Abstract
Background
The vast majority of stroke burden is attributable to its modifiable risk factors. This paper aimed to systematically summarise the evidence of Chinese herbal medicine (CHM) interventions on stroke modifiable risk factors for stroke prevention.
Methods
A literature search was conducted via the MEDLINE, CINAHL/EBSCO, SCOPUS, and Cochrane Database from 1996 to 2016. Randomised controlled trials or cross-over studies were included. Risk of bias was assessed according to the Cochrane Risk of Bias tool.
Results
A total of 46 trials (6895 participants) were identified regarding the use of CHM interventions in the management of stroke risk factors, including 12 trials for hypertension, 10 trials for diabetes, eight trials for hyperlipidemia, seven trials for impaired glucose tolerance, three trials for obesity, and six trials for combined risk factors. Amongst the included trials with diverse study design, an intervention of CHM as a supplement to biomedicine and/or a lifestyle intervention was found to be more effective in lowering blood pressure, decreasing blood glucose level, helping impaired glucose tolerance reverse to normal, and/or reducing body weight compared to CHM monotherapy. While no trial reported deaths amongst the CHM groups, some papers do report moderate adverse effects associated with CHM use. However, the findings of such beneficial effects of CHM should be interpreted with caution due to the heterogeneous set of complex CHM studied, the various control interventions employed, the use of different participants’ inclusion criteria, and low methodological quality across the published studies. The risk of bias of trials identified was largely unclear in the domains of selection bias and detection bias across the included studies.
Conclusion
This study showed substantial evidence of varied CHM interventions improving the stroke modifiable risk factors. More rigorous research examining the use of CHM products for sole or multiple major stroke risk factors are warranted.
Keywords: Chinese herbal medicine, Stroke, Risk factor, Prevention
Background
Stroke is the second foremost cause of mortality and a leading cause of serious disability worldwide [1]. The incidence of stroke continues to rise due to societal and lifestyle changes and an aging population [2]. More than 90% of the stroke burden is attributable to its modifiable risk factors such as high blood pressure, high fasting plasma glucose, and high total cholesterol [3]. These stroke risk factors are strongly inter-related and some of them are simultaneous shown as a combined risk factor in people with stroke with higher risk [4, 5]. Previous research has clearly demonstrated the benefits of treating risk factors such as hypertension, diabetes, hyperlipidemia, obesity, atrial fibrillation, or transient ischaemic attack (TIA) for reducing the prevalence of primary stroke [6, 7]. The treatments of major stroke modifiable risk factors are therefore crucial for informing stroke prevention strategies and helping achieve improved quality of life of people with those risk factors and lowered associated health care costs [3].
Chinese herbal medicine (CHM)—therapies and products made from any part of medicinal plants (e.g. leaves and roots) and some non-herb based components (e.g. shells and powdered fossil) [8]—has a history of more than 2500 years with a unique theory of diagnosis and treatment, and is considered a modality of complementary medicine in Western countries [9]. CHM has been increasingly used for a wide range of chronic diseases in China and elsewhere in the form of raw plant materials, powers, capsules, tablets and/or liquids [9–11].
Chinese herbal medicine is a field of health care that may offer potential for addressing related risk factors of stroke [12–14]. Many CHM interventions have long been used for the treatments of some stroke risk factors as individual diseases such as Type 2 diabetes [15], hypertension [8] and obesity [16]. However, the research evidence as to whether specific CHM therapies or products may be effective in reducing each individual or mixed major risk factors of stroke remains unclear. The aim of this systematic review is to assess and summarize the efficacy and safety of all relevant CHM interventions for people at greatest risk(s) of stroke.
Methods
Search strategy
Four key bibliographic databases—MEDLINE, CINAHL/EBSCO, SCOPUS, and Cochrane Database of Systematic Reviews—were searched in the systematic review. This review was designed and conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The stroke modifiable risk factors identified in this systematic review refer to high blood pressure (hypertension), high cholesterol (hyperlipidemia), irregular pulse (atrial fibrillation), TIA, high blood glucose (diabetes and impaired glucose tolerance (IGT), and overweight (obesity). The literature search employed keyword and MeSH term searches for terms relevant to ‘CHM’ and terms regarding stroke risk factors (Table 1). The combination of the search results of CHM and stroke risk factors were identified for screening. To obtain all relevant articles, reference lists of published review papers were also reviewed via Google Scholar.
Table 1.
Search terms for the systematic review
| Chinese herbal medicine | Chinese herbal medicine [MeSH Term & Keyword] OR Chinese medicine [MeSH Term & Keyword] OR Chinese herb* [Title/Abstract] OR Chinese herbal [Title/Abstract] | |
|---|---|---|
| AND | ||
| Stroke risk factors | High blood pressure | Hypertension [MeSH Term & Keyword] OR Blood pressure [MeSH Terms & Keyword] OR Hypertens* [Title/Abstract] OR Prehypertens* [Title/Abstract] OR Systolic [Title/Abstract] OR Diastolic [Title/Abstract] OR |
| High cholesterol | Cholesterol [MeSH Term & Keyword] OR Triglycerides [MeSH Term & Keyword] OR Dyslipidemia [MeSH Term & Keyword] OR Epicholesterol [Title/Abstract] OR HDL [Title/Abstract] OR LDL [Title/Abstract] OR Triglyceride* [Title/Abstract] OR Hyperlipidem* [Title/Abstract] OR Lipidem* [Title/Abstract] OR | |
| Irregular pulse | Cardiac arrhythmias [MeSH Terms & Keyword] OR Atrial fibrillation [MeSH Terms & Keyword] OR Dysrhythmia* [Title/Abstract] OR Cardiac arrhythmia* [Title/Abstract] OR | |
| Transient ischaemic attack | Transient ischaemic attack [MeSH Terms & Keyword] OR Transient ischaemic attack* [Title/Abstract] OR | |
| High blood glucose | Diabetes [MeSH Terms & Keyword] OR Mellitus [MeSH Terms & Keyword] OR Impaired glucose tolerance [MeSH Terms & Keyword] OR Diabet* [Title/Abstract] OR NIDDM [Title/Abstract] OR IDDM [Title/Abstract] OR T2DM [Title/Abstract] OR *insulin* [Title/Abstract] OR Glucose [Title/Abstract] OR | |
| Overweight | Obesity [MeSH Terms & Keyword] OR Overweight [MeSH Terms & Keyword] OR Metabolic syndrome [MeSH Terms & Keyword] OR Obes* [Title/Abstract] OR Adiposity [Title/Abstract] OR Adipos* [Title/Abstract] | |
* Truncation, refering to all records that have those letters with any ending
Study selection
The inclusion criteria of literature in the systematic review were: peer-reviewed English-language journal articles focusing upon randomized controlled trials (RCTs) or cross-over studies published in the past 20 years (1996–2016), and articles reporting primary data findings examining the efficacy and safety of any type of CHM interventions (e.g. decoction, capsule, granule, power) on one or more major modifiable risk factors of stroke. Exclusion criteria were (1) published RCT protocols of this research area; (2) quasi- or pseudo-RCTs (3) studies focusing upon the efficacy and safety of CHM for treating stroke or post-stroke symptoms; (4) studies focusing upon the efficacy and safety of CHM for treating the complications of the stroke risk factors; (5) conference abstracts; and (6) publications without abstracts.
Data extraction
Titles and abstracts of all citations identified in the initial search were imported to Endnote (Version X7) and duplicates removed. Two of the authors screened all the titles/abstracts to identify articles meeting the inclusion and exclusion criteria independently. When consensus was not reached, the full texts of these unclear papers were retrieved and assessed by these two authors. Disagreements were discussed with a third author.
Data were extracted into a pre-determined table (Table 2) and checked for coverage and accuracy by two of the authors. Any differences in data extraction and interpretation were resolved through discussion amongst all authors. Table 2 includes detailed information on study recruitment, participant characteristics, intervention groups, results of primary outcome measures, study limitations, and CHM safety.
Table 2.
Characteristics of the included studies
| Author Country Study period |
Stroke risk factor | Participants | Intervention groups | Results | Side effects | Limitations | |
|---|---|---|---|---|---|---|---|
| Treatment group(s) | Control group(s) | ||||||
| Lin et al. [18] China Sep 2001–Sep 2002 | (Primary) Hypertension |
Sample size n = 102 CHM group n = 52; 41 males and 11 females; mean age: 55 years Control group n = 50; 41 males and 9 females; mean age: 54 years Inclusion criteria SBP: 140–179 mmHg or DBP: 90–109 mmHg; TCM diagnosed for hyperactivity of the liver-yang syndrome |
Tianma gouteng decoction 150 ml/time, twice daily, 4 weeks Formulas Tianma, Niuxi, Sangjisheng, Yimucao, Yejiaoteng, Huangqi, et al. |
Nitrendipine 10 mg/time, 3 times daily, 4 weeks |
Baseline balance Yes Significantly decreased SBP and DBP of both CHM and control groups before and after treatment, without significant difference between these two groups after treatment |
No side effects | N/A |
| Li [19] China No information on study period | (Primary) Hypertension |
Sample size n = 72 CHM group n = 46; 18 males and 28 females; mean age: 54 years Control group: n = 26; 11 males and 15 females; mean age: 53 years Both groups have cases with coronary heart disease, hyperlipemia, and diabetes Inclusion criteria SBP: 140–179 mmHg or DBP: 90–109 mmHg; TCM diagnosed for flaming-up of the liver-fire syndrome |
During the intervention, no other drugs |
Baseline balance Yes An effective rate (return to the normal range of BP or ≥20 mmHg but not in the normal range) at 60.9% of hypertension in the CHM group and 15.4% in the control group; Significantly decreased cholesterol, TG, blood sugar of the CHM group before and after treatment, without significant difference compared to the control group after treatment |
CHM group: Vomiting and distension (n = 1); Slight abdominal pain and diarrhea (n = 3) | N/A | |
|
Huanglian fire-purging mixture 30 ml/time, twice daily, 4 weeks Formulas Huanglian, Gouteng, Zexie, Luhui |
Niuhuang Bolus 1–2 bolus/time, 2–3 times daily, 4 weeks | ||||||
| Ye et al. [20] China Feb 2004–Dec 2004 | (Primary) Hypertension |
Sample size n = 55 CHM group n = 28 Control group n = 27 Inclusion criteria SBP: 140–179 mmHg or DBP: 90–109 mmHg; normal LDL-C level; currently no antihypertensive medications or using antihypertensive medications for at least 6 months before screening |
Xuezhikang with Nifedipine (20 mg/time, twice daily) 1200 mg daily, 72 weeks Formulas Red yeast rice |
Placebo with Nifedipine (20 mg/time, twice daily) 1200 mg daily, 72 weeks |
Baseline balance Yes No significant differences in BP between the CHM and placebo groups after treatment; 92.8% of the CHM group and 88.9% of the placebo group reached the target BP (<140/90 mmHg) |
N/A | N/A |
| Zhao et al. [21] China No information on study period | (Primary) Hypertension |
Sample size n = 79 CHM group n = 40; 17 males and 23 females; mean age: 52 years Control group n = 39; 18 males and 21 females; mean age: 52 years Inclusion criteria SBP: 140–159 mmHg or DBP: 90–99 mmHg; no antihypertensive drugs or stopped taking antihypertensive drugs for 2 weeks; TCM diagnosed for stagnation of phlegm, blood stasis and hyperactivity of the liver-yang syndrome; age: 40–60 years |
Yinian Jiangya Yin 100 ml/time, twice daily, 15 days Formulas Gouteng, Shijueming, Yimucao, Guijia, Banxia, Zhike, et al. |
Tianma Gouteng Yin 100 ml/time, twice daily, 15 days Formulas Tianma, Gouteng, Huangqin, Yejiaoteng, Fushen, Duzhong, et al. |
Baseline balance Yes Significantly decreased SBP and DBP of both CHM and control groups before and after treatment; Significantly decreased SBP and DBP in the CHM group than those in the control group after treatment; The total effective rate at 95.0% of BP control in the CHM group, while 87.2% in the control group |
No side effects | N/A |
| Zhong et al. [22] China Jan 2006–Dec 2008 | (Primary) Hypertension |
Sample size n = 57 CHM group n = 31 Control group n = 26 Inclusion criteria SBP: 140–179 mmHg or DBP: 90–109 mmHg; daytime BP > 135/85 mmHg or nighttime BP > 120/70 mmHg; age: 18 years and older |
