Victoria and Zurzolo discuss current evidence for the emerging role of lysosomal damage and tunneling nanotubes in the intercellular propagation of prion and prion-like proteins in neurodegenerative disease.
Abstract
Progression of pathology in neurodegenerative diseases is hypothesized to be a non–cell-autonomous process that may be mediated by the productive spreading of prion-like protein aggregates from a “donor cell” that is the source of misfolded aggregates to an “acceptor cell” in which misfolding is propagated by conversion of the normal protein. Although the proteins involved in the various diseases are unrelated, common pathways appear to be used for their intercellular propagation and spreading. Here, we summarize recent evidence of the molecular mechanisms relevant for the intercellular trafficking of protein aggregates involved in prion, Alzheimer’s, Huntington’s, and Parkinson’s diseases. We focus in particular on the common roles that lysosomes and tunneling nanotubes play in the formation and spreading of prion-like assemblies.
Introduction
The pathological hallmark of a number of neurodegenerative diseases (NDs) is the deposition of protein aggregates in the brain into intracellular or extracellular inclusions. In the case of prion diseases, as well as in Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD), distinct unrelated proteins (prion protein, amyloid precursor protein [APP] and tau, huntingtin [Htt], and α-synuclein, respectively) undergo this process yet have very different conformations, subcellular localizations, and functions (Brundin et al., 2010). Upon adoption of the misfolded conformation, these proteins nonetheless share a β-sheet–rich tertiary structure, which renders them prone to the formation of intermediate oligomeric species and higher-order fibrillar aggregates. Protein aggregate accumulation is linked with fatal neuronal dysfunction and toxicity through various mechanisms, though propagation and toxicity may be mediated in distinct phases (Sandberg et al., 2011). The nature of the multimeric aggregate species responsible for toxicity is debated; several studies suggest that oligomeric species are more toxic than fibrils for many amyloid proteins (Guo and Lee, 2014) although at least for α-synuclein, this is contested (Pieri et al., 2012). Conversely, larger aggregate depositions, such as those in plaques or Lewy bodies, are suggested to be protective to the cell, acting as a sink in which toxic species are held in stable, less toxic formations (Verma et al., 2015).
These diseases are collectively referred to as protein-misfolding diseases (PMDs), of which prion diseases are the best characterized (Prusiner, 2013). The term “prion” was coined by Prusiner (1982) in the seminal paper identifying the cause of scrapie to denote a proteinaceous infectious particle that was the scrapie-causative protein agent (i.e., misfolded PrPSc or prion protein). Moreover, although the chemical identity and the topology of the different proteins involved in PMDs are distinct, they are termed “prion-like proteins,” “prionoids,” or simply “prions” in recognition of the fact that they share several features with prion protein (Prusiner, 2013; Aguzzi and Lakkaraju, 2016). These features include the ability to self-propagate by inducing the misfolding of native self-molecules and to spread intercellularly in a non–cell-autonomous fashion along the central nervous system. There is, however, much debate about whether these other proteins at the origin of PMDs are true prions (Prusiner, 2013; Aguzzi and Lakkaraju, 2016; Sanders et al., 2016). On one hand, they are prions in the sense that they have the capability to propagate misfolding (see next section), yet on the other hand, they are not, because whereas the prion protein (PrPSc) causes infectious (i.e., transmissible) diseases, most PMDs are not, sensu stricto, infectious. We adopt here the term “prion-like” to refer both to the proteins and to the respective diseases. Although this term is not limited to the proteins involved in AD, PD, and HD (Table 1), in this review, we summarize current knowledge of the prion “likeness” of misfolded Tau, amyloid β (Aβ), Htt, and α-synuclein and the mechanisms by which their intercellular transfer is mediated. We specifically focus on the relevance of two common pathways shared by these misfolded proteins that may have important roles in prion-like diseases: tunneling nanotubes (TNTs) and lysosomes (Abounit et al., 2016a,b; Rustom, 2016). We furthermore propose a universal role for TNTs, a novel mechanism of intercellular communication, in the spreading of prion-like proteins in PMDs and highlight how the endolysosomal pathway is involved in the generation of and, potentially, the propagation of these aggregates via TNTs.
Table 1. Misfolding of specific proteins causes a spectrum of neurodegenerative disorders.
| Disorder | Protein aggregate | Seeding | Intercellular transmissibility |
|---|---|---|---|
| Prion diseases (CJD, vCJD, Kuru, and FFI) | PrPSc | Yes | Yes |
| AD | Aβ, Tau | Yes | Yes |
| Tauopathies (tauopathy, progressive supranuclear palsy, Pick’s disease, and frontotemporal dementia) | Tau | Yes | Yes |
| HD | mHtt | Yes | Yes |
| Synucleinopathies (PD, DLB, MSA, and PAF) | α-Synuclein | Yes | Yes |
| ALS | TDP-43 | Yes (Polymenidou and Cleveland, 2012) | Yes (Feiler et al., 2015) |
| FUS | Yes (Polymenidou and Cleveland, 2012) | ||
| SOD | Yes (Polymenidou and Cleveland, 2012) | Yes (Pokrishevsky et al., 2016) |
Although amyotrophic lateral sclerosis is not discussed in this review, evidence suggests that proteins involved in amyotrophic lateral sclerosis are prion-like. Evidence for seeding and transmissibility of other proteins are described in detail in the text. CJD, Creutzfeldt–Jakob disease; DLB, dementia with Lewy body; FFI, fatal familial insomnia; MSA, multiple system atrophy; PAF, pure autonomic failure; SOD, superoxide dismutase; vCJD, variant Creutzfeldt–Jakob disease.
The prion-like nature of NDs
Seeding
The phenomenon of infectious template-mediated protein conversion is well described for prion diseases, a group of neurodegenerative diseases that were first considered unique because of their transmissibility across individuals and species. The causative agent, cellular prion protein PrPC, misfolds to the disease-related form PrPSc, which is capable of inducing the further conversion of naive molecules of PrPC, resulting in a pool of potentially toxic PrPSc molecules that form ordered oligomeric or fibrillar aggregates. These PrPSc oligomers and aggregates may act as “seeds” for further conversion of naive PrPC (Prusiner, 2013). The intercellular transmission of these misfolded aggregates is believed to be responsible for seeding the further conversion of naive PrPC molecules in neighboring cells, thus contributing to the overall propagation of disease.
This ability to seed conversion is at the heart of the intrinsic nature of prions (Prusiner, 2013), but is not restricted to the prion protein (Hasegawa et al., 2017). When recombinant α-synuclein fibrils were exogenously supplied to cells overexpressing soluble α-synuclein, the authors observed the recruitment and conversion of soluble α-synuclein into Lewy body–like inclusions, the hallmark of PD (Luk et al., 2009). This was later confirmed to occur in vivo in both wild type and transgenic human α-synuclein–expressing mice (Luk et al., 2012a,b; Tyson et al., 2016). Evidence also exists showing that tau and Aβ, whose misfolding and aggregation leads to protein tangles and plaques in AD and other tauopathies, can seed conversion (Hasegawa et al., 2017).
In the case of HD, the genetic expansion of a polyglutamine (polyQ) stretch in the N terminus of the Htt protein, to >35 residues, confers on it the tendency to aggregate and is causative of the disease. Aggregative tendency, and thereby disease progression, are related to the length of the repeat. Mutant Htt (mHtt) fragments as well as polyQ peptides were also shown, upon uptake, to seed conversion of a soluble Htt reporter in cells (Jansen et al., 2017).
The propensity of the misfolded proteins involved in neurodegenerative disorders to induce a chain reaction of conversion and aggregation of naive molecules seems therefore to be a shared property, regardless of primary and secondary structures, protein topology, and their subcellular localization (Aguzzi and Lakkaraju, 2016). However, how specifically this conversion occurs is not clear. Furthermore, because of low solubility and heterogeneous aggregate sizes, the detailed atomic structure of the isoform that can seed the misfolding of the normal conformer counterpart is still unknown for many NDs (Riek and Eisenberg, 2016). Although a predominant Aβ40 fibrillar form was identified from brains of prolonged duration AD clinical subtypes (Qiang et al., 2017), more heterogeneity was observed for rapid forms of the disease. More in vitro studies are thus necessary to understand how, at the molecular level, one protein conformer induces molecules of another conformer of the same protein to refold into the structure of the first. Key topics for exploration include where this occurs inside the cell and what the molecular determinants are in terms of protein sequence and the need for protein and/or lipid chaperones (Gorbenko and Kinnunen, 2006; Surewicz and Apostol, 2011).
Intercellular spreading
The pathological progression of neurodegenerative diseases is associated with the presence of misfolded aggregated proteins (and associated damage) in an expanding area of the brain, raising the question of cell-to-cell transfer. Evidence for intercellular transfer has accumulated for many prion-like proteins (Costanzo and Zurzolo, 2013; Pecho-Vrieseling et al., 2014; Calafate et al., 2015; Abounit et al., 2016b). Although the underlying mechanisms are not well understood, it is nonetheless clear that there are commonalities in the mechanisms of trafficking of these proteins along neuronal pathways. Furthermore, attempts have been made, using different cell types in vitro, to understand the role of different cells in the progression of the pathology in the brain.
Prions
Cell-to-cell transmission of infectious PrPSc aggregates has been demonstrated to occur between neuronal cells (Kanu et al., 2002; Gousset et al., 2009; Zhu et al., 2015) as well from neurons to astrocytes, suggesting a role for glia in clearance (Victoria et al., 2016). However, astrocytes are very susceptible to prion infection upon uptake of exogenously supplied PrPSc seed (Cronier et al., 2004) and, further, propagate infectivity to neurons (Cronier et al., 2012) as well as other naive astrocytes. Astrocytes may therefore be a double-edged sword in disease propagation (Victoria et al., 2016). Microglia are activated by neuronal and astrocytic infection (Marella and Chabry, 2004), and their depletion results in disease exacerbation, presumably as their phagocytic role is lost (Zhu et al., 2016). On the other hand, oligodendrocytic cells neither show susceptibility to prions nor seem capable of transferring prion infectivity (Prinz et al., 2004), though the implications of this resistance remain unclear.
