Abstract
Background
Chimeric antigen receptor (CAR) therapy has begun to demonstrate success as a novel treatment modality for hematologic malignancies. The success observed thus far has been with T cells permanently-engineered to express chimeric receptors. T cells engineered using RNA electroporation represent an alternative with the potential for similar efficacy and greater safety when initially targeting novel antigens. Neuroblastoma is a common pediatric solid tumor with the potential to be targeted using immunotherapy.
Methods
We performed xenograft studies in NSG mice assessing the efficacy of both permanently-modified and transiently-modified CAR T cells directed against the neuroblastoma antigen GD2 in both local and disseminated disease models. Disease response was monitored by tumor volume measurement and histological examination, as well as in vivo bioluminescence.
Results
RNA-modified GD2 CAR T cells mediated rapid tumor destruction and control in localized models of disease. A single infusion of lentivirally-modified GD2 CAR T cells resulted in long-term control of disseminated disease. Multiple infusions of RNA GD2 CAR T cells slowed disease progression and improved survival, but did not result in long-term disease control. Histologic examination revealed that the transiently-modified cells were unable to significantly penetrate the tumor environment, despite multiple CAR T cell infusions.
Discussion
RNA-modified GD2 CAR T cells can effectively control local neuroblastoma, and permanently-modified cells are able to control disseminated neuroblastoma in xenografted mice. Lack of long-term disease control by RNA-engineered cells resulted from an inability to penetrate the tumor microenvironment.
Keywords: immunotherapy, chimeric antigen receptors, pediatrics, solid tumors, RNA electroporation
Introduction
Neuroblastoma is the most common extra-cranial pediatric solid tumor. Derived from neuro-endocrine tissue of the sympathetic nervous system, it accounts for 9% of cancer diagnoses and 15% of cancer deaths in children (1). Current standard of care for high-risk disease consists of chemotherapy, surgery, consolidation chemotherapy followed by stem-cell transplant, tumor-directed radiation, and antibody-based therapy. This exhaustive regimen still only yields in a 3-year event-free survival of ~45% (2).
Historically, poor tumor immunogenicity and immune evasion (3, 4) have resulted in largely disappointing T cell therapy trials. Advances in understanding of T cell and tumor interaction, cell production techniques and engineering platforms, as well as the recognition of the need for host conditioning have recently allowed for significant advances. Chimeric antigen receptors (CARs) are molecules composed of an extracellular antigen-binding domain derived from the immunoglobulin variable chain (scFv) linked to intracellular T cell activating and co-stimulatory domains (5, 6). The antigen-specific scFv allows for MHC-independent tumor antigen recognition that initiates robust T cell stimulation, even in response to previously immuno-evasive malignancies (7).
In the most successful clinical trial of CARs to date, Porter et al demonstrated complete remissions lasting >3 years in two of three adults with chronic lymphocytic leukemia (CLL) using CARs directed against the B-cell antigen CD19 (8, 9). Translating this success to acute leukemia, our group recently reported the induction of complete remissions in two children with acute lymphoblastic leukemia (ALL) using the same CD19 CAR T cells (10).
The dramatic clinical responses observed in these reports have employed T cells permanently engineered to express CARs using lentiviral vectors. This process involves ex vivo exposure of harvested autologous lymphocytes to self-inactivating lentiviral vector encoding a CAR, resulting in genomic integration of the CAR transgene. While >500 patient-years of data suggest that this modification is extremely unlikely to result in insertional mutagenesis in mature lymphocytes (11), these data are from adults and the increased life-span of modified cells in children raises additional theoretical safety concerns. More importantly, when targeting solid tumor antigens the risk of on-target off-tumor toxicity becomes a significant concern. Several adverse events have demonstrated the potential risks of uncontrolled CAR T cells (12, 13), and have highlighted the need for safer CAR T cells moving forward, especially in early clinical testing (14, 15). Given these considerations, we and other groups have previously reported the development of an mRNA electroporation-based approach to induce transient CAR expression (16–18). This strategy creates an efficient CAR expression system that ensures complete loss of CAR-driven T cell activity in a predictable time frame without the need to administer other systemic agents to eliminate modified T cells. We have reported the efficacy of transiently-modified CD19 CAR T cells in a disseminated xenograft model of systemic ALL (19), and recently demonstrated enhanced efficacy of these transiently-modified cells when delivered repeatedly in an optimized dosing strategy (20). This optimized therapeutic regimen approached the anti-tumor responses observed with permanently-modified CD19 CAR T cells and demonstrated long-term disease control, suggesting that multiple infusions of transiently-modified CAR T cells may present an alternative to genome-modifying T cell engineering techniques.