During the intervention, no antiplatelet or lipid-lowering drugs and other Chinese patent medicines |
Baseline balance Yes Significantly decreased SBP and DBP in both CHM and control groups before and after treatment, without significant difference between these two groups after treatment |
N/A | N/A | |
|
Jiangya capsule with Nimodipine simulation (1 capsule simulation/time, 3 times daily) 4 capsules/time, 3 times daily, 4 weeks Formulas Dilong, Nuxi, Haizao, Tianma, Chuanxiong |
Control group 1: Integrative medicine 4 Jiangya capsule with 1 nimodipine capsule 3 times daily, 4 weeks Control group 2: Western medicine 4 Jiangya capsule simulation with 1 nimodipine capsule 3 times daily, 4 weeks |
||||||
| Yang et al. [23] Taiwan Sept 2008–Aug 2009 | (Uncontrolled primary) Hypertension |
Sample size n = 55 CHM group n = 30 Control group n = 25 Inclusion criteria sitting SBP ≥ 140 mmHg or sitting DBP ≥ 90mHg despite the conventional antihypertensive treatment; TCM diagnosed for hyperactivity of the liver-yang syndrome; age: 18–80 years |
Fufang Danshen capsule 1000 mg/time, twice daily, 12 weeks Formulas Gegen, Juhua, Danshen, Hongjingtian |
Placebo 12 weeks |
Baseline balance Yes BP control rate (SBP < 140 mmHg and DBP < 90 mmHg) at 25.5% in the CHM group and 7.3% in the placebo group; More significant decrease of SBP in the CHM group than that of the placebo group after treatment |
Mild side effects (e.g. diarrhea, fatigue, common cold) (CHM: n = 13; Control: n = 15) | Small sample size; Short study period |
| Tong et al. [24] China Mar 2010–Sep 2010 | (Mild to moderate) Hypertension |
Sample size n = 219 CHM group n = 106; 61 males and 45 females; mean age: 52 years Control group n = 113; 62 males and 51 females; mean age: 52 years Inclusion criteria SBP: 140–180 mmHg or DBP: 90–110 mmHg; age: 18–65 years; WC ≥ 85 cm (male)/80 cm (female); plus one of the following: (1) TG ≥ 1.7 mmol/l or have received antidyslipidemia treatment; (2) HDL-C < 0.9 mmol/l (male)/1.1 mmol/l (female), or have received the related treatment; (3) FPG ≥ 5.6 mmol/l, diagnosed Type 2 diabetes, or have received glycemiccontrol treatment; (4) TCM diagnosed for liver and stomach damp-heat syndrome |
Jiangzhuoqinggan 170 ml/time, twice daily, 4 weeks Formulas Huanglian, Huangbai, Gouteng, Yinyanghuo |
Irbesartan 150 mg/time, once daily, 4 weeks |
Baseline balance Yes Significantly decreased BP in both CHM and control groups before and after treatment, without significant difference between these two groups after treatment; More significant decrease of daytime and nighttime SBP and DBP in the CHM group than those in the control group after treatment; Significantly decreased WC in the CHM group before and after treatment |
N/A | Short study period; No placebo group; Small sample size |
| Wu et al. [25] China Jan 2010–May 2012 | (Primary) Hypertension |
Sample size n = 137 CHM group 1 n = 45; 31 males and 14 females; mean age: 50 years CHM group 2 n = 47; 33 males and 14 females; mean age: 48 years Control group n = 45; 29 males and 16 females; mean age: 48 years Inclusion criteria diagnosed primary hypertension for at least 3 months prior to screening; age: 18–75 years; 24 h MBP ≥ 130/80 mmHg, MBP ≥ 135/85 mmHg during waking hours, or MBP ≥ 120/70 mmHg during sleeping hours; or SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg |
CHM group 1: Bushen Qinggan granule with amlodipine (5 mg/time, twice daily) Twice daily, 8 weeks CHM group 2: Bushen Qinggan decoction with amlodipine (5 mg/time, twice daily) Twice daily, 8 weeks Formulas Tianma, Gouteng, Duzhong, Huangqin, Kudingcha |
Placebo with amlodipine (5 mg/time, twice daily) Twice daily, 8 weeks |
Baseline balance Yes Significantly decreased BP in all three groups before and after treatment; Significant decrease in the daytime SBP in the CHM group 2 than that in the other two groups after treatment; More significant decrease of BP variability in the two CHM groups than those in the placebo group, without significant difference between these two CHM groups after treatment |
N/A | N/A |
| Li et al. [26] China Jun 2007–Jan 2008 | (Isolated systolic) Hypertension |
Sample size n = 241; 98 males and 143 females; mean age: 67 years CHM group n = 80 Control group 1 n = 76 Control group 2 n = 85 Inclusion criteria diagnosed hypertension; after 1-week elution period, sitting SBP: 140–180 mmHg and sitting DBP < 90 mmHg; age: 60–80 years |
During the intervention, no other antihypertensive drugs |
Baseline balance Yes Significantly decreased SBP in all three groups before and after treatment; More significant decrease of SBP in the control group 1 than that in the CHM group and control group 2, without significant difference between the CHM group and control group 2 after treatment |
Stomach discomfort (CHM: n = 2; Control 2: n = 2); Facial flush and dizziness (Control 2: n = 1) | N/A | |
|
Jiangya capsule with Nimodipine simulation (1 capsule simulation/time, 3 times daily) 4 capsules/time, 3 times daily, 4 weeks Formulas Dilong, Nuxi, Haizao, Tianma, Chuanxiong |
Control group 1: Integrative medicine 4 Jiangya capsule with 1 nimodipine capsule 3 times daily, 4 weeks Control group 2: Western medicine 4 Jiangya capsule simulation with 1 nimodipine capsule 3 times daily, 4 weeks |
||||||
| Chen et al. [27] China 2006–2010 | (Polarized) Hypertension |
Sample size n = 125 CHM group n = 66 Control group n = 59 Inclusion criteria SBP > 140 mmHg and DBP < 70 mmHg; age: 60 years and older |
Diet, exercise, smoking/alcohol advices were provided; no other Western medicine affecting BP |
Baseline balance Yes Significantly decreased SBP and pulse pressure in the CHM group before and after treatment; Significantly decreased SBP in the control group before and after treatment; No significant difference of DBP between the two CHM capsule groups after treatment |
Dizziness and weakness (CHM: n = 5; Control: n = 4); Pretibial edema (CHM: n = 4; Control: n = 4); Facial flushing and headache (CHM: n = 4; Control: n = 4); Severe side effects (Control: n = 21) | N/A | |
|
Shiyiwei Shenqi capsule or Dengzhan Shengmai capsule with Amlodipine Besylate tablets and Irbesartan tablets 3-5 capsules/time, 2–3 times daily, 6 weeks Formulas Shiyiwei Shenqi capsule-Danggui, Xixin, Gouqi, Huangqi, Juemingzi, Lurong, et al. Dengzhan Shengmai capsule-Wuweizie, Xixin, Ginseng, Maidong |
Amlodipine Besylate tablets and Irbesartan tablets 5 mg/time, once or twice daily, 6 weeks | ||||||
| Gong et al. [28] China Apr 2007–Apr 2009 | Hypertension with cardiac damage |
Sample size n = 90 CHM group n = 32; 19 males and 13 females; mean age: 59 years Control group 1 n = 30; 18 males and 12 females; mean age: 56 years Control group 2 n = 28; 15 males and 13 females; mean age: 59 years Inclusion criteria SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg |
Co-administered medications: aspirin, β-blockers, calcium antagonists, diuretics |
Baseline balance Yes Significantly decreased SBP, DBP in all three groups before and after treatment; More significant decrease of SBP, DBP, TO, LVMI in the CHM group and control group 1 than those in the control group 2 after treatment |
Nausea and gastric discomfort (CHM: n = 3; Control 1:n = 1; Control 2: n = 2); Skin rash (Control 1: n = 1) | N/A | |
|
Xuezhikang capsule with Valsartan (80 mg/time, once daily) 600 mg/time, twice daily, 24 months Formulas Red yeast rice |
Control group 1: Valsartan 80 mg/time, once daily, 24 months Control group 2: No intervention |
||||||
| Xu et al. [29] China Jan 2006–Apr 2006 | Hypertension, hypertension with diabetes, hypertension with coronary heart disease |
Sample size n = 108 CHM group n = 55 Control group n = 53 Both groups have cases with diabetes and cases with coronary heart disease Inclusion criteria SBP > 140 mmHg or DBP > 90 mmHg; age: 40–80 years |
Qian Yang He Ji with antihypertensive angiotensin II receptor blocker therapy 35 ml/time, twice daily, 6 months Formulas Gouteng, Shengdihuang, Jili, Nvzhenzi |
Antihypertensive angiotensin II receptor blocker No information of usage |
Baseline balance Yes Significantly decreased SBP, DBP, pulse pressure, cardioankle vascular index of both CHM and control groups before and after treatment; More significant decrease of SBP, DBP, cardioankle vascular index in the CHM group than those in the control group after treatment |
CHM group: serious side effects (n = 5) | N/A |
| Chao et al. [30] China Sep 2006–Nov 2007 | Type 2 diabetes |
Sample size n = 43; age range: 18–70 Inclusion criteria newly diagnosed Type 2 diabetes; FPG ≥ 7 mmol/l and/or OGTT 2hPG ≥ 11.1 mmol/l; BMI: 23–35 kg/m2 with poor glucose level after a 1-month diet control (i.e., FPG: 7–10 mmol/l); no antidiabetic drugs before |
Diet and exercise advices were provided. During the intervention, no antidiabetic medications |
Baseline balance Yes Significantly decreased FPG, PPG, HbA1c, BMI in the CHM group before and after treatment, without significant difference between these two groups after treatment |
Moderate constipation (CHM: n = 2; Placebo: n = 2) | N/A | |
|
CHM compound 3 times daily, 3 months Formulas Huanglian, Huangqi, Rendongteng |
Placebo 3 times daily, 3 months | ||||||
| Ji et al. [31] China Dec 2007–Oct 2008 | Type 2 diabetes |
Sample size n = 627 (1) Drug naïve group; mean age: 54 years CHM group n = 153 Control group n = 150 (2) Metformin group; mean age: 55 years CHM group n = 164 Control group n = 160 Inclusion criteria diagnosed Type 2 diabetes; age: 21–70 years; BMI: 18–28 or 18–35 kg/m2 using metformin at 750 mg/day (or more) for at least 3 months before screening; stable body weight within at least 3 months before screening; FPG: 7.0–13.0 mmol/l and HbA1c >7% |
Diet and exercise advices were provided |
Baseline balance Yes In drug naı¨ve group: Significant 38% lower any hypoglycemia rate and 41% lower mild hypoglycemic episode in the CHM group than those in the control group after treatment; In Metformin group: Significant 24% lower hypoglycemia rate in the CHM group than that in the control group, without significant difference between these two groups in the mild hypoglycemic episode after treatment; In both drug naı¨ve group and Metformin groups, no significant difference of the rate of reducing HbA1c <6.5% between the CHM and control groups |
Urinary tract infection; Upper respiratory tract infection; Elevated ALT/AST; Dyslipidemia | N/A | |
|
Drug naïve group
Xiaoke pill 5–30 pills daily (according to FPG level), 48 weeks Formulas N/A |
Glibenclamide 1.25–7.5 mg daily (according to FPG level), 48 weeks | ||||||
|
Metformin group: Xiaoke pill with Metformin (250 mg/tablet) 5 tablets daily, 48 weeks Formulas N/A |
Glibenclamide with Metformin
1.25 mg daily, 48 weeks |
||||||
| Tong et al. [32] China May 2009–Dec 2009 | Type 2 diabetes |
Sample size n = 480 CHM group n = 360 Control group n = 120 Inclusion criteria early diabetic status; BMI ≥ 24 kg/m2; HbA1c ≥ 7.0%; FPG: 7.0–13.9 mmol/l or 2hPG > 11.1 mmol/l; age: 35–65 years |
During the intervention, antihyperlipidemia or antihypertensive drugs remain stable |
Baseline balance statistically different in HbA1c and 2hPG between groups Significantly decreased HbAlc, FPG, 2hPG and increased HOMA-β in both CHM and placebo groups before and after treatment; Significant higher proportion of the HbA1c reversed to normal (HbA1c ≤ 6.5%) in the CHM group (47.6%) than that in the placebo group (35.5%) after treatment; More significant decrease of HbAlc, FPG, 2hPG, body weight, BMI, WC and increase of HOMA-β in the CHM group than those in the placebo group after treatment |
Mild side effects (CHM: n = 24; Placebo: n = 7); Transient slight ALT elevation (CHM: n = 2); Transient slight AST elevation (CHM: n = 2) | Short study period; No follow-up | |
|
Tang-Min-Ling-Wan 6 g/time, 3 times daily, 12 weeks Formulas Huangqin, Huanglian, Baishao, Chenpi, Dahuang |
Placebo 6 g/time, 3 times daily, 12 weeks | ||||||
| Tu et al. [33] China No information on study period | Type 2 diabetes |
Sample size n = 80 CHM group n = 41 Control group n = 39 Inclusion criteria diagnosed Type 2 diabetes; FPG: 7.0–13.3 mmol/l or 2hPG: 11.1–22.9 mmol/l; age: 18–70 years; normal renal function |
Diet and exercise advices were provided |
Baseline balance statistically different in gender between groups No significant difference of FPG, PPG, HbA1c between the CHM and control groups after treatment |
Side effects (CHM: n = 1) | Short study period; Not double blind trial | |
|
Wumei Wan 3 packages daily, 12 weeks Formulas Huanglian, Huangbai, Ganjiang, Ginseng, Danggui, Huajiao, et al. |
Metformin 500 mg/time, twice daily, 12 weeks | ||||||
| Wu and Fan [34] China Oct 2012–Jan 2013 | Type 2 diabetes |