Different mechanisms have been implicated in intercellular PrPSc transfer (Fig. 1). Infectivity from primary astrocytes and neuronal cell lines can be released into the tissue culture medium through exosomes or other secretory mechanisms (Fevrier et al., 2004; Cronier et al., 2012; Arellano-Anaya et al., 2015). Exosomes are formed as intraluminal vesicles within multivesicular bodies (MVBs) and are released to the cell exterior by fusion of MVBs with the plasma membrane (Colombo et al., 2014). They have been implicated in the spreading of many prion-like proteins (Quek and Hill, 2017). Both PrPC and PrPSc have been found in exosomes, and exosomes are capable of inducing infection when applied on cells or injected into mouse brains (Fevrier et al., 2004). However, it is as yet unclear how efficient this is as a mode of prion propagation, as exosome-mediated infection requires exosomes to be purified and highly concentrated (Fevrier et al., 2004; Coleman et al., 2012), and not all cell types can be infected with conditioned medium from infected cells (Kanu et al., 2002). The efficiency of packaging into exosomes can also be prion-strain dependent (Arellano-Anaya et al., 2015). The mechanisms of exosome uptake and effect on acceptor cells (McKelvey et al., 2015) are furthermore unclear in this context. Furthermore, work on primary infected astrocytes suggest that although prion infectivity can be released through secretion, the efficiency of neuronal infection via this mode is much lower than when cell-cell contact is allowed (Cronier et al., 2012; Victoria et al., 2016). In this context, axonal transport and synaptic transfer have also been hypothesized in neurons (Shearin and Bessen, 2014). Furthermore, as described in the next section, TNT-mediated transfer, which depends on direct cell–cell contact, has been shown to have an important role in the intercellular spreading of PrPSc (Gousset et al., 2009).
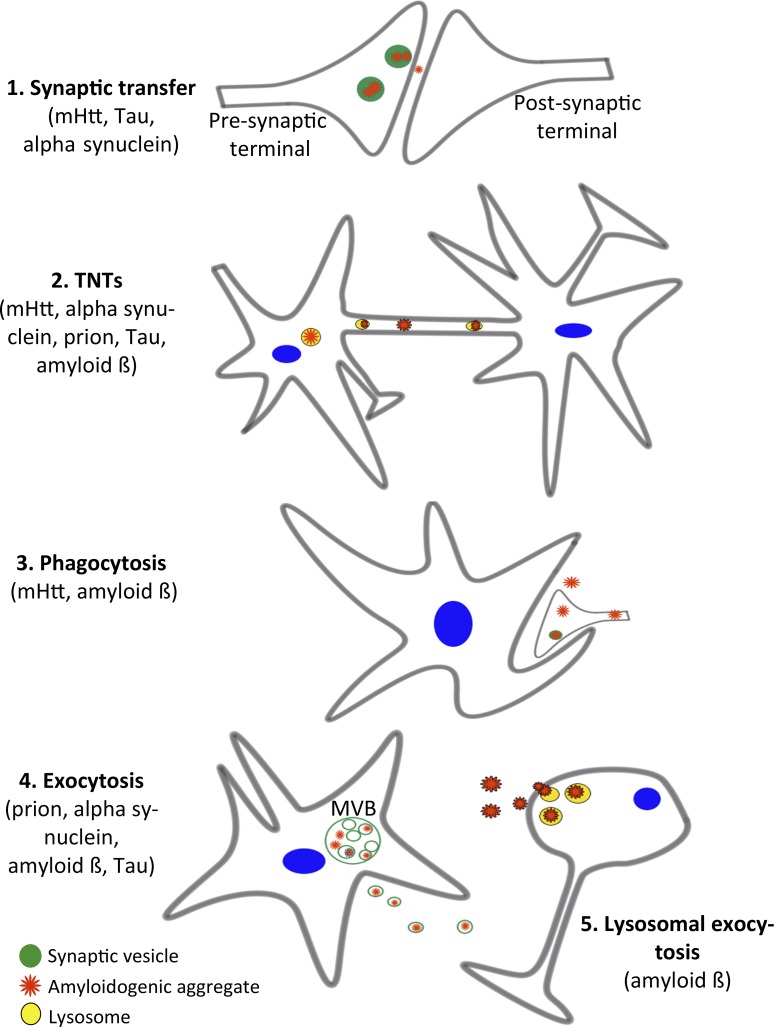
Figure 1.
Proposed routes of intercellular transfer. (1) Synaptic transfer. Misfolded proteins (red) such as mHtt, Tau, and α-synuclein, which have been shown to bind to synaptic vesicles (green), may be released from cells through synaptic vesicle fusion with the plasma membrane and thereafter be taken up by the postsynaptic neuron. (2) TNTs. TNTs have been implicated in the transfer of prion, mHtt, Aβ, tau, and α-synuclein. (3) Phagocytosis. The engulfment of aggregate-containing damaged/dying cells or synaptic terminals by glial cells may represent a mechanism that propagates transfer between cells of different types and has been demonstrated for mHtt and Aβ. This process may also occur for extracellular naked or exosomally localized aggregates in close apposition to the engulfing cell. (4) Exocytosis of exosomes. The fusion of MVBs with the plasma membrane may release exosomes containing intraluminal aggregate-containing vesicles (exosomes) that can be internalized by neighboring cells. (5) Lysosomal exocytosis. Fusion of lysosomes (yellow) with the plasma membrane can release naked aggregates (such as Aβ) into the extracellular space that may form plaques or be taken up by neighboring cells.
Aβ
Aβ are amyloid fragments resulting from the proteolytic cleavage of the amyloid precursor protein (APP) by the β and γ secretase. They accumulate, mainly extracellularly, in amyloid plaques in AD (De Strooper and Karran, 2016). Importantly, a recent seminal study reported that although a less toxic version (Aβ40) can be released extracellularly, the production of a large intracellular pool of the toxic Aβ42 is restricted to late endosomes and lysosomes because of the localization of the secretase enzyme (Sannerud et al., 2016), highlighting the importance of intracellular trafficking on the fate and toxicity of the protein.
Several studies have reported the occurrence of intercellular spreading of Aβ. Fluorescent oligomeric Aβ microinjected into hippocampal neurons spread to neighboring neurons as well as astroglia in a manner that did not appear to correspond to simple diffusive dissemination and might be cell–cell contact mediated (Nath et al., 2012). The authors reported that neuritic connections appeared to mediate interneuronal transfer and that oligomeric Aβ appeared to travel within these neurites in granules that moved at speeds similar to lysosomes and late endosomes (Nath et al., 2012). Aβ peptides were also released in exosomes (Rajendran et al., 2006), and perturbations in lysosomal function increased its presence in MVBs (Vingtdeux et al., 2007), suggesting that this organelle could be a sorting point for the eventual fate of aggregated proteins, promoting either their degradation through fusion with lysosomes or their secretion through fusion with the plasma membrane releasing exosomes. Interfering with the endosomal sorting complexes required for transport (ESCRT) machinery, which is involved in MVB biogenesis (McKelvey et al., 2015) can tip the balance between intracellular accumulation of Aβ and its exosomal release through MVBs. However, the role of ESCRT-mediated sorting of APP in MVBs remains unclear. Perturbing MVB sorting of APP via disruption of interactions with early ESCRT components, (e.g., Hrs and Tsg101) are reported both to increase secretion of Aβ (Morel et al., 2013) as well as decrease it (Choy et al., 2012; Edgar et al., 2015), whereas depletion of the late ESCRT protein CHMP-6 increases Aβ secretion (Choy et al., 2012).
Studies have also suggested that astrocytes can take up external Aβ plaques, possibly through phagocytic mechanisms (Wyss-Coray et al., 2003), suggesting a role for these cells, as well as for microglia, in Aβ clearance. Oligodendrocytes are sensitive to Aβ-induced toxicity (Lee et al., 2004) and, interestingly, NG2 cells (a subset of oligodendrocytic precursor cells) also cluster around amyloid plaques in mice brains and are capable of taking up external Aβ42 peptides (Li et al., 2013). It is possible that impaired repair of oligodendrocyte precursor cells by Aβ pathology might enhance disease progression. However, the roles of these cells in Aβ pathology need to be further explored (De Strooper and Karran, 2016).
Tau
Tau, the major component of intraneuronal neurofibrillary tangles, is a microtubule-stabilizing protein found predominantly in axons. Hyperphosphorylation or truncation of the protein correlates with its misfolding and neuropathology (Frost et al., 2009). Work in vivo and in vitro has demonstrated that pathological tau transfers between cells and in between anatomically connected brain regions, causing seeding of the protein in neurons and oligodendrocytes (Clavaguera et al., 2009, 2014; Frost et al., 2009). Evidence also exists that trans-synaptic spread of tau occurs in vivo (Liu et al., 2012; Wu et al., 2016). A recent study using a three-chambered microfluidic system demonstrated that synaptic contact facilitated trans-neuronal tau propagation, which is relevant in the context that both normal and pathogenic tau have been found localized in synaptic terminals (Calafate et al., 2015). Interestingly, misfolded tau propagates in vivo between synaptically connected neurons even in the absence of endogenous tau expression, although its neurotoxicity was reduced, suggesting that conversion is necessary for toxicity, but not for transfer (Wegmann et al., 2015). Strikingly, several studies suggest that tau is secreted as naked aggregates from cells through synaptic release and nonconventional secretion (Chai et al., 2012; Kfoury et al., 2012; Pooler et al., 2013), but exosomal release also occurs (Saman et al., 2012). Taken together, these data support the contribution of both synaptic and nonsynaptic mechanisms to the propagation of tau pathology, although increase in synaptic connections or activation enhances the process.
