CD45.2) followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. (G) Recovered total OT-II cell number (top), frequencies of OT-II Tfh cells (middle), and number of total OT-II Tfh cells were analyzed (bottom). ns, not significant. (H) Representative flow cytometry plot of OT-II cells (CD4+ B220− CD45.1+ Vα2+) analyzed for the Tfh CXCR5high PD-1high phenotype. Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are unimmunized, n = 4; (MHCII+/+ + µMT) BM chimera, n = 3; and (MHCII−/− + μMT) BM chimera, n = 5. Statistical significance was evaluated by Student’s t test. All error bars represent mean ± SEM.
Generation of B7 cKO mice
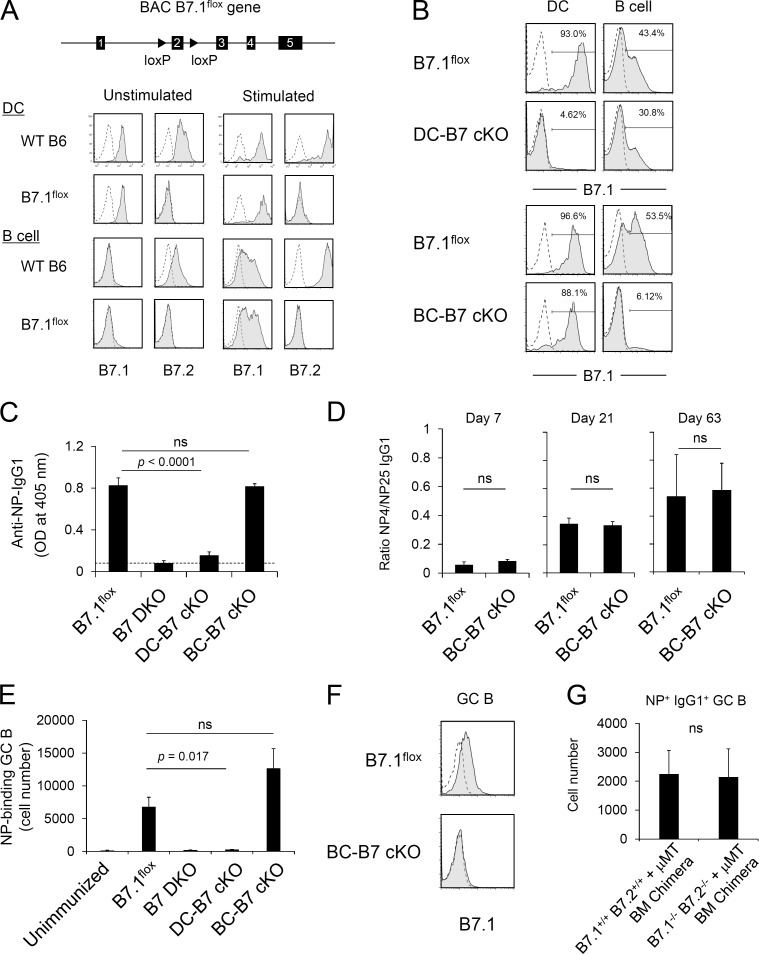
In addition to requirements for MHCII recognition in GC responses, the absence of either B7–CD28 or CD40L–CD40 co-stimulatory interactions resulted in profound defects in GC formation and antigen-specific class-switched antibody response to TD antigen immunization (Cosgrove et al., 1991; Grusby et al., 1991; Kawabe et al., 1994; Han et al., 1995; Borriello et al., 1997). Before immunization, B7.1 and B7.2 were expressed at substantially higher levels on DCs than on B cells. After immunization, B7.1 and B7.2 up-regulation was observed on GC B cells compared with non-GC follicular B cells (Fig. S3 A). In contrast, CD40 expression was slightly higher on B cells than on DCs throughout the course of GC response (Fig. S3 A). Because MHCII-dependent T cell interactions with both DCs and B cells were required for antigen-specific Tfh cell, GC B cell, and IgG1 responses, it was of interest to determine the requirements for co-stimulatory interactions of T cells with both DCs and B cells. The analysis of cell-specific CD28 co-stimulation by members of the B7 family has been constrained by the absence of models for conditional expression of the B7 ligands. Generation of conditional B7-deficient mice is made difficult by the fact that B7.1 (CD80) and B7.2 (CD86) are, to a substantial degree, functionally redundant and are encoded by closely linked genes (Borriello et al., 1997). To enable characterization of cell type–specific roles of B7, we generated B7 cKO mice by engineering a B7.1flox BAC Tg and breeding this Tg to an endogenous B7.1 and B7.2 double KO (B7.1−/−and B7.2−/−; referred to as B7 DKO) background. These mice (B7.1flox BAC Tg and B7 DKO) do not express endogenous B7.1 or B7.2 and therefore express only the B7.1flox BAC Tg (Fig. 2 A). We refer to these mice hereafter as B7.1flox. B7.1 expression by the B7.1flox BAC Tg, both ex vivo and after in vitro stimulation, showed expression patterns similar to that of endogenous B7.1 on DCs and B cells (Fig. 2 A), as well as on other cell types, including macrophages and thymic epithelial cells (Fig. S3 B). Consistent with the reported redundant roles of B7.1 and B7.2 in antibody responses to protein and adjuvant immunization (Borriello et al., 1997), reconstitution of B7 DKO mice with the B7.1flox BAC Tg resulted in IgG1 and IgG2a antibody responses to TNP-KLH immunization comparable with those of WT B6 mice (Fig. S3 C). The B7.1flox mouse was crossed to CD11c-Cre– and CD19-Cre–expressing strains (all on a B7 DKO background) to generate DC-specific (DC-B7 cKO) and B cell–specific (BC-B7 cKO) B7 KO mice, respectively. In DC-B7 cKO mice, B7.1 was completely deleted from DCs with partial but substantially less B7.1 deletion from B cells, whereas in BC-B7 cKO mice, B7.1 expression was deleted from B cells but not DCs (Fig. 2 B). These conditional B7 KO mice thus enable analysis of cell type–specific B7 function.
Figure 2.
B7 on DCs but not B cells is critical for TD antigen–specific IgG1 production. (A) Generation of B7.1flox mice. LoxP sites were inserted to flox exon 2 of the B7.1 gene on the BAC. B7.1flox BAC Tg mice were backcrossed to B7.1−/− B7.2−/− DKO mice to eliminate endogenous B7.1 and B7.2 expression. B7.1flox Tg-B7 DKO mice expressed B7.1 on splenic DCs at a level similar to that of WT B6 mice before and after LPS stimulation in vitro. Data are representative of at least three independent experiments. (B) B7.1 expression on DCs and B cells of DC-specific and B cell–specific B7 cKO mice. Splenocytes were stimulated with ConA/LPS/IL-2 for 24 h, and B7.1 expression on DCs (CD11chigh) and B cells (B220+) was analyzed. Shaded histograms indicate anti-B7.1 antibody staining of B7 cKO strains, and dashed lines indicate anti-B7.1 staining of B7.1/B7.2 DKO mice. Numbers indicate percentages of B7.1+ cells. Data are representative of three independent experiments. (C) Antigen-specific IgG1 production by B7 cKO strains. B7 cKO mice were immunized with NP-KLH/Alum. At 3 wk after immunization, serum anti-NP IgG1 titer was analyzed by ELISA. Dashed line indicates background OD value in empty wells. Each strain, n = 3. Data are representative of three independent experiments. ns, not significant. (D) Antibody affinity maturation was determined by ratio of high-affinity NP-specific IgG1 (measured by binding to NP4-BSA) to total NP-specific IgG1 (measured by binding to NP25-BSA) in serum at 7, 21, and 63 d after immunization. B7.1flox, n = 5; BC-B7cKO, n = 6 for day 7 and day 21 and n = 3 at day 63. Data are representative of three independent experiments. (E) NP-specific GC B cell development in B7 cKO strains. B7 cKO strains were immunized with NP-KLH/Alum. 1 wk after immunization, NP-binding GC B cells (B220+ GL7+ Fas+ NP-PE+) in the spleen were determined by flow cytometry. Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are B7.1flox, n = 8; B7 DKO, n = 8; DC-B7 cKO, n = 6; and BC-B7 cKO, n = 8. (F) 1 wk after NP-KLH/Alum immunization, B7.1 expression on GC B cells was analyzed. Shaded histograms indicate anti-B7.1 staining, and dashed lines indicate isotype control staining. Data are representative of three independent experiments. (G) NP-specific GC B cell development in B cell–specific B7-deficient BM chimeras. BM chimeras were made that were completely and specifically deficient in expression of B7 on B cells by reconstitution of B cell KO hosts with a mixture of B7 DKO BM and B cell KO (μMT) BM (B7.1−/− B7.2−/− + μMT). Control chimeras were reconstituted with a mixture of B7 WT BM and B cell KO (μMT) BM (B7.1+/+ B7.2+/+ + μMT). Chimeras were immunized with NP-KLH/Alum. 1 wk after immunization, NP-binding GC B cells (B220+ GL7+ CD38dull NP-PE+ IgG1+) in the spleen were determined by flow cytometry. Each group, n = 3. Data are representative of two independent experiments. Statistical significance was determined by Student’s t test for single comparison or one-way ANOVA followed by Dunnett’s method for multiple comparisons. All error bars represent the mean ± SEM.
