Schmid provides a perspective on exciting new research examining the relationship between signaling and endocytosis in cancer.
Abstract
Cell surface receptor uptake via clathrin-mediated endocytosis (CME) and subsequent intracellular sorting for degradation or recycling regulates the strength and specificity of downstream signaling. Signaling, in turn, modulates early endocytic trafficking. This reciprocal regulation of signaling and endocytosis provides opportunities for the establishment of feedback loops to enhance or suppress surface-derived signals. Recent studies suggest that dynamin-1, a presumed neuron-specific isoform of the large, membrane fission GTPase, can be activated in nonneuronal cells downstream of cancer-relevant signaling pathways and thereby function as a nexus between signaling and early endocytic trafficking. I speculate that sustained up-regulation and/or acute activation of dynamin-1 in cancer cells contributes to a program of “adaptive” CME that alters signaling to enhance cancer cell survival, migration, and proliferation.
Introduction
The plasma membrane (PM) serves as a physical barrier that separates the cytosolic milieu of the cell from the comparatively harsh external chemical environment. It also serves as a sophisticated communication platform through which cells receive and respond to messages from each other, as well as sense and respond to changes in their environment. Cell surface signaling receptors, such as receptor tyrosine kinases (RTKs), G protein–coupled receptors (GPCRs), and cytokine receptors, are activated by binding to their ligands (e.g., growth hormones, peptide agonists, and cytokines). Activated receptors then transmit messages across the PM by initiating signaling cascades in the cytosol that alter cell physiology and/or behavior.
The uptake of macromolecules across the PM, a process called endocytosis, occurs via multiple pathways, all involving the inward budding of vesicles that carry cargo (e.g., receptors and their bound ligands, membrane transporters, and adhesion molecules) into the cell (Conner and Schmid, 2003). Although endocytosis is a mechanism well known to terminate receptor signaling (Grandal and Madshus, 2008), it has also become clear that endocytosis is required for the initiation of some signaling cascades (Platta and Stenmark, 2011). Moreover, both the endocytic pathway taken by surface receptors and their intracellular fate can quantitatively and qualitatively affect the activity of downstream signaling pathways and thereby control cellular responses (Di Fiore and De Camilli, 2001; Sorkin and von Zastrow, 2009; Platta and Stenmark, 2011; Di Fiore and von Zastrow, 2014). Thus, endocytosis regulates signaling.
Several studies, described in this review, provide compelling evidence that signaling downstream of surface receptors can, in turn, regulate endocytosis and alter the intracellular itinerary of activated receptors (Puthenveedu and von Zastrow, 2006; Reis et al., 2015, 2017). The cross talk between signaling and endocytosis has implications for cancer progression, as alterations in survival, proliferative, and migratory signals are essential for metastasis. Indeed, several reviews have described how endocytosis can be “dysregulated” or “derailed” in cancer cells (Lanzetti and Di Fiore, 2008; Mosesson et al., 2008; Mellman and Yarden, 2013). These descriptors, which connote “defective” endocytosis, are supported by lists of cancer-associated mutations, translocations, or altered expression levels among components of the endocytic machinery. Recent findings, however, suggest that by taking advantage of the reciprocal cross talk between signaling and endocytosis, cancer cells elaborate mechanisms to enhance endocytosis and recycling, potentially in receptor-selective manners. Therefore, rather than defective, I propose the more deliberate term “adaptive endocytosis,” whereby evolving cancer cells specifically adopt mechanisms that quantitatively and/or qualitatively alter endocytic trafficking to enhance their survival, proliferative, and migratory properties. As described below, this perspective opens new avenues of investigation into the regulation of endocytic trafficking in both normal and cancer cells.
Clathrin-mediated endocytosis (CME) and early endosomal sorting
Several mechanistically distinct pathways exist for vesicular uptake of surface receptors (Fig. 1), but the best studied and quantitatively most significant is CME (Fig. 2). CME is initiated when the coat-forming protein clathrin is recruited to the PM by the heterotetrameric adaptor protein complex 2 (AP2) that also recognizes sorting motifs on the cytoplasmic domains of surface receptors (McMahon and Boucrot, 2011; Kirchhausen et al., 2014; Robinson, 2015). As clathrin assembles, cargo is concentrated into the inwardly growing clathrin-coated pit (CCP). With the help of numerous endocytic accessory proteins (EAPs), nascent CCPs undergo maturation until they are deeply invaginated but remain connected to the cell surface via a narrow neck. The large GTPase dynamin then assembles around these narrow necks and, with the help of curvature-generating EAPs, catalyzes membrane scission (Schmid and Frolov, 2011; Morlot and Roux, 2013; Antonny et al., 2016). The released clathrin-coated vesicles are rapidly uncoated by Hsc70, the uncoating ATPase (Rothman and Schmid, 1986), and the uncoated vesicles carry their concentrated cargo into the cell.
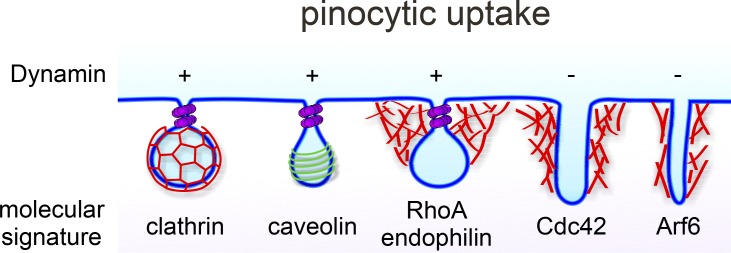
Figure 1.
Endocytosis acutely modulates the composition of the PM and is required to internalize typically receptor-bound macromolecules. Multiple mechanistically distinct pathways for pinocytosis, which involves the formation of small vesicular carriers, exist in mammalian cells. These include CME and caveolae-mediated endocytosis, which were the first discovered and remain the best-characterized pathways (Conner and Schmid, 2003; Parton and Richards, 2003). Both require the large fission GTPase, dynamin, as do a subset of clathrin and caveolin-independent endocytic and lipid-raft mediated pathways, collectively called clathrin-independent pathways (Mayor et al., 2014). Most CIE pathways are regulated by Rho-family or Arf6 GTPases that drive local actin assembly required for invagination and fission (Mooren et al., 2012; Mayor et al., 2014). Clathrin-independent and actin-dependent internalization pathways are less well understood and mediate nonspecialized/bulk/fluid phase uptake as well as internalization of glycophosphatidylinositol-anchored proteins, although specific transmembrane cargoes have also been identified (Maldonado-Báez et al., 2013). Typically, endocytic vesicles derived from these divergent pathways merge at early endosomes, although the degree of rapid recycling after uptake via different pathways can vary (Mayor et al., 2014).
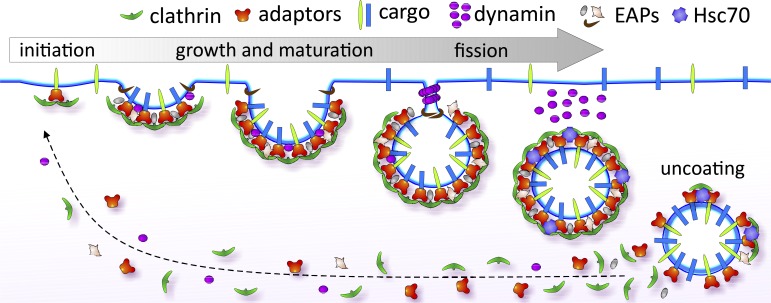
Figure 2.
CME is the major route of entry into the cell. CME is initiated by adaptor proteins that recognize sorting motifs on surface receptors (i.e., cargo) and recruit clathrin to form nascent CCPs. With the aid of numerous endocytic accessory proteins (EAPs), CCPs continue to concentrate cargo as they grow and mature. The GTPase dynamin is recruited to nascent CCPs and can regulate early stages of CCP maturation. A burst of dynamin recruitment to form collar-like structures at the necks of deeply invaginated coated pits drives membrane fission and vesicle release. An uncoating reaction recycles coat constituents and releases the vesicle for subsequent intracellular transport.
