Abstract
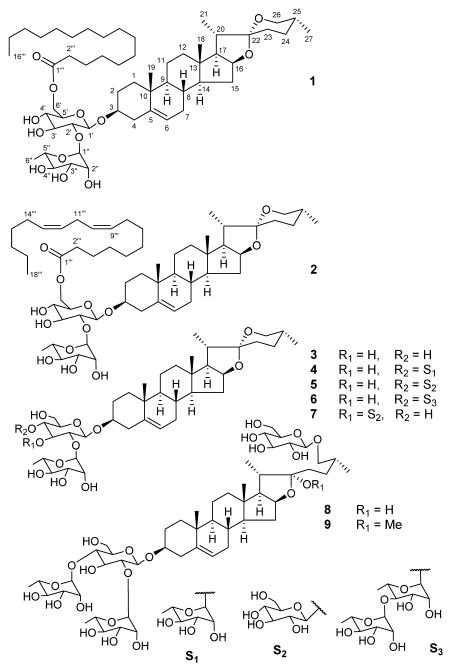
Two new fatty acid-spirostan steroid glycoside esters, progenin III palmitate (1) and progenin III linoleate (2), were isolated from the MeOH extract of Dioscorea cayenensis rhizomes. The extract also yielded seven previously known spirostan and furostan steroid glycosides (3–9). The structures of the new compounds were established as (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-palmitoyl]-O-β-D-glucopyranoside (1) and (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-linoleoyl]-O-β-D-glucopyranoside (2) by chemical and spectroscopic methods, including 1D and 2D NMR. The known compounds were identified as progenin III (3), dioscin (4), deltonin (5), asperin (6), gracillin (7), protodioscin (8)], and methyl protodioscin (9).
Keywords: Dioscorea cayenensis, Dioscoreaceae, Spirostan glycoside-fatty acid conjugates, Progenin III palmitate, Progenin III linoleate
Dioscorea, which belongs to the Dioscoreaceae family, consists of about 600 annual or perennial climbing and tuberous plants. Dioscorea is mainly distributed in tropical and sub-tropical regions. The tuberous roots of the Dioscorea species are known as yams, a few of which are cultivated for food due to their rich starch content. Steroid glycosides, particularly diosgenin-based spirostan glycosides are broadly found in most of the investigated Dioscorea species [1–4]. Diosgenin is a steroid sapogenin used as a precursor in the synthesis of many useful steroids. D. cayenensis Lam. and D. rotundata are native to tropical West Africa. The rhizomes of D. cayenensis, known as yellow yam, are used as food and as remedy for treating burns and fevers [5]. Previous phytochemical reports on D. cayenensis showed the existence of a pragnane glycoside, three spirostan glycosides, and three furostan saponins [5, 6]. In the present phytochemical study of rhizomes of D. cayenensis, seven spirostan and two furostan steroid saponins, including two previously undiscovered, progenin III palmitate (1) and progenin III linoleate (2), were isolated and identified (Figure 1). The new compounds were found to be spirostan glycosides esterified with a fatty acid unit and their structures were elucidated as (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-palmitoyl]-O-β-D-glucopyranoside (1) and (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-linoleoyl]-O-β-D-glucopyranoside (2) by chemical methods and spectroscopic analyses.
Figure 1.
Structures of isolated compounds.
The MeOH extract of the plant material was subjected to chromatography over silica gel and RP-18 silica gel for purification of compounds 1–9. The new compounds were found to be hybrids of a spirostan glycoside and a fatty acid. Compound 1 showed a protonated ion [M + H]+ at m/z 961.6615 in its HR-ESI-TOF-MS, which indicated the neutral formula of C55H92O13. The resonances in the 13C NMR spectrum of 1 were ascribed to a steroid skeleton, two sugar moieties, and a saturated fatty acid unit. A DEPT NMR spectrum was used to differentiate 13C NMR resonances as six methyl, 19 methine, and five quaternary carbons, along with remaining methylene groups.