RNA CAR T cells have demonstrated in vitro activity (21) and in vivo efficacy in localized models of solid tumors, and have similarly shown enhanced efficacy using multiple cell infusions (17, 22). Based on these findings, as well as our own experience with RNA CAR T cells in ALL, we evaluated a CAR targeting GD2, a diasialoganglioside expressed on the surface of most neuroblastomas (1) that has already been shown to be an effective target for neuroblastoma immunotherapy (23). A single chain antibody fragment (scFv) targeting GD2 was linked to the CD3ζ and 4-1BB intracellular signaling domains and tested in localized and disseminated animal models of neuroblastoma. We demonstrate that multiple infusions of RNA GD2 CAR T cells results in control of local disease, and that a single low-dose infusion of permanently-modified GD2 CAR T cells results in long-term control of disseminated neuroblastoma. Multiple infusions of RNA GD2 CAR T cells are less effective at controlling disseminated disease, and our data highlight the potential mechanism underlying this lack of efficacy. Together, these data clarify the necessary components for success of transiently-modified CAR T cells in solid tumors.
Materials and Methods
Generation of CAR constructs and RNA electroporation
CARs containing scFv domains directed against GD2 or CD19 linked to CD3ζ and 4-1BB intracellular signaling domains were produced as previously described (24, 25) (GD2-z construct was generously provided by Dr. Malcolm Brenner, Baylor College of Medicine, Houston, Texas). Development of constructs for RNA manufacture was performed as previously described (17). mScript RNA System (CellScript, Madison, WI, Catalog #MSC11625) was utilized to generate capped in vitro transcribed RNA, which was purified using an RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, Catalog #74104).
Human T cells were isolated from normal donors by the University of Pennsylvania Human Immunology Core, and expanded by incubation with microbeads coated with CD3 and CD28 stimulatory antibodies (Life Technologies, Grand Island, NY, Catalog #111.32D). When cell growth kinetics and volume suggested cells had rested down from activation they were cryopreserved. Prior to electroporation, cells were thawed, washed three times with Opti-MEM and resuspended in Opti-MEM medium at a final concentration of 1–3×108 cells/mL. T cells were then mixed with transcribed mRNA at a concentration of 10μg mRNA/0.1mL T cells and electroporated in a 2mm cuvette using an ECM830 Electro Square Wave Porator (both from Harvard Apparatus BTX, Holliston, MA, Catalog #450125, #450002). Viability post-transfection ranged from 50 – 80%, and in all cases T cells demonstrated >95% CAR expression.
Production of lentiviral vectors and T cell transduction
High-titer, replication-defective lentiviral vectors were produced using 293T human embryonic kidney cells (26). HEK293T cells were seeded at 107 cells per T150 tissue culture flask 24 hours before transfection. On the day of transfection, cells were treated with 7μg of pMDG.1, 18μg of pRSV.rev, 18μg of pMDLg/p.RRE packaging plasmids and 15μg of transfer plasmid in the presence of either Express-In Transfection Reagent (Open Biosystems, Lafayette, CO) or Lipofectamine 2000 transfection reagent (Life Technologies, Grand Island, NY, Catalog #11668019). Transfer plasmids containing CAR constructs were modified so that expression of the CAR was under control of the EF-1α promoter as previously described (25). Viral supernatants were harvested 24 hours and 48 hours after transfection and concentrated by ultracentrifugation overnight at 10,500xg. 24h after initial stimulation (described above), T cells were exposed to lentiviral vector at a concentration of 5–10 infectious particles per T cell. Cells were then counted and fed every 2 days until cryopreservation.