α-Synuclein
Like tau, α-synuclein is an intrinsically unstructured monomeric protein, which has been shown to misfold and aggregate in PD and other synuclopathies (Davidson et al., 1998). α-Synuclein is the major component of Lewy bodies, along with ubiquitin and neurofilaments (Spillantini et al., 1998). Evidence that prion-like spreading propagates the disease arose when Lewy body pathology was detected in graft tissue from PD patients who had received therapeutic grafts (Kordower et al., 2008; Li et al., 2010). Using grafts of embryonic mesencephalic tissue into mouse and rat brains expressing human α-synuclein, the protein was shown to transfer between neurons in vivo (Hansen et al., 2011; Angot et al., 2012). α-Synuclein also transfers to oligodendrocytes in ventral mesencephalon tissue grafted into rat brains (Reyes et al., 2014). Additionally, transfer of α-synuclein from neurons to astrocytes was demonstrated in vitro and in vivo, and astroglia that endocytosed the infectious seed initiated an inflammatory response in microglia, providing insight into the mechanisms of pathogenicity in PD (Lee et al., 2010). Interestingly in vitro co-cultures of primary neurons and astrocytes and organotypic brain slices confirm that α-synuclein fibrils are transferred efficiently from neurons to astrocytes but not the reverse, and support a major role of astrocytes in degradation of the protein and not in spreading of the fibrils (Loria et al., 2017). Microglial cells are capable of endocytosing α-synuclein and have the highest rate of degrading α-synuclein compared with neurons and astrocytes, consistent with a role in clearance (Lee et al., 2008). α-synuclein has been demonstrated to be released from neuronal cells in exosomes as well as in a naked form (Desplats et al., 2009; Jang et al., 2010; Danzer et al., 2012), suggesting that a secretory mechanism of transfer operates for this protein. The internalization of exosomally packaged α-synuclein has been reported to be more efficient than that of naked aggregates (Danzer et al., 2012); however, the mechanism of this uptake, whether through plasma membrane fusion or phagocytosis, is unclear. Interestingly, using microfluidic chambers, Freundt et al. (2012) observed axonal transport of exogenously uptaken α-synuclein fibrils within neurons and the subsequent transfer of these aggregates to recipient neurons in a secondary chamber. Because transfer occurred between immature neurons, this is considered to be mediated through secretion rather than synaptic contacts (Brahic et al., 2016).
mHtt
In HD, expansion of the CAG repeat in the HTT gene results in the intracellular accumulation of polyQ Htt aggregates. These aggregates were shown to be transmissible to daughter nonneuronal cells after mitosis through an undefined mechanism (Ren et al., 2009). Subsequently, transfer of mHtt fragments between neuronal cells and between primary neurons was first demonstrated in vitro to occur predominantly through cell–cell contact (Costanzo et al., 2013). Later work in vivo demonstrated that the non–cell-autonomous propagation of mHtt in the central nervous system also occurs between neurons and glial cells (Pearce et al., 2015) as well as between neurons (Pecho-Vrieseling et al., 2014; Babcock and Ganetzky, 2015). An elegant set of experiments, using both organotypic mouse brain slices and in vivo models, demonstrated that corticostriatal neuron–neuron mHtt propagation was predominantly mediated through synapses and did not appear to be secretion mediated (Pecho-Vrieseling et al., 2014). However, other modes of transmission may apply, as Drosophila melanogaster glial cells were shown to phagocytose mHtt from neurons (Pearce et al., 2015). This event was followed by recruitment of soluble Htt in the glial cytoplasm into aggregates, thus suggesting that normal protective clearance mechanisms can be subverted to spread pathogenicity.
Common pathways for different diseases
Although synaptic transfer and exosomal secretion have long been investigated as potential mechanisms of aggregate transfer, the discovery of TNTs in 2004 as a mechanism for direct cell–cell contact (Rustom et al., 2004) opened new avenues in the study of intercellular aggregate transfer. Here, we discuss two aspects of the mechanism of transfer that we propose to have a major impact on the propagation of amyloidogenic prion-like seeds: TNTs and lysosomes.
TNTs: A direct, fast route for prion-like aggregates?
TNTs and aggregate transfer
TNTs are emerging as a mechanism for long-range communication between cells (Rustom et al., 2004). These dynamic structures create direct transient cytoplasmic continuity between otherwise unconnected cells. TNTs are membranous tubes that can extend up to 100 µm in length, with diameters ranging between 50 and 800 nm, and are supported by actin and, in certain cases, microtubules (Abounit and Zurzolo, 2012). It was recently demonstrated that despite their similarity with filopodial protrusions, TNTs and filopodia are distinct structures. Indeed, although the formation of both these membrane extensions appear to rely on actin polymerization inside the protrusion, the same actin regulators (e.g., CDC42–IRSp53–VASP network and EPS8) exert contrasting effects on the two structures. This indicates that a switch in the molecular composition in common actin regulatory complexes is critical for driving the formation of either type of membrane protrusion (Delage et al., 2016).
TNTs mediate the transfer of a variety of cellular organelles, including mitochondria and lysosomes, from one cell to another (Rustom et al., 2004; Wang and Gerdes, 2015; Zhu et al., 2015). They may perform protective roles, such as correcting a genetic lysosomal defect that causes cystinosis by supplying functional lysosomes from macrophages to cystinosin-deficient cells while concomitantly mediating transfer of dysfunctional cystine-loaded lysosomes away from the diseased cell (Naphade et al., 2015). Of specific interest, they also mediate the transfer of pathogens such as viruses (Eugenin et al., 2009) and have been shown to effectively propagate PrPSc and several prion-like proteins intercellularly (Gousset et al., 2009; Wang et al., 2011; Costanzo et al., 2013; Ding et al., 2015; Abounit et al., 2016a,b). Specifically, PrPSc transfers from dendritic cells to neurons and between neurons in co-cultures through TNTs (Gousset et al., 2009), wherein it colocalized with late endosomes/lysosomes and recycling endosomes (Zhu et al., 2015), both of which contain the propitious environment for prion conversion as well as infectious seeds (Marijanovic et al., 2009).
α-Synuclein fibrils can also transfer between neuronal cells inside TNTs within lysosomes (Abounit et al., 2016b) and seed soluble α-synuclein in acceptor cells in co-culture. Notably, we found that in neuronal co-culture systems, transfer of α-synuclein was not significantly affected by transfecting acceptor cells with a dominant-negative dynamin construct shown before to inhibit α-synuclein endocytosis (Abounit et al., 2016b), thus suggesting that endocytic uptake of α-synuclein was not necessary for transfer. Although evidence also supports the localization and transfer in TNTs of Aβ, tau, and polyQ Htt (Wang et al., 2011; Costanzo et al., 2013; Abounit et al., 2016a), it is unclear whether these proteins are also associated with lysosomes, as shown for PrPSc and α-synuclein, during TNT mediated transfer.
TNT induction: A cellular stress response
Evidence shows that increasing TNT formation through the use of established TNT inducers enhances the intercellular transfer of α-synuclein and prion (Gousset et al., 2013; Abounit et al., 2016b). Congruently, inhibiting the formation of TNTs reduces intercellular transfer of these aggregates (Gousset et al., 2009; Abounit et al., 2016b). Strikingly, intracellular accumulation of the aggregates themselves induces an increase in TNT formation and thereby intercellular transfer of the respective protein to neighboring cells (Costanzo et al., 2013; Zhu et al., 2015; Abounit et al., 2016a,b). This was demonstrated directly for PrP and Htt to be caused by the pathogenic conformer, as overexpression of the native conformer did not result in increased intercellular transfer (Costanzo et al., 2013; Zhu et al., 2015). We also have direct evidence that this is also the case for αsynuclein and tau (unpublished data). Collectively, these data suggest that TNTs may be induced by the stress that accompanies intracellular aggregate buildup (Abounit et al., 2016a). Indeed, although oxidative stress increases TNT formation in neuronal cells and primary neurons (Gousset et al., 2009; Wang et al., 2011), it is also associated with the presence of aggregated proteins in neurodegeneration. Thus, we propose that reactive oxygen species (ROS), generated as a consequence of prion-like protein aggregation, may stimulate TNT formation through an as yet unknown mechanism (Fig. 2).
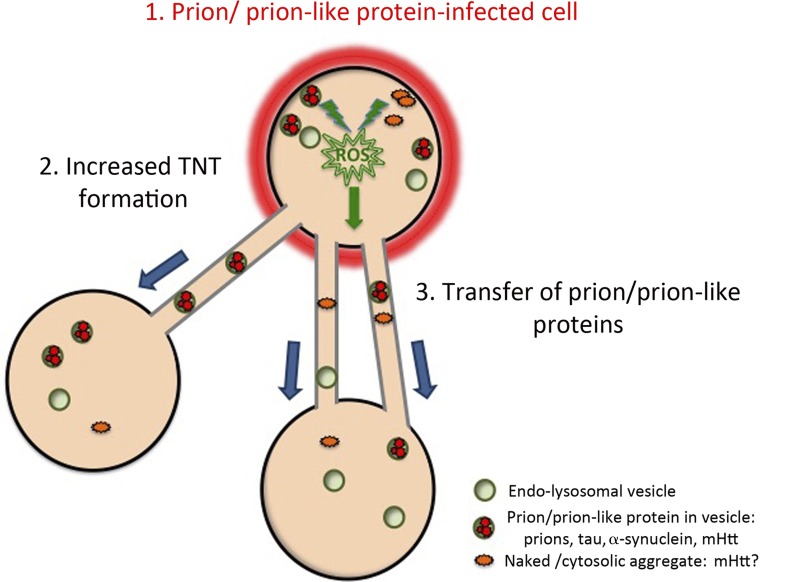
Figure 2.