The IR spectrum indicated hydroxyl and ester carbonyl functions due to absorptions at 3666 and 1738 cm−1, respectively. In the 1H NMR and 13C NMR spectra, resonances were observed for two tertiary methyls [δH/δC 0.84/16.4 (CH3-18) and 1.04/19.5 (CH3-19)], three secondary methyls [δH/δC 1.14 (d, J = 6.6 Hz)/15.1 (CH3-21), 0.69 (br. d, J = 4.8 Hz)/17.4 (CH3-27), and 1.74 (d, J = 6.0 Hz)/18.7 (CH3-6″)], a primary methyl [δH/δC 0.85 (t, J = 6.6 Hz)/14.4 (CH3-16″′)], a double bond [δH/δC 5.35 (br. s)/121.8 (CH-6) and δC 141.0 (C-5)], two oxygenated quaternary carbons [δC 109.4 (C-22) and 173.8 (C-1″′)], and two oxygenated methines [δH/δC 3.91/78.8 (CH-3) and 4.56/81.2 (CH-16)], along with β-D-glucopyranose and α-L-rhamnopynose moieties [δH 4.94 (d, J = 7.2 Hz, H-1′) and 6.30 (s, H-1″) and δC 100.7 (C-1′), 77.9 (C-2′), 79.4 (C-3′), 71.9 (C-4′), 75.0 (C-5′), 64.6 (C-6′), 102.2 (C-1″), 72.6 (C-2″), 72.8 (C-3″), 74.2 (C-4″), 69.6 (C-5″), and 18.7 (C-6″)]. The location of glucose at C-3, rhamnose at C-2′, and fatty acid at C-6′ was determined by HMBC correlations between H-1′/C-3, H-1″/C-2′, and H-6′/C-1‴, respectively. Comparative analysis of 13C NMR data of 1 with that of progenin III (3) [7, 8] also supported the position of a fatty acid moiety at C-6′ due to the downfield shift of C-6′ and the upfield shift of C-5′ resonances in 1 [9–12]. Acid hydrolysis of 1 with HCl-MeOH yielded methyl palmitate, which was identified by GCMS analysis as having the same retention time (tR for methyl palmitate: 28.6 min) as that of the standard (Sigma-Aldrich). The 1H and 13C NMR spectroscopic data assignment for compound 1 (Table 1) was based on HSQC, HMBC (Figure 2), and 1H-1H COSY spectroscopic data analyses. The NMR spectroscopic data of 1 were found to be similar to those of progenin III (3) [7, 8], except for additional resonances from the palmitoyl moiety. The stereochemistry of 1 was found to be similar to that of progenin III (3) [7, 8]. The sugars were identified as glucose and rhamnose by co-TLC (EtOAc-CHCl3-MeOH-H2O, 6:4:4:1) of standard sugars from Sigma-Aldrich and the sugar mixture obtained via acid hydrolysis of 1. The characteristic appearance of anomeric protons as a doublet (J = 7.2 Hz, H-1′) for glucopyranose and a singlet for rhamnopyranose revealed their β and α configurations, respectively. The absolute configurations of glucose and rhamnose were determined to be D and L, respectively (see Experimental). Eventually, the structure of progenin III palmitate (1) was established as (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-palmitoyl]-O-β-D-glucopyranoside.
Table 1.
1H and 13C NMR spectroscopic data of compounds 1 and 2 (pyridine-d5, 600 MHz/150 MHz).
