Abstract
Background
Depression has been associated with increased risk of death. However, there is lack of studies exploring such relationship in the context of dementia. Given the high prevalence of both depression and Alzheimer’s Disease (AD), investigating their temporal association with mortality is of public health relevance.
Methods
Longitudinal data from the WHICAP study were analyzed (1958 individuals aged ≥ 65 years). Depressive symptoms were assessed with the 10-item Center for Epidemiologic Studies Depression Scale (CES-D). Respondents were identified as having AD if they satisfied the criteria of the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Cox regressions analyses were performed to determine the association between depressive symptoms and risk of all-cause mortality using the overall sample, and by AD status.
Results
Depressive symptoms were significantly associated with higher mortality risk after adjusting for all potential covariates in the overall sample (HR = 1.22; 95% CI = 1.02, 1.46) and in individuals with incident AD (HR = 1.88; 95% CI = 1.12, 3.18).
Limitations
The CES-D does not measure clinical depression but depressive symptomatology. Since those who were exposed to known risk factors for mortality are likely to die prematurely, our results may have been skewed to the individuals with longer survival.
Conclusions
Strategies focusing on prevention and early treatment of depression in the elderly may have a beneficial effect not only on patient quality of life and disability, but may also increase survival in the context of AD.
Keywords: Depressive symptoms, Mortality, Alzheimer’s Disease, Community-based study
1. Introduction
Depression is a common mental condition among elderly individuals (Byers et al., 2010), with estimates ranging from 4.6% to 9.3% for a major depression episode and from 4.5% to 37.4% for any form of depressive disorders (Luppa et al., 2012). Depression has been consistently associated with physical and mental comorbidities, disability and increased risk of death both in clinical and epidemiological studies (Cuijpers et al., 2014; Ferrari et al., 2013; Prince et al., 2007; Saz and Dewey, 2001; Walker et al., 2015). Byers et al. (2012) found in a retrospective cohort study of male veterans that depression increased the risk of death over 40%, and Ganguli et al. (2002) showed a similar pattern in a community-based study of 1064 elder individuals. Nevertheless, there are other studies that have failed to find such association (Blazer et al., 2001; Everson-Rose et al., 2004). The inconsistency of findings may be explained by several factors, including the method for the assessment of depression, sample size, study design, follow-up period, or the control for potential confounding variables in adjusted models. That is the case for dementia, which has been rarely examined as a covariate in population or community-based studies, despite strong evidence suggesting the role of dementia as an independent risk factor for survival time and its relationship with depression (Todd et al., 2013).
The presence of depression in relation to dementia, particularly Alzheimer’s Disease (AD onwards), has been extensively studied, and it has been reported that around 50% of AD patients may suffer from a depressive episode at least once during the disease course (Chi et al., 2014). Previous evidence suggests an association between depression and the risk of incident all-cause dementia (Diniz et al., 2013). It remains unknown, however, whether depression constitutes a prodrome of dementia or an independent risk factor. Few longitudinal investigations have explicitly examined the role of depression as a potential predictor of mortality in dementia or AD samples, and they have shown mixed results: while Andersen et al. (2010) and Pimouguet et al. (2015) reported an association between depressive symptomatology and mortality, Roehr et al. (2015) did not found such association. In general, examination of survival risk associated with depression in AD samples is infrequent, with most studies focusing on the analysis of well-established risk factors (i.e. age, cardiovascular factors, or functional limitations). Given the lack of studies that explore the relationship between depression and mortality considering all confounding factors and the high prevalence of both dementia and depression, investigation of their association to mortality is of public health relevance.
The present study examine the temporal association between depressive symptoms and mortality in a community-based cohort from northern Manhattan in New York City taking into account the presence of AD, and test the same relationship in a subsample of this cohort consisting of individuals with AD.
2. Methods
2.1. Study design
Longitudinal data from the Washington Heights-Inwood Community Aging Project (WHICAP) at Columbia University Medical Center were analyzed. Information about this project is briefly summarized here as it has been described in detail elsewhere (Tang et al., 2001). WHICAP is a community-based study of aging and dementia in Medicare-eligible northern Manhattan residents aged 65 or older. The population sample was composed by a multiethnic cohort that includes Caribbean Hispanic, African-American and Caucasian (non-Hispanic) individuals. The current study included participants from the 1999 cohort. They have been followed at intervals of approximately 1.5 years. Data were collected through face-to-face structured interviews performed in either English or Spanish. Physicians conducted a standardized physical and neurological examination, as well as a neuropsychological test battery assessment. Each assessment also included data on general health status and functional ability.
The 1999 cohort had an initial sample of 2183 participants. 12 individuals with missing depression information at baseline were excluded. Of the remaining 2171 participants, we excluded 211 individuals with no follow-up visits or mortality data and 2 with missing dementia diagnosis. Thus, the final analytical sample consisted of 1958 respondents. Each participant contributed up to 15 years of follow-up from the baseline examination to death or censoring at the last evaluation. Initial analyses utilized the entire cohort. We repeated the analyses limiting them to AD cases only, analyzing prevalent and incident cases separately. Respondents who were diagnosed with AD at baseline assessment were considered prevalent AD cases (N=189). Individuals who had not dementia upon entry into the study and developed AD during follow-up were selected for the incident AD analyses (N = 169) (Fig. 1).
Fig. 1.