CAR detection on modified cells
Cells were washed and resuspended in FACS buffer (PBS + 1% bovine serum albumin), then incubated with biotin-labeled polyclonal goat anti-mouse F(ab)2 antibody (Jackson Immunoresearch, West Grove, PA, Catalog #115-066-072) at 4°C for 25 minutes, followed by two washes with FACS buffer. Cells were then incubated with R-phycoerythrin (PE) conjugated streptavidin (BD Biosciences, Franklin Lakes, NJ, Catalog #554061 for 10 minutes at 4°C and washed twice. Flow cytometry acquisition was performed on either a BD FacsCalibur or Accuri C6 Cytometer (BD Biosciences, Franklin Lakes, NJ, Catalog #342975, #653118). Analysis was performed using FlowJo software (Treestar, Inc, Ashland, OR).
Mouse Xenograft studies
6–10 week old NOD-SCID-γc−/− (NSG) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) or bred in house under an approved Institutional Animal Care and Use Committee (IACUC) protocol and maintained in pathogen-free conditions. For flank tumor studies, animals were injected subcutaneously with 2×107 SY5Y human neuroblastoma cells suspended in 0.2mL Matrigel (BD Biosciences, San Jose, CA, Catalog #354234). For disseminated tumor studies, animals were injected via tail vein with 2×106 SY5Y cells in 0.1mL sterile PBS. T cells were injected in 0.1mL sterile PBS at times indicated. Cyclophosphamide (Baxter Healthcare, Deerfield, IL, Catalog #1001995601) was injected I.P. at 60mg/kg 24 hours prior to any T cell administration following the initial infusion. Animals were monitored for signs of disease progression and overt toxicity, such as xenogeneic graft-versus-host disease, as evidenced by >10% loss in body weight, loss of fur, diarrhea, conjunctivitis and disease-related hind limb paralysis.
Measurement of flank tumors
Flank tumor measurements were made bi-weekly using electronic calipers (Fowler-Sylvac, Boston, MA, Catalog #54-200-777). Longest length and width measurements were recorded and tumor volume was calculated according to the formula ((width + length)/2)3)/2. Mice were sacrificed when tumors reached 3cm3, or when tumor burden inhibited animal activity.
Bioluminescent imaging
Disease burdens were monitored over time using a the Xenogen IVIS bioluminescent imaging system, as previously described (19, 27).
Immunohistochemistry
Histology was performed by the Pathology Core Facility at the Children’s Hospital of Philadelphia. Tumors were established and T cells administered as described, and animals were sacrificed on the day indicated. Subcutaneous tumors and tumor-infiltrated livers were excised post-mortem, and preserved in 4% paraformaldehyde. Image analysis was performed using ImageScope software (Aperio, Vista, CA). Staining was performed on a Bond Max automated staining system with the Bond Refine polymer staining kit (both Leica Microsystems, Buffalo Grove, IL). Standard protocol was followed, with the exception of the primary antibody incubation, which was extended to 1 hour at room temperature. Anti-human CD3 antibody (Dako, Carpinteria, CA, Catalog #M7254) was used at 1:50 dilution and antigen retrieval was performed with E1 retrieval solution for 20 minutes. Slides were rinsed and dehydrated through a series of ascending concentrations of ethanol and xylene. Stained slides were then digitally scanned at 20× magnification on an Aperio OS slide scanner (Aperio,Vista, CA).
Cell Line Identity Testing
Parent cell lines were genotyped by short tandem repeat (STR) analysis (28). Cell lines and samples were verified every six months, or after any genetic modification such as chimeric antigen receptor or luciferase transduction to ensure identity.
Statistical considerations
All statistical analysis was performed with Prism 4 (Graphpad Software, La Jolla, CA). For comparison among multiple groups, Kruskal-Wallis analysis was performed with Dunn Multiple Comparison tests to compare individual groups. Survival curves were compared using the log-rank test with Bonferroni correction for multiple comparisons.