In the cytosol, nascent endocytic vesicles undergo multiple rounds of homotypic fusion with each other (Dunn et al., 1989), as well as heterotypic fusion with a more stable population of early endosomes (Sigismund et al., 2008; Kalaidzidis et al., 2015) that serve as the first sorting station along the endolysosomal pathway (Fig. 3). Early endosomes are enriched in the lipid species phosphatidylinositol-3-phosphate and, in part, are functionally defined by the small GTPase Rab5 and one or both of two Rab5-interacting scaffold proteins, EEA1 (early endosome antigen 1) or APPL1 (adaptor protein containing PH domain, PTB domain and leucine zipper motif 1; Huotari and Helenius, 2011). Both so-called APPL1 and EEA1 endosomes function as cargo sorting stations, and although physically distinct, they rapidly and reciprocally exchange contents (Kalaidzidis et al., 2015). The scaffolds, in turn, regulate recruitment of other factors to the membrane that control endosomal sorting and maturation.
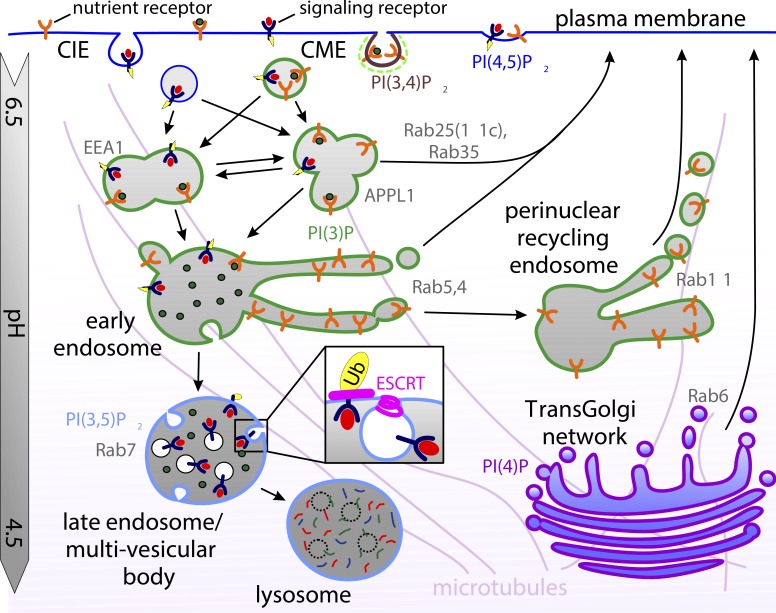
Figure 3.
Postendocytic sorting of receptors along the endolysosomal pathway. Nascent endocytic vesicles undergo multiple rounds of homotypic and heterotypic fusion at the cell periphery, forming Rab5-positive early endosomes bearing either EEA1 or APPL1 scaffold proteins. Receptors can either be recycled quickly from early endosomes in a Rab35- or Rab25-dependent manner, sorted into tubular structures that collect to form perinuclear recycling endosomes before returning the surface in a Rab11-dependent manner, or be retained in the vacuolar portion of early endosomes that migrate to the cell interior along microtubules while they mature into late endosomes in a Rab7-dependent manner. During endosome maturation, the ESCRT machinery selects ubiquitinated cargo for packaging into intraluminal vesicles forming multivesicular bodies that fuse with lysosomes, delivering their content for degradation. CIE, clathrin independent.
Incoming cargo is sorted in early endosomes along one of three pathways (Huotari and Helenius, 2011; Hsu et al., 2012). Most ligands dissociate from their receptors in the low-pH environment of the endosome. Dissociated ligands are carried within the luminal contents of early endosomes as they mature into late endosomes and migrate along microtubules to the perinuclear region of the cell before eventually fusing with lysosomes (Huotari and Helenius, 2011). Transmembrane receptors and their bound ligands are (1) retrieved from peripheral early endosomes and rapidly (∼1–3 min) recycled back to the cell surface; (2) packaged into membrane tubules that translocate to the perinuclear region, forming recycling endosomes, and then returned to the PM with slower kinetics (10–15 min); or (3) packaged into intraluminal vesicles as part of the endosomal maturation process leading to multivesicular body (MVB) formation (Fig. 3). These sorting decisions are, in part, mediated by the sequential recruitment and activation of Rab GTPases (Wandinger-Ness and Zerial, 2014) that orchestrate the machinery necessary to sort cargo and bud off recycling carriers. For example, Rab 4, 21, 25 (aka 11c), and 35 play roles in rapid recycling from early endosomes; Rab11a and b function in recycling from perinuclear recycling endosomes; and Rab7 orchestrates late endosomal maturation (Wandinger-Ness and Zerial, 2014). The formation of, and sorting into, intralumenal vesicles is mediated by the endosomal sorting complexes required for transport (ESCRT) machinery that recognizes ubiquitin-conjugated receptors (Henne et al., 2011). Packaging of signaling receptors into MVBs and their degradation in lysosomes terminates signaling and is referred to as receptor down-regulation (Eden et al., 2009).
The effect of endocytosis on signaling
Clearly, targeting receptors to the lumen of lysosomes will terminate signaling. Thus, diverting receptors from this fate and recycling them to the cell surface will prolong signaling. Although true for many RTKs, this effect has been most extensively studied in the context of the EGF receptor (EGFR; Grandal and Madshus, 2008; Sorkin and Goh, 2009), an oncogenic signaling receptor frequently up-regulated or activated in cancer cells. Here, tuning EGFR lifetime via modulation of its ubiquitination, which ultimately targets it for lysosomal degradation via the ESCRT pathway, is a strategy exploited by cancer cells to drive sustained receptor signaling. Thus, mutations in EGFR or Cbl, the E3 ligase that ubiquitinylates the EGFR, are frequently associated with cancers (Mellman and Yarden, 2013). Similarly, mitogenic stimuli, such as TGFα, which binds more weakly to EGFRs, dissociates in early endosomes, allowing unoccupied EGFR to avoid ubiquitination and be recycled to the cell surface (Longva et al., 2002). Finally, at high concentrations of EGF, activated EGFRs saturate CME and spill over into a clathrin-independent endocytic pathway (Sigismund et al., 2008). A higher percentage of EGFRs are recycled after uptake via CME than by the clathrin-independent pathways in a manner dependent on the extent of EGFR ubiquitination (Sigismund et al., 2008). Hence the magnitude of EGFR signaling is dependent on the endocytic pathway taken.
In addition to postuptake sorting decisions (i.e., recycling vs. degradation), several earlier stages of endocytosis also regulate signaling in more subtle ways (Barbieri et al., 2016). For example, recent studies have suggested that coated pits themselves may function as signaling platforms to trigger specific signaling pathways, such as receptor-mediated activation of JAK2 and Akt (Chen et al., 2012; Garay et al., 2015). Indeed, it has been suggested that longer-lived, flat clathrin lattices may serve as signaling platforms (Grove et al., 2014) in a manner analogous to caveolar microdomains (Martinez-Outschoorn et al., 2015).
Endosomes themselves can also serve as signaling platforms triggering specific downstream cascades. This activity was first demonstrated by subcellular fractionation studies showing that Grb2–mSOS–Ras signaling complexes copurified with endosomes isolated from rat liver parenchyma (Di Guglielmo et al., 1994). Subsequent studies have firmly established a role for endosomes in determining signaling strength and specificity. For example, EGFR endocytosis is required to initiate MAPK signaling (Vieira et al., 1996). APPL1 endosomes scaffold activated Akt and direct its phosphorylation toward GSK3β, whereas PM-bound Akt selectively phosphorylates TCS2, thereby activating the mTOR pathway (Schenck et al., 2008). A third endosome-associated scaffold protein, Sara (SMAD anchor for receptor activation), mediates activation of SMAD2/4 downstream of TGFβ signaling (Tsukazaki et al., 1998). Moreover, early endosomal trafficking and the multiple homo- and heterotypic fusion events driving endosomal sorting can play a direct role in determining cellular responses to activated RTKs by ensuring quantal packaging of activated EGFR into signaling endosomes (Villaseñor et al., 2015). Inactive c-Src kinase, which is downstream of many RTKs, is also highly enriched in endosomes; hence, its endosomal trafficking and activation are tightly linked (Sandilands and Frame, 2008). These data compellingly argue that rather than simply terminating signaling, endocytosis and endosomal trafficking of EGFRs (Grandal and Madshus, 2008; Sorkin and Goh, 2009; Avraham and Yarden, 2011; Ceresa, 2012) and other RTKs (Wiley and Burke, 2001) spatially and temporally regulates downstream signaling. This is accomplished by bringing activated RTKs in contact with differentially localized components of their signaling pathways and/or by controlling the duration of these interactions.