Sample size n = 152 CHM group n = 76; 48 males and 28 females; age: 48–66 years Control group n = 76; 35 males and 41 females; age: 47–68 years Inclusion criteria diabetes symptoms and any plasma glucose ≥ 11.1 mmol/l; FPG ≥ 7.0 mmol/l; 2hPG ≥ 11.1 mmol/l during OGTT |
Self-proposed Chinese herbal medicines with insulin 1 dose daily, 2 weeks Formulas Guijianyu, Zhimu, Gegen, Jineijin, Zexie, Ginseng, et al. |
Insulin injection Novolin 30R before breakfast and lunch, 2 weeks |
Baseline balance Yes Significant more 20% decrease of insulin use in the CHM group than that in the control group after treatment; Significant less treatment days and frequency of hypoglycaemia in the CHM group than those in the control group after treatment |
N/A | N/A |
| Cai et al. [35] China No information on study period | Type 2 diabetes |
Sample size n = 67 CHM group n = 37 Control group n = 30 Inclusion criteria diabetes course < 5 years, fasting serum glucose > 7.0 mmol/l and/or 11.1 mmol/l after meal |
Diet and exercise advices were provided |
Baseline balance Yes Significantly decreased serum glucose and increased insulinogenic index in the CHM group before and after treatment; Significantly increased HDL in the CHM group than that in the placebo group after treatment |
No side effects | Small sample size; Short follow-up | |
|
Lycium barbarum Poly-saccharide capsule 300 mg/day, twice daily, 3 months Formulas Gouqi |
Placebo 300 mg/day body weight, twice daily, 3 months | ||||||
| Lian et al. [36] China Apr 2013–Oct 2013 | Type 2 diabetes |
Sample size n = 186 CHM group n = 92 Control group n = 94 Inclusion criteria diagnosed type 2 diabetes; standard diet control and exercise therapy; taking metformin in a steady dose for over 3 months; HbA1c ≥ 7.0%; FPG: 7.0–13.9 mmol/l or 2hPG ≥ 11.1 mmol/l; BMI: 18–40 kg/m2; age: 18–70 years |
Diet and exercise advices were provided |
Baseline balance Yes Significantly decreased HbA1c and increased HOMA-β in the CHM group before and after treatment; More significant decrease of HbA1c, FPG, 2hPG in the CHM group than those in the placebo group after treatment |
N/A | Short study period; Small sample size | |
|
Jinlida with metformin (1500 mg/kg/day) 1 granule/time, 3 times daily, 12 weeks Formulas Shuweicao, Yinyanghuo, Ginseng, Huangjing, Cangzhu, Kushen, et al. |
Placebo with metformin (1500 mg/kg/day) 1 granule/time, 3 times daily, 12 weeks | ||||||
| Zhang et al. [37] China Jan 2011–Dec 2013 | Type 2 diabetes |
Sample size n = 219; 112 males and 107 females; age: 38–74 years CHM group n = 109 Control group n = 110 Inclusion criteria diagnosed type 2 diabetes treated with insulin alone; FPG ≥ 7.0 mmol/l or 2hPG ≥ 11.1 mmol/l; age: 18 years and older; standard food containing100 g of carbohydrate during intervention |
Shen-Qi-Formula with insulin injection (300 IU, twice daily before breakfast and dinner) 100 ml/time, 3 times daily, 12 weeks Formulas Shengdihuang, Huangqi, Zhidahuang, Ginseng, Shanzhuyu, Shuweicao, et al. |
Insulin injection 300 IU, twice daily before breakfast and dinner, 12 weeks |
Baseline balance: Yes Significantly decreased FPG, HbA1c in both CHM and control groups before and after treatment; Significantly decreased HOMA-IR and insulin usage level in the CHM group, while significantly increased insulin usage level in the control group, before and after treatment after treatment; More significant decrease of FPG, PPG, HbA1c in the CHM group than those in the control group after treatment |
Transient hypoglycemia (Control: n = 1) | N/A |
| Hu et al. [38] China No information on study period | Type 2 diabetes |
Sample size n = 112 CHM group n = 59 Control group n = 53 Inclusion criteria newly diagnosed type 2 diabetes (illness course ≤5 years); only taking metformin for treatment; age: 18–75 years; HbA1c: 6.5–9.0% despite taking two 500 mg metformin tablets daily |
Diet and exercise advices were provided |
Baseline balance Yes Significantly decreased FPG, HbA1c in both CHM and placebo groups before and after treatment; More significant decrease of FPG, HbA1c in the CHM group than those in the placebo group after treatment |
No side effects | Small sample size; No group without lifestyle intervention; Almost 25% participants lost from both groups | |
|
Jianyutangkang tablet with Metformin (1.5 g/time, 3 times daily) 3 tablets/time, 3 times daily, 26 weeks Formulas Ciwujia, Zhimu, Guijianyu |
Placebo with Metformin 1.5 g/time, 3 times daily, 26 weeks | ||||||
| Li et al. [39] China Jun 2014–Dec 2014 | Type 2 diabetes |
Sample size n = 38 CHM group n = 23 Control group n = 15 Inclusion criteria diagnosed Type 2 diabetes; not on a regimen of antidiabetic medical treatment at least 3 months before screening, or on a regimen of antidiabetic treatment no more than 3 months at any time in the past, or on a stable regimen of metformin monotherapy for at least 8 weeks; age:18–70 years; HbA1c: 7.0–10.0%; FPG ≤ 13 mmol/l; BMI: 19–30 kg/m2 |
During the intervention, metformin remains stable |
Baseline balance Yes Significantly decreased 1 h and 2 h PPG, HbA1c in the CHM group before and after treatment without significant difference between these two groups after treatment; No significant difference of FPG between the CHM and control groups after treatment |
Gastrointestinal side effects (lower in the CHM group than control group) Slightly higher liver and kidney function indices in the CHM group than those in the control group |
Short study period; Small sample size; Missing data of BMI in follow-up period | |
|
Mulberry twig alkaloid tablet with Acarbose placebo (50 mg/time, 3 times daily) 50 mg-100 mg/time, 3 times daily, 24 weeks Formulas Sangzhi |
Placebo with Acarbose (50–100 mg/time, 3 times daily) 50 mg/time, 3 times daily, 24 weeks | ||||||
| Wang et al. [40] China No information on study period | Hyperlipidemia |
Sample size n = 446 CHM group n = 324; 188 males and 136 females; mean age: 56 years Control group n = 122; 73 males and 49 females; mean age: 56 years Inclusion criteria serum TC ≥ 5.95 mmol/l, LDL-C ≥ 3.41 mmol/l, or TG: 2.26-4.52 mmol/l; HDL-C ≤ 1.04 mmol/l (male)/1.16 mmol/l (female); no medication for hyperlipidemia for more than 4 weeks and received dietary advice for 2–4 weeks |
During the intervention, no medications affecting serum lipids |
Baseline balance Yes Significantly decreased TC, LDL-C, TG in both CHM and control groups before and after treatment; More significant decrease of TC, LDL-C, TG and increase of HDL-C in the CHM group than those that in the control group after treatment; Significant higher total effective rate in the CHM group (93.2%) than that in the control group (50.8%) |
CHM group: Heartburn; flatulence; Dizziness; Exacerbation of preexisting stomachache | N/A | |
|
Monascus purpureus rice preparation 3 tablets (600 mg)/time, twice daily, 8 weeks Formulas Red yeast rice |
Jiaogulan 3 tablets (600 mg)/time, twice daily, 8 weeks Formulas Jiaogulan |
||||||
| Yang et al. [41] China Feb 2002–May 2004 | Hyperlipidemia |
Sample size n = 96 CHM group n = 56; 31 males and 25 females; mean age: 69 years Control group n = 40; 29 males and 11 females; mean age: 68 years Both groups have cases with coronary heart disease, hypertension, and cerebral vascular disease Inclusion criteria TC > 5.7 mmol/l and/or TG > 1.7 mmol/l; TCM diagnosed for phlegm-damp and blood stasis syndrome |
During the intervention, no other drugs |
Baseline balance Yes Significantly decreased TC, LDL-C in both CHM and control groups before and after treatment; Significantly decreased TG in the CHM group before and after treatment; More significant decrease of TC, LDL-C in the CHM group than those in the control group after treatment |
No side effects | N/A | |
|
Danshen Jueming granules 24 g/time, twice daily Formulas Taizishen, Danshen, Juemingzi, Shanzha, Zexie, Chenpi, et al. |
Xuezhikang capsules 0.8 g/time, 3 times daily | ||||||
| Ai et al. [42] China No information on study period | Hyperlipidemia |
Sample size n = 60 CHM group n = 30 Control group n = 30 Inclusion criteria BMI < 35 kg/m2; TC ≥ 5.72 mmol/l and TG > 4.52 mmol/l; age: 18 years and older |
During the intervention, no other lipid-modulating drugs |
Baseline balance statistically different in the serum TG level between groups Significantly decreased in the TC, LDL-C in both CHM and control groups before and after treatment; More significant decrease of TC, LDL-C in the control group than those in the CHM group after treatment |
Diarrhea (CHM: n = 8); Myalgia and epigastric discomfort (Control: n = 2) | N/A | |
|
Daming capsule 2 g/time, twice daily, 6 weeks Formulas Dahuang, Ginseng, Juemingzi, Danshen |
Pravastatin 10 mg/time, once daily, 6 weeks | ||||||
| Xu et al. [43] China No information on study period | Hyperlipidemia |
Sample size n = 77 CHM group n = 37; 17 males and 20 females; mean age: 59 years Control group n = 40; 20 males and 20 females; mean age: 61 years Inclusion criteria TC ≥ 5.72 mmol/l or TG ≥ 1.70 mmol/l or HDL-C ≤ 1.04 mmol/l (male)/1.17 mmol/l (female); TCM diagnosed for phlegm-damp and blood stasis syndrome |
During the intervention, no drugs affecting the blood lipid metabolism |
Baseline balance Yes Significantly decreased TC, TG, LDL-C, BMI in the CHM group and significantly decreased LDL-C, BMI in the control group, before and after treatment; More significant decrease of TC, TG in the CHM group than those in the control group after treatment; Significantly lower recurrence rate in the CHM group than that in the control group after treatment |
No side effects | N/A | |
|
Antihyperlipidemic decoction 150 ml/time, twice daily, 8 weeks Formulas: Yiyiren, Shengpuhuang, Zexie, Shengshanzha, Huangqi, Juemingzi, et al. |
Zhinbiticose 1050 mg/time, 3 times daily, 8 weeks | ||||||
| Hu et al. [44] Hong Kong No information on study period | Hyperlipidemia |
Sample size n = 40 CHM group n = 20; 6 males and 14 females; mean age: 58 years Control group n = 20; 10 males and 10 females; mean age: 55 years Inclusion criteria diagnosed dyslipidemia with lipid-lowering therapy or fasting LDL-C ≥ 4.1 mmol/l or TG ≥ 1.7 mmol/l; plasma LDL-C ≥ 2.6 mmol/l or ≥ 1.8 mmol/l for those with high cardiovascular risk following lipid-lowering treatment and diet or plasma TG ≥ 1.7 mmol/l following a lipid-lowering diet; age: 18 years and older |
A multiherb formula 4 capsules in the morning and 4 capsules in the evening, 12 weeks Formulas Shanzha, Zexie, Yumixu, Sangye, Lingzhi, Heshouwu |
Placebo 4 capsules in the morning and 4 capsules in the evening, 12 weeks |
Baseline balance statistically different in the LDL-C level between groups More significant decrease of LDL-C in the CHM group than that in the placebo group after treatment; No significant difference of LDL-C in the CHM group before and after treatment |
CHM group: n = 11, including one stomach upset; Placebo group: n = 12, including one acid reflux | Not balanced baseline data of the two groups; Small sample size; Lack of consideration of the different types of dyslipidemia |
| Moriarty et al. [45] USA and China Apr 2011–Aug 2012 | Hyperlipidemia |
Sample size n = 116 CHM group 1 n = 36; 6 males and 30 females; mean age: 58 years CHM group 2 n = 42; 13 males and 29 females; mean age: 56 years Control group n = 38; 11 males and 27 females; mean age: 56 years Inclusion criteria TC ≥ 13.3 mmol/l; LDL-C: 8.9-12.2 mmol/l; TG < 22.2 mmol/l; BMI < 36 kg/m2; age: 18 years and older |
During the intervention, no lipid-lowering drugs, investigational agent, medications promoting weight loss, agents affecting lipid metabolism |
Baseline balance Yes Significantly decreased LDL-C in both two CHM groups before and after treatment, without significant difference between these two groups after treatment; The total effective rates at about 48% of LDL-C by ≥30% in the two CHM groups before and after treatment, without significant difference between these two groups |
CHM groups 1, 2: n = 5, not CHM-related side effects (thyroid cancer, pulmonary embolism, fractured leg) Placebo group: n = 3 |
Not representative data; More females than males; Short treatment period | |
|
CHM group 1: Xuezhikang 1200 mg 2 capsules (300 mg) and 2 placebo daily, 12 weeks CHM group 2: Xuezhikang 2400 mg 4 capsules (300 mg) daily, 12 weeks Formulas Red yeast rice |
Placebo 4 placebo capsules daily, 12 weeks | ||||||
| Heber et al. [46] USA No information on study period | Hyperlipidemia |
Sample size n = 83; 46 males and 37 females; age: 34–78 years Inclusion criteria LDL-C > 4.14 mmol/l and TG < 2.94 mmol/l; no treatment for hypercholesterolemia before; normal liver and renal function |
Diet advices were provided |