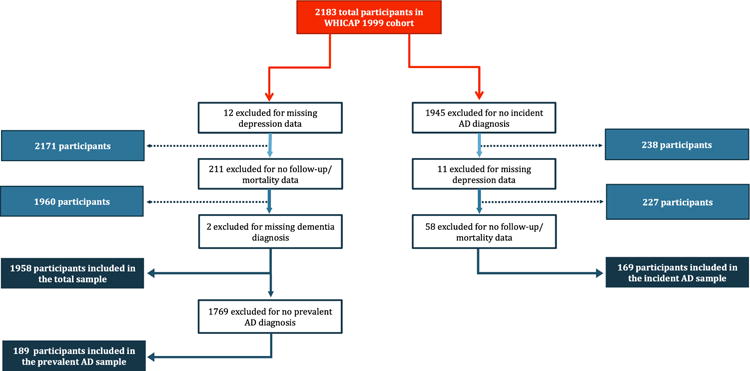
Flowchart describing selection of study sample. AD=Alzheimer’s Disease.
2.2. Ethic statement
The WHICAP study was reviewed and approved by the Institutional Review Boards of Columbia Presbyterian Medical Center and the New York State Psychiatric Institute. Written informed consent was obtained from all respondents.
2.3. Measurements
2.3.1. Outcome (Depressive symptoms)
The 10-item Center for Epidemiologic Studies Depression Scale (CES-D) (Irwin et al., 1999) was used to assess the presence and severity of depressive symptoms. Individuals were asked whether they had experienced each of 10 symptoms over the last week. Those questions answered with “yes” were endorsed with 1 point, leading to a total score that ranged from 0 to 10, with higher scores indicating greater depressive symptoms. We used two outcomes based on the CES-D: 1) The presence or absence of depressive symptoms was defined using a conventional cutoff score of ≥ 4 (CES-D depression); 2) Total CES-D score was treated as a continuous variable (CES-D severity).
Supplemental analyses were conducted by using additional questions included in the survey that evaluated the presence of DSM-V depression in the previous week. The presence or absence of depression was defined using an algorithm based on the DSM-V criteria (DSM-V depression) (American Psychiatric Association, 2013).
2.3.2. Alzheimer’s Disease diagnosis
Respondents were assessed using a standard neuropsychological battery measuring different domains: orientation (assessed with items from the Mini Mental State Examination (Folstein et al., 1975)), memory (with the multiple choice version of the Benton Visual Retention Test (BVRT) (Benton, 1955) and the Selective Reminding Test (SRT) (Buschke and Fuld, 1974)), language (measured with Boston Naming Test (Kaplan et al., 1983), Controlled Oral Word Association Test (CFL) (Benton et al., 1994), Category Naming, and the Complex Ideational Material and Repetition phrases from the Boston Diagnostic Aphasia Examination (BDAE) (Goodglass and Kaplan, 1983)), abstract reasoning (evaluated with the Wechsler Adult Intelligence Scales-Revised (WAIS-R) (Wechsler, 1981), the similarities subtest and the Identities and Oddities subtest from the Mattis Dementia Rating Scale (DRS) (Mattis, 1976)), speed of processing (with the Color Trail Making Test, Part A and B) (D’Elia et al., 1996), and visuospatial ability (assessed with the Rosen Drawing Test (Rosen, 1981) and the Benton Visual Retention Test (BVRT) (Benton, 1955)).
A consensus conference of neurologists, physicians, neuropsychologists and psychiatrists was made using all available information for the diagnosis of AD. Individuals were first identified as having dementia if they met the criteria of the DSM-V (American Psychiatric Association, 2013), requiring evidence of cognitive deficits (based on the neuropsychological scores described above), and impairment in social or occupational function in comparison to the past (measured through the Blessed Dementia Rating Scale (Blessed et al., 1968), the Schwab and England Activities of Daily Living Scale (Schwab et al., 1969), and the physician’s assessment). For the diagnosis of probable or possible AD, individuals satisfied the criteria of the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease (McKhann et al., 2011).
2.3.3. Mortality
Mortality data was obtained through follow-up interviews every 18 months and through the vital status from the National Death Index until June 2015.
2.3.4. Other measures
Sociodemographic variables included age (in years), gender, education (as the number of years in full time education), and ethnicity (Black (non-Hispanic), Hispanic, White (non-Hispanic), and other).
Apolipoprotein E (APOE-ε4) was defined as the absence of ε4 allele versus presence of either 1 or 2 ε4 alleles. Smoking status and alcohol consumption were assessed by self-report and categorized as never smoker, past and current smoker; and no alcohol use versus alcohol consumption, respectively.
Since cardiovascular factors have been widely associated with older age, dementia, and mortality (Beydoun et al., 2014), we considered the following comorbidities: diabetes mellitus, hypertension, stroke and heart disease. Comorbidities were defined through self-reported diagnosis and the use of disease-specific medications. Heart disease was defined as a history of myocardial infarction, congestive heart failure or any other heart disease (e.g. arrhythmias or angina pectoris).
2.4. Statistical analysis
Descriptive analyses of the baseline data were conducted to characterize the study sample. These analyses included frequencies, proportions, means, and standard deviations (SD). Significance tests for differences in proportions were carried out using chi-squared tests and Fisher’s exact tests as applicable (for categorical variables) and Student’s t-tests (for continuous variables).
Cox proportional hazard models were used to determine the association between depression (as assessed with the CES-D and DSM-V variables) and risk of all-cause mortality. Mortality was presented as the dichotomous outcome, and the time-to-event variable was time from baseline to death. Those individuals who did not die were censored at the time of their last follow-up. Three stages of multivariable Cox regression models were conducted: 1) In an initial model (model 1) depressive symptoms at baseline were the main predictor, controlling for demographic variables (i.e. gender, age, education, ethnicity); 2) Model 2 also included some risk factors (i.e. smoking status, alcohol consumption and APOE-ε4 –only in AD samples-); and 3) Model 3 further adjusted for medical comorbidities (i.e. diabetes mellitus, hypertension, stroke, heart disease and AD- only in the total sample-) and treatment (i.e. diabetes mellitus and hypertension), entered as categorical variables. All regression analyses were based on the sample with no missing data across all variables included.