Although a positive role for endocytosis in signaling downstream of RTKs has long been appreciated, GPCR signaling through trimeric G proteins has until recently been assumed to occur exclusively at the cell surface. This is because agonist-stimulated GPCRs interact with and activate PM-localized trimeric G proteins and then are rapidly inactivated by GPCR kinases, which recruit arrestins that prevent further activation of downstream G proteins (Moore et al., 2007). Concomitantly, arrestins also function as GPCR-specific adaptors linking them to CCPs and triggering their CME. However, recent studies have shown that several GPCRs can affect a second wave of signaling from early endosomes via two distinct mechanisms. First, arrestins can function as scaffolds in endosomes, leading to activation of MAPK signaling pathways (Lefkowitz and Shenoy, 2005; Thomsen et al., 2016). Second, conformation-specific nanobodies have detected activated GPCRs and G proteins in endosomal compartments from where they trigger a second wave of G protein–mediated signaling (Irannejad et al., 2013; Tsvetanova et al., 2015). This second wave alters not only the strength but also the specificity of downstream signaling (Tsvetanova et al., 2015; Bowman et al., 2016). Given the diversity of GPCRs, these signaling pathways naturally vary from receptor to receptor.
Together, these studies establish that divergent endocytic pathways as well as early endosomes can function both as sorting stations that determine whether activated signaling receptors are recycled or degraded and as signaling platforms that influence the strength and specificity of downstream signaling events.
The effect of signaling on endocytosis
Sorting along the endocytic pathway (whether into CCPs, for recycling from early/sorting endosomes, or for packaging into intraluminal vesicles) is determined by sorting motifs in endocytic receptors. It has generally been assumed that the sorting machinery, which recognizes and acts upon these motifs, is constitutively active. For example, clathrin-coated vesicles were thought to form at the PM at fixed rates (like “busses”), whereas the uptake of cell-surface receptors and transporters (like “passengers”) was regulated by whether or not they held “tickets.” These tickets could be provided by posttranslational modification of the receptor, such as phosphorylation, ubiquitination, or acetylation (Goh et al., 2010). Similarly, posttranslational modifications such as ubiquitination (Piper et al., 2014), sorting signals such as dileucine motifs (Bonifacino and Traub, 2003), or receptor oligomerization (Weissman et al., 1986) would target receptors for later endosomal compartments and into MVBs, whereas receptors lacking these signals were recycled along default pathways. However, there is now support for the idea that recycling is an active process (Hsu et al., 2012) and that the cellular machineries affecting endocytosis and endosomal sorting are themselves subject to regulation downstream of the same signaling receptors they regulate. Thus, CCPs and the vesicles derived from them can function more like “taxicabs,” whose rates of transport and destinations are controlled by their passengers.
That receptor trafficking is highly regulated at multiple stages along endocytic pathways was made strikingly clear when a genome-wide siRNA screen revealed that knockdown of a large fraction of the human kinome either directly or indirectly altered one or another aspect of either CME or endosomal trafficking (Pelkmans et al., 2005). A second, more recent siRNA screen has confirmed that multiple kinase activities impinge on diverse endocytic pathways and alter endosomal trafficking and the steady-state distribution of endosomal compartments (Liberali et al., 2014).
Endocytosis is also regulated by Ras and Rho family GTPases. Thus, growth factor receptor signaling and subsequent activation of Rho-family GTPases have long been known to trigger membrane ruffling and macropinocytosis (Bar-Sagi and Feramisco, 1986; Orth and McNiven, 2006). Rho-family GTPases are also required for multiple clathrin-independent and actin-dependent endocytic pathways (Mayor et al., 2014). In contrast, activation of Rho and Rac can inhibit CME, independent of their effects on actin assembly (Lamaze et al., 1996). A potential mechanism was revealed when synaptojanin, a PI(4,5)P2-phosphatase, was shown to be an effector of Rac. Rac-dependent recruitment of synaptojanin led to reduced PI(4,5)P2 levels at the PM, thus inhibiting CME (Malecz et al., 2000). Signaling pathways influencing endosomal trafficking likely impinge on Rab GTPases, given their central role in these processes.
Signaling receptors and “customized” coated pits
Increasing evidence suggests that signaling receptors themselves can influence their own endocytic trafficking in both positive and negative ways. The first example of this regulation was the demonstration that clathrin can be tyrosine phosphorylated downstream of EGFR activation via the Src kinase, leading to redistribution of clathrin to the cell periphery and accelerated endocytosis of EGFR (Wilde et al., 1999). More recent studies have shown that a subset of PDZ domain–containing GPCRs (Puthenveedu and von Zastrow, 2006) and ubiquitination of GPCRs (Henry et al., 2012) can specifically alter the maturation kinetics of the CCPs in which they reside. The former mechanism appeared to be related to PDZ-dependent interactions of receptors with the actin cytoskeleton, thereby delaying CCP maturation and the recruitment of dynamin. These findings also provided the first compelling evidence for the existence of cargo-selective, compositionally distinct subsets of CCPs.
A second example derives from dynamin isoform–specific requirements for CME of TRAIL (TNF-related apoptosis-inducing ligand)–activated death receptors (DRs). Reis et al. (2017) confirmed previous findings (Kohlhaas et al., 2007) that CME of TRAIL–DR complexes suppresses apoptotic signaling in TRAIL-resistant cancer cell lines. However, and surprisingly, siRNA knockdown of dynamin-2 (Dyn2), the presumed ubiquitously expressed isoform had no effect, whereas knockdown of Dyn1, the presumed neuron-specific isoform, was as effective at suppressing apoptotic signaling as knockdown of either clathrin or AP2. Remarkably, these dynamin-isoform–specific effects could be ascribed to cargo-selective CME. Thus, TRAIL–DR uptake was Dyn1 dependent but Dyn2 independent, whereas in the same cells, CME of constitutively internalized transferrin receptors was Dyn2 dependent but Dyn1 independent.
Collectively, these studies highlight a previously unappreciated complexity as to how endocytosis and subsequent vesicle trafficking can regulate receptor signaling and how, in turn, signaling receptors can actively regulate their own endocytic trafficking, in part through activation of dynamin.
Dynamin, a master regulator of CME
As described above, dynamin is best known for its role in catalyzing membrane scission at late stages of CME to release clathrin-coated vesicles into the cytosol. However, increasing evidence suggests that dynamin also functions to regulate earlier stages of CCP maturation. The first evidence of a regulatory role of dynamin came from the finding that CME was accelerated in cells overexpressing Dyn1 mutants defective in self-assembly and, hence, assembly-stimulated GTPase activity (Sever et al., 1999, 2000). Given that dynamin-catalyzed membrane fission is rapid (<5 s) compared with the median lifetime of a CCP (∼60 s), these dynamin mutants must have been accelerating early rate-limiting steps in CCP maturation, albeit through unknown mechanisms. Although met with considerable skepticism (Kirchhausen, 1999; van der Bliek, 1999; Yang and Cerione, 1999; Marks et al., 2001), other mutations in the same region of dynamin have since been shown to accelerate CME (Faelber et al., 2011).