Baseline balance Yes Significantly decreased TC, TG, LDL-C in the CHM group before and after treatment; More significant decrease of TC, LDL-C in the CHM group than those in the placebo group after treatment |
Placebo group: Rash (n = 1); Headaches (n = 1); Concurrent development of pneumonia (n = 1) | N/A | |
|
Red yeast rice capsule 1 capsule (600 mg), 2.4 g daily, 12 weeks Formulas Red yeast rice |
Rice powder placebo capsule 1 capsule (600 mg), 2.4 g daily, 12 weeks | ||||||
| Lin et al. [47] Taiwan Dec 2001–Jan 2003 | Hyperlipidemia |
Sample size n = 79 CHM group n = 39; 23 males and 16 females; mean age: 46 years Control group n = 40; 22 males and 18 females; mean age: 47 years Inclusion criteria TC ≥ 6.22 mmol/l; LDL-C ≥ 4.14 mmol/l; TG ≤ 4.52 mmol/l; age: 18–65 years; BMI < 30 kg/m2; no lipid-lowering drugs 4 weeks before screening |
Diet advices were provided |
Baseline balance Yes Significantly decreased TC, TG, LDL-C in the CHM group before and after treatment; More significant decrease of TC, TG, LDL-C in the CHM group than those in the placebo group after treatment |
CHM group: Drug-related side effects (n = 6) | No record of diets of the participants | |
|
Monascus purpureus Went rice 1 capsule (600 mg)/time, twice daily, 8 weeks Formulas Red yeast rice |
Rice powder placebo 1 capsule (600 mg)/time, twice daily, 8 weeks | ||||||
| Wei et al. [48] China Mar 2006–Sep 2007 | Impaired glucose tolerance |
Sample size n = 140 CHM group n = 70; 31 males and 39 females; mean age: 51 years Control group n = 70; 32 males and 38 females; mean age: 51 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l; age: 25–70 years; BMI: 18.5–35.0 kg/m2; no IGT treatment before; TCM diagnosed for spleen-stomach dampness-heat syndrome |
Tang No.1 granule with IGT knowledge education 2 packets/time, twice daily, 6 months Formulas: Dangshen, Fushen, Huangqi, Shanyao, Huangqin, Huanglian, et al. |
IGT knowledge education |
Baseline balance Yes Significantly decreased FPG, 2hPG, HbA1c, TG, HOMA-IR in the CHM group before and after treatment;More significant decrease of FPG, 2hPG, HbA1c, TG, HOMA-IR in the CHM group than those in the control group after treatment;More patients with IGT reversed to normal in the CHM group (19.1%) than that in the control group (3.1%) |
No side effects | N/A |
| Gao et al. [49] China No information on study period | Impaired glucose tolerance |
Sample size n = 510 CHM group n = 255; 110 males and 145 females; mean age: 49 years Control group n = 255; 112 males and 143 females; mean age: 51 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l after OGTT and FPG > 7.0 mmol/l; age: 25–75 years; BMI: 20–35 kg/m2 |
Co-administered medications: calcium antagonists, α blockers or ACE antagonists, or β-blockers or thiazide for hypertension control |
Baseline balance Yes Significantly decreased 2hPG, HbA1c, BMI, FIN, HOMA-IR in the CHM group before and after treatment; More significant decrease of FPG, 2hPG, HbA1c, FIN, HOMA-IR in the CHM group than those in the control group after treatment; More patients with IGT reversed to normal in the CHM group (29.1%) than those in the control group (13.6%) after treatment; Lower risk of IGT patients progressing to Type 2 diabetes in the CHM group (22.2%) than that in the placebo group (43.9%) |
Mild abdominal distension (CHM: n = 4; Control: n = 3) | Small sample size; Short follow-up | |
|
Tangzhiping granule with Standard health care advice 5 g/time, twice daily, 5 days a week Formulas Huanglian, Sangbaipi, Gegen |
Standard health care advice | ||||||
| Fang et al. [50] China No information on study period | Impaired glucose tolerance |
Sample size n = 514 CHM group n = 257; 136 males and 121 females; mean age: 55 years Control group n = 257; 142 males and 115 females; mean age: 55 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l and FPG < 7.0 mmol/l; TCM diagnosed for spleen deficiency and dampness syndrome; age: 25–70 years; no IGT treatment before; no participation in clinical trials within the 3 months before screening |
Shenzhu Tiaopi granule with lifestyle intervention 8.8 g/time, twice daily, 12 months Formulas N/A |
Lifestyle intervention |
Baseline balance Yes More patients with IGT reversed to normal in the CHM group (42.2%) than that in the control group (32.9%); Lower risk of IGT patients progressing to Type 2 diabetes in the CHM group (8.5%) than that in the placebo group (15.3%) |
CHM group: n = 9 Placebo group: n = 5 Gastrointestinal reactions were the most common side effects |
Short follow-up; No consensus about efficacy of the CHM approach |
| Lian et al. [51] China Aug 2008–Mar 2010 | Impaired glucose tolerance |
Sample size n = 420 CHM group n = 210; 98 males and 112 females; mean age: 53 years Control group n = 210; 106 males and 104 females; mean age: 52 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l after OGTT and FPG > 7.0 mmol/l; age: 25–70 years; no IGT treatment before; no participation in clinical trials within the 3 months before screening |
Diet and exercise advices were provided |
Baseline balance Yes More patients with IGT reversed to normal in the CHM group (63.1%) than that in the control group (46.6%); Lower risk of IGT patients progressing to Type 2 diabetes in the CHM group (18.2%) than that in the placebo group (29.3%) |
CHM group: n = 15 Placebo group: n = 11 Gastrointestinal reactions were the most common side effects |
Short study period; No data on plasma insulin and HbA1c; Small sample size | |
|
Tianqi capsule 5 capsules/time, 3 times daily, 12 months Formulas Huangqi, Nvzhenzi, Huanglian, Tianhuafen, Shihu, Jixueteng, et al. |
Placebo 5 capsules/time, 3 times daily, 12 months | ||||||
| Huang et al. [52] China Mar 2013–Jul 2015 | Impaired glucose tolerance |
Sample size n = 120 CHM group n = 60; 31 males and 29 females; mean age: 52 years Control group n = 60; 35 males and 25 females; mean age: 51 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l and FPG < 7.0 mmol/l; age: 30–70 years; no diabetes history; normal blood test, urine, stool, liver and renal function |
Tangyiping granules with lifestyle intervention 10 g/time, twice daily, 12 weeks Formulas Huangqi, Baishao, Huanglian, Danshen, Banxia, Gegen |
Lifestyle intervention |
Baseline balance Yes Significantly decreased 2hPG, HbA1c, HOMA-IR, TG in the CHM group before and after treatment; More significant decrease of 2hPG, HbA1c, HOMA-IR, TG in the CHM group than those in the control group after treatment; More patients with IGT reversed to normal in the CHM group (58.3%) than that in the control group (26.7%); Lower risk of IGT patients progressing to Type 2 diabetes in the CHM group (16.7%) than that in the placebo group (31.7%) |
No severe side effects | Small sample size; Short follow-up; Insufficient outcome measures |
| Shi et al. [53] China Apr 2014–Oct 2014 | Impaired glucose tolerance |
Sample size n = 61 CHM group n = 32; 17 males and 15 females; mean age: 47 years Control group n = 29; 14 males and 15 females; mean age: 50 years Inclusion criteria 2hPG: 7.8–11.1 mmol/l after OGTT and FPG < 7.0 mmol/l; age: 20–80 years; BMI: 18–30 kg/m2 |
Diet, exercise, smoking/alcohol consumption advices were provided; no other CHM products with similar function |
Baseline balance Yes Significantly decreased FPG, 2hPG, HbA1c, HOMA-IR, BMI in the CHM group before and after treatment; More significant decrease of HbA1c, 2hPG, HOMA-IR in the CHM group than those in the control group after treatment; Lower risk of IGT patients progressing to Type 2 diabetes in the CHM group (6.2%) than that in the placebo group (17.2%); More patients with IGT reversed to normal in the CHM group (43.8%) than that in the control group (6.9%) |
Gastrointestinal reactions (n = 2) | Short study period; Small sample size | |
|
Jinlida granule 1 granule (9 g)/time, 3 times daily, 12 weeks Formulas Ginseng, Fuling, Cangzhu, Gegen, Huangjing, Zhimu, et al. |
No drug intervention | ||||||
| Grant et al. [54] Australia Jun 2007–Dec 2009 | Impaired glucose tolerance |
Sample size n = 71 CHM group n = 39; 15 males and 24 females; mean age: 58 years Control group n = 32; 18 males and 14 females; mean age: 60 years Inclusion criteria FPG < 7.0 mmol/l and 2hPG: 7.8–11.0 mmol/l; age: 18 years and older |
Jiangtang Xiaozhi 3 capsules/time, 3 times daily, 16 weeks Formulas Nvzhenzi, Huangqi, Huanglian, Kunbu, Lizhihe, Jianghuang |
Placebo 3 capsules/time, 3 times daily, 16 weeks |
Baseline balance Yes More significant decrease of fasting insulin, HDL in the CHM group than those in the placebo group after treatment; No information on the efficacy of CHM before and after treatment |
CHM group: moderate dizziness (n = 1) | Short study period; Small sample size |
| Pan et al. [55] China Jul 2003–Aug 2003 | Obesity |
Sample size n = 78 CHM group n = 40; 18 males and 22 females; mean age: 41 years Control group n = 38; 17 males and 21 females; mean age: 41 years Inclusion criteria BMI ≥ 25 kg/m2; age: 20–50 years |
Dietary powder 1 package (9 g)/time, twice daily, 7 weeks Formulas Lotus rhizome, Green tea, Sanqi |
Placebo 1 package (9 g)/time, twice daily, 7 weeks |
Baseline balance Yes Significantly decreased body mass, percentage of body fat, BMI, WC, HC in the CHM group before and after treatment; More significant decrease of body mass, percentage of body fat, BMI, WC, HC in the CHM group than those in the placebo group |
Irritability (CHM: n = 1; Placebo: n = 1); Nausea (CHM: n = 2; Placebo: n = 1); Constipation (Placebo: n = 2) | N/A |
| Zhou et al. [56] China May 2010–Feb 2011 | Obesity |
Sample size n = 134 CHM group n = 70; 31 males and 39 females; mean age: 40 years Control group n = 64; 29 males and 35 females; mean age: 40 years Inclusion criteria BMI: 28–40 kg/m2; WC ≥ 85 cm (male)/80 cm (female); age: 18–60 years; TCM diagnosed for qi and phlegm stasis syndrome |
Xin-Ju-Xiao-Gao-Fang (full-dose) 170 ml decoction/time, twice daily, 24 weeks Formulas Dahuang, Zhishi, Huanglian, Juemingzi |
Xin-Ju-Xiao-Gao-Fang (10% of full-dose) 170 mL decoction/time, twice daily, 24 weeks |
Baseline balance Yes More significant decrease of body weight, WC, HC, FIN in the CHM group than those in the control group after treatment |
Minor side effects (e.g. skin rash) (CHM: n = 4; Control: n = 3) | Short study period; No follow-up; No true placebo group |
| Lenon et al. [57] Australia No information on study period | Obesity |
Sample size n = 117 CHM group n = 59; 10 males and 49 females; mean age: 39 years Control group n = 58; 10 males and 48 females; mean age: 40 years Inclusion criteria BMI ≥ 30 kg/m2; age: 18–60 years |
During the intervention, no other medications for obesity management |
Baseline balance Yes Significantly decreased body weight, BMI, body fat in the CHM group and increased body weight, BMI, body fat in the placebo group, before and after treatment; More significant decrease of body weight, BMI in the CHM group than those in the placebo group after treatment |
Nausea (CHM: n = 4) Headache (CHM: n = 9) Decrease of appetite (Placebo: n = 2) |
N/A | |
|
Chinese herbal medicine formula RCM-104 4 capsules/time, 3 times daily, 12 weeks Formulas Green tea, Juemingzi, Huaihua |
Placebo 4 capsules/time, 3 times daily, 12 weeks | ||||||
| Hioki et al. [58] Japan No information on study period | Obesity and impaired glucose tolerance |
Sample size n = 81; mean age: 54 years CHM group n = 41 Control group n = 40 Inclusion criteria FPG < 7.0 mmol/l and 2hPG: 7.8–11.1 mmol/l after OGTT |
Diet and exercise advices were provided |
Baseline balance Yes Significantly decreased body weight, WC, HC, TC,TG, LDL-C in both CHM and placebo groups before and after treatment; Significantly decreased fasting insulin, HOMA-IR in the CHM group before and after treatment; More significant decrease of WC in the CHM group than that in the placebo group after treatment |
CHM group: Loose bowels (n = 3) | N/A | |
|
Bofu-tsusho-san 3 times daily, 24 weeks Formulas Jingjie, Bohe, Shigao, Gancao, Lianqiao, Mahuang, et al. |
Placebo 3 times daily, 24 weeks | ||||||
| Gao & Hu [59] China No information on study period | Type 2 diabetes and hyperlipidemia |
Sample size n = 80 CHM group n = 40; 22 males and 18 females; mean age: 59 years Control group n = 40; 20 males and 20 females; mean age: 59 years Inclusion criteria FPG > 7.0 mmol/l and blood PG > 6.1 mmol/l |
During the intervention, hypoglycemic agents remain stable |
Baseline balance Yes Significantly decreased TC, TG, LDL-C and increased HDL-C in the CHM group before and after treatment, without significant difference compared to the control group after treatment |
Control group: Slight elevation of ALT (n = 2) | N/A | |
|
Taizhi’an capsule with Simvastatin (10 mg daily) 0.9 g/time, 3 times daily, 12 weeks Formulas N/A |
Simvastatin 20 mg daily, 12 weeks | ||||||