Model of prion-like protein aggregate–induced formation of TNTs and intercellular spreading. The aggregation and accumulation of prions or prion-like proteins in an “infected” cell (1) induces an increase in TNT number via an unknown mechanism, e.g., via ROS-induced stress pathways (2). The prion/prion-like aggregates may then be propagated via TNTs from infected cells to naive cells (3), wherein they effect the conversion and seeding of naive self-protein molecules. Adapted from Abounit et al. (2016a).
It is also well known that actin changes its polymerization status as a consequence of different types of stress (Schiffhauer and Robinson, 2017). For example, a cofilin–actin rod stress response whereby cofilin saturates actin filaments causing them to bundle into rod structures occurs under a variety of stress conditions and specifically in PMDs (including AD, PD, and HD; Munsie and Truant, 2012). Another possibility for stimulating TNT formation could be the up-regulation of membrane biosynthetic genes through the unfolded protein response (UPR), which is activated in protein misfolding diseases (Moreno et al., 2013). Alternatively, stress mediated through lysosomes may be involved. As discussed in more detail in the Intercellular transfer of aggregates via lysosomal dysregulation section, prion-like aggregates are dispatched to lysosomes for degradation, but their accumulation causes lysosomal damage that may induce a stress-response pathway (Butler and Bahr, 2006). This in turn could result in increased numbers of TNTs, thus favoring the transfer of damaged lysosomes and prion-like aggregates from one cell to another and contribute to the spreading of the pathogenic aggregates (Fig. 2). We propose that TNTs may represent a common “rescue” mechanism adopted by the cell to rid itself of toxic material such as protein aggregates or damaged organelles. Meanwhile, because it has been shown that TNTs can also rescue functions by transferring healthy organelles such as lysosomes and mitochondria to damaged cells (Naphade et al., 2015; Rustom, 2016), we can envisage that a reciprocal transfer of such organelles from healthy to damaged cells can also occur. An alternative hypothesis could therefore be that stressed cells send out TNTs to be rescued and that the aggregates would simply hijack these highways to transfer and spread between cells. Essential experiments to understand the direction of transfer (uni- or bidirectional) and the mechanism of TNT formation, TNT structure, and TNT composition are needed to understand which of these non–mutually exclusive hypotheses is correct.
Lysosomal impairment in NDs: A link with intercellular propagation?
Risk factors in ND
Although more than 90% of NDs are sporadic, genome-wide association studies and whole-genome and whole-exome sequencing have identified different loci correlated with an increased risk for late-onset PMDs. These unbiased analyses surprisingly converge on abnormalities in the endolysosomal pathway in early, preclinical stages of the diseases (Peric and Annaert, 2015; Abeliovich and Gitler, 2016). Variants of endocytic regulators with increased risk for late-onset AD include sortilin-related receptor 1 (SORL1), phosphatidylinositol-binding clathrin assembly protein (PICALM), bridging integrator 1 (BIN1), and CD2-associated protein (CD2AP).
There is also accumulating evidence that failure of the retromer complex could be a risk factor for NDs (Morel et al., 2013; Small and Petsko, 2015). The retromer complex plays a primary role in sorting endosomal cargo back to the cell surface for reuse, to the TGN, or, alternatively, to the lysosomes (Seaman, 2012). Defects such as haploinsufficiency or mutations in one or several units of the retromer complex have been associated with AD and PD in addition to other NDs (Wen et al., 2011; Zimprich et al., 2011; Zavodszky et al., 2014). Furthermore, reduced retromer levels were found in several parts of the brains of patients with AD or PD, suggesting that reduced retromer abundance may have been associated with these conditions, and retromer has also been suggested to play a role in Creutzfeldt-Jakob disease (Kipkorir et al., 2014). These retromer deficiencies could potentially lead to lysosomal dysfunction, which in turn can be both a cause and a consequence of NDs.
Lysosomes and aggregate-mediated pathology
Prion and prion-like proteins are targeted to the lysosome for degradation through both endocytic and autophagosomal routes, and these pathways are implicated in pathogenic protein conversion (Victoria and Zurzolo, 2015). As discussed in detail in the next section, aggregate accumulation in this organelle can cause its dysfunction through different mechanisms, such as membrane permeabilization and loss of lysosomal integrity (Fig. 3), and lysosomes may be involved in intercellular aggregate transfer, including through lysosomal exocytosis of their contents, or travel through TNTs for direct cell–cell transfer (Annunziata et al., 2013; Abounit et al., 2016b). How the aggregates transfer in lysosomes is also a question of interest. Upon endocytosis, α-synuclein and prion colocalize with lysosomal proteins, presumably within the organelle (Freeman et al., 2013; Victoria et al., 2016; Loria et al., 2017). However, cytosolic aggregates of α-synuclein are also translocated through the lysosomal membrane through chaperone-mediated autophagy, and evidence indicates that pathogenic α-synuclein mutants bind to receptors on the cytosolic face of lysosomal membrane but are poorly translocated to the interior for degradation (Cuervo et al., 2004). Thus, aggregates may also associate with the cytosolic face of lysosomes, where they may interfere with lysosomal translocation function and signaling complexes. In neurons, internalized tau is found in lysosomes and may travel through axons in these compartments (Wu et al., 2013). A growing body of evidence further indicates that tau is degraded by the autophagic–lysosomal system and that disturbances to this system result in the enhanced formation of tau aggregates (Hamano et al., 2008). Furthermore, truncated tau products are described to remain initially associated to the cytosolic surface of the lysosomal membrane. This would favor oligomerization at or near the surface of the organelle, and eventually resulting in membrane disruption, lysosomal leakage, and release of other fragments into the cytosol, where they can induce aggregation (Wang et al., 2009). From these and many other studies (Alvarez-Erviti et al., 2011; Lee et al., 2013; Söllvander et al., 2016), it is evident that perturbations in the protein degradation machinery have consequences for the intercellular transfer of infectious aggregates. Lysosomal dysfunction is associated with many NDs in two different ways: (1) impairment of lysosomal function (e.g., because of genetic factors or ageing) leads to increased protein aggregation, and (2) accumulation of aggregated proteins leads to lysosomal impairment, thus originating a vicious circle (Fraldi et al., 2016).
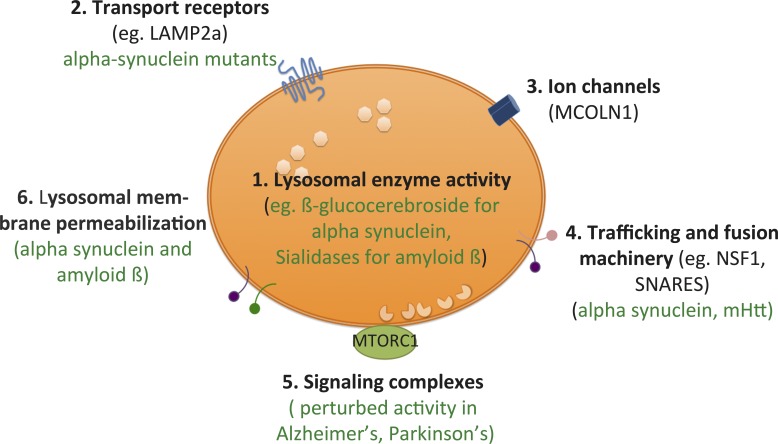
Figure 3.
Multiple factors within lysosome structures can affect proteopathic disease propagation. Lysosomes are complex vesicular structures that regulate cellular homeostasis as well as perform a significant part of cellular degradation. (1) Lysosomal enzyme activity may decrease with the natural processes of aging or by the accumulation of resistant protein aggregates within the structure. The resultant diminished degradative capacity can lead to increased intracellular burden, not only of the toxic aggregate but also of partially degraded/oxidized lipids such as cholesterol, resulting in lysosomal stress and buildup of ROS. Insufficiency in β-glucocerebroside, for example, is implicated in α-synuclein accumulation. (2) The cytosolic tails of transport receptor proteins such as Lamp2a are involved in the recognition of protein substrates of chaperone-mediated autophagy. Recognition of the misfolded protein results in translocation of the protein across the lysosomal membrane to be degraded. However, misfolded α-synuclein mutant aggregates can bind to Lamp2a receptors and block their ability to transport, thereby causing decreased degradation and increased cytosolic burden of aggregates that can result in neurotoxicity through a variety of pathways. Other receptors have yet to be investigated. (3) Calcium channels such as MCOLN1 are involved in mediating lysosomal exocytosis. They have been identified as being affected in lysosomal storage diseases, dysregulating normal Ca2+ homeostasis in the cell. Although Ca2+ homeostasis is disturbed in these diseases, it remains unclear whether channels like these are directly perturbed by the buildup of protein. (4) Proteins involved in membrane fusion, such as NSF1, have been shown to be important for clearance of toxic protein aggregates such as Htt. These proteins may regulate fusion with autophagosomes or with the plasma membrane for lysosomal exocytosis. Aggregate buildup on the surface also affects trafficking of these vesicles. (5) Lysosome–nucleus signaling is regulated by nutrient-sensing machinery, which includes the mTORC1 complex. Binding of the master transcription factor TFEB to mTORC1 normally sequesters TFEB in the cytoplasm. However, upon certain signals such as nutrient starvation, TFEB is released, whereupon it enters the nucleus and up-regulates genes involved in lysosome biogenesis, lysosomal exocytosis and autophagy. Synuclein has been shown to bind directly to TFEB, and therefore, cytosolic aggregates can potentially dysregulate this signaling during neurodegenerative disease. (6) Direct breaching of the integrity of the lysosomal membrane by aggregates has been proposed to cause lysosomal damage, release of aggregates into the cytosol thereby leading to propagation of seeding as well as causing apoptosis through the release of cathepsins into the cytoplasm. Adapted from Settembre et al. (2013).