Although most metazoans encode a single dynamin isoform, vertebrates encode three: Dyn1, which is highly abundant in neurons; Dyn2, which is ubiquitously expressed; and Dyn3, which is expressed in testes, lung, and neurons. In keeping with their tissue-specific expression patterns, Dyn1-knockout mice die perinatally because of defects in rapid synaptic vesicle recycling at nerve terminals (Ferguson et al., 2007), whereas Dyn2-knockout mice are embryonic lethal (Ferguson et al., 2009). Knockout of Dyn3, which appears to be partially redundant with Dyn1, has no detectable phenotype (Raimondi et al., 2011). Interestingly, although Dyn3 can fully restore rapid synaptic vesicle recycling in Dyn1null hippocampal neurons, Dyn2 cannot (Ferguson et al., 2007). Correspondingly, Dyn1 was less effective at rescuing CME in Dyn2null mouse fibroblasts than Dyn2 (Liu et al., 2008). This functional difference is in part caused by different biochemical properties of Dyn1 and Dyn2 (Liu et al., 2011). Studies showed that Dyn1 is a powerful membrane curvature generator capable of deforming membranes and catalyzing fission on its own, whereas Dyn2 is a curvature sensor that likely functions synergistically with other curvature-generating proteins to catalyze fission (Neumann and Schmid, 2013). In addition, neuronal Dyn1 is subject to regulation by phosphorylation and dephosphorylation that links its rapid activation to calcium influx during neurotransmission (Smillie and Cousin, 2005; Graham et al., 2007; Clayton et al., 2010).
Contrary to the aforementioned assumptions regarding tissue distribution of dynamin isoforms, a closer look at mRNA expression level data reveals that although Dyn1 mRNA is highly enriched in neuronal tissues (Fig. 4 a, inset), Dyn1and Dyn2 mRNAs are otherwise equally abundant in most tissues and cells (Fig. 4 a). Nonetheless, despite near-equal levels of expression, Dyn1 appears not to be active in most nonneuronal cells. Thus, siRNA-mediated knockdown (e.g., Huang et al., 2004) or conditional knockout (Liu et al., 2008) of Dyn2 is sufficient to potently inhibit CME. Recent studies have provided an explanation for this paradox in that Dyn1 is maintained in a phosphorylated and inactive state in nonneuronal cells (Fig. 4 b), as it is in the resting synapse (Clayton et al., 2010), by the constitutively active kinase GSK3β (Reis et al., 2015). Acute inhibition of GSK3β in nonneuronal retinal pigment epithelial cells activates Dyn1 and accelerates CME because of increased rates of CCP initiation and maturation. Importantly, the effects of GSK3β inhibition were entirely dependent on Dyn1, but not Dyn2, expression (Reis et al., 2015). These data establish that dynamins, potentially in an isoform-specific manner, can regulate even the earliest stages of CME, although the mechanisms remain unknown.
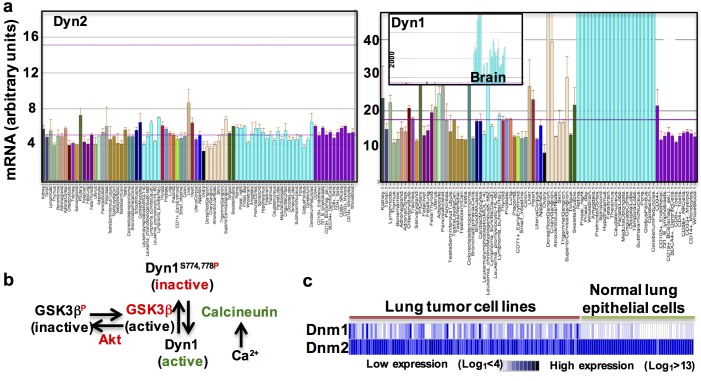
Figure 4.
Dynamin-1 and -2 are expressed in all tissues, but Dyn1 is differentially regulated. (a) Dyn2 and Dyn1 mRNA levels measured in various tissues (BioGPS.com). It has been generally assumed that Dyn1 is a neuron-specific isoform. This misperception was based on mRNA expression data like that shown in the inset. At this scale, Dyn1 mRNA, which is highly expressed in brain, is not detected in other tissues. However, when displayed at the same scale, Dyn1 and Dyn2 mRNAs are equally expressed in most tissues (mean ± SEM). (b) Dyn1 is regulated by phosphorylation/dephosphorylation, both at the synapse (Smillie and Cousin, 2005; Graham et al., 2007) and in nonneuronal cells (Reis et al., 2015, 2017). (c) Dyn1, but not Dyn2, is frequently up-regulated in lung tumor cell lines as compared with normal lung epithelial cells (unpublished data).
Altered early endocytic trafficking in cancer cells
Many properties of metastatic cancer cells (e.g., proliferation, angiogenesis, survival, and migration) are driven by altered signaling, in particular from cell surface signaling receptors. Thus, given the reciprocal relationship between signaling and endocytosis, it is not surprising that endocytic trafficking is altered in cancer cells to affect changes in signaling pathways to enhance tumorigenesis and metastasis. Indeed, cancer cell–specific changes in the sorting decisions to recycle or degrade surface signaling proteins and/or integrins, which promote oncogenic signaling and cell migration, have been extensively studied and previously reviewed (Caswell et al., 2009; Mellman and Yarden, 2013; Di Fiore and von Zastrow, 2014; Barbieri et al., 2016). Several mechanisms for these changes have been identified, many of which converge on the Rab-family of small GTPases and their effectors known to regulate early endosomal trafficking (Caswell et al., 2009). Thus, many cancers exhibit increased expression of small GTPases that control recycling from early endosomes (e.g., Rab25, Rab35, and Arf6) and shunt signaling receptors away from degradative pathways and into recycling ones (Porther and Barbieri, 2015). Moreover, coordinate increases in expression of APPL1, Rab5a, and EEA1 are strongly correlated with metastatic prostate cancers (Johnson et al., 2015). Other cancer cells have mutations in the ubiquitination or ESCRT machinery that targets signaling receptors for degradation. Mutations in the signaling receptors themselves (e.g., EGFR or cMet) can ablate sorting signals for endocytosis or ubiquitination, hence enabling them to maintain their proliferative, survival, or migratory signaling activity on the cell surface (Lanzetti and Di Fiore, 2008; Mosesson et al., 2008; Mellman and Yarden, 2013). Interestingly, expression of mutant p53, which is associated with many cancers, enhances rapid recycling of integrins, cMET, and EGFR without affecting the slower recycling of transferrin receptors (Muller et al., 2009, 2013). This rapid, cargo-selective recycling from early endosomes enhances tumor cell migration and invasion (Muller et al., 2009). The mechanisms by which mutant p53 activates cargo-selective recycling remain largely unknown.
Dyn1 activation in cancer cells alters CME and early endocytic trafficking
In contrast to the well-studied changes in endosomal sorting and trafficking, much less is known about cancer cell–specific changes in CME. Indeed, there are few studies reporting cancer-linked changes in expression levels or mutations in known components of the CME machinery (Floyd and De Camilli, 1998; Lanzetti and Di Fiore, 2008; Mellman and Yarden, 2013), supporting the prevailing view that CME is a constitutive process. However, as we lack sufficient knowledge about which factors regulate CME, identifying how cancer cell–specific alterations impinge on this regulation is difficult. Recent findings, however, have provided new insight into the regulation of CME, and they point to the GTPase dynamin.
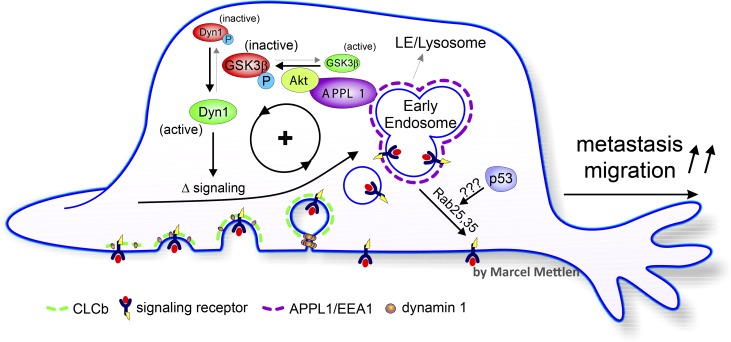
As discussed above, acute activation of Dyn1 in retinal pigment epithelial cells results in enhanced rates of CCP initiation, more rapid and less regulated CCP maturation, and consequently more rapid CME (Reis et al., 2015). Interestingly, GSK3β, the kinase that negatively regulates Dyn1, is itself negatively regulated by Akt, an oncogenic kinase frequently activated in cancer cells, especially those with mutations in the tumor suppressor PTEN. Moreover, the Akt–GSK3β kinase cascade is selectively initiated on early endosomes in association with the APPL1 scaffold (Reis et al., 2015). Concomitant with Dyn1 activation, peripheral APPL1-positive endosomes accumulate and increased APPL1-dependent activation of Akt was observed (Chen et al., 2017; Reis et al., 2017). These data point to the existence of a positive feedback loop from Dyn1, through APPL1/Akt, to promote Dyn1-dependent changes in CME dynamics (Fig. 5).