| Poppel et al. [60] Netherlands May 2012–Mar 2013 | Hyperlipidemia and hypertension |
Sample size n = 20; 14 males and 6 females; mean age: 58 years CHM group n = 9 Control group n = 11 Inclusion criteria fasting LDL-C > 3.5 mmol/l and/or TG > 1.7 mmol/l; age: 40–70 years; SBP > 140 mmHg and/or DBP > 90 mmHg despite taking antihypertensive drugs |
Danshen capsules 4 capsules (500 mg)/time, 3 time daily, 4 weeks Formulas Danshen |
Placebo 4 capsules (500 mg)/time, 3 time daily, 4 weeks |
Baseline balance Yes Significantly increased LDL-C in the CHM group before and after treatment, without significant difference compared to the placebo group; No significant difference of BP between the CHM and placebo groups after treatment after treatment |
CHM group: Headache (n = 5); Dizziness (n = 3); Change in stool frequency (n = 3); Flatulence (n = 2); Peripheral facial nerve paralysis (n = 1) | Carry-over effect |
| Chu et al. [61] China Jan 2008–Dec 2009 | Metabolic syndrome |
Sample size n = 90 CHM group n = 60; 28 males and 32 females; mean age: 51 years Control group n = 30; 13 males and 17 females; mean age: 50 years Inclusion criteria diagnosed central obesity; WC > 90 cm (male)/80 cm (female) and/or BMI > 25 kg/m2; fasting blood glucose ≥ 6.1 mmol/l and/or 2hPG ≥ 7.8 mmol/l or having diabetes history; TG > 1.7 mmol/l and/or HDL-C < 0.9 mmol/l(male)/1.0 mmol/l (female); age: 18–70 years |
Diet and exercise advices were provided; During the intervention, no other CHM with hypoglycemic, lipid-lowering and antihypertensive effects |
Baseline balance Yes Significantly decreased BMI, waist-to-hip ratio, TC, TG, LDL-C, 2hPG and increased HDL-C in the CHM group before and after treatment; More significant decrease of BMI, TC, LDL-C, 2hPG and increase of HDL-C in the CHM group than those in the placebo group after treatment |
CHM group: Diarrhea (n = 1) | N/A | |
|
Pu’er tea extract capsules 4 capsules/time, twice daily, 3 months Formulas Pu’er tea |
Placebo 4 capsules/time, twice daily, 3 months | ||||||
| Chen et al. [62] China Oct 2011–Oct 2012 | Hypertension and metabolic syndrome |
Sample size n = 43 CHM group n = 22; 14 males and 8 females; mean age: 49 years Control group n = 21; 14 males and 7 females; mean age: 49 years Inclusion criteria diagnosed metabolic syndrome; average BP > 135/85 mmHg when awake and > 120/75 mmHg during sleep or SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg; age: 18–65 years |
Diet and exercise intervention were provided |
Baseline balance Yes Significantly decreased body weight, WC, BMI, FPG, 2hPG, FIN, HOMA-IR, SBP, DBP, daytime SBP, daytime DBP, nighttime SBP in the CHM group before and after treatment; More significant decrease of WC, waist-to-hip ratio, 2hPG, HOMA-IR, FIN, SBP, DBP, daytime SBP and DBP than those in the placebo group after treatment |
CHM group: Skin allergy (n = 2) | N/A | |
|
Yiqi Huaju formula 1 bag/time, twice daily, 12 weeks Formulas Huangqi, Zexie, Huanglian, Yinchen, Puhuang |
Placebo 12 weeks | ||||||
| Azushima et al. [63] Japan Jun 2010–Mar 2013 | Hypertension and obesity |
Sample size n = 106 CHM group n = 54; 28 males and 26 females; mean age: 59 years Control group n = 52; 29 males and 23 females; mean age: 60 years Inclusion criteria diagnosed hypertension with a history of antihypertensive treatment more than 4 weeks; BMI > 25 kg/m2; age: 20–79 years |
Diet and exercise advices were provided |
Baseline balance Yes Significantly decreased daytime SBP, daytime DBP, body weight, BMI in the CHM group before and after treatment; More significant decrease of daytime SBP, body weight, BMI in the CHM group than those in the control group after treatment |
CHM group: Gastric irritation (n = 1); Constipation (n = 1); Elevation of serum hepatic enzyme level (n = 1) | Not a double-blinded placebo-controlled study; Short study period | |
|
Bofu-tsusho-san with Antihypertensive therapy 2.5 g/time, once daily, 24 weeks Formulas Jingjie, Bohe, Shigao, Mahuang, Gancao, Lianqiao, et al. |
Antihypertensive therapy No further information | ||||||
2hPG 2-hour postprandial glucose, BP blood pressure, BMI body mass index, DBP diastolic blood pressure, FIN fasting plasma insulin, FPG Fasting plasma glucose, HbA1c glycated hemoglobin, HC hip circumferences, HDL high-density lipoprotein, HDL-C high-density lipoprotein cholesterol, HOMA-β homeostatic model assessment β-cell function, HOMA-IR homeostatic model assessment insulin resistance, IGT Impaired glucose tolerance, LDL-C low-density lipoprotein cholesterol, LVMI left ventricular mass index, MBP mean blood pressure, OGTT oral glucose tolerance test, PPG postprandial plasma glucose, SBP systolic blood pressure, TC total cholesterol, TG triglyceride, TO original heart rate, WC waist circumference
Quality assessment
Two authors independently assessed the methodological quality of the included studies using the Cochrane risk of bias criteria [17]. The characteristics of RCTs that might be related to selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other bias were evaluated. Disagreements regarding the risks of bias of some studies were resolved through discussion amongst these two authors (Table 3).
Table 3.
Risk of bias assessment of the included studies using the Cochrane risk of bias tool
| Author, Country, Publication year | Stroke risk factor | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|---|
| Lin et al. [18], China, 2004 | (Primary) Hypertension | Unclear | Unclear | High risk | Unclear | Low risk | Unclear | Unclear |
| Li [19], China, 2005 | (Primary) Hypertension | Unclear | Unclear | High risk | Unclear | Unclear | Unclear | Unclear |
| Ye et al. [20], China, 2009 | (Primary) Hypertension | Unclear | Unclear | Low risk | Low risk | Unclear | Low risk | Unclear |
| Zhao et al. [21], China, 2010 | (Primary) Hypertension | Unclear | Unclear | Low risk | Unclear | Unclear | Unclear | Unclear |
| Zhong et al. [22], China, 2011 | (Primary) Hypertension | Low risk | High risk | High risk | Unclear | Low risk | Low risk | Unclear |
| Yang et al. [23], Taiwan, 2012 | (Uncontrolled primary) Hypertension | Low risk | Unclear | High risk | Low risk | Unclear | Low risk | High risk |
| Tong et al. [24], China, 2013 | Hypertension | Low risk | High risk | High risk | Low risk | Unclear | Low risk | Unclear |
| Wu et al. [25], China, 2014 | (Primary) Hypertension | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | Unclear |
| Li et al. [26], China, 2010 | (Isolated systolic) Hypertension | Low risk | Unclear | Low risk | Unclear | High risk | Unclear | Unclear |
| Chen et al. [27], China, 2012 | (Polarized) Hypertension | Low risk | Unclear | High risk | Unclear | Unclear | Unclear | High risk |
| Gong et al. [28], China, 2010 | Hypertension with cardiac damage | Unclear | Unclear | High risk | Unclear | Low risk | Unclear | Unclear |
| Xu et al. [29], China, 2013 | Hypertension, hypertension with diabetes, hypertension with coronary heart disease | Unclear | Unclear | High risk | Unclear | Unclear | Low risk | High risk |
| Chao et al. [30], China, 2009 | Type 2 diabetes | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Ji et al. [31], China, 2013 | Type 2 diabetes | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Unclear |
| Tong et al. [32], China, 2013 | Type 2 diabetes | Low risk | Unclear | Low risk | Low risk | High risk | Unclear | Unclear |
| Tu et al. [33], China, 2013 | Type 2 diabetes | Low risk | Low risk | High risk | Unclear | Low risk | Low risk | Unclear |
| Wu & Fan [34], China, 2014 | Type 2 diabetes | Unclear | Unclear | High risk | Unclear | Unclear | Unclear | Unclear |
| Cai et al. [35], China, 2015 | Type 2 diabetes | Low risk | Unclear | Low risk | Unclear | Low risk | Low risk | Unclear |
| Lian et al. [36], China, 2015 | Type 2 diabetes | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Zhang et al. [37], China, 2015 | Type 2 diabetes | Low risk | Low risk | High risk | Unclear | Unclear | Low risk | Unclear |
| Hu et al. [38], China, 2016 | Type 2 diabetes | Low risk | High risk | Low risk | Low risk | High risk | Low risk | Unclear |
| Li et al. [39], China, 2016 | Type 2 diabetes | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Wang et al. [40], China, 1997 | Hyperlipidemia | Low risk | Unclear | High risk | Low risk | Low risk | Low risk | Unclear |
| Yang et al. [41], China, 2006 | Hyperlipemia | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High risk |
| Ai et al. [42], China, 2009 | Hyperlipemia | High risk | High risk | High risk | High risk | Unclear | Low risk | High risk |
| Xu et al. [43], China, 2009 | Hyperlipemia | Unclear | Unclear | High risk | Unclear | Unclear | Unclear | Unclear |
| Hu et al. [44], Hong Kong, 2014 | Hyperlipemia | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | High risk |
| Moriarty et al. [45], USA & China, 2014 | Hyperlipemia | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk | Unclear |
| Heber et al. [46], USA, 1999 | Hyperlipidemia | Unclear | Unclear | Low risk | Unclear | Low risk | Low risk | High risk |
| Lin et al. [47], Taiwan, 2005 | Hyperlipidemia | High risk | Unclear | Low risk | Low risk | Low risk | Low risk | High risk |
| Wei et al. [48], China, 2008 | Impaired glucose tolerance | High risk | Unclear | High risk | Unclear | Low risk | Unclear | Unclear |
| Gao et al. [49], China, 2013 | Impaired glucose tolerance | Low risk | Unclear | High risk | Unclear | Low risk | Low risk | Unclear |
| Fang et al. [50], China, 2014 | Impaired glucose tolerance | Unclear | Unclear | High risk | Unclear | Low risk | Low risk | Unclear |
| Lian et al. [51], China, 2014 | Impaired glucose tolerance | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear |
| Huang et al. [52], China, 2016 | Impaired glucose tolerance | Low risk | Low risk | High risk | Unclear | Low risk | Low risk | Unclear |
| Shi et al. [53], China, 2016 | Impaired glucose tolerance | Low risk | Unclear | High risk | Unclear | High risk | Low risk | Unclear |
| Grant et al. [54], Australia, 2013 | Impaired glucose tolerance | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | High risk |
| Pan et al. [55], China, 2005 | Obesity | Low risk | Unclear | Low risk | Unclear | Low risk | Unclear | High risk |
| Zhou et al. [56], China, 2014 | Obesity | Low risk | Unclear | Low risk | Unclear | Unclear | Low risk | Unclear |
| Lenon et al. [57], Australia, 2012 | Obesity | Unclear | Low risk | Low risk | Unclear | Low risk | Low risk | Unclear |
| Hioki et al. [58], Japan, 2004 | Obesity and impaired glucose tolerance | Low risk | High risk | Low risk | Unclear | Unclear | Low risk | High risk |
| Gao & Hu [59], China, 2006 | Type 2 diabetes and hyperlipidemia | Unclear | Unclear | High risk | Unclear | Low risk | Low risk | Unclear |
| Poppel et al. [60], Netherlands, 2015 | Hyperlipidemia and hypertension | High risk | Unclear | Low risk | Unclear | Low risk | Low risk | High risk |
| Chu et al. [61], China, 2011 | Metabolic syndrome | High risk | Unclear | Low risk | Unclear | Low risk | Low risk | Unclear |
| Chen et al. [62], China, 2013 | Hypertension and metabolic syndrome | Low risk | Unclear | Low risk | Unclear | High risk | Low risk | Unclear |
| Azushima et al. [63], Japan, 2015 | Hypertension and obesity | Low risk | Unclear | High risk | High risk | Low risk | Low risk | High risk |
Results
The systematic review reported in this paper has been registered on the PROSPERO (International prospective register of systematic reviews, #CRD42017060107). The PRISMA flowchart of literature search and study/article selection has been shown in Fig. 1. A total of 2377 papers were identified (2374 via database searches and three additional papers via Google Scholar). After removing duplicates, a total of 2065 papers remained for review. From amongst these, 70 manuscripts were identified for full review following title and abstract screening. Further screening of the full texts identified 46 publications (reporting on 46 RCTs) as eligible for final inclusion in the systematic review. Twelve of the included articles report on the efficacy of CHM for hypertension (1340 participants), 10 for diabetes (2004 participants), eight for hyperlipidaemia (997 participants), seven for IGT (1805 participants), three for obesity (329 participants), and six for the combination of several stroke risk factors (420 participants). No manuscript reported on a trial investigating the efficacy of CHM interventions for the stroke risk factor of transient ischemic attack or atrial fibrillation as a primary outcome. The characteristics of included studies with regards to the CHM interventions for hypertension, diabetes, hyperlipidaemia, IGT, obesity, and combined stroke risk factors are summarized in Table 2.