Intercellular transfer of aggregates via lysosomal dysregulation
Apart from mediating degradation, lysosomes are significant hubs for stress signaling to the nucleus and mitochondria and regulate a variety of cellular processes, from nutrient homeostasis to plasma membrane repair to oxidative stress signaling and apoptosis (Butler and Bahr, 2006; Lim and Zoncu, 2016). The intracellular accumulation of amyloidogenic proteins, diminished degradative capacity, and damage to the lysosome membrane caused by their buildup (Wyss-Coray et al., 2003; Freeman et al., 2013; Abounit et al., 2016b) can therefore potentially misregulate lysosomes and their signaling pathways (Fig. 3), leading to their intercellular propagation by various means in an attempt to relieve the cell of their toxic burden. Diminishing lysosomal capacity can induce a compensatory increase of exosomal release (Vingtdeux et al., 2007; Alvarez-Erviti et al., 2011). Deficiency or mutations in the lysosomal hydrolase β-glucocerebrosidase, which cause the lysosomal storage disease known as Gaucher’s disease, have been linked to PD and cause lysosomal dysfunction and increased α-synuclein aggregate accumulation (Bae et al., 2015), which may result in increased intercellular transfer.
There are several indications that perturbations of the autophagy–lysosomal pathway increase extracellular vesicle/exosome release, in contexts such as lysosomal storage diseases, viral infections, and neurodegenerative diseases (Eitan et al., 2016). The study by Annunziata et al. (2013) demonstrated that lysosomal enzymatic deficiency resulted in not only impaired processing of Aβ but also increased lysosomal exocytosis through the oversialylation of Lamp1, which acts as a signal for exocytosis. Indeed, the lysosomal accumulation of most of these amyloidogenic proteins causes abnormal morphology and physical damage (Rockenstein et al., 2005; Freeman et al., 2013; Domert et al., 2014; Victoria et al., 2016). Notwithstanding, there are few data regarding whether lysosomal exocytosis is a generalized mechanism of extracellular release of these aggregates. Lysosomal exocytosis is regulated by increased Ca2+ levels; intriguingly, Ca2+ homeostasis is perturbed by Aβ, α-synuclein, and mHtt (Danzer et al., 2007; Lim et al., 2008; Small, 2009).
Lysosomal stress/damage caused by the accumulation of amyloidogenic proteins may also contribute to TNT formation (Abounit et al., 2016a,b). A recent model proposed that TNTs could be an intermediate cell survival response to cope with increased oxidative stress, allowing the bidirectional transfer of material between cells in an attempt to rescue damage before cell death (Rustom, 2016). Dissection of signaling pathways involved in TNT formation identified several molecules that overlap between mitochondrial and lysosomal signaling, notably the mTOR (Martini-Stoica et al., 2016; Rustom, 2016) and Akt–PI3K signaling pathways (Fig. 4; Wang et al., 2011). Oxidative stress, although associated with the mitochondria, is also regulated by lysosomal damage (Butler and Bahr, 2006). The coupling of mitochondria and lysosomes in regulating oxidative stress (the mitochondria–lysosome axis) could therefore be involved in regulating TNT formation induced by protein aggregates (Fig. 3). The intracellular accumulation of prions, α-synuclein, mHtt, and tau has been shown to increase TNT formation (Costanzo et al., 2013; Zhu et al., 2015; Abounit et al., 2016a,b), and these proteins are associated with increased ROS production and oxidative stress (Kim et al., 2015). Evidence suggests that lysosomes are damaged in various ways by these proteins, including diminished lysosomal degradation (Wyss-Coray et al., 2003; Cuervo et al., 2004; Butler and Bahr, 2006; Wang et al., 2009; Jackson et al., 2014) and lysosomal membrane permeabilization and rupture in the case of Aβ and synuclein and tau (Ditaranto et al., 2001; Wang et al., 2009; Freeman et al., 2013). Diminished lysosomal capacity results in the lysosomal buildup of oxidized proteins and other damaged material, causing lysosomal stress which can cause increased feedback loops of mitochondrial dysfunction and ROS production (Cleeter et al., 2013), as well as increased autophagy, a phenotype that is well documented in neurodegenerative disease (Butler and Bahr, 2006). Lysosomal membrane permeabilization may also trigger oxidative stress and eventual apoptosis (Ghosh et al., 2011). Aggregate-damaged lysosomes therefore may contribute to the localized ROS production proposed to be involved in TNT formation (Figs. 2 and 3).
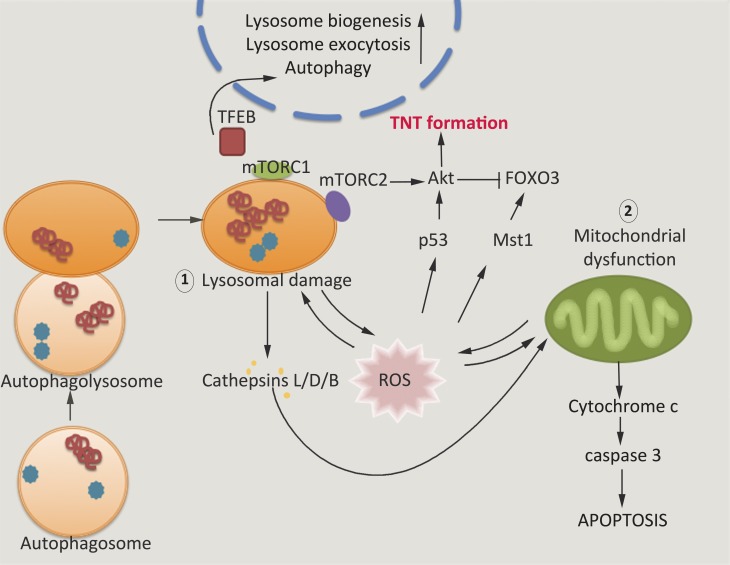
Figure 4.
Signaling pathways that can cross talk with TNT formation. The Akt-dependent TNT formation pathway cross talks with signaling complexes from the lysosome and the mitochondria. Increased oxidative stress, through increased ROS formation from aggregates within the cytosol or from lysosomal aggregation of protein, can cause mitochondrial dysfunction and lysosomal dysfunction, which act in a feedback loop to create increased ROS. Increased localized ROS is believed to be responsible for TNT formation (Rustom, 2016) through the Akt pathway. Lysosomal stress can also alter TFEB signaling, which is directly responsible for increasing lysosomal exocytosis (Martini-Stoica et al., 2016). Direct interaction between α-synuclein and TFEB has been demonstrated (Decressac et al., 2013), and thus, cytosolic aggregates may influence TFEB signaling in a way that affects its intercellular transfer.
There are several other indications that incomplete or insufficient lysosomal activity is particularly important in creating neurotoxicity and neurodegeneration. Transcription factor EB (TFEB), a major regulator of the autophagy–lysosomal pathway, is a cytosolic transcription factor that can associate with the lysosome surface under normal conditions (Sardiello et al., 2009; Martina et al., 2012). It is itself regulated by the mTOR pathway and, upon nutrient starvation or other lysosomal stresses, translocates to the nucleus, up-regulating lysosome biogenesis and exocytosis (Settembre et al., 2011, 2012; Martina et al., 2012; Medina et al., 2015). Several studies point to it being affected in PD patients and, to a less well-defined extent, in AD pathologies (Martini-Stoica et al., 2016). Restoring lysosomal activity through the overexpression of TFEB has been shown to rescue neurotoxicity resulting from tau, Htt, and α-synuclein aggregation (Tsunemi et al., 2012; Decressac et al., 2013; Polito et al., 2014). Congruently, miRNA repression of TFEB exacerbated synuclein-mediated toxicity in dopaminergic neurons (Decressac et al., 2013). Additionally, TFEB-induced astrocytic clearance reduced external amyloid plaque deposition (Xiao et al., 2014), highlighting a link between impaired degradation and disease propagation through extracellular release of toxic material. It is therefore increasingly clear that perturbations of degradation or clearance organelles, especially the lysosome, affect intercellular transfer. This is especially significant in light of the emerging role of this organelle as a signaling hub that regulates cellular homeostasis, and so it is tempting to consider them as new therapeutic targets. However, it should be noted that these pathways are not solely responsible for toxicity and that responses like the UPR are also up-regulated. Therapeutic treatment targeting the UPR, for instance, has been shown to relieve prion infections in mice (Moreno et al., 2013); therefore, several cellular pathways are simultaneously affected. Additionally, therapeutics aimed at increasing or rescuing lysosomal activity should also take into account the role of this organelle in the production of amyloidogenic aggregates (Pasternak et al., 2003; Wang et al., 2009; Sannerud et al., 2016).
Conclusion and perspectives
Recent data suggest that transfer of PrPSc, α-synuclein, tau, polyQ aggregates, and Aβ assemblies between co-cultured neuronal cells and primary neurons occurs through TNTs. Despite differences in structure, they are all prion-like, supporting the hypothesis that other prion-like protein aggregates may use TNTs as one mechanism of intercellular transmission. We propose that TNTs have a relevant role in the spreading of the pathology of prions and other NDs. As intracellular accumulation of misfolded proteins causes lysosomal impairments, cells try to dispose of damaged material through TNTs, which enhances their formation. We also postulate that accumulation of aggregates in lysosomes changes their signaling/positioning, making them more prone to entering the newly formed TNTs. As a consequence of lysosomal transfer and damage, proteins escape from lysosomes, leading to further seeding and propagation of the diseases (Fig. 2).
Characterizing the mechanism of TNT formation and how misfolded protein aggregates trigger their formation along with their intercellular transport, as well as the mechanisms leading to lysosomal impairment, may lead to new therapeutic avenues to inhibit the spreading of prion-like proteins and the progression of these devastating, fatal diseases.
Acknowledgments
We thank Drs. Ayşegül Dilsizoğlu-Şenol, Yuan-Ju Wu, and Frida Loria Salinas for their critical reading of the manuscript.