Figure 5.
Adaptive CME in cancer cells. Activation and/or up-regulation of Dyn1 enhances the rate of CCP initiation and maturation and increases the number of APPL1-positive endosomes through as yet unknown mechanisms. APPL1 scaffolding of Akt on endosomes in turn enhances Akt signaling to activate Dyn1, creating a positive feedback loop that alters signaling from cell surface receptors. Other cancer-related, mutant p53-dependent mechanisms can enhance receptor recycling to prolong signaling. Thus, during tumor progression, adaptive CME can contribute to tumor progression and cancer cell metastasis. LE, late endosome; P, phosphorylated Dyn1 or GSK3β.
Consistent with this, we recently showed that CME in H1299 non–small cell lung cancer (NSCLC) cells is dependent on Dyn1 and sensitive to Akt inhibitors. CRISPR knockout and reconstitution experiments with wild-type and a nonphosphorylatable, and thus constitutively active, mutant of Dyn1 established that the effect of Akt on CME was dependent on Dyn1 (Reis et al., 2015). Moreover, Dyn1 is overexpressed in many cancers, including NSCLC (Fig. 4 c), breast cancer, and acute myeloid leukemia cells. Importantly, given that Dyn1 is tightly regulated by phosphorylation/dephosphorylation, its expression levels do not necessarily correlate with activity.
Clathrin itself provides a second example of an isoform-specific regulation of CME by cancer cells. Clathrin triskelions are composed of three clathrin heavy chains and three clathrin light chains (CLCs), of which there are two ∼60% identical isoforms (Brodsky et al., 1991), with as yet unknown distinct functions. CLCa is the predominant isoform in most tissues, whereas CLCb is predominant in brain and neuroendocrine cells (Acton et al., 1993). CLCb is also preferentially up-regulated in many cancer cell types, and histological studies showed that CLCb expression increased in more aggressive human lung cancers and their metastases (Chen et al., 2017). NSCLC lines expressing only CLCa or CLCb (sCLCa and sCLCb cells, respectively) were generated using CRISPR knockout technology. Alternatively, CLCb was overexpressed in NSCLC lines to invert the ratio of CLCa to CLCb and generate switched CLCb (swCLCb) cells. CME of EGFR was accelerated in sCLCb or swCLCb cells relative to parental or sCLCa cells, as was the rate of rapid recycling of EGFR, but not transferrin receptors. There was also an increase in the abundance of APPL1-positive early endosomes, enhanced Akt signaling, and activation of Dyn1. Indeed, Dyn1 expression levels increased in both sCLCb and swCLCb cells (Chen et al., 2017). Strikingly, the increased rates of CME, EGFR recycling, and numbers of APPL1 endosomes in these swCLCb or sCLCb cells were all dependent on Dyn1 expression. These experiments provided a second example of a positive feedback loop in which altered CME and the accumulation of APPL1 endosomes leads to the amplification of Akt signaling and Dyn1 activation, which in turn alters CME (Fig. 5). Interestingly, Akt and GSK3β were prominent hits in a recent screen for signaling molecules affecting endocytic trafficking in HeLa cells (Liberali et al., 2014).
Thus, CME and early endocytic trafficking in cancer cells could be subject to regulation in a cargo-selective manner downstream of RTKs that signal through Akt and APPL1 endosomes, a speculation yet to be directly tested. However, in several TRAIL-resistant cancer cell lines, TRAIL-activated death receptors are specifically internalized by CME in a Dyn1-dependent manner to suppress apoptotic signaling and enhance cancer cell survival (Reis et al., 2017). Interestingly, Dyn1 is not activated downstream of TRAIL–DR through an Akt–GSK3β signaling cascade. Rather, DR actives endoplasmic reticulum–associated ryanodine receptors, leading to an increase in intracellular calcium levels and activation of the Ca2+-dependent phosphatase, calcineurin, which dephosphorylates and activates Dyn1 (Reis et al., 2017; Fig. 4).
“Adaptive” CME and its implications for the evolving cancer cell
The clathrin-mediated uptake of transferrin continues unabated in cells expressing a truncated α-adaptin subunit lacking the appendage domain (ΔαAD cells; Motley et al., 2006; Aguet et al., 2013) that recruits numerous EAPs (Praefcke et al., 2004). CME is maintained in ΔαAD cells not because of functional redundancy with other adaptors, as previously thought (Schmid and McMahon, 2007), but via activation of Dyn1 (Reis et al., 2015). Activated Dyn1 increases the rate of CCP initiation and results in more rapid and dysregulated CCP maturation, which together account for apparently normal rates of transferrin uptake. Indeed, small (<100 µm) flat clathrin lattices accumulate in ΔαAD cells indicative of a role for EAPs in efficient curvature generation and CCP maturation. That these aberrant CCPs are rapidly turned over is evidence for the existence of an active endocytic checkpoint (Aguet et al., 2013).
Interestingly, these qualitative versus quantitative changes in CME in ΔαAD cells resulted in significant changes in cell physiology that reveal an intimate association between CME with early endosomal trafficking. Thus, ΔαAD cells exhibited an accumulation of APPL1-positive early endosomes, more rapid recycling of TfnR from early endosomes to the PM, increased signaling downstream of activated EGFR, increased rates of proliferation, and constitutively activated Akt (Reis et al., 2015). The APPL1 scaffold was required for Akt activation, which in turn was required for Dyn1 activation. Thus, by means of a positive feedback loop, dependent on Dyn1 activation, ΔαAD cells have adapted to their mutated state, altering both CME and signaling.
It has long been know that early endocytic trafficking can be altered in cancer cells as a result of mutation or dysregulated expression of components of the endocytic trafficking or sorting machinery (Lanzetti and Di Fiore, 2008; Mosesson et al., 2008; Mellman and Yarden, 2013). Might cancer cells, like the ΔαAD cells, qualitatively adapt their endocytic trafficking pathways either in response to these randomly acquired mutations or as a consequence of altered signaling?
Indeed, the preceding examples provide direct evidence for alterations in CME downstream of signaling receptors and pathways frequently up-regulated in cancer cells. This “outside-in” regulation of CME sets up a feedback loop in which alterations in early endocytic trafficking can, in turn, sustain signaling downstream of, for example, EGFRs to enhance cell proliferation, migration, and metastasis (Chen et al., 2017) or suppress apoptotic DR signaling (Reis et al., 2017) to enhance cell survival. Extending these observations, a recent siRNA screen conducted in HeLa and A431 adenocarcinoma cells identified tyrosine kinases, as well as several MAPKs, Akt, GSK3β, and JAK3 as regulators of early endocytic trafficking (Liberali et al., 2014). In particular, Akt and JAK3 were shown to regulate the number and lifetimes of CCPs, potentially through effects on Dyn2 recruitment (the authors did not investigate Dyn1). That stress-induced and cytokine-activated kinases can alter CME suggests that the evolving cancer cells might “adapt” in response to different environmental cues and/or be selected for fitness and metastatic potential based on their endocytic activities.