B7 expression on DCs but not B cells is critical for an antigen-specific GC B cell response and IgG1 production
To analyze cell type–specific requirements for B7 expression in GC and IgG1 responses, mice with selective deletion of B7 in B cells or DCs were immunized with NP-KLH/Alum and antigen-specific GC B cells, and IgG1 production was evaluated. NP-specific IgG1 production was strongly dependent on B7 as indicated by the lack of IgG1 production in B7 DKO mice (Fig. 2 C). In the absence of Cre, B7.1flox mice responded with robust IgG1 production (Fig. 2 C). Interestingly, IgG1 production was nearly absent in DC-B7 cKO mice (Fig. 2 C), demonstrating a strict requirement for B7 expressed on DCs in this response. In contrast, NP-specific IgG1 levels measured 21 and 63 d after immunization were undiminished in BC-B7 cKO mice (Fig. 2 C and Fig. S3 D), indicating that B7 expression on B cells was not required for this response. Notably, affinity maturation of NP-specific IgG1, regarded as an outcome of iterative Tfh–B cell interaction during the GC response, was equivalent in BC-B7 cKO and B7.1flox mice through 63 d after immunization (Fig. 2 D). CD11c-Cre or CD19-Cre had no effect on NP-specific IgG1 production in the absence of floxed B7, excluding off-target effects of Cre expression (Fig. S3 E). We also analyzed the requirement for B7 expression in the generation of antigen-specific GC B cells. Consistent with the observed effects on serum IgG1 responses, NP-specific GC B cell development was decreased in DC-B7 cKO mice to the level seen in the complete absence of B7 (B7 DKO), whereas the response of BC-B7 cKO mice was not diminished from that seen in Cre-negative mice (Fig. 2 E). The NP-specific GC B cell response of BC-B7 cKO mice was similarly undiminished at low-dose antigen (10 µg NP-KLH/Alum) immunization conditions (Fig. S3 F). Light zone and dark zone GC B cell composition (Victora et al., 2010) was also similar between BC-B7 cKO and undeleted control mice (Fig. S3 G). The antibody response observed in the BC-B7 cKO mice was not mediated by escapee B7.1+ B cells that had failed to undergo Cre-mediated deletion because B7.1 was not detected on GC B cells of the BC-B7 cKO mice (Fig. 2 F). As shown in Fig. 2 A, the B7 cKO BAC Tg mice generated in these experiments were constructed by introducing a floxed B7.1 BAC Tg to an endogenous B7.1 and B7.2 DKO background. Comparison of Cre-positive and Cre-negative cKO mice thus assessed the functional requirement for B7.1 in the absence of B7.2. To further test the requirement for B7 on B cells for GC B cell development, BM chimeras were made that expressed endogenous B7.1 and B7.2 on all cell types or that were completely deficient in the expression of both B7.1 and B7.2 only on B cells. B cell KO hosts were reconstituted with a mixture of B7 (B7.1 and B7.2) WT BM and B cell KO (µMT) BM (B7.1+/+ B7.2+/+ + µMT) or with a mixture of B7 DKO BM and B cell KO (µMT) BM (B7.1−/− B7.2−/− + µMT; Fig. S3 H). The BM chimeras in which all B cells were B7 DKO (B7.1−/− B7.2−/− + µMT) gave numbers of NP-specific IgG1+ GC B cells comparable with those in control mixed BM chimeras (B7.1+/+ B7.2+/+ + µMT), in which all B cells expressed both B7.1 and B7.2 (Fig. 2 G). These results demonstrate that B7 expression by DCs, but not B cells, is critical for an antigen-specific GC B cell response and high-affinity IgG1 production.
B7 expression by DCs, but not B cells, is important for antigen-specific T cell proliferation and Tfh cell development
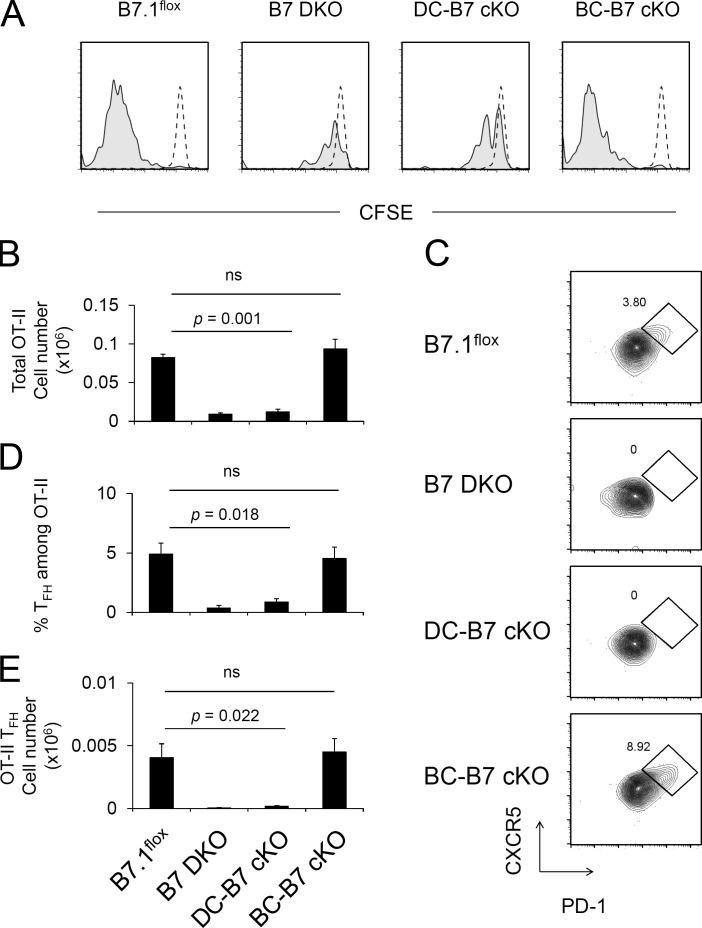
To assess the B7 dependence of antigen-specific T cell activation and Tfh cell development, OT-II T cells (CD45.1) were transferred to control and B7 cKO mice (CD45.2), and the recipient mice were immunized with NP-OVA/Alum. To analyze the expansion of antigen-specific T cells, OT-II T cells were labeled with CFSE, and dye dilution was assayed at day 10 after NP-OVA/Alum immunization. The transferred OT-II cells underwent robust antigen-dependent cell division and expansion in the B7.1flox and BC-B7 cKO host mice (Fig. 3, A and B). In contrast, OT-II T cell expansion did not occur in B7 DKO or DC-B7 cKO host mice (Fig. 3, A and B). We next assessed the generation of CXCR5high PD-1high GC Tfh cells within the OT-II population. GC Tfh cells were similar in number in B7.1flox and BC-B7 cKOs but were severely reduced in B7 DKO and DC-B7 cKO recipient mice (Fig. 3, C–E). These results indicated that B7 expression by DCs was critical for antigen-specific CD4+ T cell expansion and GC Tfh cell development, but B7 on B cells was dispensable for these T cell responses.
Figure 3.
Antigen-specific T cell activation and Tfh cell development require B7 on DCs but not B cells. (A) B7 cKO strains (CD45.2) were adoptively transferred with CFSE-labeled OT-II T cells (CD45.1) and immunized with NP-OVA/Alum 1 d later. Spleens were harvested on day 10, and CFSE dilution was analyzed by flow cytometry. Dashed-line and shaded histograms indicate transferred OT-II T cells in unimmunized and immunized mice, respectively. Data are representative of three independent experiments. (B–E) OT-II cell transfer was done as described in A but without CFSE staining. Recovered total OT-II cell number (B), FACS analysis of Tfh (CXCR5high PD-1high) OT-II cells (C), frequency of Tfh cells among OT-II (D), and total OT-II Tfh cell number were analyzed at days 8–10 after immunization (E). Data presented are the combined result of four independent experiments (mean ± SEM). The total numbers of mice in the four combined experiments are B7.1flox, n = 8; B7 DKO, n = 9; DC-B7 cKO, n = 8; and BC-B7 cKO, n = 11. Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison.