Fig. 1.
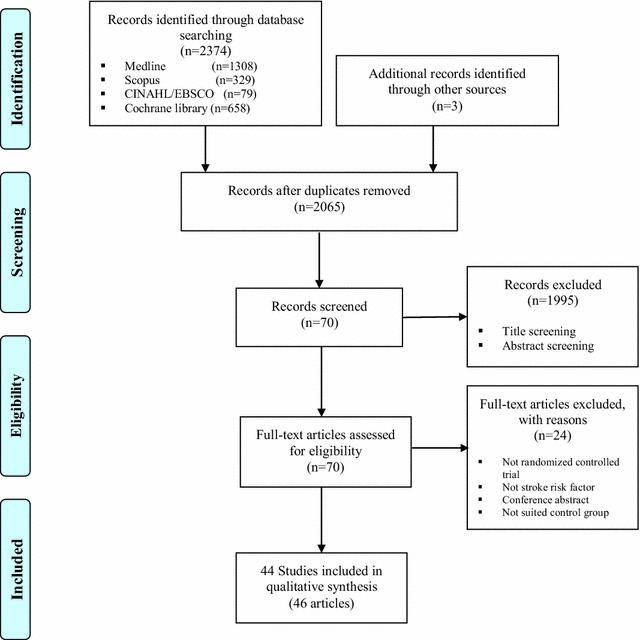
PRISMA flowchart of literature search and study selection
Hypertension
Eight RCTs were focused upon primary (essential) hypertension [18–25], one with isolated systolic [26], one with elder polarized hypertension [27], and two with hypertension and related cardiovascular diseases [28, 29]. Of the 12 RCTs on CHM for hypertension, 11 RCTs originated from China [18–22, 24–29]. Amongst the hypertension-focused RCTs, one RCT compared ‘CHM, biomedicine plus lifestyle’ intervention with ‘biomedicine plus lifestyle’ intervention [27] and showed significant decreased systolic blood pressure (SBP) before and after treatment of both intervention groups and a similar effect on controlling SBP between these two groups after treatment. Another two RCTs compared two different CHM interventions using different inclusion criteria of people with hypertension [19, 21]—these studies both reported a significant decrease of SBP and diastolic blood pressure (DBP) via all the CHM interventions examined with higher effective rate of treatments in the CHM groups than those in the control groups. Another three RCTs compared ‘CHM’ interventions with ‘biomedicine’ interventions and employed consistent inclusion criteria regarding SBP (140–179 mmHg) and DBP (90–109 mmHg) of participants, reporting a statistically significant decrease of SBP and DBP before and after treatment of both groups and a similar effect on controlling SBP and DBP between these two groups after treatment [18, 22, 24]. Another six RCTs compared ‘CHM plus biomedicine’ interventions with ‘biomedicine alone’ or ‘biomedicine plus placebo’ interventions [20, 23, 25, 26, 28, 29]. It is noteworthy that two of these six trials [20, 28] examined the efficacy of the same CHM products (Xuezhikang capsule) at different dose levels, demonstrating a significant decrease of SBP and DBP before and after treatment of both intervention groups and a silimar effect on SBP and DBP control between these two groups after treatment. Also amongst these six RCTs, three were three-armed RCTs which compared either ‘CHM plus biomedicine’ intervention versus ‘biomedicine/no intervention’, ‘CHM’ interventions versus ‘CHM plus biomedicine’ or ‘placebo plus biomedicine’ intervention, or two types of preparations of a ‘CHM plus biomedicine’ intervention versus ‘placebo plus biomedicine’ intervention [25, 26, 28], showing inconsistent findings with regards to the decrease of SBP or DBP amongst the three groups after treatment. Gouteng (钩藤) [18, 19, 21, 24, 25, 29] and Tianma (天麻) [18, 22, 25–27] were the most frequently used Chinese herbs in the hypertension-focused RCTs included, and all the CHM interventions using Gouteng and/or Tianma reported significant pre-post effectiveness regarding the decrease of SBP (and/or DBP) level. Also, Gouteng was the principal CHM formula constituent amongst four out of six hypertension-focused RCTs presenting between-group effectiveness of the investigated CHM interventions on the decrease of SBP (and/or DBP) levels compared to control interventions [21, 24, 25, 29]. In addition, the sample size of hypertension-focused RCTs ranged from 55 to 219. Six hypertension-focused RCTs did not provide the age and gender profile of the participants in either CHM group or control group [20, 22, 23, 26, 27, 29]. The duration of the hypertension-focused trials ranged from 2 weeks to 24 months, with the majority of trials conducted between 4 and 12 weeks.
Eight hypertension-focused RCTs reported safety-related information and no deaths were noted [18, 19, 21, 23, 26–29]. One trial reported five cases of serious side effects of the ‘CHM plus biomedicine’ intervention group [29]. One trial (sample: 55) reported 13 mild side effects in the ‘CHM plus biomedicine’ intervention group and 15 in the ‘placebo plus biomedicine’ control group [23]. Only two of the papers reporting results from hypertension-focused RCTs listed any study limitations including small sample size and short study period [23, 24]. As for risk of bias in the hypertension-focused RCTs, three papers provided information on the allocation concealment [22, 24, 25] and four on the blinding of outcome assessment [20, 23–25]. Additionally, only three trials reported double-blinding of participants and personnel involved [20, 21, 26].
Diabetes
All of the 10 included diabetes-focused RCTs were focusing upon patients diagnosed with Type 2 diabetes mellitus and all these RCTs were conducted in China [30–39]. Amongst the 10 RCTs examining the efficacy of CHM on controlling the glucose level of patients with diabetes, four RCTs compared ‘CHM’ intervention to ‘placebo’ [32], ‘CHM plus biomedicine’ intervention to ‘placebo plus biomedicine’ intervention [39], and further, ‘CHM plus lifestyle’ intervention to ‘placebo plus lifestyle’ intervention [30, 35]. These four trials indicated more significant decreased glucose level [e.g. fasting plasma glucose (FPG), 2-hour postprandial glucose (2hPG), glycated hemoglobin (HbA1c)] by using CHM products when compared to the placebos after treatment, while this significant between-group variance in the decrease of glucose level showed no statistical significance when both CHM interventions and placebos were used concurrently with biomedicine or lifestyle intervention. Also amongst these 10 diabetes-focused RCTs, ‘CHM plus biomedicine’ intervention was compared to ‘biomedicine’ intervention, showing a more significant decrease of insulin usage by the CHM plus biomedicine treatment after treatment [34]. Also, after treatment, ‘CHM, biomedicine plus lifestyle’ interventions were found to achieve a more significant decrease of FPG, HbA1c, or hypoglycemia when compared to either ‘biomedicine plus lifestyle’ intervention [31, 37] or ‘placebo, biomedicine plus lifestyle’ intervention [36, 38]. Of the nine diabetes-focused RCTs providing CHM formulas, Huanglian (黄连) was the most common Chinese herb [30, 32–34, 36], followed by Ginseng (人参) [33, 34, 36, 37], Shanzhuyu (山茱萸) [34, 36, 37], Dahuang (大黄) [32, 34, 37], and Huangqi (黄芪) [30, 34, 37]. The CHM interventions examined in three out of five diabetes-focused RCTs, showing significant between-group effectiveness on the decrease of glucose level, indicated that the combination of these five commonly used Chinese herbs played a vital role for the efficacy of type 2 diabetes management [34, 36, 37]. All diabetes-focused RCTs defined inclusion criteria of diabetes based on different FPG, 2hPG, and/or HbA1c levels, and all the tested CHM products used in these RCTs were different. The sample size of the diabetes-focused RCTs ranged from 43 to 627. Only one RCT provided the age and gender profile of participants in the CHM and control groups [35]. The duration of the trials ranged from 2 weeks to 12 months, with the majority of trials conducted between 3–12 months.
Only two diabetes-focused RCTs failed to report safety-related information and no death were noted [34, 36]. The side effects of CHM products reported in the diabetes-focused RCTs are generally moderate, such as constipation, gastrointestinal disorders, and urinary tract infection. However, three diabetes-focused RCTs showed that CHM interventions caused slightly abnormal liver and kidney function after 3, 6, and 12 months, respectively [31, 32, 39]. Six diabetes-focused RCTs have specified their study limitations, with a short study period being the most common issue, followed by small sample size and no/short follow-up period [32, 33, 35, 36, 38, 39]. As for risk of bias of the diabetes-focused RCTs, one trial failed to use the random sequence generation method [34], three trials did not report information on allocation concealment [32, 34, 35], four trials failed to apply a double-blinding method [31, 33, 34, 37], and four trials did not provide details on the blinding outcome assessment [33–35, 37].
Hyperlipidemia
Half of the eight RCTs on CHM for the treatment of hyperlipidemia originated from China [40–43]. Amongst the hyperlipidemia-focused RCTs, two compared ‘CHM’ interventions with ‘biomedicine’ interventions [42, 43], two compared different ‘CHM’ interventions [40, 41], two compared ‘CHM’ interventions with ‘placebos’ [44, 45] and two compared ‘CHM plus lifestyle’ interventions with ‘placebo plus lifestyle’ interventions [46, 47]. Although the inclusion criteria of people with hyperlipidemia shown in the included hyperlipidemia-focused RCTs are limited to the total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and/or body mass index (BMI) levels, the threshold value of these indices are diverse across the RCTs. It is worth noting that Monascus purpureus rice preparation (Xuezhikang capsule in Chinese) of which the main ingredient is red yeast rice, was tested in four hyperlipidemia-focused RCTs [40, 45–47]. The effects of the red yeast rice products are not consistent across these four RCTs. When the ‘red yeast rice product plus lifestyle’ intervention was compared with ‘placebo plus lifestyle’ intervention, a more significant decrease of TC and LDL-C was found in the red yeast rice product group after treatment. However, there was no significant improvement in TC or LDL-C amongst those receiving the red yeast rice product alone when compared to placebo alone. Amongst the rest four hyperlipidemia-focused RCTs, Danshen (丹参) [41–43], Juemingzi (决明子) [41–43], Zexie (泽泻) [41, 43, 44], and/or Shanzha (山楂) [41, 43, 44] were the main constituents of the CHM formulas examined and three of these trials reported the significant between-group effectiveness of the investigated CHM interventions on the decrease of TC, LDL-C, and/or TG levels [41, 43, 44] compared to control interventions. The sample size of the hyperlipidemia-focused RCTs ranged from 40 to 446. Only two hyperlipidemia-focused RCTs did not provide the age and gender profile of the participants in CHM and control groups [42, 46]. The duration of the trials ranged from 6 weeks to 12 months while one trial did not specify the study period.
All hyperlipidemia-focused RCTs reported safety-related information and no deaths were noted. Three trials specified their side effects in the CHM intervention groups, including heartburn/flatulence [40], diarrhea [42], and stomach upset [40, 44]. Three hyperlipidemia-focused RCTs reported their study limitations including small sample size, lack of balanced baseline data between the CHM and control groups and no record of the participants’ dietary control [44, 45, 47]. As for risk of bias of the hyperlipidemia-focused RCTs, five trials did not use the random sequence generation method [41–43, 46, 47], only two trials specified the appropriate allocation concealment [44, 45], and six trials failed to employ the blinding of outcome assessment [41–46].