Based on the previously reported effects modifications for the association between depression and mortality with other main variables (Elderon and Whooley, 2013), we tested whether there were significant interactions between heart disease, stroke and AD with depression. We found a significant interaction between DSM-V depression and AD that was added to the final adjusted model for that depression outcome.
Additionally, two separate analyses were conducted in 1) individuals who presented AD at the baseline assessment (prevalent AD); and 2) individuals who were non-demented at initial evaluation and developed AD throughout the follow-up (incident AD). In the latter case, the time-to-event variable was time from diagnosis of AD to death or last follow-up, both depressive symptoms and age were considered at the time of AD diagnosis, and covariates were included at any time prior to or including the AD diagnosis visit.
Proportional hazard assumptions were statistically tested with Cox-Snell residuals in all full adjusted models. No violations of the assumptions were found. Hazard ratios (HR), 95% confidence intervals (CI), and p values are reported. Data analyses were performed using IBM SPSS statistics 21.
3. Results
3.1. Sample characteristics
Baseline characteristics of the study sample, according to the depression variable based on CES-D, are illustrated in Table 1. In this sample of 1958 individuals, 409 (20.9%) respondents presented depressive symptoms and 992 (50.7%) died during the study period. Mean age at baseline was 77 years (SD = 7.1) and there were more females than males (67.2% vs. 32.8%). Overall, subjects with depressive symptoms differed from the non-depressed sample in age, gender, education, ethnicity, stroke and heart disease. In the prevalent AD sample, compared to individuals with non depressive symptoms, those with depressive symptoms were significantly more likely to be female. In the incident AD sample, subjects who presented depressive symptoms did not differ from those non-depressed in any demographic-clinical variable with the exception of alcohol consumption and heart disease. Table 2 displays demographic and clinical characteristics based on the depression variable that followed the DSM-V criteria.
Table 1.
Baseline characteristics of respondents according to depressive symptoms.
| Characteristics | Total sample (n=1958) |
p* | Prevalent AD sample (n=189) |
p* | Incident AD sample (n=169) |
p* | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non depressive symptoms (n =1549) |
Depressive symptoms (n =409) |
Non depressive symptoms (n =128) |
Depressive symptoms (n=61) |
Non depressive symptoms (n=125) |
Depressive symptoms (n=44) |
|||||
| Age (M, sd) | 76.8 (7.0) | 77.7 (7.2) | 0.02 | 83.4 (8.2) | 83.3 (7.9) | 0.92 | 84.6 (7.2) | 83.6 (6.3) | 0.83 | |
| Gender (n, %) | < 0.001 | 0.01 | 0.56 | |||||||
| Female | 992 (64.0%) | 323 (79.0%) | 83(64.8%) | 51 (83.6%) | 85 (68.0%) | 32 (72.7%) | ||||
| Male | 557 (36.0%) | 86 (21.0%) | 45 (35.2%) | 10 (16.4%) | 40 (32.0%) | 12 (27.3%) | ||||
| Education (M, sd) | 10.7 (4.8) | 9.1 (4.7) | < 0.001 | 7.3 (4.5) | 6.2 (4.1) | 0.11 | 7.8 (5.2) | 7.6 (4.8) | 0.23 | |
| Ethnicity (n, %) | < 0.001 | 0.29 | 0.36 | |||||||
| White | 494 (31.9%) | 106 (25.9%) | 17 (13.3%) | 7 (11.5%) | 23 (18.4%) | 11 (25.0%) | ||||
| Black | 549 (35.4%) | 100 (24.5%) | 51 (39.8%) | 18 (29.5%) | 35 (28.0%) | 7 (15.9%) | ||||
| Hispanic | 483 (31.2%) | 198 (48.4%) | 60 (46.9%) | 36 (59.0%) | 66 (52.8%) | 26 (59.1%) | ||||
| Other | 23 (1.5%) | 5 (1.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 0 (0.0%) | ||||
| Smoking status (n, %) | 0.44 | 0.85 | 0.14 | |||||||
| No smoking | 642 (50.2%) | 173 (53.6%) | 51 (59.3%) | 21 (58.3%) | 55 (47.8%) | 27 (64.3%) | ||||
| Past smoking | 501 (39.1%) | 114 (35.3%) | 28 (32.6%) | 13 (36.1%) | 57 (49.6%) | 15 (35.7%) | ||||
| Current smoking | 137 (10.7%) | 36 (11.1%) | 7 (8.1%) | 2 (5.6%) | 3 (2.6%) | 0 (0.0%) | ||||
| Alcohol consumption (n, %) | 89 (5.8%) | 16 (4.0%) | 0.15 | 4 (3.2%) | 0 (0.0%) | 0.31 | 18 (14.4%) | 1 (2.3%) | 0.03 | |
| Diabetes (n, %) | 296 (19.4%) | 82 (20.5%) | 0.63 | 28 (22.2%) | 8 (14.0%) | 0.20 | 27 (21.6%) | 14 (31.8%) | 0.17 | |
| Hypertension (n, %) | 957 (63.0%) | 272 (68.0%) | 0.06 | 91 (73.4%) | 44 (77.2%) | 0.59 | 103 (82.4%) | 35 (79.5%) | 0.67 | |
| Stroke (n, %) | 131 (8.6%) | 49 (12.4%) | 0.02 | 16 (12.7%) | 11 (19.3%) | 0.24 | 27 (21.6%) | 9 (20.5%) | 0.87 | |
| Heart disease (n, %) | 416 (27.2%) | 134 (33.5%) | 0.01 | 35 (27.8%) | 16 (28.1%) | 0.97 | 55 (44.0%) | 27 (61.4%) | 0.05 | |
| APOE4 (n, %) | 366 (26.6%) | 102 (29.4%) | 0.30 | 38 (37.3%) | 18 (38.3%) | 0.90 | 31 (27.7%) | 14 (32.6%) | 0.55 | |
| Mortality (n, %) | 768 (49.6%) | 224 (54.8%) | 0.06 | 99 (77.3%) | 45 (73.8%) | 0.59 | 73 (58.4%) | 30 (68.2%) | 0.25 | |
Abbreviations: AD-Alzheimer’s Disease; sd = standard deviation; CES-D: Center for Epidemiologic Studies Depression Scale; M=mean; n=number (frequencies).