Generation of CD40 cKO mice
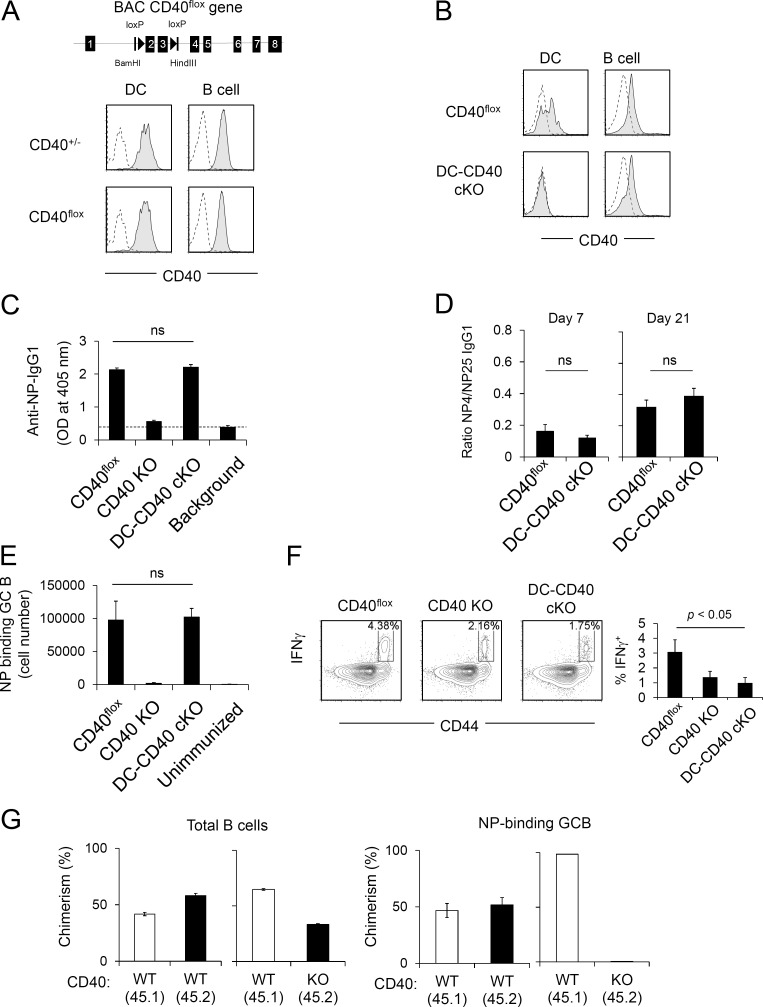
We next examined the cell type–specific requirements for CD40 in GC responses. We and others have previously reported that CD40 on B cells is required for TD antibody responses and GC formation, using BM chimera strategies (Lee et al., 2003; Lumsden et al., 2003), but the absence of conditional CD40-deficient mice prevented evaluation of CD40 function on other cell types, such as DCs, which express CD40 during the course of GC response (Fig. S3 A) or in nonchimeric experimental systems. We therefore generated CD40 cKO mice using a strategy similar to that described in Fig. 2 A for B7 cKO mice (Fig. 4 A). In CD40flox BAC Tg mice, CD40 expression was derived from only the CD40flox BAC Tg because the CD40 KO background of these mice eliminated expression of endogenous CD40. We refer to these mice (CD40flox BAC Tg and CD40−/−) as CD40flox mice hereafter. CD40 expression levels on activated B cells and DCs in CD40flox mice were equivalent to those in heterozygous CD40+/− mice expressing a single copy of the endogenous CD40 gene (Fig. 4 A). CD40flox mice were crossed to CD11c-Cre Tg mice (on an endogenous CD40 KO background) to generate DC-specific CD40 cKO (DC-CD40 cKO) mice. In these mice, CD40 cell surface expression was deleted specifically on DCs but not B cells (Fig. 4 B).
Figure 4.
CD40 on B cells but not DCs is required for TD antigen–specific IgG production. (A) Generation of CD40flox mice. Exons 2 and 3 of the CD40 gene on BAC were floxed by loxP sites (top). CD40flox BAC Tg mice were backcrossed to CD40 KO mice to eliminate endogenous CD40 expression. CD40flox BAC Tg expression on LPS-stimulated DCs and B cells is shown. (B) CD40 expression on LPS-stimulated splenic DCs and B cells of CD40flox, CD40flox × CD11c-Cre Tg (DC-CD40 cKO), and CD40 KO mice. Splenocytes were stimulated with LPS for 48 h, and CD40 expression on B cells (B220+) and DCs (CD11c+) was analyzed by flow cytometry. Filled histograms show anti-CD40 antibody staining of CD40flox or DC-CD40 cKO DCs and B cells. Dashed lines show anti-CD40 antibody staining of CD40 KO. (A and B) Data are representative of four independent experiments. (C) Antigen-specific IgG1 production of DC-CD40 cKO mice. Mice were immunized with NP-KLH/Alum, and serum was collected after 3 wk. Serum titer of anti-NP IgG1 was determined by ELISA. Dashed line indicates background OD value in empty wells. Data are combined results of four independent experiments. The total numbers of mice in the four combined experiments are CD40flox, n = 8; CD40 KO, n = 7; and DC-CD40 cKO, n = 6. ns, not significant. (D) Antibody affinity maturation was determined by the ratio of high-affinity NP-specific IgG1 to total NP-specific IgG1 in serum at 7 and 21 d after immunization. The total numbers of mice in the three combined experiments are CD40flox, n = 8; and DC-CD40 cKO, n = 6. (E) Mice were immunized with NP-KLH/Alum. At day 8 after immunization, the absolute number of NP-specific GC B cells (B220+ GL7+ CD38dull NP-PE+) in the spleen was determined by flow cytometry. (D and E) Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are CD40flox, n = 4; CD40 KO, n = 4; DC-CD40 cKO, n = 5; and unimmunized, n = 3. (F) Ex vivo IFN-γ production of purified splenic CD4+ T cells after stimulation with PMA and ionomycin for 2 h. Representative FACS plots are shown. The graph is a combined result of three independent experiments. The total numbers of mice in the three combined experiments are n = 3 for each strain. (G) WT (CD45.1) + CD40 KO (CD45.2) mixed BM chimera mice were immunized with NP-KLH/Alum, and NP-specific GC B cells were analyzed 1 wk later. Each group, n = 4. Data are representative of two independent experiments. Statistical significance was determined by Student’s t test. All error bars represent means ± SEM.
CD40 on B cells, but not on DCs, is required for an antigen-specific GC B cell response and IgG1 production
To analyze DC-specific roles of CD40 for IgG1 responses and GC B cell induction, mice were immunized with NP-KLH/Alum. Equivalent NP-specific IgG1 production was observed in CD40flox and DC-CD40 cKO mice, whereas this response was completely abrogated in CD40 KO mice (Fig. 4 C). NP-specific IgG1 produced in DC-CD40 cKO mice showed similar affinity maturation to that in CD40flox mice (Fig. 4 D). Likewise, NP-specific GC B cell numbers were not significantly different between DC-CD40 cKO and CD40flox mice, whereas NP-specific GC B cells were essentially absent in CD40 KO mice (Fig. 4 E). Composition of light zone/dark zone GC B cells (Victora et al., 2010) was also similar between DC-CD40 cKO and CD40flox mice (Fig. S4 A). These results indicate that CD40 expression on DCs is not required for efficient GC B cell and antibody responses. To confirm the functional deletion of CD40 on DCs, we examined the in vivo induction of IFN-γ–producing Th1 CD4+ T cells, previously shown to be a sensitive measure of CD40-dependent DC function (Schulz et al., 2000; Fujii et al., 2004). We observed that IFN-γ–producing CD4+ T cells were decreased in DC-CD40 cKO mice to a level similar to that of CD40 KO mice (Fig. 4 F), confirming the effectiveness of CD40 deletion from DCs.
We similarly tested the requirement for CD40 on B cells by generating B cell–specific CD40-deficient (BC-CD40 cKO) mice through crosses of CD40flox to the CD19-Cre strain. BC-CD40 cKO mice generated NP-specific IgG1 and NP-binding GC B responses similar to those of Cre-negative CD40flox mice (Fig. S4, B and C). This response was mediated by expansion of an initially small population of CD40+ escapee B cells that had failed to undergo Cre-mediated deletion and had selectively expanded and differentiated to GC B cells in BC-CD40 cKO mice (Fig. S4, C and D). This strong selective pressure suggested a critical role for CD40 on B cells in GC B cell development. The cell-intrinsic role of CD40 on B cells for antigen-specific GC B development was further analyzed using a mixed BM chimera strategy (Fig. S4 E). CD40 WT + CD40 KO mixed chimeras had similar proportions of peripheral B cells derived from CD40 WT and CD40 KO BM (Fig. 4 G, left). In contrast, antigen-specific GC B cells were essentially all of CD40 WT origin (Fig. 4 G, right). Together, these results demonstrate that CD40 expression on B cells is critical for GC B development and IgG1 production, whereas CD40 expression by DCs is not required for these same responses.