Impaired glucose tolerance
The seven RCTs on CHM for the treatment of IGT originated from China (n = 6) [48–53] and Australia (n = 1) [54]. Amongst the IGT-focused RCTs, one compared ‘CHM’ with ‘placebo’ [54], five compared ‘CHM plus lifestyle’ interventions with ‘lifestyle’ interventions alone [48–50, 52, 53], and one compared ‘CHM plus lifestyle’ intervention with ‘placebo plus lifestyle’ intervention [51]. The inclusion criteria regarding the 2hPG level remain stable (7.8–11.0 mmol/l) while the FPG level is either <7.0 or >7.0 mmol/l across all the IGT-focused RCTs. Additionally, all the tested CHM products within the IGT-focused RCTs are different. Despite the variation in the inclusion criteria and CHM products, the results on the effects of CHM interventions are consistent throughout all IGT-focused trials. Specifically, more people with IGT reversed to normal in the CHM group (range 19.1–63.1%) compared to those in the control group (range 3.1–46.6%) and less people with IGT progressed to Type 2 diabetes in the CHM group (range 6.2–22.2%) compared to those in the control group (range 15.3–43.9%). Of the six IGT-focused RCTs with detailed CHM formulas, five reported the significant between-group effectiveness of the investigated CHM interventions regarding the decrease of FPG, 2hPG, and/or HbA1c levels compared to control interventions [48, 49, 52–54] and Huanglian (黄连) and Gegen (葛根) were the only Chinese herbs both included in these five IGT-focused trials. The sample size of the IGT-focused RCTs ranged from 61 to 514, and all these RCTs provided the age and gender profile of participants in the CHM and control groups (897 males, 939 females, mean age 53 years with the range from 47 to 60 years). The duration of the IGT-focused trials ranged from 3 to 12 months.
All IGT-focused RCTs reported safety-related information and no deaths were noted. The most common side effects reported in the CHM groups were dizziness, gastrointestinal reactions, and abdominal distension. Almost all IGT-related RCTs provided information on their study limitations including a short study period and short follow-up period as well as small sample size. As for risk of bias of the IGT-focused RCTs, three trials provided information about the allocation concealment [51, 52, 54], two trials provided details on the blinding of outcome assessment [51, 54], and two trials reported double-blinding of participants and personnel [51, 54].
Obesity
Two RCTs on CHM for the treatment of obesity originated from China [55, 56] and one from Australia [57]. The three obesity-focused trials compared three different CHM products with their placebos. BMI is the key indicator of the inclusion criteria of all obesity-focused RCTs included. However, the threshold value of BMI was set differently across these trials. Amongst the obesity-focused RCTs, CHM products all showed more decrease of body weight than placebos after treatment. Green tea (绿茶) [55, 57] and Juemingzi (决明子) [56, 57] were the Chinese herbs included in two CHM formulas amongst these three obesity-focused trials. The sample size of the obesity-focused RCTs ranged from 78 to 134 and all these RCTs provided the age and gender profile of participants in the CHM and placebo groups. There were 115 males and 214 females across all the obesity-focused RCTs with a mean age of 40 years, ranging from 39 to 41 years. The duration of the obesity-focused trials ranged from 7 weeks to 6 months.
All obesity-focused RCTs reported safety-related information and no death were noted. CHM interventions were reported more side effects than the placebos, including nausea, headache, and skin rash. One obesity-focused RCT indicated the study limitations including short study period, no follow-up period, and no true placebo group [56]. As for risk of bias of the obesity-focused RCTs, all trials reported the double-blinding of participants and personnel while these trials failed to provide any details of the blinding of outcome assessment.
Combined stroke risk factors
Six RCTs exploring the efficacy of CHM on one or more of the stroke risk factors were identified in the systematic review. Specifically, one trial examined the ‘CHM plus lifestyle’ intervention for the treatment of ‘IGT and obesity’ compared to ‘placebo plus lifestyle’ intervention, showing significant efficacy on both IGT and obesity before and after treatment and a significant effect on obesity control between groups after treatment [58]; Two trials examined the ‘CHM plus biomedicine’ interventions for the treatment of ‘diabetes and hyperlipidemia’ and ‘hypertension and hyperlipidemia’ compared to the ‘biomedicine’ intervention [59] and ‘placebo plus biomedicine’ intervention [60], respectively—both of these studies found similar effect on the combined stroke risk factors between groups after treatment. Moreover, three trials examined the ‘CHM, biomedicine plus lifestyle’ interventions for the treatment of ‘metabolic syndrome’ [61], ‘hypertension and metabolic syndrome’ [62], and ‘hypertension and obesity’ [63] compared to the ‘biomedicine plus lifestyle’ interventions with or without placebo, respectively, indicating significant effects on all included stroke risk factors by the CHM interventions compared to the control groups after treatment. Except the Bofu-tsusho-san (防风通圣散) used in two trials, all the other CHM interventions involved exploring a combination of multiple stroke risk factors were different and therefore it is unable to report the commonly used Chinese herbs which are vital for the efficacy of combined stroke risk factors across these six RCTs. The sample size of the RCTs focused upon combined stroke risk factors ranged from 20 to 106, and two of these RCTs failed to provide the age and gender profile of participants in the CHM and control groups [58, 60]. The duration of the RCTs exploring the combined stroke risk factors ranged from 4 to 6 months.
All RCTs focusing upon combined stroke risk factors reported safety-related information and no deaths were noted. Five out of these six RCTs reported that side effects only occurred in the CHM group [58, 60–63] including headache, dizziness, gastrointestinal reactions, and skin allergy. Only two RCTs focusing upon combined stroke risk factors identified their study limitations [60, 63], including failure to double-blind the RCT, short study period and carry-over effect. As for risk of bias of the RCTs focusing upon combined stroke risk factors, no trial reported appropriate allocation concealment and blinding of outcome assessment, and two trials were found to have a high risk of bias regarding the random sequence generation [60, 61].
Discussion
This paper reports the first comprehensive systematic review of the literature concerning the use of CHM amongst people at greatest risk(s) of stroke. A number of significant findings from our review are important for future evidence-based planning and priority setting for research in stroke prevention.
Our analyses show some positive efficacy and safety evidence of varied CHM interventions in lowering high blood pressure, high blood glucose, high cholesterol, high body BMI and a combination of multiple stroke risk factors. Importantly, our findings indicate that, compared to biomedicine alone/lifestyle modification alone/biomedicine plus lifestyle intervention, CHM monotherapy may be not sufficient enough for people to obtain their treatment goals when treating hypertension, diabetes, and hyperlipidemia, while an intervention of CHM as a supplement to biomedicine and/or a lifestyle intervention is more effective in lowering the levels of SBP/DBP, glucose, BMI, TC, 2hPG, and/or HbA1c. These findings from our review are in line with previous systematic reviews on CHM for cardiovascular diseases [12–14]. In addition, the evidence reported in the papers included with regards to the successful reversion from elevated blood glucose level to normal by using CHM interventions suggests that some CHM products, in combination with a lifestyle intervention, could be considered a potential effective therapeutic regimen for IGT, and these findings are consistent with a Cochrane review on CHM for IGT published in 2009 [13]. Although many RCTs identified in our review demonstrate the therapeutic benefits of CHM in people with a number of stroke risk factors, there is a lack of replicable evidence on CHM use in combined stroke risk factors. It is worth noting that a CHM product (red yeast rice preparation), a medicinal food [64], has been used several times not only for the management of hypertension but also for hyperlipidemia. However, the control interventions of all RCTs examining the efficacy of this rice preparation are different. Therefore, no trial included in our review paper has tested exactly the same CHM and control interventions for the treatment of any stroke risk factor(s).
Our findings show a large variation in the sample size and study period across the included RCTs. The potential risks of bias have been reported in the domains of allocation concealment, the blinding of participants and personnel, and/or the blinding of outcome assessment in the included RCTs. Most included trials have reported their safety information. No serious adverse events were noted although some studies showed some moderate side effects in the CHM groups.
Stroke risk factors vary by ethnic groups and such disparities may influence the etiology of stroke and the implementation of stroke prevention programs [65]. Nevertheless, the majority of studies on CHM use for stroke risk factors included in this review were conducted in China on Chinese populations. As such, the results shown in our review paper may not always be directly applicable to populations at risk of stroke in other countries beyond China. Furthermore, CHM is often composed of a number of herbs and is prescribed based on the unique Chinese medicine theory—syndrome differentiation. The replicability of these trial designs without Chinese medicine practitioners is therefore difficult.
There are some limitations to our systematic review that should be mentioned. Generalisability of the results from this systematic review is limited. Meanwhile, the overall ‘unclear’ reporting of research methodology in the included RCTs may limit the quality of the results reported in this review. In addition, our review was restricted to English peer-reviewed journal articles.
Conclusion
Although the findings in this systematic review with regards to the effect of CHM for stroke modifiable risk factors should be interpreted with caution, the potential therapeutic benefits of CHM as a treatment—particularly in combination with biomedicine and/or lifestyle intervention—for different stroke risk factors needs to be further examined by conducting rigorous trials. Future research should be designed and implemented with adequate sample size, detailed reporting of the allocation concealment method, sufficient application of double-blinding with an adequate placebo and blinding of outcome assessment, and long-term follow-up in different countries. Moreover, it is important for future research on this topic to pay attention to potential drug-herb interactions as a major safety issue in trial design when participants need to take one or more co-administered biomedicine as well as CHM products.
Authors’ contributions
DS designed the study. WP, CF and JF conducted the literature search. WP and RL extracted and interpreted the data. WP drafted the manuscript and prepared tables and figures. JA and DS contributed to the critical revisions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data used in this systematic review are fully available in the public domain.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This systematic review was funded by the Nancy and Vic Allen Stroke Prevention Fund.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 2hPG
2-hour postprandial glucose
- BMI
body mass index
- BP
blood pressure
- CHM
Chinese herbal medicine
- DBP
diastolic blood pressure
- FIN
fasting plasma insulin
- FPG
fasting plasma glucose
- HbA1c
glycated hemoglobin
- HC
hip circumferences
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- HOMA-β
homeostatic model assessment β-cell function
- HOMA-IR
homeostatic model assessment insulin resistance
- IGT
impaired glucose tolerance
- LDL-C
low-density lipoprotein cholesterol
- LVMI
left ventricular mass index
- MBP
mean blood pressure
- OGTT
oral glucose tolerance test
- PPG
postprandial plasma glucose
- RCT
randomized controlled trial
- SBP
systolic blood pressure
- TC
total cholesterol
- TG
triglyceride
- TIA
transient ischaemic attack
- TO
original heart rate
- WC
waist circumference
Contributor Information
Wenbo Peng, Email: Wenbo.Peng@uts.edu.au.
Romy Lauche, Email: Romy.Lauche@uts.edu.au.
Caleb Ferguson, Email: Caleb.Ferguson@uts.edu.au.
Jane Frawley, Email: Jane.Frawley@uts.edu.au.
Jon Adams, Email: Jon.Adams@uts.edu.au.
David Sibbritt, Phone: +61 2 9514 4172, Email: David.Sibbritt@uts.edu.au.
References
- 1.Kim J, Fann DY, Seet RC, Jo DG, Mattson MP, Arumugam TV. Phytochemicals in ischemic stroke. Neuromol Med. 2016;18:283–305. doi: 10.1007/s12017-016-8403-0. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76:S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 4.Holloway RG, Benesch C, Rush SR. Stroke prevention: narrowing the evidence-practice gap. Neurology. 2000;54:1899–1906. doi: 10.1212/WNL.54.10.1899. [DOI] [PubMed] [Google Scholar]
- 5.Straus SE, Majumdar SR, McAlister FA. New evidence for stroke prevention: scientific review. JAMA. 2002;288:1388–1395. doi: 10.1001/jama.288.11.1388. [DOI] [PubMed] [Google Scholar]
- 6.Collaboration Blood Pressure Lowering Treatment Trialists’. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955–1964. doi: 10.1016/S0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary prevention of ischemic stroke: a guideline from the American heart association/American stroke association stroke council: cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: The American academy of neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Xiong X. Outcome measures of Chinese herbal medicine for hypertension: an overview of systematic reviews. Evid Based Complement Alternat Med. 2012;2012:7. doi: 10.1155/2012/697237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Zhang J, Zhao W, Zhang Y, Zhang L, Shang H. Cochrane systematic reviews of Chinese herbal medicines: an overview. PLoS ONE. 2011;6:e28696. doi: 10.1371/journal.pone.0028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Complementary and Integrative Health. Traditional Chinese medicine: in depth. 2013. https://nccih.nih.gov/health/whatiscam/chinesemed.htm. Accessed Oct 2013.