| Position | δC | 1 | δC | 2 |
|---|---|---|---|---|
| δHa (HMQC) | δHa (HMQC) | |||
| 1 | 37.7 | 1.82 m, 1.12 m | 37.7 | 1.84 m, 1.11 m |
| 2 | 30.1 | 2.20 m, 1.90 m | 30.1 | 2.21 m, 1.91 m |
| 3 | 78.8 | 3.91 m | 78.8 | 3.95 m |
| 4 | 39.1 | 2.79 br. d (12.6), 2.70 t (12.6) | 39.2 | 2.75 br. d (12.6), 2.82 t (12.6) |
| 5 | 141.0 | 141.0 | ||
| 6 | 121.8 | 5.35 br. s | 121.8 | 5.35 br. s |
| 7 | 32.4 | 1.88, 1.44 | 32.4 | 1.87, 1.47 |
| 8 | 31.8 | 1.62 m | 31.8 | 1.63 m |
| 9 | 50.4 | 0.94 m | 50.4 | 0.95 m |
| 10 | 37.2 | 37.2 | ||
| 11 | 21.2 | 1.53, 1.44 | 21.2 | 1.52, 1.45 |
| 12 | 40.0 | 1.72, 1.14 | 40.0 | 1.73, 1.15 |
| 13 | 40.5 | 40.6 | ||
| 14 | 56.7 | 1.09 m | 56.8 | 1.10 m |
| 15 | 32.3 | 2.05, 1.51 | 32.3 | 2.06, 1.52 |
| 16 | 81.2 | 4.56 m | 81.2 | 4.56 m |
| 17 | 63.0 | 1.82 | 63.0 | 1.83 |
| 18 | 16.4 | 0.84 s | 16.4 | 0.86 s |
| 19 | 19.5 | 1.04 s | 19.5 | 1.07 s |
| 20 | 42.0 | 1.96 m | 42.0 | 1.98 m |
| 21 | 15.1 | 1.14 d (6.6) | 15.1 | 1.16 d (6.6) |
| 22 | 109.4 | 109.4 | ||
| 23 | 31.9 | 1.72, 1.66 | 31.9 | 1.71, 1.65 |
| 24 | 29.2 | 1.56, 1.31 | 29.3 | 1.57, 1.33 |
| 25 | 30.6 | 1.58 | 30.7 | 1.59 |
| 26 | 66.9 | 3.58 br. d (10.2), 3.49 t (10.2) | 66.9 | 3.59 br. d (10.2), 3.51 t (10.2) |
| 27 | 17.4 | 0.69 br. d (4.8) | 17.4 | 0.70 br. d (4.2) |
| 1′ | 100.7 | 4.94 d (7.2) | 100.7 | 5.00 d (7.0) |
| 2′ | 77.9 | 4.21 | 77.8 | 4.25 |
| 3′ | 79.4 | 4.25 | 79.4 | 4.27 |
| 4′ | 71.9 | 3.96 | 71.9 | 3.97 |
| 5′ | 75.0 | 3.99 | 75.0 | 4.00 |
| 6′ | 64.6 | 4.92, 4.75 | 64.6 | 4.94, 4.79 |
| 1″ | 102.2 | 6.30 s | 102.2 | 6.37 s |
| 2″ | 72.6 | 4.79 br. s | 72.6 | 4.80 br. s |
| 3″ | 72.8 | 4.59 dd (3.0, 9.6) | 72.9 | 4.62 dd (2.4, 9.3) |
| 4″ | 74.2 | 4.35 t (9.6) | 74.2 | 4.36 t (9.3) |
| 5″ | 69.6 | 4.93 | 69.6 | 4.96 |
| 6″ | 18.7 | 1.74 d (6.0) | 18.7 | 1.77 d (6.6) |
| Pal-1‴ | 173.8 | |||
| 2‴ | 34.5 | 2.35 m | ||
| 3‴ | 25.4 | 1.63 m | ||
| 14‴ | 32.2 | 1.26 | ||
| 15‴ | 23.0 | 1.29 | ||
| 16‴ | 14.4 | 0.85 t (6.6) | ||
| Lin-1‴ | 173.7 | |||
| 2‴ | 34.5 | 2.36 m | ||
| 3‴ | 25.4 | 1.64 m | ||
| 8‴, 14‴ | 27.6 | 2.12 | ||
| 9‴, 10‴, 12‴, 13‴ | 128.4, 128.5, 130.4, 130.5 | 5.51-5.46 | ||
| 11‴ | 26.1 | 2.90 | ||
| 16‴ | 32.2 | 1.28 | ||
| 17‴ | 23.0 | 1.29 | ||
| 18‴ | 14.4 | 0.88 t (6.0) | ||
| Other CH2 | 29.3–30.3 | 1.35-1.16 | 29.3–30.3 | 1.35-1.16 |
Multiplicity is not clear for some resonances due to overlapping, chemical shifts are in ppm, J in parenthesis are in Hertz.
Figure 2.