Future prospects and open questions
The perspective of adaptive versus defective CME in cancer cells opens up many new avenues of investigation and many unanswered questions. First, how prevalent is adaptive CME in cancer cells, and are there other signaling pathways that alter CME, in perhaps cargo-selective manners? Do all of these signaling pathways impinge on Dyn1, or are other components of the CME machinery, including Dyn2, subject to regulation? Most studies on CME have focused on abundant receptors, whose ligands are readily and commercially available (i.e., transferrin and EGF). Indeed, a systematic analysis of the rates of CME in NSCLC cell lines revealed no consistent changes (Elkin et al., 2015), perhaps because only transferrin endocytosis was measured. Moreover, these biochemical assays would not have revealed qualitative differences CCP dynamics and the regulation CME that might affect signaling downstream of surface receptors. The focus on defective versus adaptive CME may also explain the dearth of mutations in the CME endocytic machinery linked to cancers (Lanzetti and Di Fiore, 2008; Mellman and Yarden, 2013). Instead, adaptive CME could result from even subtle changes in levels of expression (Johnson et al., 2015) or, in the case of Dyn1, activation of components of the endocytic machinery through posttranslational modifications not easily detected by current genomic analyses. This raises the question as to which and whether other kinases might regulate CME in a cancer cell–specific manner.
Importantly, the studies described in the previous section using cancer cell lines must be validated using primary human tumor-derived cells and/or by comparative histochemical analyses of expression levels and/or activation states of components of the endocytic machinery in normal tissue, tumors, and tumor-derived metastases. Whether changes in endocytic activity can be a prognostic marker for cancer progression remains to be seen.
The discovery that Dyn1 functions as a nexus between signaling and early endocytic trafficking also raises many new questions. What are the mechanisms that confer these isoform-specific functions? How does Dyn1 accelerate CCP initiation and maturation? What are its downstream effectors? What is the role of Dyn1 in early endosomal recycling and in APPL1 endosome maturation and function? How does phosphorylation at the many sites identified in Dyn1, but not Dyn2 (Graham et al., 2007), regulate Dyn1 function in nonneuronal cells? Does Dyn2 also regulate CME, and is it also subject to regulation? Until now, research on dynamin has focused on the protein’s now well-established role in membrane fission (Schmid and Frolov, 2011; Morlot and Roux, 2013; Antonny et al., 2016). The observations described here will hopefully shift research toward addressing these less-studied aspects of dynamin function. The neuron-specific and other components of adaptive CME identified in subsequent studies might be targets for therapeutic intervention to limit cancer cell aggressiveness and inhibit metastasis.
Finally, decades worth of studies of virally or bacterially infected cells have revealed much to cell biologists regarding the inner workings of the cell. Similarly, the pathological state of the evolved cancer cell, in comparison to nontransformed cells, can also be a gold mine to cell biologists in revealing hidden aspects of cellular function. Given the intimate relationship between signaling and endocytosis, the intensity of this relationship in the cancer cells, in comparison with nontransformed cells, may be especially informative for studies on the regulation of early endocytic trafficking.
Acknowledgments
I am grateful to Dr. Marcel Mettlen for illustrations, all Schmid laboratory members for helpful discussions, and to this paper’s referee, who provided helpful comments and discussions that greatly improved the review. Boning Gao performed the analyses of dynamin expression in normal and cancer-derived lung cells. Several colleagues at University of Texas Southwestern also provided helpful comments on the manuscript, including Maralice Conacci-Sorrell, Joe Goldstein, Mike Henne, Jim Malter, David Russell, and Jerry Shay.
Research in the Schmid laboratory was supported by National Institutes of Health R01 grants GM42455, GM73165 (with G. Danuser), and MH61345, as well as by Cancer Prevention and Research Institute of Texas grant RP150573 and Welch Foundation grant I-1823.
The author declares no competing financial interests.
Footnotes
Abbreviations used:
- AD
- appendage domain
- AP2
- adaptor protein complex 2
- CCP
- clathrin-coated pit
- CLC
- clathrin light chain
- CME
- clathrin-mediated endocytosis
- DR
- death receptor
- EAP
- endocytic accessory protein
- EEA1
- early endosome antigen 1
- EGFR
- EGF receptor
- ESCRT
- endosomal sorting complexes required for transport
- GPCR
- G protein–coupled receptor
- MVB
- multivesicular body
- NSCLC
- non–small cell lung cancer
- PM
- plasma membrane
- RTK
- receptor tyrosine kinase
- sw
- switched
- TRAIL
- TNF-related apoptosis-inducing ligand
References
- Acton S.L., Wong D.H., Parham P., Brodsky F.M., and Jackson A.P.. 1993. Alteration of clathrin light chain expression by transfection and gene disruption. Mol. Biol. Cell. 4:647–660. 10.1091/mbc.4.6.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet F., Antonescu C.N., Mettlen M., Schmid S.L., and Danuser G.. 2013. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell. 26:279–291. 10.1016/j.devcel.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B., Burd C., De Camilli P., Chen E., Daumke O., Faelber K., Ford M., Frolov V.A., Frost A., Hinshaw J.E., et al. . 2016. Membrane fission by dynamin: What we know and what we need to know. EMBO J. 35:2270–2284. 10.15252/embj.201694613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R., and Yarden Y.. 2011. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12:104–117. 10.1038/nrm3048 [DOI] [PubMed] [Google Scholar]
- Barbieri E., Di Fiore P.P., and Sigismund S.. 2016. Endocytic control of signaling at the plasma membrane. Curr. Opin. Cell Biol. 39:21–27. 10.1016/j.ceb.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., and Feramisco J.R.. 1986. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 233:1061–1068. 10.1126/science.3090687 [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., and Traub L.M.. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447. 10.1146/annurev.biochem.72.121801.161800 [DOI] [PubMed] [Google Scholar]
- Bowman S.L., Shiwarski D.J., and Puthenveedu M.A.. 2016. Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling. J. Cell Biol. 214:797–806. 10.1083/jcb.201512068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F.M., Hill B.L., Acton S.L., Näthke I., Wong D.H., Ponnambalam S., and Parham P.. 1991. Clathrin light chains: Arrays of protein motifs that regulate coated-vesicle dynamics. Trends Biochem. Sci. 16:208–213. 10.1016/0968-0004(91)90087-C [DOI] [PubMed] [Google Scholar]
- Caswell P.T., Vadrevu S., and Norman J.C.. 2009. Integrins: Masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10:843–853. 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- Ceresa B.P. 2012. Spatial regulation of epidermal growth factor receptor signaling by endocytosis. Int. J. Mol. Sci. 14:72–87. 10.3390/ijms14010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.H., Chien F.C., Lee S.P., Chan W.E., Lin I.H., Liu C.S., Lee F.J., Lai J.S., Chen P., Yang-Yen H.F., and Yen J.J.. 2012. Identification of a novel function of the clathrin-coated structure at the plasma membrane in facilitating GM-CSF receptor-mediated activation of JAK2. Cell Cycle. 11:3611–3626. 10.4161/cc.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.H., Bendris N., Hsiao Y.J., Reis C.R., Mettlen M., Chen H.Y., Yu S.L., and Schmid S.L.. 2017. Crosstalk between CLCb/Dyn1-mediated adaptive clathrin-mediated endocytosis and epidermal growth factor receptor signaling increases metastasis. Dev. Cell. 40:278–288. 10.1016/j.devcel.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E.L., Sue N., Smillie K.J., O’Leary T., Bache N., Cheung G., Cole A.R., Wyllie D.J., Sutherland C., Robinson P.J., and Cousin M.A.. 2010. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat. Neurosci. 13:845–851. 10.1038/nn.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S.D., and Schmid S.L.. 2003. Regulated portals of entry into the cell. Nature. 422:37–44. 10.1038/nature01451 [DOI] [PubMed] [Google Scholar]