C. Zurzolo was supported by the Agence Nationale de Recherche (grants Joint Programme–Neurodegenerative Disease Neutargets: ANR-14-JPCD-0002-01 and ANR-16 CE160019-01 NEUROTUNN) and the Equipe Fondation Recherche Médicale 2014 (grant DEQ20140329557). G. Soraya Victoria was supported by Bourse Pasteur-Roux, Institut Pasteur, Paris.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Aβ
- amyloid β
- AD
- Alzheimer’s disease
- APP
- amyloid precursor protein
- ESCRT
- endosomal sorting complexes required for transport
- HD
- Huntington’s disease
- MVB
- multivesicular body
- ND
- neurodegenerative disease
- PD
- Parkinson’s disease
- PMD
- protein-misfolding disease
- polyQ
- polyglutamine
- ROS
- reactive oxygen species
- TFEB
- transcription factor EB
- TNT
- tunneling nanotube
- UPR
- unfolded protein response
References
- Abeliovich A., and Gitler A.D.. 2016. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 539:207–216. 10.1038/nature20414 [DOI] [PubMed] [Google Scholar]
- Abounit S., and Zurzolo C.. 2012. Wiring through tunneling nanotubes: From electrical signals to organelle transfer. J. Cell Sci. 125:1089–1098. 10.1242/jcs.083279 [DOI] [PubMed] [Google Scholar]
- Abounit S., Bousset L., Loria F., Zhu S., de Chaumont F., Pieri L., Olivo-Marin J.C., Melki R., and Zurzolo C.. 2016b Tunneling nanotubes spread pathogenic α-synuclein fibrils by intercellular trafficking of lysosomes. EMBO J. 35:2120–2138. 10.15252/embj.201593411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abounit S., Wu J.W., Duff K., Victoria G.S., and Zurzolo C.. 2016a Tunneling nanotubes: A possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion. 10:344–351. 10.1080/19336896.2016.1223003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A., and Lakkaraju A.K.. 2016. Cell biology of prions and prionoids: A status report. Trends Cell Biol. 26:40–51. 10.1016/j.tcb.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L., Seow Y., Schapira A.H., Gardiner C., Sargent I.L., Wood M.J., and Cooper J.M.. 2011. Lysosomal dysfunction increases exosome-mediated α-synuclein release and transmission. Neurobiol. Dis. 42:360–367. 10.1016/j.nbd.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E., Steiner J.A., Lema Tomé C.M., Ekström P., Mattsson B., Björklund A., and Brundin P.. 2012. α-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One. 7:e39465 10.1371/journal.pone.0039465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata I., Patterson A., Helton D., Hu H., Moshiach S., Gomero E., Nixon R., and d’Azzo A.. 2013. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat. Commun. 4:2734 10.1038/ncomms3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano-Anaya Z.E., Huor A., Leblanc P., Lehmann S., Provansal M., Raposo G., Andréoletti O., and Vilette D.. 2015. Prion strains are differentially released through the exosomal pathway. Cell. Mol. Life Sci. 72:1185–1196. 10.1007/s00018-014-1735-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D.T., and Ganetzky B.. 2015. Transcellular spreading of huntingtin aggregates in the Drosophila brain. Proc. Natl. Acad. Sci. USA. 112:E5427–E5433. 10.1073/pnas.1516217112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.J., Yang N.Y., Lee C., Lee H.J., Kim S., Sardi S.P., and Lee S.J.. 2015. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp. Mol. Med. 47:e153 10.1038/emm.2014.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Bousset L., Bieri G., Melki R., and Gitler A.D.. 2016. Axonal transport and secretion of fibrillar forms of α-synuclein, Aβ42 peptide and HTTExon 1. Acta Neuropathol. 131:539–548. 10.1007/s00401-016-1538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Melki R., and Kopito R.. 2010. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 11:301–307. 10.1038/nrm2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D., and Bahr B.A.. 2006. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid. Redox Signal. 8:185–196. 10.1089/ars.2006.8.185 [DOI] [PubMed] [Google Scholar]
- Calafate S., Buist A., Miskiewicz K., Vijayan V., Daneels G., de Strooper B., de Wit J., Verstreken P., and Moechars D.. 2015. Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Reports. 11:1176–1183. 10.1016/j.celrep.2015.04.043 [DOI] [PubMed] [Google Scholar]
- Chai X., Dage J.L., and Citron M.. 2012. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol. Dis. 48:356–366. 10.1016/j.nbd.2012.05.021 [DOI] [PubMed] [Google Scholar]
- Choy R.W., Cheng Z., and Schekman R.. 2012. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid β (Aβ) production in the trans-Golgi network. Proc. Natl. Acad. Sci. USA. 109:E2077–E2082. 10.1073/pnas.1208635109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Bolmont T., Crowther R.A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A.K., Beibel M., Staufenbiel M., et al. 2009. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11:909–913. 10.1038/ncb1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Grueninger F., and Tolnay M.. 2014. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology. 76(Pt A):9–15. 10.1016/j.neuropharm.2013.08.037 [DOI] [PubMed] [Google Scholar]
- Cleeter M.W., Chau K.Y., Gluck C., Mehta A., Hughes D.A., Duchen M., Wood N.W., Hardy J., Mark Cooper J., and Schapira A.H.. 2013. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem. Int. 62:1–7. 10.1016/j.neuint.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B.M., Hanssen E., Lawson V.A., and Hill A.F.. 2012. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J. 26:4160–4173. 10.1096/fj.11-202077 [DOI] [PubMed] [Google Scholar]
- Colombo M., Raposo G., and Théry C.. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30:255–289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Costanzo M., and Zurzolo C.. 2013. The cell biology of prion-like spread of protein aggregates: Mechanisms and implication in neurodegeneration. Biochem. J. 452:1–17. 10.1042/BJ20121898 [DOI] [PubMed] [Google Scholar]
- Costanzo M., Abounit S., Marzo L., Danckaert A., Chamoun Z., Roux P., and Zurzolo C.. 2013. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci. 126:3678–3685. 10.1242/jcs.126086 [DOI] [PubMed] [Google Scholar]
- Cronier S., Laude H., and Peyrin J.M.. 2004. Prions can infect primary cultured neurons and astrocytes and promote neuronal cell death. Proc. Natl. Acad. Sci. USA. 101:12271–12276. 10.1073/pnas.0402725101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronier S., Carimalo J., Schaeffer B., Jaumain E., Béringue V., Miquel M.C., Laude H., and Peyrin J.M.. 2012. Endogenous prion protein conversion is required for prion-induced neuritic alterations and neuronal death. FASEB J. 26:3854–3861. 10.1096/fj.11-201772 [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Stefanis L., Fredenburg R., Lansbury P.T., and Sulzer D.. 2004. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science. 305:1292–1295. 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- Danzer K.M., Haasen D., Karow A.R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., and Kostka M.. 2007. Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27:9220–9232. 10.1523/JNEUROSCI.2617-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer K.M., Kranich L.R., Ruf W.P., Cagsal-Getkin O., Winslow A.R., Zhu L., Vanderburg C.R., and McLean P.J.. 2012. Exosomal cell-to-cell transmission of α synuclein oligomers. Mol. Neurodegener. 7:42 10.1186/1750-1326-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W.S., Jonas A., Clayton D.F., and George J.M.. 1998. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273:9443–9449. 10.1074/jbc.273.16.9443 [DOI] [PubMed] [Google Scholar]
- Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., and Björklund A.. 2013. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl. Acad. Sci. USA. 110:E1817–E1826. 10.1073/pnas.1305623110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage E., Cervantes D.C., Pénard E., Schmitt C., Syan S., Disanza A., Scita G., and Zurzolo C.. 2016. Differential identity of filopodia and tunneling nanotubes revealed by the opposite functions of actin regulatory complexes. Sci. Rep. 6:39632 10.1038/srep39632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., and Lee S.J.. 2009. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl. Acad. Sci. USA. 106:13010–13015. 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., and Karran E.. 2016. The cellular phase of Alzheimer’s disease. Cell. 164:603–615. 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- Ding X., Ma M., Teng J., Teng R.K., Zhou S., Yin J., Fonkem E., Huang J.H., Wu E., and Wang X.. 2015. Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure. Oncotarget. 6:24178–24191. 10.18632/oncotarget.4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditaranto K., Tekirian T.L., and Yang A.J.. 2001. Lysosomal membrane damage in soluble Aβ-mediated cell death in Alzheimer’s disease. Neurobiol. Dis. 8:19–31. 10.1006/nbdi.2000.0364 [DOI] [PubMed] [Google Scholar]
- Domert J., Rao S.B., Agholme L., Brorsson A.C., Marcusson J., Hallbeck M., and Nath S.. 2014. Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis. 65:82–92. 10.1016/j.nbd.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Edgar J.R., Willén K., Gouras G.K., and Futter C.E.. 2015. ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-β accumulation. J. Cell Sci. 128:2520–2528. 10.1242/jcs.170233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E., Suire C., Zhang S., and Mattson M.P.. 2016. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 32:65–74. 10.1016/j.arr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin E.A., Gaskill P.J., and Berman J.W.. 2009. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: A potential mechanism for intercellular HIV trafficking. Cell. Immunol. 254:142–148. 10.1016/j.cellimm.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiler M.S., Strobel B., Freischmidt A., Helferich A.M., Kappel J., Brewer B.M., Li D., Thal D.R., Walther P., Ludolph A.C., et al. 2015. TDP-43 is intercellularly transmitted across axon terminals. J. Cell Biol. 211:897–911. 10.1083/jcb.201504057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., and Raposo G.. 2004. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA. 101:9683–9688. 10.1073/pnas.0308413101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A., Klein A.D., Medina D.L., and Settembre C.. 2016. Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. 39:277–295. 10.1146/annurev-neuro-070815-014031 [DOI] [PubMed] [Google Scholar]