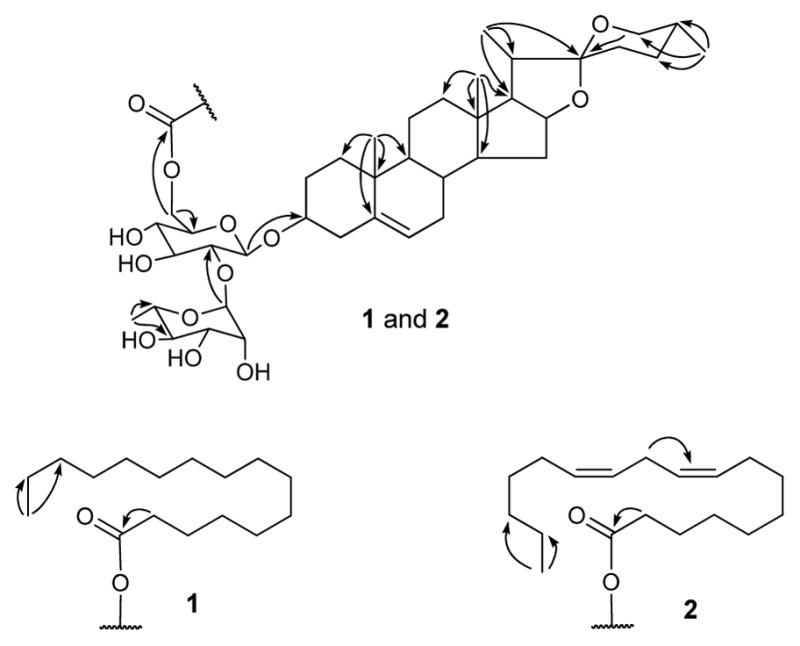
Key HMBC correlations of 1 and 2.
Compound 2 was obtained as a white powder and showed a protonated ion [M + H]+ at m/z 985.6612 (calcd for C57H92O13 + H, 985.6616) in the HR-ESI-TOF-MS, which corresponded to the molecular formula of C57H92O13. The hydroxyl and carbonyl functions were deduced from the absorptions at 3626 and 1737 cm−1 in the IR spectrum. The 1H and 13C NMR data assignment (Table 1) in 2 was based on DEPT, COSY, HSQC, and HMBC spectra (Figure 2) and was found to be similar to that of 1, except for the additional resonances in 2 of two olefin bonds [δC 128.4, 128.5, 130.4, and 130.5 and δH 5.51–5.46 (CH-9‴, 10‴, 12‴, and 13‴)]. Acid hydrolysis followed by GCMS analysis of 2, as described for 1, indicated a linoleoyl moiety (tR for methyl linoleate: 36.2 min), which was located at C-6′ by HMBC correlations of H-6′ with ester carbonyl carbon. The configuration of olefin bonds in the fatty acid chain was found to be cis from the typical absorption band at 725 cm−1 in the IR spectrum (about 967 cm−1 in the case of trans double bond) and characteristic 13C NMR resonances of methylene carbons (δC 26–28) adjacent to double bonds (δC 32–33 for trans double bond). Thus, the structure of progenin III linoleate (2) was elucidated as (25R)-spirost-5-en-3β-yl O-α-L-rhamnopyranosyl-(1→2)-[6-O-linoleoyl]-O-β-D-glucopyranoside.
The known compounds were identified as progenin III (3) [7, 8], dioscin (4) [7, 8], deltonin (5) [8], asperin (6) [5], gracillin (7) [7, 13], protodioscin (8) [14], and methyl protodioscin (9) [15]. The known compounds have previously been reported to possess in vitro antifungal activity against different strains [16, 17].
Experimental
General experimental procedures
IR spectra were acquired on a Bruker Tensor 27 spectrophotometer. Specific rotations were measured at ambient temperature using a Rudolph Research Analytical Autopol IV automatic polarimeter. HR-ESI-TOF-MS data were obtained on an Agilent Series 1100 SL mass spectrometer, whereas NMR spectra were recorded on a Varian Unity Inova 600 MHz NMR spectrometer. Chromatography was performed using silica gel (40 μm for flash chromatography, 60 Å, J. T. Baker), reversed-phase RP-C18 silica gel (Polarbond, JT Baker), and Sephadex LH-20 (Sigma). TLC was carried out on aluminum-backed plates pre-coated with silica gel F254 (20 × 20 cm, 200 μm, 60 Å, Merck). Visualization was accomplished by spraying with 5% vanillin (Sigma) solution in conc. H2SO4-EtOH (5:95), followed by heating. HPLC was carried out on a Waters Alliance 2695, equipped with a 996 photodiode array detector (Waters Corp., Milford, MA), with Waters Empower-2 software. A Luna C 18 column (150 x 4.6 mm, 5 μm particle size, Phenomenex Inc., Torrance, CA) was protected with a 2 cm LC-18 guard column (Phenomenex Inc.). GCMS analyses were carried out on an Agilent Technologies 5975C GC and an Agilent Technologies 7890A MS using an Agilent DB-5ms column (30 m × 0.25 mm × 0.25 μm). The solvents (Fisher) used for HPLC and other chromatographic procedures were of HPLC and certified grades, respectively. Sugar standards were purchased from Sigma-Aldrich.