Results
mRNA GD2 CAR T cells mediate rapid neuroblastoma cell killing in vivo
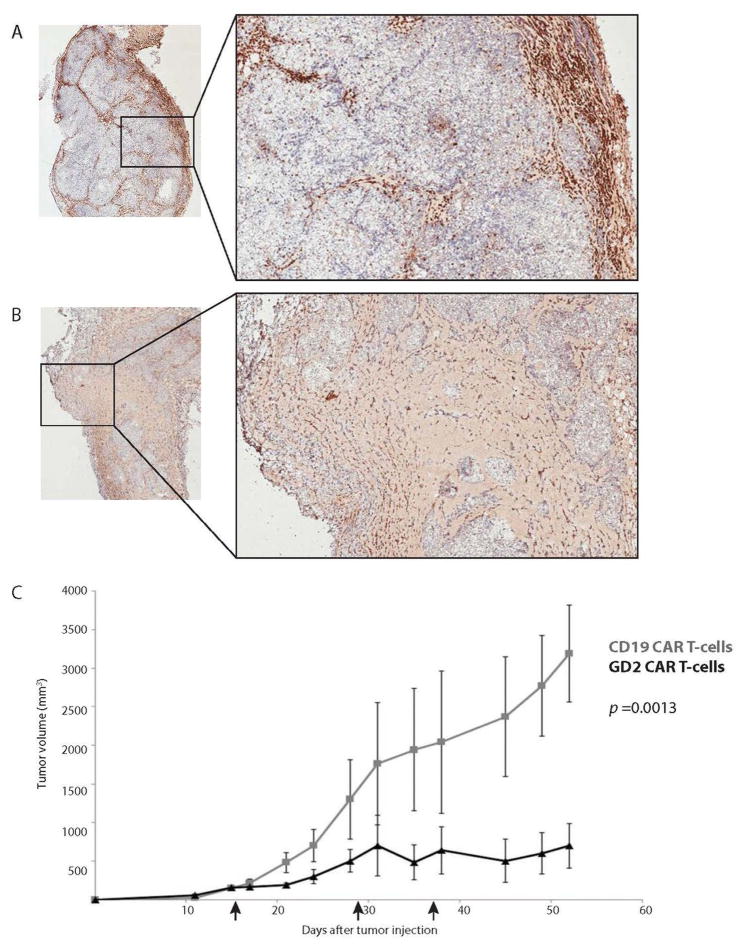
Subcutaneous injection of tumor cells in the mouse flank is a well-established model of solid tumor therapy in mice. To assess the anti-tumor efficacy of our transiently-modified GD2 CAR T cell in a solid tumor model of neuroblastoma, NOD/SCID/γ−/− (NSG) mice were injected subcutaneously with 2×107 SY5Y (a GD2+ human neuroblastoma cell line) cells, and given two weeks to establish large flank tumors. On day 15, 5×106 human T cells electroporated with RNA encoding either a GD2 or CD19 CAR bearing the 4-1BB and CD3ζ signaling domains (GD2-BB/z or CD19-BB/z) were injected into the tumor. On day 20, several mice were sacrificed and tumors were excised and probed for the presence of human T cells by immunohistochemical analysis. Intratumoral injection of RNA GD2 CAR T cells resulted in rapid necrosis of target neuroblastoma, with T cells diffusely present throughout the tumor environment (Figure 1A). RNA CD19 CAR T cells, on the other hand, did not alter the tumor architecture after intratumoral injection, and quickly diffused out of the tumor and localize to the periphery of the tissue (Figure 1B). Mice not sacrificed for histology received two additional intra-tumoral (I.T.) injections of 1.5×107 cells each, and RNA GD2 CAR T cells mediated significant tumor control while RNA CD19 CAR T cells permitted rapid tumor growth (Figure 1C, p = 0.013).
Figure 1. RNA GD2 CART-cells mediate rapid destruction of neuroblastoma cells and control tumor growth.