- Freeman D., Cedillos R., Choyke S., Lukic Z., McGuire K., Marvin S., Burrage A.M., Sudholt S., Rana A., O’Connor C., et al. 2013. α-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One. 8:e62143 10.1371/journal.pone.0062143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt E.C., Maynard N., Clancy E.K., Roy S., Bousset L., Sourigues Y., Covert M., Melki R., Kirkegaard K., and Brahic M.. 2012. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 72:517–524. 10.1002/ana.23747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B., Jacks R.L., and Diamond M.I.. 2009. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284:12845–12852. 10.1074/jbc.M808759200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M., Carlsson F., Laskar A., Yuan X.M., and Li W.. 2011. Lysosomal membrane permeabilization causes oxidative stress and ferritin induction in macrophages. FEBS Lett. 585:623–629. 10.1016/j.febslet.2010.12.043 [DOI] [PubMed] [Google Scholar]
- Gorbenko G.P., and Kinnunen P.K.. 2006. The role of lipid-protein interactions in amyloid-type protein fibril formation. Chem. Phys. Lipids. 141:72–82. 10.1016/j.chemphyslip.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Gousset K., Schiff E., Langevin C., Marijanovic Z., Caputo A., Browman D.T., Chenouard N., de Chaumont F., Martino A., Enninga J., et al. 2009. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11:328–336. 10.1038/ncb1841 [DOI] [PubMed] [Google Scholar]
- Gousset K., Marzo L., Commere P.H., and Zurzolo C.. 2013. Myo10 is a key regulator of TNT formation in neuronal cells. J. Cell Sci. 126:4424–4435. 10.1242/jcs.129239 [DOI] [PubMed] [Google Scholar]
- Guo J.L., and Lee V.M.. 2014. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 20:130–138. 10.1038/nm.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T., Gendron T.F., Causevic E., Yen S.H., Lin W.L., Isidoro C., Deture M., and Ko L.W.. 2008. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur. J. Neurosci. 27:1119–1130. 10.1111/j.1460-9568.2008.06084.x [DOI] [PubMed] [Google Scholar]
- Hansen C., Angot E., Bergström A.L., Steiner J.A., Pieri L., Paul G., Outeiro T.F., Melki R., Kallunki P., Fog K., et al. 2011. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 121:715–725. 10.1172/JCI43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Nonaka T., and Masuda-Suzukake M.. 2017. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol. Ther. 172:22–33. 10.1016/j.pharmthera.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Jackson W.S., Krost C., Borkowski A.W., and Kaczmarczyk L.. 2014. Translation of the prion protein mRNA is robust in astrocytes but does not amplify during reactive astrocytosis in the mouse brain. PLoS One. 9:e95958 10.1371/journal.pone.0095958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A., Lee H.J., Suk J.E., Jung J.W., Kim K.P., and Lee S.J.. 2010. Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions. J. Neurochem. 113:1263–1274. [DOI] [PubMed] [Google Scholar]
- Jansen A.H., Batenburg K.L., Pecho-Vrieseling E., and Reits E.A.. 2017. Visualization of prion-like transfer in Huntington’s disease models. Biochim. Biophys. Acta. 1863:793–800. 10.1016/j.bbadis.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Kanu N., Imokawa Y., Drechsel D.N., Williamson R.A., Birkett C.R., Bostock C.J., and Brockes J.P.. 2002. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 12:523–530. 10.1016/S0960-9822(02)00722-4 [DOI] [PubMed] [Google Scholar]
- Kfoury N., Holmes B.B., Jiang H., Holtzman D.M., and Diamond M.I.. 2012. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 287:19440–19451. 10.1074/jbc.M112.346072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.H., Kim J.E., Rhie S.J., and Yoon S.. 2015. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24:325–340. 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipkorir T., Tittman S., Botsios S., and Manuelidis L.. 2014. Highly infectious CJD particles lack prion protein but contain many viral-linked peptides by LC-MS/MS. J. Cell. Biochem. 115:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., and Olanow C.W.. 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14:504–506. 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- Lee H.J., Suk J.E., Bae E.J., and Lee S.J.. 2008. Clearance and deposition of extracellular α-synuclein aggregates in microglia. Biochem. Biophys. Res. Commun. 372:423–428. 10.1016/j.bbrc.2008.05.045 [DOI] [PubMed] [Google Scholar]
- Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S., Hwang D., Masliah E., and Lee S.J.. 2010. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 285:9262–9272. 10.1074/jbc.M109.081125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Cho E.D., Lee K.W., Kim J.H., Cho S.G., and Lee S.J.. 2013. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 45:e22 10.1038/emm.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.T., Xu J., Lee J.M., Ku G., Han X., Yang D.I., Chen S., and Hsu C.Y.. 2004. Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J. Cell Biol. 164:123–131. 10.1083/jcb.200307017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.Y., Englund E., Widner H., Rehncrona S., Björklund A., Lindvall O., and Brundin P.. 2010. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov. Disord. 25:1091–1096. 10.1002/mds.23012 [DOI] [PubMed] [Google Scholar]
- Li W., Tang Y., Fan Z., Meng Y., Yang G., Luo J., and Ke Z.J.. 2013. Autophagy is involved in oligodendroglial precursor-mediated clearance of amyloid peptide. Mol. Neurodegener. 8:27 10.1186/1750-1326-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.Y., and Zoncu R.. 2016. The lysosome as a command-and-control center for cellular metabolism. J. Cell Biol. 214:653–664. 10.1083/jcb.201607005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., and Carafoli E.. 2008. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 283:5780–5789. 10.1074/jbc.M704704200 [DOI] [PubMed] [Google Scholar]
- Liu L., Drouet V., Wu J.W., Witter M.P., Small S.A., Clelland C., and Duff K.. 2012. Trans-synaptic spread of tau pathology in vivo. PLoS One. 7:e31302 10.1371/journal.pone.0031302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F., Vargas J.Y., Bousset L., Syan S., Salles A., Melki R., and Zurzolo C.. 2017. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. In press. [DOI] [PubMed] [Google Scholar]
- Luk K.C., Song C., O’Brien P., Stieber A., Branch J.R., Brunden K.R., Trojanowski J.Q., and Lee V.M.. 2009. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 106:20051–20056. 10.1073/pnas.0908005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K.C., Kehm V.M., Zhang B., O’Brien P., Trojanowski J.Q., and Lee V.M.. 2012a Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209:975–986. 10.1084/jem.20112457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., and Lee V.M.. 2012b Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 338:949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella M., and Chabry J.. 2004. Neurons and astrocytes respond to prion infection by inducing microglia recruitment. J. Neurosci. 24:620–627. 10.1523/JNEUROSCI.4303-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijanovic Z., Caputo A., Campana V., and Zurzolo C.. 2009. Identification of an intracellular site of prion conversion. PLoS Pathog. 5:e1000426 10.1371/journal.ppat.1000426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina J.A., Chen Y., Gucek M., and Puertollano R.. 2012. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 8:903–914. 10.4161/auto.19653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini-Stoica H., Xu Y., Ballabio A., and Zheng H.. 2016. The autophagy-lysosomal pathway in neurodegeneration: A TFEB perspective. Trends Neurosci. 39:221–234. 10.1016/j.tins.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K.J., Powell K.L., Ashton A.W., Morris J.M., and McCracken S.A. Exosomes: Mechanisms of uptake. J. Circ. Biomark; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D.L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., et al. 2015. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17:288–299. 10.1038/ncb3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E., Chamoun Z., Lasiecka Z.M., Chan R.B., Williamson R.L., Vetanovetz C., Dall’Armi C., Simoes S., Point Du Jour K.S., McCabe B.D., et al. 2013. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat. Commun. 4:2250 10.1038/ncomms3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J.A., Halliday M., Molloy C., Radford H., Verity N., Axten J.M., Ortori C.A., Willis A.E., Fischer P.M., Barrett D.A., and Mallucci G.R.. 2013. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5:206ra138 10.1126/scitranslmed.3006767 [DOI] [PubMed] [Google Scholar]
- Munsie L.N., and Truant R.. 2012. The role of the cofilin-actin rod stress response in neurodegenerative diseases uncovers potential new drug targets. BioArchitecture. 2:204–208. 10.4161/bioa.22549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naphade S., Sharma J., Gaide Chevronnay H.P., Shook M.A., Yeagy B.A., Rocca C.J., Ur S.N., Lau A.J., Courtoy P.J., and Cherqui S.. 2015. Brief reports: Lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells. 33:301–309. 10.1002/stem.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S., Agholme L., Kurudenkandy F.R., Granseth B., Marcusson J., and Hallbeck M.. 2012. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J. Neurosci. 32:8767–8777. 10.1523/JNEUROSCI.0615-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak S.H., Bagshaw R.D., Guiral M., Zhang S., Ackerley C.A., Pak B.J., Callahan J.W., and Mahuran D.J.. 2003. Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane. J. Biol. Chem. 278:26687–26694. 10.1074/jbc.M304009200 [DOI] [PubMed] [Google Scholar]
- Pearce M.M., Spartz E.J., Hong W., Luo L., and Kopito R.R.. 2015. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat. Commun. 6:6768 10.1038/ncomms7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E., Rieker C., Fuchs S., Bleckmann D., Esposito M.S., Botta P., Goldstein C., Bernhard M., Galimberti I., Müller M., et al. 2014. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat. Neurosci. 17:1064–1072. 10.1038/nn.3761 [DOI] [PubMed] [Google Scholar]