The role of CD40 on DCs and B cells for antigen-specific T cell proliferation and Tfh cell development
To determine the cell type–specific role of CD40 for in vivo antigen-dependent T cell proliferation and Tfh cell differentiation, OT-II T cells (CD45.1) were transferred to recipients (CD45.2), which differed in their expression of CD40, followed by NP-OVA/Alum immunization. A week after immunization, there was no significant difference in the number of recovered OT-II cells in CD40flox, CD40 KO, and DC-CD40 cKO mice, indicating the absence of a requirement for CD40 in overall OT-II T cell expansion in response to antigen (Fig. 5 A and Fig. S4 F). In marked contrast, the percentage of GC Tfh cells among OT-II cells (Fig. 5, B and C) and the absolute number of the OT-II GC Tfh cells were drastically decreased in CD40 KO mice (Fig. 5 D), whereas those in DC-CD40 cKO were significantly greater than those in CD40 KO mice and intermediate between the numbers in CD40flox and CD40 KO mice (Fig. 5, B–D). These results indicate that other non-DC CD40–expressing APCs are critical for inducing and maintaining a GC Tfh cell population that can support GC B cell responses and antigen-specific IgG1 production (Fig. 4, C–E). Because CD40+ escapee B cells in BC-CD40 cKO mice expanded to mediate normal GC B cell development and IgG1 production (Fig. S4, B–D), we were unable to test the contribution of CD40 signaling in B cells for Tfh cell development with this experimental design (Fig. S4, G–I). We therefore used an alternative approach to generate chimeric mice in which CD40 was selectively absent on all B cells and in which the role of CD40 on B cells could be tested in the generation of Tfh cells. A mixture of BM from B cell KO (μMT) and CD40 KO donors was used to reconstitute irradiated B cell KO host mice, generating chimeras (CD40−/− + μMT) in which all B cells were constitutively CD40 deficient (Fig. S4 J). OT-II T cells were transferred to these chimeric mice, and Tfh cell development was evaluated after NP-OVA/Alum immunization. Transferred OT-II cells expanded to an equivalent extent in the presence or absence of CD40 expression on B cells (Fig. 5 E). In contrast, GC Tfh cell phenotype OT-II cells were observed only in control chimeras (CD40+/+ + μMT) containing CD40-expressing B cells, but not in B cell–specific CD40 KO (CD40−/− + µMT) chimeras (Fig. 5, F–H), indicating that CD40 on B cells was critical for Tfh cell development.
Figure 5.
Antigen-specific T cell activation and Tfh cell development require CD40 on B cells but not DCs. (A–D) Naive OT-II CD4+ T cells were transferred to CD40 cKO mice followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. Recovered total OT-II cell number (A), FACS plot of OT-II (CD4+ B220− CD45.1+ Vα2+) cells analyzed for Tfh CXCR5high PD-1high phenotype (B), frequency of Tfh cells among OT-II (C), and total OT-II Tfh cell number were determined (D). Data are combined from three independent experiments. The total numbers of mice in the three combined experiments are CD40flox, n = 8; CD40 KO, n = 8; and DC-CD40 cKO, n = 11. ns, not significant. (E–H) A mixture of BM from B cell KO and CD40 KO donors was used to reconstitute irradiated B cell KO host mice, generating chimeras in which all B cells were constitutively CD40 deficient (CD40−/− + µMT). Control chimeras received a mixture of BM from B cell KO and WT donors (CD40+/+ + µMT). OT-II T cells were transferred to recipient BM chimera mice (CD45.2) followed by NP-OVA/Alum immunization 1 d later, and splenic OT-II cells were analyzed at day 8 after immunization. Recovered OT-II cell number (E), FACS plot of OT-II (CD4+ CD45.1+ Vα2+) cells analyzed for the Tfh CXCR5highPD-1high phenotype (F), frequency of OT-II Tfh cells among total CD4+ T cells (G), and the number of Tfh phenotype OT-II cells were analyzed (H). Data presented are the combined result of three independent experiments. The total numbers of mice in the three combined experiments are unimmunized, n = 3; (CD40+/+ + µMT) BM chimera, n = 3; and (CD40−/− + μMT) BM chimera, n = 5. Statistical significance was determined by Student’s t test. All error bars represent means ± SEM.
The demonstration of a cell-autonomous requirement for CD40 on B cells during GC B cell responses (Fig. 4 G) is consistent with previous demonstrations of direct signaling through CD40 on B cells (Grewal and Flavell, 1998; Quezada et al., 2004). In contrast, it is not clear whether signaling through CD40L on T cells plays a functional role in T cell responses. We therefore tested whether there is a cell-autonomous requirement for CD40L expression on T cells for Tfh cell generation. A mixture of CD40L WT (CD45.1) and CD40L KO (CD45.1/CD45.2) OT-II cells was transferred to WT B6 (CD45.2) host mice and followed by NP-OVA/Alum immunization. CD40L WT and CD40L KO OT-II cells expanded equivalently and generated similar numbers of GC Tfh cells (Fig. S4, K–M). Therefore, cell-autonomous expression of CD40L on T cells is not necessary for T cell expansion and Tfh cell differentiation.
CD40 and B7 on distinct cell populations cooperate to support the GC response
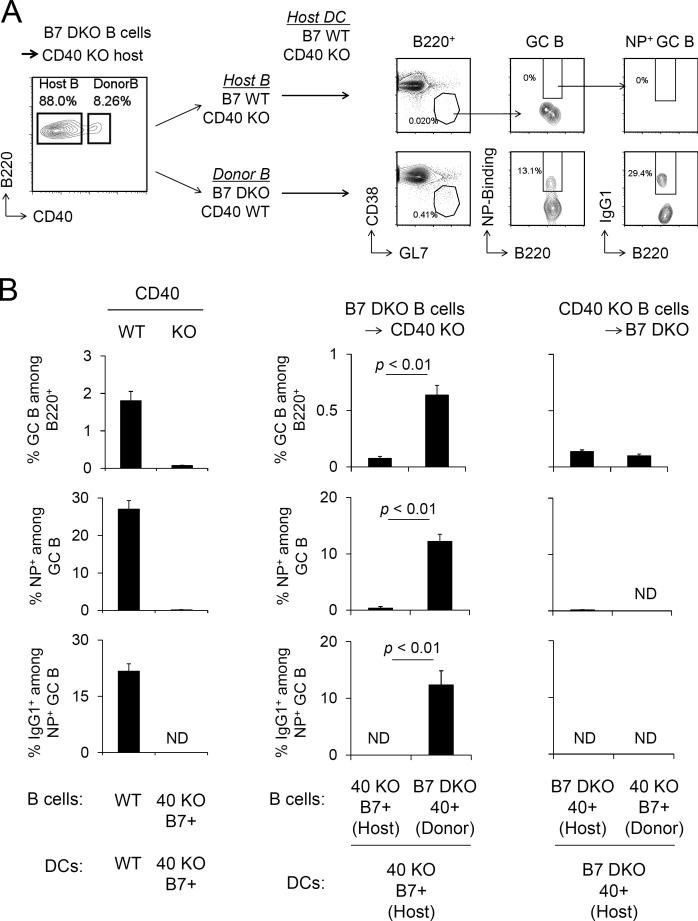
Our results indicate that antigen-specific GC B cell responses require both B7 on DCs and CD40 on B cells, but neither CD40 on DCs nor B7 on B cells is necessary. To test directly whether GC responses could be induced in the absence of both CD40 on DCs and B7 on B cells, B7 DKO (B7.1−/−, B7.2−/−, CD40+/+) B cells were adoptively transferred to CD40 KO (B7.1+/+, B7.2+/+, CD40−/−) recipients, which were then immunized with NP-OVA/Alum. Under these experimental conditions, 8–10% of splenic B cells in the CD40 KO host were CD40+ and thus corresponded to adoptively transferred B7 DKO B cells, whereas 90% of B cells were of host origin (Fig. 6 A). DCs and other cell types in CD40 KO hosts were CD40 KO but B7 WT (B7.1+/+, B7.2+/+, CD40−/−). Strikingly, essentially all NP-specific IgG1+ class-switched GC B cells were detected in the donor CD40+ B7− B cell compartment in CD40 KO recipient mice (Fig. 6, A and B). Of note, the ability of donor B7 DKO B cells (B7.1−/−, B7.2−/−, CD40+/+) to develop antigen-specific GC B cells in CD40 KO recipient mice was comparable with that of donor B7 WT B cells (B7.1+/+, B7.2+/+, CD40+/+), indicating that there is no requirement for B7.1 or B7.2 on B cells in this response (Fig. S5 A). In contrast, the reciprocal transfer of CD40 KO B cells (B7.1+/+, B7.2+/+, CD40−/−) into B7 DKO (B7.1−/−, B7.2−/−, CD40+/+) hosts failed to support a GC response to the same immunization (Fig. 6 B). These results demonstrate that neither CD40 on DCs nor B7.1/B7.2 on B cells is required for GC B cell responses. CD40+ B7− B cells and a distinct population of B7+ CD40− DCs were capable of efficient cooperation in GC responses, with no requirement for expression of both B7 and CD40 on the same cell (Fig. 6 and Fig. S5).