Plant material
The plant material (rhizomes), purchased from the local market, was identified by Dr Aruna Weerasooriya, a plant taxonomist at the National Center for Natural Products Research, University of Mississippi, where a specimen (No. 9462) has been deposited. The plant material was sliced, air dried at 20°C, and ground into powder.
Extraction and isolation
The powder (1.1 kg) was extracted with methanol (1.5 L × 3) at 35 °C. Following removal of the solvent, a gummy extract (18 g) was obtained. An aliquot (17 g) was separated by normal phase column chromatography (NPCC) using silica gel (12″ × 2″) into 8 fractions (A1–A8) with EtOAc-CHCl3-MeOH-H2O (10:6:4:1) [A1 (300 mL, 571 mg), A2 (200 mL, 1.2 g), A3 (200 mL, 474 mg), A4 (100 mL, 914 mg), A5 (100 mL, 895 mg), A6 (100 mL, 652 mg), and EtOAc-CHCl3-MeOH-H2O (6:4:4:1), A7 (200 mL, 1.9 g), and A8 (400 mL, 9.4 g)]. Fraction A2 was divided into 8 subfractions (A2A–A2H) by NPCC [silica gel (36″ × 1.0″), CHCl3-MeOH (19:1, 1.0 L), (9:1, 1.0 L), and (0:1, 1.0 L)]. Compounds 1 (3.4 mg) and 2 (8.6 mg) were obtained from subfractions A2G (71 mg) and A2F (61 mg), respectively, by NPCC [silica gel (20″ × 0.5″), EtOAc-CHCl3-MeOH-H2O (15:8:4:1, 0.5 L)]. The MeOH soluble part (70 mg) of fraction A3 was subjected to NPCC [silica gel (20″ × 0.5″), CHCl3-MeOH-H2O (8:2:0.25, 250 mL)] to purify compound 3 (15.4 mg). Compound 4 (66.0 mg) was purified from fraction A4 by NPCC [silica gel (40″ × 1″), CHCl3-MeOH-H2O (8:2:0.25), 2.0 L] and reversed phase column chromatography (RPCC) [RP-18 silica gel (18″ × 0.5″), acetone-H2O (7:3), 0.5 L]. Compounds 5 (39.2 mg), 6 (89.0 mg), and 7 (81.3 mg) were obtained from the MeOH soluble part of fraction A5 by NPCC [silica gel (40″ × 1″), CHCl3-MeOH-H2O (8:2:0.25), 2.0 L]. Fraction A8 yielded compounds 8 and 9 (25.0 mg) by RPCC [RP-18 silica gel (30″ × 1″), acetone-H2O (1:1), 0.5 L] and NPCC [silica gel (40″ × 1″), CHCl3-MeOH-H2O (7:3:0.5), 2.0 L].
Sugar determination
Compound 1 (1.0 mg) was dissolved in 0.5 mL of 2 N HCl in dioxane-H2O (1:1) and heated at 80°C for 3 h. The mixture was diluted with H2O (1.0 mL), neutralized with NH4OH, and extracted with EtOAc (2 × 2 mL). The residue obtained after drying the aqueous layer was dissolved in pyridine (0.5 mL), and 0.1 M cysteine methyl ester hydrochloride in pyridine (0.5 mL) was added. The reaction mixture was heated at 60°C for 1h. An equal volume of phenyl isothiocyanate in pyridine (10 mg/mL) was added and heated at 60°C for 1h. The mixture was filtered and analyzed by reversed-phase HPLC. Acetonitrile with 0.1% HOAc (A) and H2O with 0.1% HOAc (B) were used as the mobile phase at a flow rate of 1 mL/min with the following gradient: 10 % A for 20 min and 55 % A for 25 min. The response was recorded at 250 nm. The process was repeated for compound 2. The standard sugar (Sigma-Aldrich) derivatives were prepared identically and analyzed by HPLC under similar conditions. A pair of isomers (major & minor) was detected in each case. D-glucose and L-rhamnose in both compounds were identified by comparing the retention times of their derivatives with those of authentic sugar samples [D-glucose: 12.8 min (minor)/15.5 min (major), L-glucose: 13.3 min (minor)/15.2 min (major), and L-rhamnose: 14.6 min (minor)/17.4 min (major)] [18].