Note-Depressive symptoms variable was based on the CES-D questionnaire, using a cutoff of ≥ 4.
Chi-squared and fisher’s exact tests (for categorical variables) and t-tests (for continuous variables) were carried out to compare across depressive symptoms.
Table 2.
Baseline characteristics of respondents according to depression status.
| Characteristics | Total sample (n=1933) |
p* | Prevalent AD sample (n=185) |
p* | Incident AD sample (n=174) |
p* | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No depression (n=1836) |
Depression (n=97) |
No depression (n=166) |
Depression (n=19) |
No depression (n=156) |
Depression (n=18) |
|||||
| Age (M, sd) | 76.9 (7.0) | 77.7 (7.8) | 0.31 | 83.3 (7.8) | 83.1 (9.6) | 0.90 | 84.5 (7.0) | 82.7 (5.4) | 0.29 | |
| Gender (n, %) | < 0.001 | 0.06 | 0.71 | |||||||
| Female | 1213 (66.1%) | 83 (85.6%) | 114 (68.7%) | 17 (89.5%) | 106 (67.9%) | 13 (72.2%) | ||||
| Male | 623 (33.9%) | 14 (14.4%) | 52 (31.3%) | 2 (10.5%) | 51 (32.1%) | 5 (27.8%) | ||||
| Education (M, sd) | 10.4 (4.8) | 8.7 (4.7) | 0.001 | 6.9 (4.3) | 6.9 (4.9) | 0.99 | 7.9 (5.2) | 5.7 (3.6) | 0.03 | |
| Ethnicity (n, %) | 0.004 | 0.30 | 0.02 | |||||||
| White | 561 (30.6%) | 30 (30.9%) | 21 (12.6%) | 3 (15.8%) | 31 (19.9%) | 2 (11.1%) | ||||
| Black | 623 (33.9%) | 18 (18.6%) | 65 (39.2%) | 4 (21.0%) | 43 (27.6%) | 0 (0.0%) | ||||
| Hispanic | 627 (34.2%) | 46 (47.4%) | 80 (48.2%) | 12 (63.2%) | 81 (51.9%) | 16 (88.9%) | ||||
| Other | 25 (1.3%) | 3 (3.1%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | ||||
| Smoking status (n, %) | 0.91 | 0.67 | 0.8 | |||||||
| No smoking | 776 (50.8%) | 39 (52.0%) | 67 (61.5%) | 7 (50.0%) | 74 (51.0%) | 10 (55.6%) | ||||
| Past smoking | 586 (38.3%) | 29 (38.7%) | 34 (31.2%) | 6 (42.9%) | 68 (46.9%) | 8 (44.4%) | ||||
| Current smoking | 167 (10.9%) | 7 (9.3%) | 8 (7.3%) | 1 (7.1%) | 3 (2.1%) | 0 (0.0%) | ||||
| Alcohol consumption (n, %) | 100 (5.5%) | 5 (5.2%) | 0.90 | 3 (1.8%) | 1 (5.3%) | 0.36 | 20 (12.8%) | 0 (0.0%) | 0.23 | |
| Diabetes (n, %) | 351 (19.2%) | 28 (28.9%) | 0.02 | 32 (19.4%) | 4 (21.1%) | 0.77 | 36 (23.1%) | 9 (50.0%) | 0.02 | |
| Hypertension (n, %) | 1167 (63.9%) | 61 (62.9%) | 0.83 | 120 (73.6%) | 15 (78.9%) | 0.79 | 127 (81.4%) | 16 (88.9%) | 0.74 | |
| Stroke (n, %) | 165 (9.1%) | 17 (17.5%) | 0.01 | 24 (14.5%) | 5 (26.3%) | 0.19 | 32 (20.5%) | 6 (33.3%) | 0.23 | |
| Heart disease (n, %) | 513 (28.0%) | 35 (36.1%) | 0.09 | 41 (24.8%) | 10 (52.6%) | 0.01 | 73 (46.8%) | 11 (61.1%) | 0.25 | |
| APOE4 (n, %) | 439 (26.9%) | 25 (29.4%) | 0.62 | 51 (38.6%) | 6 (40.0%) | 0.92 | 40 (28.2%) | 6 (35.3%) | 0.58 | |
| Mortality (n, %) | 921 (50.2%) | 55 (56.7%) | 0.21 | 130 (78.3%) | 11 (57.9%) | 0.08 | 95 (60.9%) | 11 (61.1%) | 0.98 | |
Abbreviations: AD=Alzheimer’s Disease; sd = standard deviation; M=mean; n=number (frequencies).