Figure 6.
GC responses are elicited by CD40 KO DCs and B7 KO B cells. (A) B cells were purified from B7 DKO mice and adoptively transferred to CD40 KO mice. Host mice were immunized with NP-OVA/Alum 1 d after transfer. 8 d later, splenocytes were analyzed for antigen-specific GC B population. (B) Frequency of GC B cells, NP-binding GC B cells in each B cell compartment, and frequency of IgG1+ cells among NP-binding GC B cells were analyzed. Data presented are the combined result of three independent experiments (mean ± SEM). The total numbers of mice in the three combined experiments are WT, n = 7; CD40 KO, n = 6; B7 DKO B cells into CD40 KO mice, n = 8; and CD40 KO B cells into B7 DKO mice, n = 3. Statistical significance was determined by Student’s t test.
Discussion
T cell–dependent GC responses require coordinated interactions of T cells with antigen-presenting B cells and DCs. We used histocytometry to add to the existing data on the tissue localization of T cell–APC interactions during GC responses to primary vaccine immunization with protein antigen and adjuvant. Our experiments identified spatially and temporally distinct patterns of physical interactions of T cells with both populations of APCs. Although B7- and CD40-dependent co-stimulatory pathways, in addition to cognate MHCII–TCR interactions, are essential for GC responses (Kawabe et al., 1994; Han et al., 1995; Ferguson et al., 1996; Borriello et al., 1997), a comprehensive analysis of cell type–specific pathways has been hindered by the absence of models for conditional expression of B7 or CD40. We generated cKO models for both B7 and CD40 and used these genetic lines, together with conditional MHCII KOs and BM chimeric strategies, to analyze pathways involved in TD GC and antibody responses. Our results indicated that recognition of antigen presented by MHCII expressed by both DCs and B cells is necessary for the generation of antigen-specific Tfh cells, GC B cells, and antibody responses. Notably, the cellular requirements for B7 and CD40 expression were, in contrast, distinct: B7 expression on DCs was required for GC responses, but there was no requirement for B7 expression on B cells; reciprocally, CD40 was required on B cells but not DCs for the generation of antigen-specific Tfh cells, GC B cells, and sustained high-affinity class-switched antibody responses. These findings support a model in which, contrary to current paradigms, distinct cell populations engage in the major co-stimulatory interactions necessary for effective primary Tfh cell and GC responses. There is in fact neither a requirement for expression of B7 on B cells nor a requirement for expression of both B7 and CD40 on the same cell under conventional vaccination conditions (Fig. S5 B).
Tfh cell differentiation and GC formation have been described as a multistep process (Victora and Nussenzweig, 2012; Crotty, 2014; Vinuesa et al., 2016). Initiation of Tfh cell differentiation has been suggested to begin with priming of a naive CD4+ T cell by antigen-presenting DCs. Subsequent signals determine Tfh cell differentiation fate in response to factors, including cytokines, most specifically IL-6, which induce Bcl6 and its downstream target events and chemokine-driven migration to the B cell follicle (Crotty, 2014; Vinuesa et al., 2016). Another cytokine, IL-2, has a negative role on Tfh cell development (Choi et al., 2011; Crotty, 2014; Vinuesa et al., 2016). It was recently shown that DCs present in the outer T cell zone promote Tfh cell differentiation by quenching IL-2 availability in this distinct lymphoid niche (Li et al., 2016). Later stages of Tfh cell differentiation occur through interactions with antigen-specific B cells in the follicle, where the efficiency of antigen presentation to Tfh cells is critical in the selection of B cells (Crotty, 2014; Vinuesa et al., 2016). Those B cells that, as a consequence of activation-induced cytidine deanimase (AID)–dependent SHM, express progressively higher BCR affinity for antigen and therefore interact most effectively with Tfh cells via antigen presentation are selectively driven through cycles of proliferation and interzonal migration (Victora and Nussenzweig, 2012). These later events in the GC response, which are reflected in affinity maturation of BCRs and antibody production, thus appear to depend heavily on Tfh cell interactions with GC B cells.
We explored the requirement for recognition of antigen presented by MHCII on B cells and DCs in primary GC responses using mixed BM chimeras as well as MHCII cKO mice. Consistent with previous studies (Shimoda et al., 2006; Deenick et al., 2010), MHCII expression by B cells was important for GC B cell and antibody responses. We found no evidence of bystander B cell responses driven in a noncognate fashion by cytokines or CD40L from T cells activated by neighboring MHCII+ antigen-presenting B cells. In addition, we identified a requirement for MHCII expressed on DCs for GC B cell and antibody responses, indicating a nonredundant role for T cell–DC interactions, consistent with the proposed role of DCs in the initial antigen-specific stimulation of naive CD4+ T cells (Itano and Jenkins, 2003). Although it has been reported that B cells are also capable of stimulating primary T cell responses (Morris et al., 1994; Constant, 1999; Evans et al., 2000; Rodríguez-Pinto and Moreno, 2005), this may occur only under conditions in which B cells express BCRs with sufficient antigen affinity to mediate efficient presentation to T cells. These conditions may exist during later stages of the GC response and, in fact, appear to underlie the selection of B cells expressing high-affinity BCRs for specific antigen (Victora and Nussenzweig, 2012).
Given the apparent requirement for cognate T cell interactions with both B cells and DCs during the course of a GC response, it was of interest to define cooperative co-stimulatory signaling events involved in productive T cell interactions with each of these populations. A requirement for CD28-B7 co-stimulation in GC and TD antibody responses had been established by studies of constitutive deficiency in these pathways, as well as by the effect of blocking antibodies in vivo (Han et al., 1995; Borriello et al., 1997). The experiments reported here used mice conditionally deficient for B7, allowing direct tests of the requirement for B7 on B cells or DCs. We found a strict requirement for B7 on DCs for antigen-specific TD antibody, Tfh cell, and GC B cell responses. Thus, MHCII-dependent activation of T cells by DCs for clonal expansion and subsequent differentiation to Tfh cells appears to require B7 co-stimulation, consistent with the reported co-stimulatory requirement for T cell functional activation (Esensten et al., 2016), in particular naive T cells, in a variety of settings. In contrast, there was no requirement for B7 expression by B cells in T cell clonal expansion or Tfh cell development despite the demonstrated requirement for recognition of pMHCII on B cells in Tfh cell generation. Notably, we also found that B7 expression on B cells was dispensable for WT levels of class-switched antibody responses and for affinity maturation, which has been shown to be dependent on iterative interactions between Tfh and GC B cells. These findings indicate that effective communication between T cells and pMHCII-bearing B cells leading to Tfh cell generation, GC B cell differentiation, and affinity maturation during antigen-specific GC responses does not require B7 co-stimulation. This result is consistent with our previous finding in WT + B7 DKO BM chimeras, which suggested that there is no requirement for cell-autonomous B7 expression on B cells for TD antibody responses but did not address the overall requirement for B7 expression on B cells because of the presence of B7 WT B cells in these chimeras and did not assess GC formation or the GC origin of these responses (Lumsden et al., 2003). Recent publications have suggested that B cells have a cell-intrinsic requirement for expression of CD80 and/or CD86 for differentiation into GC B cells (Salek-Ardakani et al., 2011; Wing et al., 2014). Interestingly, the data that were presented in support of that suggestion did not measure the antigen specificity of the GC response being characterized. A recent B7-blocking study suggested B7’s role in supporting the Tfh population after GC formation (Linterman et al., 2014), but it is not clear whether this resulted from B7 blockage at the T–DC or T–B cell interaction, and B7 at the DC–T cell interaction might still be required at a relatively later stage of GC response to support Tfh cell differentiation and functions (León et al., 2012). Recent studies analyzing the role of T follicular regulatory cells (Tf reg cells) in GC responses proposed that Tf reg cells might function through CTLA-4–mediated down-regulation of B7 on B cells (Sage et al., 2014; Wing et al., 2014). However, our results indicate that there is no requirement for B7.1 or B7.2 on B cells for antigen-specific Tfh generation, GC B development, or high-affinity antibody production, and analysis of the mechanism mediating Tf reg cell function in antigen-specific GC responses will require further study (Sage and Sharpe, 2015).