NSG mice were implanted with 2×107 SY5Y neuroblastoma cells subcutaneously in the flank and given 3 weeks to establish disease. Mice were then injected intra-tumorally with 5×106 (A) RNA CD19 CART-cells or (B) RNA GD2 CART-cells, and tumors were excised 5 days later and immuno-stained for human CD3. RNA CD19 CART-cells do not effect tumor architecture and passively filter out of the tumor to the periphery of the tissue, while RNAGD2 CART-cells mediate rapid destruction of SY5Y cells and disperse throughout the tumor. (Inlay 2× magnification, main image 6× magnification). (C) RNA GD2 CART-cells mediate long-term disease suppression as compared toRNACD19 CART-cells (black arrows represent cell infusions, 5×106 on day 15,1.5×107on days 29 and 36).
Intravenous injection of neuroblastoma cell lines results in a reproducible model of disseminated neuroblastoma
Subcutaneous tumors have many anatomic and physiologic differences when compared to spontaneous intra-abdominal tumors, several of which have direct bearing on the physiology of cellular therapies. Previous studies of CAR T cells in solid tumors, such as epithelial ovarian cancer (29), have employed a peritoneal tumor model in which tumor cells are injected directly into the peritoneum to establish disease along the internal peritoneal wall, mimicking natural disease. We sought to develop a model that would more closely mimic the clinical setting of disseminated neuroblastoma in which CAR T cells are likely to be given. SY5Y neuroblastoma cells were engineered to express click beetle green luciferase (SY5Y-CBG) prior to delivery, which allowed for longitudinal observation of a disseminated tumor burden. Tumor cells were injected intravenously and animals were observed for the establishment and progression of systemic disease. Within 2 hours of cell delivery, bioluminescent signal was observed in the lungs, and then rapidly disseminated (Supplemental Figure 1). As early as day 7, disease was detected in the abdomen, and was subsequently observed in the bone marrow and lymph nodes. Necropsy revealed that this abdominal signal resulted from hepatic infiltration of neuroblastoma, causing diffuse nodular hepatic disease.
Permanently-modified GD2 CAR T cells mediate regression of disseminated neuroblastoma
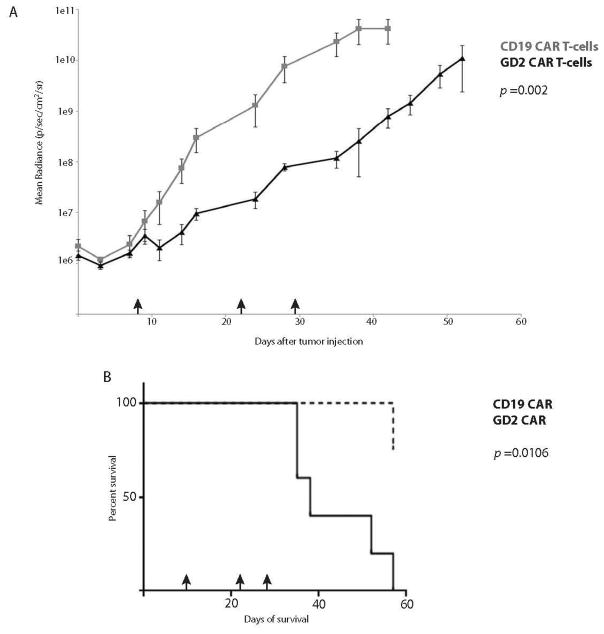
We first tested the efficacy of permanently-modified GD2 CAR T cells in our disseminated model of neuroblastoma. CAR T cells were engineered using a well-established lentiviral vector transduction technique (9, 25). NSG mice were given 2×106 SY5Y-CBG cells via tail vein and given 1–2 weeks to establish a significant disease burden (>106 photons/sec/cm2/sr), after which time CAR T cells were delivered systemically. Animals were imaged twice weekly to monitor disease burden. A single infusion of as few as 106 permanently-modified GD2 CAR T cells was sufficient to suppress and control disseminated neuroblastoma long-term, while tumor progression occurred after systemically-delivered control (CD19) CAR T cells, excluding an allogeneic effect (Figure 2A, p <0.001). Not surprisingly, the disease control observed had a significant impact on overall animal survival (Figure 2B, p <0.001). Interestingly, all mortality in animals treated with GD2 CAR T cells resulted from xenogeneic GVHD, with 75% of GVHD mortality occurring in disease remission and the remaining 25% with minimal disease burdens (<2×107 photons/sec/cm2/sr).