Note=Depression status was based on an algorithm that followed DSM-V criteria.
Chi-squared and fisher’s exact (for categorical variables) and t-tests (for continuous variables) were carried out to compare across depression status.
3.2. Depressive symptoms and the risk of mortality
Overall, respondents were followed for an average of 5.6 years (SD = 4.5; maximum 14.4 years). Mean survival time from baseline assessment was 8.4 years (95% CI = 7.89, 8.86) for those individuals with depressive symptoms and 9.4 years (95% CI = 9.09, 9.65) for the subjects with non depressive symptoms. When restricting the analyses to the AD sample, mean survival time for those individuals with prevalent AD was 5.3 years (95% CI = 4.72, 5.91) at a mean age of 83.4 years (SD = 8.04), while for the incident cases of AD mean survival time after AD onset was 5.1 years (95% CI = 4.49, 5.65) at a mean age of 84.2 years (SD = 6.9). For those individuals that, apart from suffering AD, presented depressive symptoms, mean survival time was 5.7 years (95% CI = 4.72, 5.91) for respondents with prevalent AD and depressive symptoms (versus 5.1 years; 95% CI = 4.41, 5.8 for those without depressive symptoms), and 4.6 years (95% CI = 3.44, 5.71) for respondents with incident AD and depressive symptoms (versus 5.2 years; 95% CI = 4.57, 5.9 for those without depressive symptoms).
Table 3 shows the adjusted hazard ratios of Cox regression analyses of the mortality risk associated with depressive symptoms in the total sample as well as in each of the AD groups. In the total sample, CES-D depression was significantly associated with higher mortality risk after adjusting for demographic variables (HR = 1.32; 95% CI = 1.13, 1.54). This association remained significant when we added risk factors to the model (HR = 1.31 95% CI = 1.10, 1.57), and after the inclusion of all potential covariates (HR = 1.22; 95% CI = 1.02, 1.46). Similar results were observed when we considered CES-D severity. In the fully adjusted model (model 3), each additional unit increase in CES-D severity was associated with a higher risk of mortality (HR = 1.04; 95% CI = 1.01, 1.08). The association between depressive symptoms and mortality was not significant in the respondents with prevalent AD using either of the CES-D depression variables (Model 3: HR = 1.07; 95% CI = 0.57, 2.03 for CES-D depression; Model 3: HR = 1.06; 95% CI = 0.94, 1.19 for CES-D severity). The sample with incident AD consisted of 169 individuals and 103 died during follow-up. In this group, respondents with depressive symptoms had more than 50% increased risk of mortality compared to those who had non depressive symptoms in the fully adjusted model (HR = 1.88; 95% CI = 1.12, 3.18). This association was also significant when depressive symptoms were analyzed as CES-D severity (Model 3: HR = 1.12; 95% CI = 1.01, 1.23).
Table 3.
Adjusted hazard ratios of the effect of depressive symptoms on all-cause-mortality among older adults. Cox regression models.
| Characteristics | Total sample
|
Prevalent AD sample
|
Incident AD sample
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 1a | Model 2b | Model 3c | Model 1a | Model 2b | Model 3c | |
|
| |||||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| CES-D depression (Ref. No) | 1.32*** (1.13, 1.54) |
1.31** (1.10, 1.57) |
1.22* (1.02, 1.46) |
0.88 (0.61, 1.28) |
1.05 (0.57, 1.94) |
1.07 (0.57, 2.03) |
1.70* (1.08, 2.67) |
1.74* (1.08, 2.67) |
1.88* (1.12,3.18) |
| CES-D severity(continuous) | 1.07*** (1.03, 1.1) |
1.06** (1.02, 1.1) |
1.04** (1.01, 1.08) |
0.99 (0.92, 1.06) |
1.06 (0.95, 1.19) |
1.06 (0.94, 1.19) |
1.13* (1.02, 1.21) |
1.11* (1.00, 1.22) |
1.12* (1.01, 1.23) |
Abbreviations: AD = Alzheimer’s Disease; CES-D: Center for Epidemiologic Studies Depression Scale; CI=Confidence Interval; HR= Hazard Ratio.
Note: Definition of the CES-D variables were based on the CES-D questionnaire, using a cutoff of ≥ 4.
Model 1 adjusted for age, gender, education and ethnicity.
Model 2 adjusted for age, gender, education, ethnicity, smoking status, alcohol consumption and APOE4 (only in the AD samples).
Model 3 adjusted for age, gender, education, ethnicity, smoking status, alcohol consumption, APOE4 (only in the AD samples), diabetes mellitus, hypertension, stroke, heart disease and prevalent AD (only in the total sample).
p < 0.05
p < 0.01
p < 0.001.
We found a similar pattern of findings when using the DSM-V depression variable, except for the incident AD cases. These individuals showed no excess risk of mortality associated with depression (Table 4). Moreover, a term interaction between DSM-V depression and AD was added to the fully-adjusted model for the overall sample. The interaction revealed that DSM-V depression had a greater effect on mortality for individuals without prevalent AD versus prevalent AD (HR = 0.39; 95% CI = 0.17, 0.92).
Table 4.