The requirement for CD28 co-stimulation in T cell activation has been shown to be critically dependent on the strength of primary TCR-mediated antigenic signaling, with strong TCR signaling associated with a decreased requirement for co-stimulation (Acuto and Michel, 2003). During the course of GC responses, the initial T cell response appears to be initiated by a DC as an APC. In this interaction, the relatively low efficiency of antigen presentation may be associated with the limited strength of TCR signaling and hence a strong requirement for B7-dependent co-stimulation by DCs. Later in the GC response, Tfh cells interact with GC B cells, which undergo SHM through iterative interaction with Tfh, resulting in increased BCR affinity for antigen and consequently enhanced efficiency of antigen presentation and strength of TCR signaling (Victora and Nussenzweig, 2012). Under the conditions of primary GC responses that we have analyzed, efficient pMHCII presentation by B cells may obviate the requirement for B7 co-stimulation by B cells. The observed absence of B7 dependence may also reflect additional parameters of the activation or differentiation state of the Tfh cells that are interacting with B cells. Inducible T cell co-stimulator (ICOS) molecules induced on activated T cells by B7–CD28 co-stimulation (McAdam et al., 2000) were shown to be critical for Tfh cell development and the GC response (Choi et al., 2011) through interaction with ICOS ligand on B cells (Nurieva et al., 2008; Xu et al., 2013), possibly supplanting the requirement for B7 co-stimulation in Tfh cells interacting with GC B cells. Also of interest is the recent study showing that PD-1 can function to suppress signaling through CD28 rather than through the TCR (Hui et al., 2017; Kamphorst et al., 2017). Down-regulation of CD28 signaling in PD-1high GC Tfh cells by PD-1 ligands on GC B cells may therefore obviate any role for co-stimulation through B7 on GC B cells. Our finding that B7 expression on B cells is not required for antigen-specific GC responses is based on a primary conventional protein/adjuvant vaccination model. It remains to be determined whether co-stimulatory requirements differ for other vaccine strategies or infection models.
GC B cell responses and TD antibody responses are strongly dependent on CD40, as initially demonstrated by the inhibitory effects of blocking anti-CD40 antibodies and studies of CD40-deficient mice (Kawabe et al., 1994; Han et al., 1995). Use of CD40 cKO mice in addition to chimera strategies in this study has permitted analysis of the requirement for CD40 on B cells or DCs in the generation of Tfh cells and productive GC responses. Cell type–specific requirements for CD40 were in fact strikingly reciprocal to the requirement for B7. CD40 expression on B cells was required for GC and antibody responses, but these responses were not affected by deletion of CD40 from DCs. The requirement for CD40 expression on B cells was cell autonomous, as reflected in mixed BM chimeras. This suggests that CD40 functions by signaling through this molecule on B cells, consistent with previous in vitro and in vivo studies demonstrating a role of CD40 in signaling B cell proliferation and differentiation (Inaba et al., 1995; Grewal and Flavell, 1998; Lumsden et al., 2003). In contrast, our data from studies of cotransferred CD40L WT and CD40L KO T cells indicated that CD40L expression on a T cell is not cell autonomously required for expansion and Tfh cell differentiation of that T cell in this experimental setting. However, the overall requirement for CD40L signaling in Tfh generation is not clear because a role for CD40L signaling on the cotransferred CD40L WT T cells cannot be excluded.
Collectively, these findings support a model in which requirements for co-stimulatory signaling involving B7 and CD40 are segregated to distinct cells and components of the primary GC response (Fig. S5 B), providing a strong parallel to the differential adhesive molecular requirements for T–DC versus T–B cell interactions revealed in studies of SAP-deficient animals (Qi et al., 2008; Cannons et al., 2010). Initial activation of CD4+ T cells is dependent on cognate interactions with antigen-presenting DCs. Generation of expanded T cell populations upon such antigen recognition requires co-stimulation of the T cells by B7 expressed by the DC, without an essential role of CD40. Subsequent generation of GC Tfh cells and the function of these Tfh cells in supporting GC B cell proliferation, differentiation, class switch recombination, SHM, and affinity maturation are all dependent on pMHC cognate interactions between Tfh cells and B cells and require CD40 but not B7 expressed on B cells.
Our results challenge previously proposed models for the interaction of B7–CD28 and CD40L–CD40 co-stimulatory pathways in the activation and differentiation of T cells. It has been suggested that CD40L on activated T cells signals through CD40 on APCs to up-regulate B7 on those APCs, providing positive feedback between B7–CD28 and CD40L–CD40 co-stimulatory pathways (Roy et al., 1995; Grewal and Flavell, 1998; Quezada et al., 2004). However, our findings indicate that there is in fact no requirement for the expression of both B7 and CD40 on the same cell in mediating efficient GC responses. Rather, each co-stimulatory pathway has distinct cell type–specific roles in these TD responses (Fig. S5 B).
These findings have implications for opportunities to modulate humoral immunity at each stage of the GC response through cell-specific targeting of distinct co-stimulatory pathways. Interfering with B7–CD28 co-stimulation should be effective in preventing antigen-specific CD4+ T cell clonal expansion and subsequent Tfh cell differentiation by acting at the initial stage of the response mediated by T cell–DC interactions. B7–CD28 blockade would be less effective at later stages of the GC response that are mediated by T–B cell interactions, where we show that B7 plays a negligible role. In contrast, interfering with the CD40 pathway should not impact CD4+ T cell clonal expansion mediated by T cell–DC interaction, but would be effective at inhibiting T–B cell interactions in developing and developed GC reactions at later stages.
Materials and methods
Mice
C57BL/6 and B6.CD45.1 mice were obtained from the National Institutes of Health. B7.1/B7.2 DKO (B7 DKO), CD40 KO, B cell KO (IgM KO, μMT), MHCII KO, MHCII(I-Ab)flox/flox, Igha congenic, CD11c-Cre-Tg, CD11c-EGFP-Cre-Tg, CD19-Cre knock-in, and OT-II TCR Tg mice were purchased from Jackson Laboratory. BM chimera mice were generated by reconstitution of 107 total T cell–depleted BM cells from donor mice to irradiated (950 rad) host mice i.v. Mice were maintained in accordance with National Institutes of Health guidelines. All animal experiments were approved by the National Cancer Institute and the National Institute of Allergy and Infectious Diseases Animal Care and Use Committees.
Immunofluorescence and confocal microscopy
Draining LNs and spleens were harvested and fixed with PLP buffer (0.05 M phosphate buffer containing 0.1 M l-lysine, pH 7.4, 2 mg/ml NaIO4, and 10 mg/ml paraformaldehyde) for 12 h. After fixation, tissues were incubated in 30% sucrose for 6 h before embedding in optimum cutting temperature compound (Tissue-Tek). 30-μm sections were cut on a cryostat (CM3050S; Leica) and adhered to slides (Super Frost Plus Gold; Electron Microscopy Services). Frozen sections were permeabilized and blocked for 1–2 h in PBS containing 0.3% Triton X-100 (Sigma-Aldrich), 1% normal mouse serum, 1% BSA, and 10% normal goat serum. Sections were stained with directly conjugated antibodies for a minimum of 5 h at room temperature or 12 h at 4°C in a humidity chamber in the dark. Anti–mouse CD80, CD86, CD45.1, CD4, and B220 antibodies were purchased from BioLegends. Anti–mouse CD11c antibody was purchased from Thermo Fisher. Anti-Bcl6 antibody was purchased from BD Biosciences. Cell nuclei were visualized with JOJO-1 (Thermo Fisher). Stained slides were mounted with Fluoromount G (eBioscience) and sealed with a glass coverslip. Each section was visually inspected by epifluorescent light microscopy, and several representative sections from different lymphoid organs were acquired using a confocal microscope (SP8; Leica) and 40× objective (numerical aperture 1.3).
Histocytometry
For histocytometric analysis of OT-II cells, we developed a seven-color panel consisting of the following fluorophores: brilliant violet 421, brilliant violet 510, Alexa Fluor 488, JOJO-1, Alexa Fluor 594, Alexa Fluor 647, and Alexa Fluor 700. Fluorophore emission was collected on separate detectors with sequential laser excitation used to minimize spectral spillover. The channel dye separation module within the LAS AF software (Leica) was then used to correct for any residual spillover. Representative tile scans were taken at a voxel density of 1,024 × 1,024 and 1-µm z step. Threshold identification, voxel gating, surface creation, masking, and signal segmentation were performed as previously described (Gerner et al., 2012, 2015; Radtke et al., 2015). Channel statistics for all surfaces were exported into Excel (Microsoft) and converted to a CSV file for direct visualization in FlowJo v10.1r5 (Tree Star). Mean voxel intensities for all channels were plotted on a linear scale and used for gating distinct leukocyte populations. Position data were also exported into FlowJo and used to gate B cell follicles, T cell zones, GCs, T–B cell borders, and white pulp regions in the spleen. To determine the phenotype of APCs in association with OT-II cells, CD11c and B220 fluorescence intensities were examined on OT-II cell surfaces (CD45.1+CD4+) as a surrogate for OT-II association with CD11c+ DCs or B220+ B cells as described previously for OT-I cells (Gerner et al., 2015; Radtke et al., 2015).