- 11.Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004;3:CD003642. doi: 10.1002/14651858.CD003642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant SJ, Bensoussan A, Chang D, Kiat H, Klupp NL, Liu JP, et al. Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev. 2009;4:CD006690. doi: 10.1002/14651858.CD006690.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu ZL, Li GQ, Bensoussan A, Kiat H, Chan K, Liu JP. Chinese herbal medicines for hypertriglyceridaemia. Cochrane Database Syst Rev. 2013;6:CD009560. doi: 10.1002/14651858.CD009560.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong X, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med. 2012;40:877–886. doi: 10.1142/S0192415X12500656. [DOI] [PubMed] [Google Scholar]
- 16.Sui Y, Zhao H, Wong V, Brown N, Li X, Kwan A, et al. A systematic review on use of Chinese medicine and acupuncture for treatment of obesity. Obes Rev. 2012;13:409–430. doi: 10.1111/j.1467-789X.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Xing Z, Cai C, Tan H, Zhang C. Effects of tianma gouteng decoction on the plasma endothelin of patients with primary hypertension of hyperactivity of the liver yang. Chin J Clin Rehabil. 2004;27:5992–5993. [Google Scholar]
- 19.Li Y. A clinical study on haunglian fire-purging mixture in treatment of 46 cases of primary hypertension. J Tradit Chin Med. 2005;25:29–33. [PubMed] [Google Scholar]
- 20.Ye P, Wu C, Sheng L, Li H. Potential protective effect of long-term therapy with Xuezhikang on left ventricular diastolic function in patients with essential hypertension. J Altern Complement Med. 2009;15:719–725. doi: 10.1089/acm.2008.0599. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Liu Y, Guan Y, Liu N. Effect of Yinian Jiangya Yin on primary hypertension in early stage—a clinical observations on 40 patients. J Tradit Chin Med. 2010;30:171–175. doi: 10.1016/S0254-6272(10)60035-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhong G, Chen M, Luo Y, Xiang L, Xie Q, Li Y, et al. Effect of Chinese herbal medicine for calming Gan (肝) and suppressing hyperactive yang on arterial elasticity function and circadian rhythm of blood pressure in patients with essential hypertension. Chin J Integr Med. 2011;17:414–420. doi: 10.1007/s11655-011-0761-6. [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Wei J, Lee M, Chen C, Ueng K. A randomized, double-blind, placebo-controlled study to evaluate the efficacy and tolerability of Fufang Danshen (Salvia miltiorrhiza) as add-on antihypertensive therapy in Taiwanese patients with uncontrolled hypertension. Phytother Res. 2012;26:291–298. doi: 10.1002/ptr.3548. [DOI] [PubMed] [Google Scholar]
- 24.Tong X, Lian F, Zhou Q, Xu L, Ji H, Xu G, et al. A prospective multicenter clinical trial of Chinese herbal formula JZQG (Jiangzhuoqinggan) for hypertension. Am J Chin Med. 2013;41:33–42. doi: 10.1142/S0192415X13500031. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Zhang J, Zhao Y, Chen J, Liu Y. Chinese herbal medicine bushen qinggan formula for blood pressure variability and endothelial injury in hypertensive patients: a randomized controlled pilot clinical trial. Evid Based Complement Alternat Med. 2014;2014:7. doi: 10.1155/2014/804171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Liu L, Zhao W, Liu J, Yao M, Han Y, et al. Traditional Chinese versus integrative treatment in elderly patients with isolated systolic hypertension: a multicenter, randomized, double-blind controlled trial. J Integr Med. 2010;8:410–416. doi: 10.3736/jcim20100503. [DOI] [PubMed] [Google Scholar]
- 27.Chen SL, Liu XY, Xu WM, Mei WY, Chen XL. Clinical study of Western medicine combined with Chinese medicine based on syndrome differentiation in the patients with polarized hypertension. Chin J Integr Med. 2012;18:746–751. doi: 10.1007/s11655-012-1231-7. [DOI] [PubMed] [Google Scholar]
- 28.Gong C, Huang SL, Huang JF, Zhang ZF, Luo M, Zhao Y, et al. Effects of combined therapy of Xuezhikang Capsule and Valsartan on hypertensive left ventricular hypertrophy and heart rate turbulence. Chin J Integr Med. 2010;16:114–118. doi: 10.1007/s11655-010-0114-z. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Yan H, Yao MJ, Ma J, Jia JM, Ruan FX, et al. Cardioankle vascular index evaluations revealed that cotreatment of ARB Antihypertension medication with traditional Chinese medicine improved arterial functionality. J Cardiovasc Pharmacol. 2013;61:355–360. doi: 10.1097/FJC.0b013e31827afddf. [DOI] [PubMed] [Google Scholar]
- 30.Chao M, Zou D, Zhang Y, Chen Y, Wang M, Wu H, et al. Improving insulin resistance with traditional Chinese medicine in type 2 diabetic patients. Endocrine. 2009;36:268–274. doi: 10.1007/s12020-009-9222-y. [DOI] [PubMed] [Google Scholar]
- 31.Ji L, Tong X, Wang H, Tian H, Zhou H, Zhang L, et al. Efficacy and safety of traditional chinese medicine for diabetes: a double-blind, randomised, controlled trial. PLoS ONE. 2013;8:e56703. doi: 10.1371/journal.pone.0056703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong XL, Wu ST, Lian FM, Zhao M, Zhou SP, Chen XY, et al. The safety and effectiveness of TM81, a Chinese herbal medicine, in the treatment of type 2 diabetes: a randomized double-blind placebo-controlled trial. Diabetes Obes Metab. 2013;15:448–454. doi: 10.1111/dom.12051. [DOI] [PubMed] [Google Scholar]
- 33.Tu X, Xie C, Wang F, Chen Q, Zuo Z, Zhang Q, et al. Fructus Mume formula in the treatment of type 2 diabetes mellitus: A randomized controlled pilot trial. Evid Based Complement Alternat Med. 2013;2013:8. doi: 10.1155/2013/787459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Fan H. The research for the clinical curative effect through combing traditional Chinese medicine with insulin to cure diabetes. Pak J Pharm Sci. 2014;27:1057–1061. [PubMed] [Google Scholar]
- 35.Cai H, Liu F, Zuo P, Huang G, Song Z, Wang T, et al. Practical application of antidiabetic efficacy of Lycium barbarum polysaccharide in patients with type 2 diabetes. Med Chem. 2015;11:383–390. doi: 10.2174/1573406410666141110153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian F, Tian J, Chen X, Li Z, Piao C, Guo J, et al. The efficacy and safety of Chinese herbal medicine Jinlida as add-on medication in type 2 diabetes patients ineffectively managed by metformin monotherapy: a double-blind, randomized, placebo-controlled, multicenter trial. PLoS ONE. 2015;10:e0130550. doi: 10.1371/journal.pone.0130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Liu Y, Xiong D, Xie C. Insulin combined with Chinese medicine improves glycemic outcome through multiple pathways in patients with type 2 diabetes mellitus. J Diabetes Investig. 2015;6:708–715. doi: 10.1111/jdi.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Zhou X, Liu P, Wang B, Duan D, Guo D. A comparison study of metformin only therapy and metformin combined with Chinese medicine jianyutangkang therapy in patients with type 2 diabetes: a randomized placebo-controlled double-blind study. Complement Ther Med. 2016;24:13–18. doi: 10.1016/j.ctim.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Huang X, Ye H, Chen Y, Yu J, Yang J, et al. Randomized, double-blinded, double-dummy, active-controlled, and multiple-dose clinical study comparing the efficacy and safety of Mulberry Twig (Ramulus Mori, Sangzhi) Alkaloid Tablet and Acarbose in individuals with type 2 diabetes mellitus. Evid Based Complement Alternat Med. 2016;2016:8. doi: 10.1155/2016/7121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Lu Z, Chi J, Wang W, Su M, Kou W, et al. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional Chinese medicine. Curr Ther Res. 1997;58:964–978. doi: 10.1016/S0011-393X(97)80063-X. [DOI] [Google Scholar]
- 41.Yang H, Han L, Sheng T, He Q, Liang J. Effects of replenishing qi, promoting blood circulation and resolving phlegm on vascular endothelial function and blood coagulation system in senile patients with hyperlipemia. J Tradit Chin Med. 2006;26:120–124. [PubMed] [Google Scholar]
- 42.Ai J, Zhao L, Lu Y, Cai B, Zhang Y, Yang B. A randomized, multicentre, open-label, parallel-group trial to compare the efficacy and safety profile of daming capsule in patients with hypercholesterolemia. Phytother Res. 2009;23:1039–1042. doi: 10.1002/ptr.2654. [DOI] [PubMed] [Google Scholar]
- 43.Xu CF, Lin XR, Wang YK. Clinical observation on hyperlipemia treated with antihyperlipidemic decoction. J Tradit Chin Med. 2009;29:121–124. doi: 10.1016/S0254-6272(09)60047-9. [DOI] [PubMed] [Google Scholar]
- 44.Hu M, Zeng W, Tomlinson B. Evaluation of a Crataegus-based multiherb formula for dyslipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evid Based Complement Alternat Med. 2014;2014:365742. doi: 10.1155/2014/365742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moriarty PM, Roth EM, Karns A, Ye P, Zhao SP, Liao Y, et al. Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo-controlled study. J Clin Lipidol. 2014;8:568–575. doi: 10.1016/j.jacl.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69:231–236. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- 47.Lin C, Li T, Lai M. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrinol. 2005;153:679–686. doi: 10.1530/eje.1.02012. [DOI] [PubMed] [Google Scholar]
- 48.Wei Y, Hong YZ, Ye X. Effect of Tang No.1 granule in treating patients with impaired glucose tolerance. Chin J Integr Med. 2008;14:298–302. doi: 10.1007/s11655-008-0298-7. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Zhou H, Zhao H, Feng X, Feng J, Li Y, et al. Clinical research of traditional Chinese medical intervention on impaired glucose tolerance. Am J Chin Med. 2013;41:21–32. doi: 10.1142/S0192415X1350002X. [DOI] [PubMed] [Google Scholar]
- 50.Fang Z, Zhao J, Shi G, Shu Y, Ni Y, Wang H, et al. Shenzhu Tiaopi granule combined with lifestyle intervention therapy for impaired glucose tolerance: a randomized controlled trial. Complement Ther Med. 2014;22:842–850. doi: 10.1016/j.ctim.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Lian F, Li G, Chen X, Wang X, Piao C, Wang J, et al. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J Clin Endocrinol Metab. 2014;99:648–655. doi: 10.1210/jc.2013-3276. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Yang Q, Wang H, Xu Y, Peng W, Jiang Y. Long-term clinical effect of Tangyiping Granules (糖异平颗粒) on patients with impaired glucose tolerance. Chin J Integr Med. 2016;22:653–659. doi: 10.1007/s11655-016-2512-3. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y, Liu W, Zhang X, Su W, Chen N, Lu S, et al. Effect of Chinese herbal medicine Jinlida granule in treatment of patients with impaired glucose tolerance. Chin Med J. 2016;129:2281–2286. doi: 10.4103/0366-6999.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant SJ, Chang DH, Liu J, Wong V, Kiat H, Bensoussan A. Chinese herbal medicine for impaired glucose tolerance: a randomized placebo controlled trial. BMC Complement Altern Med. 2013;13:104. doi: 10.1186/1472-6882-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan L, Li D, Lei M, Zhang L, Zhou L. Preparation-containing node of Lotus Rhizome, green tea and Panax notoginseng for obese adults. Chin J Clin Rehabil. 2005;15:231–233. [Google Scholar]
- 56.Zhou Q, Chang B, Chen X, Zhou S, Zhen Z, Zhang L, et al. Chinese herbal medicine for obesity: a randomized, double-blinded, multicenter, prospective trial. Am J Chin Med. 2014;42:1345–1356. doi: 10.1142/S0192415X14500840. [DOI] [PubMed] [Google Scholar]
- 57.Lenon GB, Li KX, Chang Y-H, Yang AW, Da Costa C, Li CG, et al. Efficacy and safety of a Chinese herbal medicine formula (RCM-104) in the management of simple obesity: a randomized, placebo-controlled clinical trial. Evid Based Complement Alternat Med. 2012;2012:435702. doi: 10.1155/2012/435702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hioki C, Yoshimoto K, Yoshida T. Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin Exp Pharmacol Physiol. 2004;31:614–619. doi: 10.1111/j.1440-1681.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- 59.Gao F, Hu XF. Effect of Taizhi’an capsule combined with Simvastatin on hyperlipidemia in diabetic patients. Chin J Integr Med. 2006;12:24–28. doi: 10.1007/BF02857425. [DOI] [PubMed] [Google Scholar]
- 60.Poppel PC, Breedveld P, Abbink EJ, Roelofs H, Heerde W, Smits P, et al. Salvia miltiorrhiza root water-extract (Danshen) has no beneficial effect on cardiovascular risk factors. a randomized double-blind cross-over trial. PLoS ONE. 2015;10:e0128695. doi: 10.1371/journal.pone.0128695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu SL, Fu H, Yang JX, Liu GX, Dou P, Zhang L, et al. A randomized double-blind placebo-controlled study of Pu’er tea extract on the regulation of metabolic syndrome. Chin J Integr Med. 2011;17:492–498. doi: 10.1007/s11655-011-0781-4. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Fu DY, He YM, Fu XD, Xu YQ, Liu Y, et al. Effects of Chinese herbal medicine Yiqi Huaju Formula on hypertensive patients with metabolic syndrome: a randomized, placebo-controlled trial. J Integr Med. 2013;11:184–194. doi: 10.3736/jintegrmed2013031. [DOI] [PubMed] [Google Scholar]
- 63.Azushima K, Tamura K, Haku S, Wakui H, Kanaoka T, Ohsawa M, et al. Effects of the oriental herbal medicine Bofu-tsusho-san in obesity hypertension: a multicenter, randomized, parallel-group controlled trial (ATH-D-14-01021.R2) Atherosclerosis. 2015;240:297–304. doi: 10.1016/j.atherosclerosis.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 64.Lee C, Jan M, Yu M, Lin C, Wei J, Shih H. Relationship between adiponectin and leptin, and blood lipids in hyperlipidemia patients treated with red yeast rice. Forsch Komplementmed. 2013;20:197–203. doi: 10.1159/000351455. [DOI] [PubMed] [Google Scholar]
- 65.Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors. Stroke. 2008;39:2204–2210. doi: 10.1161/STROKEAHA.107.507285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this systematic review are fully available in the public domain.