Adjusted hazard ratios of the effect of depression on all-cause-mortality among older adults. Cox regression models.
| Characteristics | Total sample
|
Prevalent AD sample
|
Incident AD sample
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | Model 1a | Model 2b | Model 3c | Model 1a | Model 2b | Model 3c | |
|
| |||||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| DSM-V depression (Ref. No) | 1.47** (1.11, 1.95) | 1.47* (1.07, 2.03) | 1.50* (1.06, 2.14) | 0.70 (0.36, 1.36) | 0.72 (0.28, 1.86) | 0.66(0.25, 1.79) | 1.52(0.79, 2.91) | 2.03(0.98, 4.24) | 1.91(0.88, 4.13) |
Abbreviations: AD = Alzheimer’s Disease; CI=Confidence Interval; HR= Hazard Ratio.
Note: Definition of “DSM-V depression” was based on questions that follow DSM-V criteria for the presence of a major depressive episode.
Model 1 adjusted for age, gender, education and ethnicity.
Model 2 adjusted for age, gender, education, ethnicity, smoking status, alcohol consumption and APOE4 (only in the AD samples).
Model 3 adjusted for age, gender, education, ethnicity, smoking status, alcohol consumption, APOE4 (only in the AD samples), diabetes mellitus, hypertension, stroke, heart disease and, prevalent AD and an interaction term between depression and prevalent AD (only in the total sample).
p< 0.05.
p < 0.01.
4. Discussion
In this community-based study of elders in Northern Manhattan, we found that depressive symptoms were independently associated with increased risk of mortality after controlling for the presence of AD and other confounders. Moreover, we observed an association between depressive symptoms and survival in a subsample of incident AD individuals. To the best of our knowledge, this is the first community-based study that explicitly explores the role of depressive symptoms in a sample of individuals with AD.
4.1. Depression and mortality in the general population
A recent meta-analysis by Walker et al. (2015) showed that individuals with mental disorders present excess mortality rates compared to the general population. Moreover, depression, either characterized by a clinical diagnosis of depression or depressive symptomatology, has been consistently associated with increased risk of death in the elderly (Cuijpers et al., 2014). For example, the results of a study by Schulz et al. (2000) showed that more severe depressive symptoms, as assessed with the CES-D, were associated with higher mortality in a large population-based sample of older adults from the United States (US). Schoevers et al. (2009), in a 10-year follow-up study of a community living elderly from Amsterdam, found that severity and chronicity of depression were also associated with mortality.
Additionally, there is evidence of an association between depression and subsequent mortality in cardiovascular patients (Peters et al., 2010; Vinkers et al., 2004). It has been suggested that the effects of depression on mortality may be due to its relationship with vascular disease (Elderon and Whooley, 2013; Hare et al., 2014). If this is the case, the influence of depression on mortality should disappear if the presence of cardiovascular disease is taken into account. In our study, the effects of depressive symptoms on mortality were not altered after controlling for major cardiovascular risk factors, which indicate that depressive symptoms are an independent predictor of mortality in the general population.
4.2. Depression and mortality in the context of AD
Our second aim was to estimate the effect of depressive symptoms on survival in AD individuals. Depression is common among older individuals with dementia, with estimates up to 50% at any point of the disease course (Chi et al., 2014). Recent studies have suggested that late-life depression might constitute a prodromal phase for subsequent dementia (Saczynski et al., 2010), and this may lead to excess mortality in those individuals with co-occurrence of depression and dementia (Bellelli et al., 2008).
Regarding the relationship between depression and mortality in AD individuals, literature is scarce and results are mixed. A systematic review by Guehne et al. (2005) pointed out that very little information is available on depressive symptomatology when studying mortality in dementia, and most studies have not examined depression as a potential predictor for survival (Todd et al., 2013). Moreover, methodological differences in the few studies that explored depression hinder comparisons between them (Ganguli et al., 2002).
We identified a significant relationship between depressive symptoms and survival in the incident AD sample. Prior reports have found that depressive symptoms were associated with increased risk of mortality in individuals with incident dementia, while others found no evidence for that relationship. For instance, a prospective longitudinal study involving six urban areas in Germany found no significant association between depressive symptoms and mortality in a sample of individuals with incident dementia (Roehr et al., 2015). Andersen et al. (2010), whose results are focused on a population-based cohort of both prevalent and incident dementia samples, reported similar findings. Alternatively, Lopez et al. (2000) examined the possible additive effect of AD and depression to survival in 267 patients diagnosed with possible AD. Their results suggested that history of depression associated with the onset of AD decreased time of survival. Our results accord with those of Pimouguet et al. (2015), who found that depressive symptomatology, assessed with the CES-D, was a significant predictor for mortality in a Cox model that also adjusted for demographics and medical conditions in a population-based cohort of 253 individuals with incident dementia. On the other hand, the DSM-V depression variable was not associated with mortality in that sample, which could be explained by the small sample of respondents that met the criteria for a major depressive episode. Interestingly, these results are in accordance with the meta-analyses conducted by Cuijpers and Smit (2002) in elders without dementia, whose findings showed that the rates for mortality were higher in subclinical depression compared to individuals with a major depressive disorder. A different explanation of these results may be related with the apathy syndrome. Apathy is the most common neuropsychiatric symptom in dementia and it has been found in association with increased functional impairment, faster functional decline and poorer prognosis in AD patients (Landes et al., 2001). A study of Vilalta-Franch et al. (2013) demonstrated that apathy had an increased mortality risk in a cohort study of 491 patients with probable Alzheimer’s disease (HR = 1.99; 95% CI = 1.15, 3.45). Since apathy and depression often co-occur in AD and recent evidence suggests that apathy could be defined as an AD subtype with greater clinical severity (Vilalta-Franch et al., 2013; Zhao et al., 2016), our results could be the reflection of an undetected apathy syndrome.