- Di Fiore P.P., and De Camilli P.. 2001. Endocytosis and signaling: An inseparable partnership. Cell. 106:1–4. 10.1016/S0092-8674(01)00428-7 [DOI] [PubMed] [Google Scholar]
- Di Fiore P.P., and von Zastrow M.. 2014. Endocytosis, signaling, and beyond. Cold Spring Harb. Perspect. Biol. 6:a016865 10.1101/cshperspect.a016865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo G.M., Baass P.C., Ou W.J., Posner B.I., and Bergeron J.J.. 1994. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 13:4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.W., McGraw T.E., and Maxfield F.R.. 1989. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 109:3303–3314. 10.1083/jcb.109.6.3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E.R., White I.J., and Futter C.E.. 2009. Down-regulation of epidermal growth factor receptor signalling within multivesicular bodies. Biochem. Soc. Trans. 37:173–177. 10.1042/BST0370173 [DOI] [PubMed] [Google Scholar]
- Elkin S.R., Bendris N., Reis C.R., Zhou Y., Xie Y., Huffman K.E., Minna J.D., and Schmid S.L.. 2015. A systematic analysis reveals heterogeneous changes in the endocytic activities of cancer cells. Cancer Res. 75:4640–4650. 10.1158/0008-5472.CAN-15-0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faelber K., Posor Y., Gao S., Held M., Roske Y., Schulze D., Haucke V., Noé F., and Daumke O.. 2011. Crystal structure of nucleotide-free dynamin. Nature. 477:556–560. 10.1038/nature10369 [DOI] [PubMed] [Google Scholar]
- Ferguson S.M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., Raimondi A., Gong L.W., Ariel P., Paradise S., et al. . 2007. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 316:570–574. 10.1126/science.1140621 [DOI] [PubMed] [Google Scholar]
- Ferguson S.M., Raimondi A., Paradise S., Shen H., Mesaki K., Ferguson A., Destaing O., Ko G., Takasaki J., Cremona O., et al. . 2009. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev. Cell. 17:811–822. 10.1016/j.devcel.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S., and De Camilli P.. 1998. Endocytosis proteins and cancer: A potential link? Trends Cell Biol. 8:299–301. 10.1016/S0962-8924(98)01316-6 [DOI] [PubMed] [Google Scholar]
- Garay C., Judge G., Lucarelli S., Bautista S., Pandey R., Singh T., and Antonescu C.N.. 2015. Epidermal growth factor-stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol. Biol. Cell. 26:3504–3519. 10.1091/mbc.E14-09-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh L.K., Huang F., Kim W., Gygi S., and Sorkin A.. 2010. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 189:871–883. 10.1083/jcb.201001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.E., Anggono V., Bache N., Larsen M.R., Craft G.E., and Robinson P.J.. 2007. The in vivo phosphorylation sites of rat brain dynamin I. J. Biol. Chem. 282:14695–14707. 10.1074/jbc.M609713200 [DOI] [PubMed] [Google Scholar]
- Grandal M.V., and Madshus I.H.. 2008. Epidermal growth factor receptor and cancer: Control of oncogenic signalling by endocytosis. J. Cell. Mol. Med. 12(5a, 5A):1527–1534. 10.1111/j.1582-4934.2008.00298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J., Metcalf D.J., Knight A.E., Wavre-Shapton S.T., Sun T., Protonotarios E.D., Griffin L.D., Lippincott-Schwartz J., and Marsh M.. 2014. Flat clathrin lattices: Stable features of the plasma membrane. Mol. Biol. Cell. 25:3581–3594. 10.1091/mbc.E14-06-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., and Emr S.D.. 2011. The ESCRT pathway. Dev. Cell. 21:77–91. 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Henry A.G., Hislop J.N., Grove J., Thorn K., Marsh M., and von Zastrow M.. 2012. Regulation of endocytic clathrin dynamics by cargo ubiquitination. Dev. Cell. 23:519–532. 10.1016/j.devcel.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu V.W., Bai M., and Li J.. 2012. Getting active: Protein sorting in endocytic recycling. Nat. Rev. Mol. Cell Biol. 13:323–328. [DOI] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., and Sorkin A.. 2004. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279:16657–16661. 10.1074/jbc.C400046200 [DOI] [PubMed] [Google Scholar]
- Huotari J., and Helenius A.. 2011. Endosome maturation. EMBO J. 30:3481–3500. 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R., Tomshine J.C., Tomshine J.R., Chevalier M., Mahoney J.P., Steyaert J., Rasmussen S.G., Sunahara R.K., El-Samad H., Huang B., and von Zastrow M.. 2013. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 495:534–538. 10.1038/nature12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I.R., Parkinson-Lawrence E.J., Keegan H., Spillane C.D., Barry-O’Crowley J., Watson W.R., Selemidis S., Butler L.M., O’Leary J.J., and Brooks D.A.. 2015. Endosomal gene expression: A new indicator for prostate cancer patient prognosis? Oncotarget. 6:37919–37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaidzidis I., Miaczynska M., Brewińska-Olchowik M., Hupalowska A., Ferguson C., Parton R.G., Kalaidzidis Y., and Zerial M.. 2015. APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. J. Cell Biol. 211:123–144. 10.1083/jcb.201311117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. 1999. Cell biology. Boa constrictor or rattlesnake? Nature. 398:470–471. 10.1038/18989 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Owen D., and Harrison S.C.. 2014. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6:a016725 10.1101/cshperspect.a016725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas S.L., Craxton A., Sun X.M., Pinkoski M.J., and Cohen G.M.. 2007. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J. Biol. Chem. 282:12831–12841. 10.1074/jbc.M700438200 [DOI] [PubMed] [Google Scholar]
- Lamaze C., Chuang T.H., Terlecky L.J., Bokoch G.M., and Schmid S.L.. 1996. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 382:177–179. 10.1038/382177a0 [DOI] [PubMed] [Google Scholar]
- Lanzetti L., and Di Fiore P.P.. 2008. Endocytosis and cancer: An ‘insider’ network with dangerous liaisons. Traffic. 9:2011–2021. 10.1111/j.1600-0854.2008.00816.x [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J., and Shenoy S.K.. 2005. Transduction of receptor signals by β-arrestins. Science. 308:512–517. 10.1126/science.1109237 [DOI] [PubMed] [Google Scholar]
- Liberali P., Snijder B., and Pelkmans L.. 2014. A hierarchical map of regulatory genetic interactions in membrane trafficking. Cell. 157:1473–1487. 10.1016/j.cell.2014.04.029 [DOI] [PubMed] [Google Scholar]
- Liu Y.W., Surka M.C., Schroeter T., Lukiyanchuk V., and Schmid S.L.. 2008. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol. Biol. Cell. 19:5347–5359. 10.1091/mbc.E08-08-0890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.W., Neumann S., Ramachandran R., Ferguson S.M., Pucadyil T.J., and Schmid S.L.. 2011. Differential curvature sensing and generating activities of dynamin isoforms provide opportunities for tissue-specific regulation. Proc. Natl. Acad. Sci. USA. 108:E234–E242. 10.1073/pnas.1102710108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longva K.E., Blystad F.D., Stang E., Larsen A.M., Johannessen L.E., and Madshus I.H.. 2002. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 156:843–854. 10.1083/jcb.200106056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Báez L., Williamson C., and Donaldson J.G.. 2013. Clathrin-independent endocytosis: A cargo-centric view. Exp. Cell Res. 319:2759–2769. 10.1016/j.yexcr.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecz N., McCabe P.C., Spaargaren C., Qiu R., Chuang Y., and Symons M.. 2000. Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 10:1383–1386. 10.1016/S0960-9822(00)00778-8 [DOI] [PubMed] [Google Scholar]
- Marks B., Stowell M.H.B., Vallis Y., Mills I.G., Gibson A., Hopkins C.R., and McMahon H.T.. 2001. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 410:231–235. 10.1038/35065645 [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn U.E., Sotgia F., and Lisanti M.P.. 2015. Caveolae and signalling in cancer. Nat. Rev. Cancer. 15:225–237. 10.1038/nrc3915 [DOI] [PubMed] [Google Scholar]
- Mayor S., Parton R.G., and Donaldson J.G.. 2014. Clathrin-independent pathways of endocytosis. Cold Spring Harb. Perspect. Biol. 6:a016758 10.1101/cshperspect.a016758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., and Boucrot E.. 2011. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12:517–533. 10.1038/nrm3151 [DOI] [PubMed] [Google Scholar]