Figure 2. Lentivirally-modified GD2 CAR T-cells control disseminated neuroblastoma long-term and prolong animal survival.
Mice with disseminated neuroblastoma were given a single dose of 1×107 CAR T-cells (day 14, black arrow; CD19 cells 56% CAR+, GD2 cells 10.9% CAR+). (A) One dose of GD2 CAR T-cells mediated sustained disease control long-term. (B) Overall survival of animals treated with either CD19 or GD2 CAR T-cells. (C) Disease-related mortality of animals treated with either CD19 or GD2 CAR T-cells. 5/8 animals treated with GD2 CAR T-cells died in disease remission of xenogeneic graft-versus-host disease and 3/8 died with controlled minimal disease burdens of GVHD.
Multiple infusions of RNA GD2 CAR T cells delay disease progression, but do not eradicate disseminated neuroblastoma
We next used this disseminated model system to assess the efficacy of RNA GD2 CAR T cells against neuroblastoma. Disseminated disease was established as described, and transiently-modified T cells were delivered I.V. Based on our experience with ALL (20), we undertook a multiple infusion strategy using RNA-electroporated T cells. Mice were treated initially with 5×106 cells on day 8, and 1.5×107 on days 22 and 29 after tumor delivery. We observed a transient decline in disease burden after the initial infusion, and modest disease stabilization with the second two infusions (Figure 3A). While this strategy significantly slowed disease progression (p = 0.002), it was unable to control disease long-term. This delayed progression did, however, prolong animal survival (Figure 3B, p = 0.0106).
Figure 3. Multiple infusions of RNA GD2 CART-cells delay disease progression and enhance animal survival.
Mice with disseminated neuroblastoma were given an initial infusion of 5×106 CAR T-cells on day 8, followed by two infusions of 1.5×107 CAR T-cells on days 22 and 29 (black arrows). (A) An initial dose of GD2 CAR T-cells demonstrates a modest reduction in disease burden, and the second two infusions mediated transient disease stabilization, resulting in a cumulative delay in disease progression over time. (B) Three infusions of RNA GD2 CART-cells enhance overall animal survival.
Visual representations of disease burden and response to therapy highlight the variations in anti-tumor activity between transiently and permanently-modified CAR T cells (Figure 4). Animals treated with lentivirally-modified GD2 CAR T cells demonstrate rapid loss of bioluminescent signal with maintained disease suppression long-term. Interestingly, these animals occasionally have focal disease recurrence, which is lost on subsequent evaluation. This likely represents the long-term, anti-tumor T cell memory of permanently-modified CAR T cells. As was demonstrated in the quantitative bioluminesecent graph (Figure 3B), animals treated with GD2 RNA CAR T cells have an initial decline in disease burden after the first cell infusion, followed by transient stabilizations with the subsequent two infusions.
Figure 4. Effect of GD2 CAR T-cells on disseminated neuroblastoma.
(A) An initial infusion of RNA GD2 CAR T-cells reduces disease burden, while the subsequent infusions modestly stabilize disease. (B) Lentiviral GD2 CAR T-cells mediate long-term disease control. On day 28 a transient disease recurrence appears, but is eliminated, highlighting the memory response of permanently-modified CAR T-cells.