Generation of B7flox and CD40flox BAC Tg mice
BAC DNA (RP23-206M14 for B7.1 and RP23-413G19 for CD40) was obtained from the BACPAC Resource Center (Children’s Hospital Oakland Research Institute). Each loxP site was inserted by standard BAC recombineering methods (https://ncifrederick.cancer.gov/research/brb/recombineeringinformation.aspx). The engineered BAC DNA was linearized by NotI digestion and purified and injected into C57BL/6-fertilized eggs. Founder lines positive for BAC Tgs were backcrossed to constitutive B7 DKO or CD40 KO strains to generate B7.1flox BAC Tg (endogenous B7.1 and B7.2 DKO) and CD40flox BAC Tg (endogenous CD40 KO) mice.
Immunizations and adoptive transfer studies
Mice were immunized i.p. with 100 µg NP-KLH or NP-OVA (Biosearch Technologies) mixed with Imject Alum (Pierce). For adoptive transfer experiments, CD45.1 OT-II Tg CD4+ T cells were purified with a MACS CD4+ T cell isolation kit (Miltenyi Biotec), and 5 × 105 cells were transferred i.v. to host mice 1 d before immunization. Splenic B cells were purified with MACS pan–B cell isolation kits (Miltenyi Biotec), and 3 × 107 cells were transferred i.v. to host mice 1 d before immunization.
Flow cytometry
Cells were washed with FACS buffer (HBSS containing 0.2% BSA and 0.05% Azide), treated with anti-FcR (24G2), and then stained with specific antibodies. Anti–mouse CD4, CD8, PD-1, CXCR5, B220, CD19, Fas, GL7, CD38, CD11c, B7.1, B7.2, CD40, CXCR4, CD83, TCR-Vα2, TCR-Vβ5, CD45.1, and CD45.2 antibodies were purchased from BD Biosciences. NP-PE was purchased from Biosearch Technologies. Propidium iodide was purchased from Sigma-Aldrich. For intracellular cytokine staining, splenic CD4+ T cells were stimulated with PMA and ionomycin for 2 h, and cells were fixed and permeabilized with the BD Fix/Perm kit (BD Biosciences) according to the manufacturer’s instructions and then stained with anti–IFN-γ (BD Biosciences) and isotype control antibody for 30 min. Data were collected with a FACS Calibur II, FACS LSR II, FACS Fortessa, or FACS Aria III flow cytometer (BD Biosciences) and analyzed with FlowJo software.
ELISA
NP-specific IgG1 was measured by ELISA. In brief, NP25-BSA (Biosearch Technologies) was coated on ELISA plates (Immulon 4HBX; Thermo Fisher) overnight. The plates were then washed with ELISA wash buffer (0.5% Tween in PBS), serially diluted sera were applied to the plates, and plates were incubated 2 h at room temperature. Anti–mouse IgG1 HRP (Southern Biotech) was used to detect NP-specific IgG1. To measure IgG1 affinity maturation, antibody titer was determined with NP4-BSA and NP25-BSA. For allotype-specific IgG1 detection, anti–mouse IgG1a or IgG1b-biotin antibody (BD Biosciences) was used and followed by a streptavidin-HRP (BD Biosciences) reaction. After a wash step, 2,2'-Azino-di-(3-ethylbenzthiazoline-6-sulfonate) (ABTS) substrate (KPL) was added to the wells, and enzyme reaction was stopped by ABTS HRP Stop Solution (KPL). Optical density at 405 nm was measured with Fluostar Optima plate reader and software (BMG Labtech).
Statistical analysis
Student’s t test with two-tailed distributions was performed for statistical analyses with single comparisons. For multiple comparisons, statistical analysis was performed with one-way ANOVA followed by Dunnett’s multiple comparison. P-values <0.05 were considered statistically significant.
Online supplemental material
Fig. S1 is histocytometry analysis of GC response. Fig. S2 is supplemental data related to Fig. 1. Fig. S3 is supplemental data related to Fig. 2. Fig. S4 is supplemental data related to Fig. 4 and Fig. 5. Fig. S5 is supplemental data related to Fig. 6.
Acknowledgments
We thank Alfred Singer, Pam Schwartzberg, Karen Hathcock, and Joy Williams for their thoughtful comments and review of this manuscript.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health. M. Watanabe was supported by the Uehara Memorial Foundation.
The authors declare no competing financial interests.
Author contributions: M. Watanabe and C. Fujihara generated Tg mice, performed experiments, and analyzed and interpreted data. A.J. Radtke performed the experiments and analyzed and interpreted data. Y.J. Chiang and S. Bhatia contributed to the generation of Tg mice. A.J. Radtke and R.N. Germain were involved in critical discussions throughout and contributed to the writing of the manuscript. R.J. Hodes supervised the project. M. Watanabe, C. Fujihara, and R.J. Hodes wrote the manuscript.
Footnotes
Abbreviations used:
- cKO
- conditional KO
- DKO
- double KO
- dLN
- draining LN
- GC
- germinal center
- μMT
- μ mutation
- NP
- 4-hydroxy-3-nitrophenylacetyl
- pMHCII
- peptide-MHCII
- SHM
- somatic hypermutation
- TD
- T helper cell dependent
- Tg
- transgenic
References
- Acuto O., and Michel F.. 2003. CD28-mediated costimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 3:939–951. 10.1038/nri1248 [DOI] [PubMed] [Google Scholar]
- Armitage R.J., Fanslow W.C., Strockbine L., Sato T.A., Clifford K.N., Macduff B.M., Anderson D.M., Gimpel S.D., Davis-Smith T., Maliszewski C.R., et al. . 1992. Molecular and biological characterization of a murine ligand for CD40. Nature. 357:80–82. 10.1038/357080a0 [DOI] [PubMed] [Google Scholar]
- Bakdash G., Sittig S.P., van Dijk T., Figdor C.G., and de Vries I.J.. 2013. The nature of activatory and tolerogenic dendritic cell-derived signal II. Front. Immunol. 4:53 10.3389/fimmu.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello F., Sethna M.P., Boyd S.D., Schweitzer A.N., Tivol E.A., Jacoby D., Strom T.B., Simpson E.M., Freeman G.J., and Sharpe A.H.. 1997. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 6:303–313. 10.1016/S1074-7613(00)80333-7 [DOI] [PubMed] [Google Scholar]
- Cannons J.L., Qi H., Lu K.T., Dutta M., Gomez-Rodriguez J., Cheng J., Wakeland E.K., Germain R.N., and Schwartzberg P.L.. 2010. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 32:253–265. 10.1016/j.immuni.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.S., Kageyama R., Eto D., Escobar T.C., Johnston R.J., Monticelli L., Lao C., and Crotty S.. 2011. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 34:932–946. 10.1016/j.immuni.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant S.L. 1999. B lymphocytes as antigen-presenting cells for CD4+ T cell priming in vivo. J. Immunol. 162:5695–5703. [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., and Mathis D.. 1991. Mice lacking MHC class II molecules. Cell. 66:1051–1066. 10.1016/0092-8674(91)90448-8 [DOI] [PubMed] [Google Scholar]
- Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity. 41:529–542. 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick E.K., Chan A., Ma C.S., Gatto D., Schwartzberg P.L., Brink R., and Tangye S.G.. 2010. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 33:241–253. 10.1016/j.immuni.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esensten J.H., Helou Y.A., Chopra G., Weiss A., and Bluestone J.A.. 2016. CD28 costimulation: From mechanism to therapy. Immunity. 44:973–988. 10.1016/j.immuni.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.E., Munks M.W., Purkerson J.M., and Parker D.C.. 2000. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J. Immunol. 164:688–697. 10.4049/jimmunol.164.2.688 [DOI] [PubMed] [Google Scholar]
- Ferguson S.E., Han S., Kelsoe G., and Thompson C.B.. 1996. CD28 is required for germinal center formation. J. Immunol. 156:4576–4581. [PubMed] [Google Scholar]
- Fujii S., Liu K., Smith C., Bonito A.J., and Steinman R.M.. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199:1607–1618. 10.1084/jem.20040317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner M.Y., Kastenmuller W., Ifrim I., Kabat J., and Germain R.N.. 2012. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 37:364–376. 10.1016/j.immuni.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner M.Y., Torabi-Parizi P., and Germain R.N.. 2015. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 42:172–185. 10.1016/j.immuni.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Goenka R., Barnett L.G., Silver J.S., O’Neill P.J., Hunter C.A., Cancro M.P., and Laufer T.M.. 2011. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 187:1091–1095. 10.4049/jimmunol.1100853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal I.S., and Flavell R.A.. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135. 10.1146/annurev.immunol.16.1.111 [DOI] [PubMed] [Google Scholar]
- Grusby M.J., Johnson R.S., Papaioannou V.E., and Glimcher L.H.. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 253:1417–1420. 10.1126/science.1910207 [DOI] [PubMed] [Google Scholar]
- Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., and Kelsoe G.. 1995. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 155:556–567. [PubMed] [Google Scholar]