- Mellman I., and Yarden Y.. 2013. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 5:a016949 10.1101/cshperspect.a016949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.A., Milano S.K., and Benovic J.L.. 2007. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69:451–482. 10.1146/annurev.physiol.69.022405.154712 [DOI] [PubMed] [Google Scholar]
- Mooren O.L., Galletta B.J., and Cooper J.A.. 2012. Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81:661–686. 10.1146/annurev-biochem-060910-094416 [DOI] [PubMed] [Google Scholar]
- Morlot S., and Roux A.. 2013. Mechanics of dynamin-mediated membrane fission. Annu. Rev. Biophys. 42:629–649. 10.1146/annurev-biophys-050511-102247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson Y., Mills G.B., and Yarden Y.. 2008. Derailed endocytosis: An emerging feature of cancer. Nat. Rev. Cancer. 8:835–850. 10.1038/nrc2521 [DOI] [PubMed] [Google Scholar]
- Motley A.M., Berg N., Taylor M.J., Sahlender D.A., Hirst J., Owen D.J., and Robinson M.S.. 2006. Functional analysis of AP-2 alpha and mu2 subunits. Mol. Biol. Cell. 17:5298–5308. 10.1091/mbc.E06-05-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P.A., Caswell P.T., Doyle B., Iwanicki M.P., Tan E.H., Karim S., Lukashchuk N., Gillespie D.A., Ludwig R.L., Gosselin P., et al. . 2009. Mutant p53 drives invasion by promoting integrin recycling. Cell. 139:1327–1341. 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- Muller P.A., Trinidad A.G., Timpson P., Morton J.P., Zanivan S., van den Berghe P.V., Nixon C., Karim S.A., Caswell P.T., Noll J.E., et al. . 2013. Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene. 32:1252–1265. 10.1038/onc.2012.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S., and Schmid S.L.. 2013. Dual role of BAR domain-containing proteins in regulating vesicle release catalyzed by the GTPase, dynamin-2. J. Biol. Chem. 288:25119–25128. 10.1074/jbc.M113.490474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth J.D., and McNiven M.A.. 2006. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 66:11094–11096. 10.1158/0008-5472.CAN-06-3397 [DOI] [PubMed] [Google Scholar]
- Parton R.G., and Richards A.A.. 2003. Lipid rafts and caveolae as portals for endocytosis: New insights and common mechanisms. Traffic. 4:724–738. 10.1034/j.1600-0854.2003.00128.x [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., and Zerial M.. 2005. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 436:78–86. 10.1038/nature03571 [DOI] [PubMed] [Google Scholar]
- Piper R.C., Dikic I., and Lukacs G.L.. 2014. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb. Perspect. Biol. 6:a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platta H.W., and Stenmark H.. 2011. Endocytosis and signaling. Curr. Opin. Cell Biol. 23:393–403. 10.1016/j.ceb.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Porther N., and Barbieri M.A.. 2015. The role of endocytic Rab GTPases in regulation of growth factor signaling and the migration and invasion of tumor cells. Small GTPases. 6:135–144. 10.1080/21541248.2015.1050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke G.J., Ford M.G., Schmid E.M., Olesen L.E., Gallop J.L., Peak-Chew S.Y., Vallis Y., Babu M.M., Mills I.G., and McMahon H.T.. 2004. Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 23:4371–4383. 10.1038/sj.emboj.7600445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu M.A., and von Zastrow M.. 2006. Cargo regulates clathrin-coated pit dynamics. Cell. 127:113–124. 10.1016/j.cell.2006.08.035 [DOI] [PubMed] [Google Scholar]
- Raimondi A., Ferguson S.M., Lou X., Armbruster M., Paradise S., Giovedi S., Messa M., Kono N., Takasaki J., Cappello V., et al. . 2011. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 70:1100–1114. 10.1016/j.neuron.2011.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C.R., Chen P.H., Srinivasan S., Aguet F., Mettlen M., and Schmid S.L.. 2015. Crosstalk between Akt/GSK3β signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 34:2132–2146. 10.15252/embj.201591518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C.R., Chen P.H., Bendris N., and Schmid S.L.. 2017. TRAIL-death receptor endocytosis and apoptosis are selectively regulated by dynamin-1 activation. Proc. Natl. Acad. Sci. USA. 114:504–509. 10.1073/pnas.1615072114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. 2015. Forty years of clathrin-coated vesicles. Traffic. 16:1210–1238. 10.1111/tra.12335 [DOI] [PubMed] [Google Scholar]
- Rothman J.E., and Schmid S.L.. 1986. Enzymatic recycling of clathrin from coated vesicles. Cell. 46:5–9. 10.1016/0092-8674(86)90852-4 [DOI] [PubMed] [Google Scholar]
- Sandilands E., and Frame M.C.. 2008. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 18:322–329. 10.1016/j.tcb.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., and Zerial M.. 2008. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 133:486–497. 10.1016/j.cell.2008.02.044 [DOI] [PubMed] [Google Scholar]
- Schmid E.M., and McMahon H.T.. 2007. Integrating molecular and network biology to decode endocytosis. Nature. 448:883–888. 10.1038/nature06031 [DOI] [PubMed] [Google Scholar]
- Schmid S.L., and Frolov V.A.. 2011. Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 27:79–105. 10.1146/annurev-cellbio-100109-104016 [DOI] [PubMed] [Google Scholar]
- Sever S., Muhlberg A.B., and Schmid S.L.. 1999. Impairment of dynamin’s GAP domain stimulates receptor-mediated endocytosis. Nature. 398:481–486. 10.1038/19024 [DOI] [PubMed] [Google Scholar]
- Sever S., Damke H., and Schmid S.L.. 2000. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150:1137–1148. 10.1083/jcb.150.5.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., and Di Fiore P.P.. 2008. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell. 15:209–219. 10.1016/j.devcel.2008.06.012 [DOI] [PubMed] [Google Scholar]
- Smillie K.J., and Cousin M.A.. 2005. Dynamin I phosphorylation and the control of synaptic vesicle endocytosis. Biochem. Soc. Symp. 72:87–97. 10.1042/bss0720087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., and Goh L.K.. 2009. Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 315:683–696. 10.1016/j.yexcr.2008.07.029 [DOI] [PubMed] [Google Scholar]
- Sorkin A., and von Zastrow M.. 2009. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10:609–622. 10.1038/nrm2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A.R., Plouffe B., Cahill T.J. III, Shukla A.K., Tarrasch J.T., Dosey A.M., Kahsai A.W., Strachan R.T., Pani B., Mahoney J.P., et al. . 2016. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 166:907–919. 10.1016/j.cell.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang T.A., Davison A.F., Attisano L., and Wrana J.L.. 1998. SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell. 95:779–791. 10.1016/S0092-8674(00)81701-8 [DOI] [PubMed] [Google Scholar]
- Tsvetanova N.G., Irannejad R., and von Zastrow M.. 2015. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J. Biol. Chem. 290:6689–6696. 10.1074/jbc.R114.617951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A.M. 1999. Is dynamin a regular motor or a master regulator? Trends Cell Biol. 9:253–254. 10.1016/S0962-8924(99)01591-3 [DOI] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze C., and Schmid S.L.. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 274:2086–2089. 10.1126/science.274.5295.2086 [DOI] [PubMed] [Google Scholar]
- Villaseñor R., Nonaka H., Del Conte-Zerial P., Kalaidzidis Y., and Zerial M.. 2015. Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes. eLife. 4:06156 10.7554/eLife.06156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A., and Zerial M.. 2014. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 6:a022616 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A.M., Klausner R.D., Rao K., and Harford J.B.. 1986. Exposure of K562 cells to anti-receptor monoclonal antibody OKT9 results in rapid redistribution and enhanced degradation of the transferrin receptor. J. Cell Biol. 102:951–958. 10.1083/jcb.102.3.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A., Beattie E.C., Lem L., Riethof D.A., Liu S.H., Mobley W.C., Soriano P., and Brodsky F.M.. 1999. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 96:677–687. 10.1016/S0092-8674(00)80578-4 [DOI] [PubMed] [Google Scholar]
- Wiley H.S., and Burke P.M.. 2001. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2:12–18. 10.1034/j.1600-0854.2001.020103.x [DOI] [PubMed] [Google Scholar]
- Yang W., and Cerione R.A.. 1999. Endocytosis: Is dynamin a ‘blue collar’ or ‘white collar’ worker? Curr. Biol. 9:R511–R514. 10.1016/S0960-9822(99)80323-6 [DOI] [PubMed] [Google Scholar]