Regarding prevalent cases of AD, several studies on the relationship between depression and mortality found no association between depressive symptomatology and survival (Andersen et al., 2010; Larson et al., 2004; Schaufele et al., 1999). In contrast, Burns et al. (1991) and Butler et al. (2004) observed, in clinic-based settings, a significant increase of the risk of dying when both depression and prevalent dementia co-occurred. We found no significant association between depressive symptoms and mortality in individuals with prevalent AD after full adjustment for other variables. These findings may be explained by survival bias, as those with rapidly progressing disease may not have been included in our study or they would have died before the next follow-up evaluation due to earlier mortality (Helzner et al., 2008). Moreover, given that AD respondents were at different stages of disease progression at study entry, the non-significant results may be explained by differences in the severity of the underlying AD pathology. Thus, we feel that these results should be interpreted with caution.
The mechanisms for the association of depression to increased mortality remain unclear. However, diverse explanations have been suggested. From a psychological perspective, it is common that depressed individuals disengage from preventive and therapeutic health behaviors (Schulz et al., 2000). Depression has been related to unhealthy lifestyle factors such as physical inactivity, smoking habits or poor diet, which would lead to a greater frequency of chronic conditions in depressed people (van Gool et al., 2003). From a biological perspective, explanations have been related with changes in the neuroendocrine, immune and inflammatory systems (Anisman, 2009; Chi et al., 2014). Reduced heart rate variability or greater catecholamine levels have been also explored as possible pathways between depression, cardiovascular events and mortality (Elderon and Whooley, 2013). With regard to AD, Chi et al. (2014) indicated that many factors may contribute to explain the mechanism of depression in AD, including genetic factors, neuroanatomic changes, vascular risk factors, and the imbalance of neurotransmitters. Moreover, it has been hypothesized that the co-occurrence of both depression and dementia would play a role as an additive factor for mortality (Bellelli et al., 2008; Mehta et al., 2003).
4.3. Strengths and limitations
The strengths of our study include the use of large community-representative data, with a varied ethnic sample and socioeconomic background of elderly people, which result in a greater ecological validity. Furthermore, this study included a comprehensive neuropsychological battery to aid in the clinical diagnosis by experts in dementia. In addition, our analyses were adjusted for potential confounders, allowing for a more accurate examination of the relationship between depressive symptoms and mortality over time. These results should be also interpreted in light of several limitations. First, the CES-D does not measure clinical depression but depressive symptomatology, and it was collected retrospectively through self-report that may result in recall or reporting bias. Moreover, depressive information collected from AD individuals may have decreased accuracy because of poor recall, especially in severe AD respondents. As a consequence, it might turn into an error of estimation of the exact associations between depressive symptoms and survival risk. Second, it is possible that our results have been potentially skewed to the individuals with longer survival, since those who were exposed to known risk factors for mortality are likely to die prematurely. Third, we diagnosed AD for incident AD cases at the time of follow-up assessment, which may result in some inaccuracy of the incidence dates. However, this method is commonly used in community and population-based studies. Finally, we did not explore the specific causes of death. Nevertheless, information from death certificates or caregivers is often unreliable, since the true reasons for death are commonly underreported.
5. Conclusions
The results of the present study suggest that depressive symptomatology constitutes an independent risk factor that lead to excess mortality in the general population, and support further investigation to elucidate the mechanisms that explain this association. On the other hand, independent confirmation of our findings regarding the relationship between depressive symptoms and survival in the context of dementia is necessary.
Since depression is one of the most disabling diseases, strategies focused on the development of effective and affordable approaches to reduce incident-cases would have beneficial effects not only related with a better quality of life and reduced disability, but also with a longer survival.
Acknowledgments
Elvira Lara’s work is supported by the FPU predoctoral grant (FPU13/03573) from the Spanish Ministry of Education, Culture and Sports. Elvira Lara thanks to Dr. Nicole Shupf for providing help in the data management.
Role of funding source
The study was supported by grants from the National Institute on Aging (NIH) AG037212, AG007370, AG034189, and AG042483. The funding source had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
Conflict of interest
Dr Josep Maria Haro has been consultant of Elli Lilly and Co, Roche, Lundbeck and Otsuka. For the remaining authors, none were declared.
Author contributions
EL: undertook the statistical analysis and wrote the main body of the manuscript; MT: assisted in the data analysis and approved the final version to be published; JM: conceived and designed the experiments, commented for intellectual content and approved the final version to be published; JMH: commented for intellectual content, critically revised the paper and approved the final version to be published; YS: conceived and designed the experiments, commented for intellectual content, critically revised the paper and approved the final version to be published.