Antigen-targeted RNA CAR T cells are unable to penetrate the tumor microenvironment
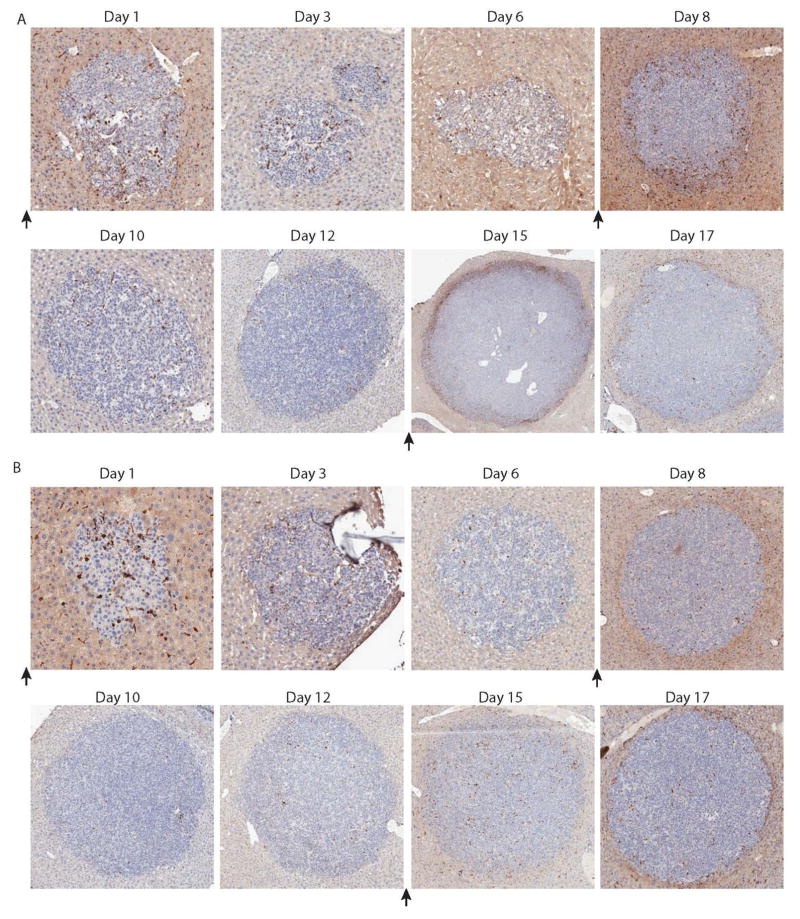
In an effort to better understand the dynamics of the T cell:tumor interaction in our disseminated solid tumor model, we examined tumor sites using immunohistochemical analysis. Animals were given the standard 1–2 weeks to establish disease after I.V. tumor injection, and then given either a single infusion of lentivirally-modified CAR T cells or three infusions of RNA CAR T cells, each separated by one week as in previously described studies. Animals were then sacrificed on the day indicated, and excised livers were sent for histopathological examination and immunohistochemical staining for human CD3. Animals receiving permanently-modified GD2 CAR T cells had rapid infiltration into tumor sites by day 3, and demonstrated a significant T cell presence in all tumor sites by day 5 (Figure 5A). Permanently-modified control CD19 CAR T cells did not demonstrate any significant tumor infiltration (Figure 5B). Alternatively, by day 3 after infusion of the first dose of RNA-modified GD2 CAR T cells there was minimal infiltration into the tumor site, and this T cell presence had largely dissipated by day 6. 24 hours after the second infusion of RNA GD2 CAR T cells (on day 7), there was a minor accumulation of T cells around the periphery of the tumor, which has again largely dissipated by day 10. This pattern is again observed after the third cell infusion, with a peripheral accumulation around the edge of the tumor within 24 hours, which was quickly lost (Figure 6A). As expected, control RNA CD19 CAR T cells did not demonstrate any significant infiltration (Figure 6B).
Figure 5. Permanently-modified GD2 CAR T-cells demonstrate rapid tumor infiltration.
Animals were sacrificed on the days indicated and livers examined histologically after staining for human CD3. Tumor sites boxed in the 1× magnification are shown in the 10× magnification. (A) Within 3 days of T-cell infusion, GD2 CART-cells have infiltrated into tumor sites. By day 5, gross examination reveals significant T-cell infiltration and activity within tumors. (B) CD! 9 CAR T-cells do not penetrate into tumor sites.