- Peric A., and Annaert W.. 2015. Early etiology of Alzheimer’s disease: Tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol. 129:363–381. 10.1007/s00401-014-1379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L., Madiona K., Bousset L., and Melki R.. 2012. Fibrillar α-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys. J. 102:2894–2905. 10.1016/j.bpj.2012.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrishevsky E., Grad L.I., and Cashman N.R.. 2016. TDP-43 or FUS-induced misfolded human wild-type SOD1 can propagate intercellularly in a prion-like fashion. Sci. Rep. 6:22155 10.1038/srep22155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito V.A., Li H., Martini-Stoica H., Wang B., Yang L., Xu Y., Swartzlander D.B., Palmieri M., di Ronza A., Lee V.M., et al. 2014. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol. Med. 6:1142–1160. 10.15252/emmm.201303671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M., and Cleveland D.W.. 2012. Prion-like spread of protein aggregates in neurodegeneration. J. Exp. Med. 209:889–893. 10.1084/jem.20120741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler A.M., Phillips E.C., Lau D.H., Noble W., and Hanger D.P.. 2013. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14:389–394. 10.1038/embor.2013.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Montrasio F., Furukawa H., van der Haar M.E., Schwarz P., Rülicke T., Giger O.T., Häusler K.G., Perez D., Glatzel M., and Aguzzi A.. 2004. Intrinsic resistance of oligodendrocytes to prion infection. J. Neurosci. 24:5974–5981. 10.1523/JNEUROSCI.0122-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S.B. 1982. Novel proteinaceous infectious particles cause scrapie. Science. 216:136–144. 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. 2013. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 47:601–623. 10.1146/annurev-genet-110711-155524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., Yau W.M., Lu J.X., Collinge J., and Tycko R.. 2017. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature. 541:217–221. 10.1038/nature20814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek C., and Hill A.F.. 2017. The role of extracellular vesicles in neurodegenerative diseases. Biochem. Biophys. Res. Commun. 483:1178–1186. 10.1016/j.bbrc.2016.09.090 [DOI] [PubMed] [Google Scholar]
- Rajendran L., Honsho M., Zahn T.R., Keller P., Geiger K.D., Verkade P., and Simons K.. 2006. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 103:11172–11177. 10.1073/pnas.0603838103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P.H., Lauckner J.E., Kachirskaia I., Heuser J.E., Melki R., and Kopito R.R.. 2009. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 11:219–225. 10.1038/ncb1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J.F., Rey N.L., Bousset L., Melki R., Brundin P., and Angot E.. 2014. α-Synuclein transfers from neurons to oligodendrocytes. Glia. 62:387–398. 10.1002/glia.22611 [DOI] [PubMed] [Google Scholar]
- Riek R., and Eisenberg D.S.. 2016. The activities of amyloids from a structural perspective. Nature. 539:227–235. 10.1038/nature20416 [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Schwach G., Ingolic E., Adame A., Crews L., Mante M., Pfragner R., Schreiner E., Windisch M., and Masliah E.. 2005. Lysosomal pathology associated with α-synuclein accumulation in transgenic models using an eGFP fusion protein. J. Neurosci. Res. 80:247–259. 10.1002/jnr.20446 [DOI] [PubMed] [Google Scholar]
- Rustom A. 2016. The missing link: Does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 6:160057 10.1098/rsob.160057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A., Saffrich R., Markovic I., Walther P., and Gerdes H.H.. 2004. Nanotubular highways for intercellular organelle transport. Science. 303:1007–1010. 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A.C., Alvarez V.E., Lee N.C., and Hall G.F.. 2012. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287:3842–3849. 10.1074/jbc.M111.277061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M.K., Al-Doujaily H., Sharps B., Clarke A.R., and Collinge J.. 2011. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 470:540–542. 10.1038/nature09768 [DOI] [PubMed] [Google Scholar]
- Sanders D.W., Kaufman S.K., Holmes B.B., and Diamond M.I.. 2016. Prions and protein assemblies that convey biological information in health and disease. Neuron. 89:433–448. 10.1016/j.neuron.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud R., Esselens C., Ejsmont P., Mattera R., Rochin L., Tharkeshwar A.K., De Baets G., De Wever V., Habets R., Baert V., et al. 2016. Restricted location of PSEN2/γ-secretase determines substrate specificity and generates an intracellular Aβ pool. Cell. 166:193–208. 10.1016/j.cell.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S., et al. 2009. A gene network regulating lysosomal biogenesis and function. Science. 325:473–477. [DOI] [PubMed] [Google Scholar]
- Schiffhauer E.S., and Robinson D.N.. 2017. Mechanochemical signaling directs cell-shape change. Biophys. J. 112:207–214. 10.1016/j.bpj.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N. 2012. The retromer complex: Endosomal protein recycling and beyond. J. Cell Sci. 125:4693–4702. 10.1242/jcs.103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S.U., Huynh T., Medina D., Colella P., et al. 2011. TFEB links autophagy to lysosomal biogenesis. Science. 332:1429–1433. 10.1126/science.1204592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Zoncu R., Medina D.L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M.C., et al. 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31:1095–1108. 10.1038/emboj.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Fraldi A., Medina D.L., and Ballabio A.. 2013. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14:283–296. 10.1038/nrm3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearin H., and Bessen R.A.. 2014. Axonal and transynaptic spread of prions. J. Virol. 88:8640–8655. 10.1128/JVI.00378-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.H. 2009. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem. Res. 34:1824–1829. 10.1007/s11064-009-9960-5 [DOI] [PubMed] [Google Scholar]
- Small S.A., and Petsko G.A.. 2015. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat. Rev. Neurosci. 16:126–132. 10.1038/nrn3896 [DOI] [PubMed] [Google Scholar]
- Söllvander S., Nikitidou E., Brolin R., Söderberg L., Sehlin D., Lannfelt L., and Erlandsson A.. 2016. Accumulation of amyloid-β by astrocytes result in enlarged endosomes and microvesicle-induced apoptosis of neurons. Mol. Neurodegener. 11:38 10.1186/s13024-016-0098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., and Goedert M.. 1998. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA. 95:6469–6473. 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W.K., and Apostol M.I.. 2011. Prion protein and its conformational conversion: A structural perspective. Top. Curr. Chem. 305:135–167. 10.1007/128_2011_165 [DOI] [PubMed] [Google Scholar]
- Tsunemi T., Ashe T.D., Morrison B.E., Soriano K.R., Au J., Roque R.A., Lazarowski E.R., Damian V.A., Masliah E., and La Spada A.R.. 2012. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med. 4:142ra97 10.1126/scitranslmed.3003799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson T., Steiner J.A., and Brundin P.. 2016. Sorting out release, uptake and processing of α-synuclein during prion-like spread of pathology. J. Neurochem. 139(Suppl 1):275–289. 10.1111/jnc.13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M., Vats A., and Taneja V.. 2015. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 18:138–145. 10.4103/0972-2327.144284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria G.S., and Zurzolo C.. 2015. Trafficking and degradation pathways in pathogenic conversion of prions and prion-like proteins in neurodegenerative diseases. Virus Res. 207:146–154. 10.1016/j.virusres.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Victoria G.S., Arkhipenko A., Zhu S., Syan S., and Zurzolo C.. 2016. Astrocyte-to-neuron intercellular prion transfer is mediated by cell-cell contact. Sci. Rep. 6:20762 10.1038/srep20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V., Hamdane M., Loyens A., Gelé P., Drobeck H., Bégard S., Galas M.C., Delacourte A., Beauvillain J.C., Buée L., and Sergeant N.. 2007. Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 282:18197–18205. 10.1074/jbc.M609475200 [DOI] [PubMed] [Google Scholar]
- Wang X., and Gerdes H.H.. 2015. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22:1181–1191. 10.1038/cdd.2014.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Martinez-Vicente M., Krüger U., Kaushik S., Wong E., Mandelkow E.M., Cuervo A.M., and Mandelkow E.. 2009. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. 18:4153–4170. 10.1093/hmg/ddp367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cui J., Sun X., and Zhang Y.. 2011. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18:732–742. 10.1038/cdd.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S., Maury E.A., Kirk M.J., Saqran L., Roe A., DeVos S.L., Nicholls S., Fan Z., Takeda S., Cagsal-Getkin O., et al. 2015. Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J. 34:3028–3041. 10.15252/embj.201592748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Tang F.L., Hong Y., Luo S.W., Wang C.L., He W., Shen C., Jung J.U., Xiong F., Lee D.H., et al. 2011. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J. Cell Biol. 195:765–779. 10.1083/jcb.201105109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.W., Herman M., Liu L., Simoes S., Acker C.M., Figueroa H., Steinberg J.I., Margittai M., Kayed R., Zurzolo C., et al. 2013. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 288:1856–1870. 10.1074/jbc.M112.394528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.W., Hussaini S.A., Bastille I.M., Rodriguez G.A., Mrejeru A., Rilett K., Sanders D.W., Cook C., Fu H., Boonen R.A., et al. 2016. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19:1085–1092. 10.1038/nn.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., and Husemann J.. 2003. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 9:453–457. 10.1038/nm838 [DOI] [PubMed] [Google Scholar]
- Xiao Q., Yan P., Ma X., Liu H., Perez R., Zhu A., Gonzales E., Burchett J.M., Schuler D.R., Cirrito J.R., et al. 2014. Enhancing astrocytic lysosome biogenesis facilitates Aβ clearance and attenuates amyloid plaque pathogenesis. J. Neurosci. 34:9607–9620. 10.1523/JNEUROSCI.3788-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodszky E., Seaman M.N., Moreau K., Jimenez-Sanchez M., Breusegem S.Y., Harbour M.E., and Rubinsztein D.C.. 2014. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 5:3828 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Herrmann U.S., Falsig J., Abakumova I., Nuvolone M., Schwarz P., Frauenknecht K., Rushing E.J., and Aguzzi A.. 2016. A neuroprotective role for microglia in prion diseases. J. Exp. Med. 213:1047–1059. 10.1084/jem.20151000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Victoria G.S., Marzo L., Ghosh R., and Zurzolo C.. 2015. Prion aggregates transfer through tunneling nanotubes in endocytic vesicles. Prion. 9:125–135. 10.1080/19336896.2015.1025189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A., Benet-Pagès A., Struhal W., Graf E., Eck S.H., Offman M.N., Haubenberger D., Spielberger S., Schulte E.C., Lichtner P., et al. 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89:168–175. 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]