Fatty acid determination
A solution of 1 (0.5 mg) in 0.5 mL of 2 N HCl in MeOH was heated at 60°C for 15 min. After cooling, the reaction mixture was partitioned between MeOH and hexanes (1.0 mL each). The process was repeated for compound 2 and the hexanes layers containing the fatty acid methyl esters were analyzed by GCMS [Capillary Column: DB-5ms (30 m × 0.25 mm × 0.25 μm); carrier gas He; pressure: 19.98 psi; injection volume 1 μL; split ratio 20:1; injection temperature 250°C; detector temperature 280°C; column temperature [80°C for 2 min, 5°C/min up to 170°C, 1°C/min up to 190°C, 6°C/min up to 260°C, 260°C for 10 m]. The peaks corresponding to methyl palmitate and methyl linoleate were observed at tR 28.6 min and 36.2 min, respectively.
Progenin III palmitate (1)
Colorless solid.
[α]D25: – 64.6 (c 0.13, MeOH).
IR (NaCl): 3666 (OH), 2924, 1738 (C=O), 1658, 1548, 1467, 1378, 1177, 1052 cm−1.
1H NMR (pyridine-d5, 600 MHz): Table 1
13C NMR (pyridine-d5, 150 MHz): Table 1.
HR-ESI-TOF-MS (+ve ion mode): m/z [M + H]+ calcd for C55H92O13 + H, 961.6616; found: 961.6615.
Progenin III linoleate (2)
Colorless solid.
[α]D25: – 53.9 (c 0.2, MeOH).
IR (NaCl): 3626 (OH), 2925, 1737 (C=O), 1603, 1515, 1453, 1377, 1144, 1050, 725 cm−1.
1H NMR (pyridine-d5, 600 MHz): Table 1
13C NMR (pyridine-d5, 150 MHz): Table 1.
HR-ESI-TOF-MS (+ve ion mode): m/z [M + H]+ calcd for C57H92O13 + H, 985.6616; found: 985.6612.
Acknowledgments
This publication was made possible by Grant Number P50AT006268 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health. Additional funding was also provided by the United States Food and Drug Administration (FDA), Grant Number 5U01FD004246. The authors are thankful to Dr Bharathi Avula for mass analyses and Dr Aruna Weerasooriya for providing an authentic plant sample. The authors would like to thank Dr Jon Parcher for editorial assistance.
References
- 1.Hu C-C, Lin J-T, Liu S-C, Yang D-J. A spirostanol glycoside from wild yam (Dioscorea villosa) extract and its cytostatic activity on three cancer cells. Journal of Food and Drug Analysis. 2007;15:310–315. [Google Scholar]
- 2.Liu X-T, Wang Z-Z, Xiao W, Zhao H-W, Hu J, Yu B. Cholestane and spirostane glycosides from the rhizomes of Dioscorea septemloba. Phytochemistry. 2008;69:1411–1418. doi: 10.1016/j.phytochem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Shen P, Wang S-L, Liu X-K, Yang C-R, Cai B, Yao X-S. A new ergostanol saponin from Dioscorea deltoidea Wall var. orbiculata. Journal of Asian Natural Products Research. 2002;4:211–215. doi: 10.1080/10286020290024013. [DOI] [PubMed] [Google Scholar]
- 4.Yin J, Kouda K, Tezuka Y, Tran QL, Miyahara T, Chen Y, Kadota S. Steroidal glycosides from the rhizomes of Dioscorea spongiosa. Journal of Natural Products. 2003;66:646–650. doi: 10.1021/np0205957. [DOI] [PubMed] [Google Scholar]
- 5.Sautour M, Mitaine-Offer AC, Miyamoto T, Dongmo A, Lacaille-Dubois MA. Antifungal steroid saponins from Dioscorea cayenensis. Planta Medica. 2004;70:90–92. doi: 10.1055/s-2004-815467. [DOI] [PubMed] [Google Scholar]
- 6.Sautour M, Mitaine-Offer A-C, Miyamoto T, Dongmo A, Lacaille-Dubois M-A. A new steroidal saponin from Dioscorea cayenensis. Chemical and Pharmaceutical Bulletin. 2004;52:1353–1355. doi: 10.1248/cpb.52.1353. [DOI] [PubMed] [Google Scholar]