- Haynes N.M., Allen C.D., Lesley R., Ansel K.M., Killeen N., and Cyster J.G.. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179:5099–5108. 10.4049/jimmunol.179.8.5099 [DOI] [PubMed] [Google Scholar]
- Hui E., Cheung J., Zhu J., Su X., Taylor M.J., Wallweber H.A., Sasmal D.K., Huang J., Kim J.M., Mellman I., and Vale R.D.. 2017. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 355:1428–1433. 10.1126/science.aaf1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Inaba K., Fukuba Y., Mori S., Haruna H., Doi H., Adachi Y., Iwai H., Hosaka N., Hisha H., et al. . 1995. Activation of thymic B cells by signals of CD40 molecules plus interleukin-10. Eur. J. Immunol. 25:1244–1248. 10.1002/eji.1830250517 [DOI] [PubMed] [Google Scholar]
- Itano A.A., and Jenkins M.K.. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. 10.1038/ni957 [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., and Crotty S.. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 325:1006–1010. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst A.O., Wieland A., Nasti T., Yang S., Zhang R., Barber D.L., Konieczny B.T., Daugherty C.Z., Koenig L., Yu K., et al. . 2017. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 355:1423–1427. 10.1126/science.aaf0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M.L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984–993. 10.1038/nri1246 [DOI] [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., and Kikutani H.. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1:167–178. 10.1016/1074-7613(94)90095-7 [DOI] [PubMed] [Google Scholar]
- Kerfoot S.M., Yaari G., Patel J.R., Johnson K.L., Gonzalez D.G., Kleinstein S.H., and Haberman A.M.. 2011. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 34:947–960. 10.1016/j.immuni.2011.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano M., Moriyama S., Ando Y., Hikida M., Mori Y., Kurosaki T., and Okada T.. 2011. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 34:961–972. 10.1016/j.immuni.2011.03.025 [DOI] [PubMed] [Google Scholar]
- Lee B.O., Moyron-Quiroz J., Rangel-Moreno J., Kusser K.L., Hartson L., Sprague F., Lund F.E., and Randall T.D.. 2003. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J. Immunol. 171:5707–5717. 10.4049/jimmunol.171.11.5707 [DOI] [PubMed] [Google Scholar]
- León B., Ballesteros-Tato A., Browning J.L., Dunn R., Randall T.D., and Lund F.E.. 2012. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat. Immunol. 13:681–690. 10.1038/ni.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lu E., Yi T., and Cyster J.G.. 2016. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 533:110–114. 10.1038/nature17947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman M.A., Denton A.E., Divekar D.P., Zvetkova I., Kane L., Ferreira C., Veldhoen M., Clare S., Dougan G., Espéli M., and Smith K.G.. 2014. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. eLife. 3 10.7554/eLife.03180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Gerner M.Y., Van Panhuys N., Levine A.G., Rudensky A.Y., and Germain R.N.. 2015. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 528:225–230. 10.1038/nature16169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J.M., Williams J.A., and Hodes R.J.. 2003. Differential requirements for expression of CD80/86 and CD40 on B cells for T-dependent antibody responses in vivo. J. Immunol. 170:781–787. 10.4049/jimmunol.170.2.781 [DOI] [PubMed] [Google Scholar]
- McAdam A.J., Chang T.T., Lumelsky A.E., Greenfield E.A., Boussiotis V.A., Duke-Cohan J.S., Chernova T., Malenkovich N., Jabs C., Kuchroo V.K., et al. . 2000. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 165:5035–5040. 10.4049/jimmunol.165.9.5035 [DOI] [PubMed] [Google Scholar]
- Morris S.C., Lees A., and Finkelman F.D.. 1994. In vivo activation of naive T cells by antigen-presenting B cells. J. Immunol. 152:3777–3785. [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., and Dong C.. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149. 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., and Dong C.. 2009. Bcl6 mediates the development of T follicular helper cells. Science. 325:1001–1005. 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S.L., and Tarlinton D.M.. 2011. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 12:472–477. 10.1038/ni.2019 [DOI] [PubMed] [Google Scholar]
- Pape K.A., Kouskoff V., Nemazee D., Tang H.L., Cyster J.G., Tze L.E., Hippen K.L., Behrens T.W., and Jenkins M.K.. 2003. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J. Exp. Med. 197:1677–1687. 10.1084/jem.20012065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M., Pagán A.J., Igyártó B.Z., Taylor J.J., and Jenkins M.K.. 2011. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 35:583–595. 10.1016/j.immuni.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., Cannons J.L., Klauschen F., Schwartzberg P.L., and Germain R.N.. 2008. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 455:764–769. 10.1038/nature07345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Jarvinen L.Z., Lind E.F., and Noelle R.J.. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307–328. 10.1146/annurev.immunol.22.012703.104533 [DOI] [PubMed] [Google Scholar]
- Radtke A.J., Kastenmüller W., Espinosa D.A., Gerner M.Y., Tse S.W., Sinnis P., Germain R.N., Zavala F.P., and Cockburn I.A.. 2015. Lymph-node resident CD8α+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathog. 11:e1004637 10.1371/journal.ppat.1004637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Pinto D., and Moreno J.. 2005. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur. J. Immunol. 35:1097–1105. 10.1002/eji.200425732 [DOI] [PubMed] [Google Scholar]
- Roy M., Aruffo A., Ledbetter J., Linsley P., Kehry M., and Noelle R.. 1995. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur. J. Immunol. 25:596–603. 10.1002/eji.1830250243 [DOI] [PubMed] [Google Scholar]
- Sage P.T., and Sharpe A.H.. 2015. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 36:410–418. 10.1016/j.it.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage P.T., Paterson A.M., Lovitch S.B., and Sharpe A.H.. 2014. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 41:1026–1039. 10.1016/j.immuni.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S., Choi Y.S., Rafii-El-Idrissi Benhnia M., Flynn R., Arens R., Shoenberger S., Crotty S., Croft M., and Salek-Ardakani S.. 2011. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J. Immunol. 186:5294–5303. 10.4049/jimmunol.1100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O., Edwards A.D., Schito M., Aliberti J., Manickasingham S., Sher A., and Reis e Sousa C.. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. 10.1016/S1074-7613(00)00045-5 [DOI] [PubMed] [Google Scholar]
- Shimoda M., Li T., Pihkala J.P., and Koni P.A.. 2006. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. J. Immunol. 176:2122–2133. 10.4049/jimmunol.176.4.2122 [DOI] [PubMed] [Google Scholar]
- Singer A., and Hodes R.J.. 1983. Mechanisms of T cell-B cell interaction. Annu. Rev. Immunol. 1:211–241. 10.1146/annurev.iy.01.040183.001235 [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Koide S., Witmer M., Crowley M., Bhardwaj N., Freudenthal P., Young J., and Inaba K.. 1988. The sensitization phase of T-cell-mediated immunity. Ann. NY Acad. Sci. 546:80–90. 10.1111/j.1749-6632.1988.tb21622.x [DOI] [PubMed] [Google Scholar]
- Tubo N.J., Pagán A.J., Taylor J.J., Nelson R.W., Linehan J.L., Ertelt J.M., Huseby E.S., Way S.S., and Jenkins M.K.. 2013. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 153:785–796. 10.1016/j.cell.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora G.D., and Nussenzweig M.C.. 2012. Germinal centers. Annu. Rev. Immunol. 30:429–457. 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., and Nussenzweig M.C.. 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 143:592–605. 10.1016/j.cell.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa C.G., Linterman M.A., Yu D., and MacLennan I.C.. 2016. Follicular helper T cells. Annu. Rev. Immunol. 34:335–368. 10.1146/annurev-immunol-041015-055605 [DOI] [PubMed] [Google Scholar]
- Wing J.B., Ise W., Kurosaki T., and Sakaguchi S.. 2014. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 41:1013–1025. 10.1016/j.immuni.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Xu H., Li X., Liu D., Li J., Zhang X., Chen X., Hou S., Peng L., Xu C., Liu W., et al. . 2013. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 496:523–527. 10.1038/nature12058 [DOI] [PubMed] [Google Scholar]
- Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., et al. . 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 31:457–468. 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Zotos D., and Tarlinton D.M.. 2012. Determining germinal centre B cell fate. Trends Immunol. 33:281–288. 10.1016/j.it.2012.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.