References
- Andersen K, Lolk A, Martinussen T, Kragh-Sørensen P. Very mild to severe dementia and mortality: a 14-year follow-up – The odense study. Dement Geriatr Cogn Disord. 2010;29:61–67. doi: 10.1159/000265553. [DOI] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fifth. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Bellelli G, Frisoni GB, Turco R, Trabucchi M. Depressive symptoms combined with dementia affect 12-months survival in elderly patients after rehabilitation post-hip fracture surgery. Int J Geriatr Psychiatry. 2008;23:1073–1077. doi: 10.1002/gps.2035. [DOI] [PubMed] [Google Scholar]
- Benton AL. The Visual Retention Test. the Psychological Corporation; New York: 1955. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Manual for the Multilingual Aphasia Examination. third. AJA Associates; Iowa: 1994. [Google Scholar]
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Hybels CF, Pieper CF. The association of depression and mortality in elderly persons: a case for multiple, independent pathways. J Gerontol. 2001;56:505–509. doi: 10.1093/gerona/56.8.m505. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of senile change in the cerebral grey matter of elderly subjects. Br J Psychol. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Burns A, Lewis G, Jacoby R, Levy R. Factors affecting survival in Alzheimer’s disease. Psychol Med. 1991;21:363–370. doi: 10.1017/s0033291700020468. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Butler R, Orrell M, Ukoumunne OC, Bebbington P. Life events and survival in dementia: a 5-year follow-up study. Aust N Z J Psychiatry. 2004;38:702–705. doi: 10.1111/j.1440-1614.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20:664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: the national comorbidity survey replication. Arch Gen Psychiatry. 2010;67:489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S, Yu JT, Tan MS, Tan L. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J Alzheimers Dis. 2014;42:739–755. doi: 10.3233/JAD-140324. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- D’Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test Professional Manual. Psychological Assessment Resources; Odessa: 1996. [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:511–523. doi: 10.1016/j.pcad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: confounding effects of health status. Psychosom Med. 2004;66:823–830. doi: 10.1097/01.psy.0000145903.75432.1f. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford AH. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch Gen Psychiatry. 2002;59:1046–1052. doi: 10.1001/archpsyc.59.11.1046. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and related Disorders. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Guehne U, Riedel-Heller S, Angermeyer MC. Mortality in dementia. Neuroepidemiology. 2005;25:153–162. doi: 10.1159/000086680. [DOI] [PubMed] [Google Scholar]
- Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult. Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s Disease. J Am Geriatr Soc. 2001;49:1700–1707. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, Kukull WA. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk W, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Meltzer C, Wisniewski S, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- Luppa M, Sikorski C, Luck T, Ehreke L, Konnopa A, Wiese B, Weyerer S, König HH, Riedel-Heller SG. Age-and gender-specific prevalence of depression in latest-life- Systematic review and meta-analysis. J Affect Disord. 2012;136:212–221. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu TB, editors. Geriatric Psychiatry. Grune & Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, Yaffe K, Langa KM, Sands L, Whooley MA, Covinsky KE. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. J Gerontol A Biol Sci Med Sci. 2003;58:461–467. doi: 10.1093/gerona/58.5.m461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Pinto E, Beckett N, Swift C, Potter J, McCormack T, Junes M, Grimley-Evans J, Fletcher A, Bulpitt C. Association of depression with subsequent mortality, cardiovascular morbidity and incident dementia in people aged 80 and over and suffering from hypertension. Data from the Hypertension in the Very Elderly Trial (HYVET) Age Ageing. 2010;39:439–445. doi: 10.1093/ageing/afq042. [DOI] [PubMed] [Google Scholar]
- Pimouguet C, Delva F, Le Goff M, Stern Y, Pasquier F, Berr C, Tzourio C, Dartigues JF, Helmer C. Survival and early recourse to care for dementia: a population based study. Alzheimers Dement. 2015;11:385–393. doi: 10.1016/j.jalz.2014.04.512. [DOI] [PubMed] [Google Scholar]
- Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- Roehr S, Luck T, Bickel H, Brettschneider C, Ernst A, Fuchs A, Heser K, König HH, Jessen F, Lange C, Mösch E, Pentzek M, Steinmann S, Weyerer S, Werle J, Wiese B, Scherer M, Maier W, Riedel-Heller SG. Mortality in incident dementia – results from the German study on aging, cognition, and Dementia in primary care patients. Acta Psychiatr Scand. 2015;132:257–269. doi: 10.1111/acps.12454. [DOI] [PubMed] [Google Scholar]
- Rosen W. The Rosen Drawing Test. Veterans Administration Medical Center; Bronx, New York: 1981. [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the framingham heart study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saz P, Dewey ME. Depression, depressive symptoms and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:622–630. doi: 10.1002/gps.396. [DOI] [PubMed] [Google Scholar]
- Schaufele M, Bickel H, Weyerer S. Predictors of mortality among demented elderly in primary care. Int J Geriatr Psychiatry. 1999;14:946–956. doi: 10.1002/(sici)1099-1166(199911)14:11<946::aid-gps45>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Geerlings MI, Deeg DJH, Holwerda TJ, Jonker C, Beekman ATF. Depression and excess mortality: evidence for a dose response relation in community living elderly. Int J Geriatr Psychiatry. 2009;24:169–176. doi: 10.1002/gps.2088. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- Schwab JF, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Billingham FH, Donaldson MC, editors. Third Symposium on Parkinson’s Disease. Churchill Livingstone; Edinburgh: 1969. pp. 152–157. [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013;28:1109–1124. doi: 10.1002/gps.3946. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32:81–87. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- Vilalta-Franch J, Calvó-Perxas L, Garre-Olmo J, Turró-Garriga O, López-Pousa S. Apathy syndrome in Alzheimer’s disease epidemiology: prevalence, incidence, persistence, and risk and mortality factors. J Alzheimer’s Dis. 2013;33:535–543. doi: 10.3233/JAD-2012-120913. [DOI] [PubMed] [Google Scholar]
- Vinkers DJ, Stek ML, Gussekloo J, van der Mast RC, Westendorp RGJ. Does depression in old age increase only cardiovascular mortality? The Leiden 85-plus study. Int J Geriatr Psychiatry. 2004;19:852–857. doi: 10.1002/gps.1169. [DOI] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications. JAMA Psychiatry. 2015;72:334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Test. The Psychological Corporation; New York: 1981. [Google Scholar]
- Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Xu W, Li JQ, Wang J, Lai TJ, Yu JT. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]