- 7.Hu K, Dong A, Yao X, Kobayashi H, Iwasaki S. Antineoplastic agents; I. Three spirostanol glycosides from rhizomes of Dioscorea collettii var. hypoglauca. Planta Medica. 1996;62:573–575. doi: 10.1055/s-2006-957978. [DOI] [PubMed] [Google Scholar]
- 8.Hayes PY, Lambert LK, Lehmann R, Penman K, Kitching W, De VJJ. Spectral assignments and reference data, complete 1H and 13C assignments of the four major saponins from Dioscorea villosa (wild yam) Magnetic Resonance in Chemistry. 2007;45:1001–1005. doi: 10.1002/mrc.2071. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y-N, Chai X-Y, Xu Z-R, Bi D, Ren H-Y, Zhao M, Tu P-F. Three new phenolic glycosides and a new triterpenoid from the stems of Scolopia chinensis. Planta Medica. 2010;76:358–361. doi: 10.1055/s-0029-1186157. [DOI] [PubMed] [Google Scholar]
- 10.Shikishima Y, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O. Phenylbutanoids and stilbene derivatives of Rheum maximowiczii. Phytochemistry. 2001;56:377–381. doi: 10.1016/s0031-9422(00)00370-8. [DOI] [PubMed] [Google Scholar]
- 11.Wei G-Q, Zheng Y-N, Li W, Liu W-C, Lin T, Zhang W-Y, Chen H-F, Zeng J-Z, Zhang X-K, Chen Q-C. Structural modification of ginsenoside Rh2 by fatty acid esterification and its detoxification property in antitumor. Bioorganic and Medicinal Chemistry Letters. 2012;22:1082–1085. doi: 10.1016/j.bmcl.2011.11.104. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Nakamura N, Hattori M, Yang X-W, Komatsu K, Qiu M-H. New triterpenoid saponins from the roots of Sinocrassula asclepiadea. Chemical and Pharmaceutical Bulletin. 2004;52:230–237. doi: 10.1248/cpb.52.230. [DOI] [PubMed] [Google Scholar]
- 13.Zou C-C, Hou S-J, Shi Y, Lei P-S, Liang X-T. The synthesis of gracillin and dioscin: two typical representatives of spirostanol glycosides. Carbohydrate Research. 2003;338:721–727. doi: 10.1016/s0008-6215(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 14.Shao Y, Poobrasert O, Kennelly EJ, Chin CK, Ho CT, Huang MT, Garrison SA, Cordell GA. Steroidal saponins from Asparagus officinalis and their cytotoxic activity. Planta Medica. 1997;63:258–262. doi: 10.1055/s-2006-957667. [DOI] [PubMed] [Google Scholar]
- 15.Bah M, Gutierrez DM, Escobedo C, Mendoza S, Isela RJ, Rojas A. Methylprotodioscin from the Mexican medical plant Solanum rostratum (Solanaceae) Biochemical Systematics and Ecology. 2004;32:197–202. [Google Scholar]
- 16.Yang C-R, Zhang Y, Jacob MR, Khan SI, Zhang Y-J, Li X-C. Antifungal activity of C-27 steroidal saponins. Antimicrobial Agents and Chemotherapy. 2006;50:1710–1714. doi: 10.1128/AAC.50.5.1710-1714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai S, Murata T, Fujioka S, Murata E, Goto M. Crude drugs and oriental crude drug preparations by bioassay. XXI. Search for biologically active plant ingredients by means of antimicrobial tests. 3. Antifungal principles of Dioscorea species. Takeda Kenkyusho Nempo. 1967;26:66–75. [Google Scholar]
- 18.Ali Z, Khan IA. Alkyl phenols and saponins from the roots of Labisia pumila (Kacip Fatimah) Phytochemistry. 2011;72:2075–2080. doi: 10.1016/j.phytochem.2011.06.014. [DOI] [PubMed] [Google Scholar]