Figure 6. RNA GD2 CART-cells localize to, but do not infiltrate, disseminated tumors.
Animals with established disseminated tumors were sacrificed on days indicated and examined histologically after staining for human CD3. Black arrows indicate infusion of 1×107 T-cells. (A) With small tumors, some RNA GD2 CART-cells are able to penetrate and remain within tumor sites in the first 3 days of injection, however subsequent injections demonstrate peripheral localization without infiltration. (B) Initial passive infilatration by CD19 CART-cells is lost by day 3, and subsequent injections do not result in localization to tumor sites.
Discussion
High-risk neuroblastoma remains a significant cause of cancer death in children. While we have seen dramatic improvement in the treatment of both solid and liquid pediatric tumors, survival rates for both have plateaued since the mid-1990s (30). Patients with relapsed neuroblastoma are largely incurable, although temporary control of disease can be achieved for some patients. Thus a new therapeutic paradigm is required to improve survival. Not until recently has engineered T cell therapy begun to meet its potential. Integration of the powerful cytotoxicity, significant in vivo proliferation and surveillance mechanisms of memory T cells with the antigen-specificity endowed by CARs has created a platform for the success of cell therapy. As we have suggested, the recent successes of CAR therapy (8–10) may primarily be attributed to improved cell manufacturing processes that generates T cells armed with rapid and potent in vivo effector and proliferative capacity (Barrett and Singh et al, Cytotherapy, 2014, in press).
Previous reports have demonstrated efficacy of GD2 CAR T cells against disseminated GD2+ melanoma using two infusions of 107 CAR T cells each (31). In the only other study to our knowledge of CAR T cells in a model of systemic solid malignancy, 2×107 PSMA-directed T cells demonstrated efficacy in a disseminated model of prostate cancer (32). In this study, we were able to demonstrate disease eradication in more than half of animals and disease suppression in the rest of the animals treated using a single infusion of only 106 cells, 5% the doses used in the previous studies of CAR T cells in solid tumors. Not only were these cells able to control disease, but we also observed massive cell expansion driven by antigen-specific activation and expansion. Indeed, in some cases the T cell expansion was high enough to cause death of some animals from xenogeneic graft-versus-host disease. T cells that could not recognize tumor (CD19 CAR T cells) did not proliferate to the same degree, as evidenced by the lack of GVHD in these animals. Both of these observations highlight the significant proliferative capacity of our CAR T cells, an attribute endowed by a combination of cell manufacturing processes and CAR signaling constructs. The ability to use fewer cells to achieve clinical efficacy is of particular importance in pediatric patients, who undergo extensive chemotherapy which creates significant lymphodepletion as part of standard treatment for many malignancies. Reducing the number of cells needed for treatment would dramatically enhance the number of patients eligible for this therapy.
GD2 serves as an exciting target in neuroblastoma given its narrow expression profile. However, as is the case with most solid tumor antigens, on-target off-tumor toxicity remains a persistent issue, as GD2 is also expressed on sensory nerves. Clinical experience has demonstrated that antibody therapy directed at GD2 can result in significant neuropathic pain (33, 34). This toxicity is managed clinically during infusion of the antibody, and when the infusion ends the toxicity resolves. Previous clinical studies with first generation GD2/ζ CAR T cells found that of 19 patients followed for more than 5 years, only two had local pain at site of tumor necrosis and one had unexplained local pain (35), with no patient experiencing pain greater than grade 3. While suggestive that the physiology of this toxicity may be distinct when using an antibody versus a cell-based therapy using a single chain Fv without an Fc domain, this remains a potential on-target off-tumor toxicity of highly active GD2 CAR T cells. Our cell production techniques, structure of CAR signaling domains, in vivo expansion and persistence all differ from those described in previous clinical trials (Barrett and Singh et al, Cytotherapy, 2014, in Press). For this reason we have been very interested in an RNA-based approach.
Having demonstrated cure of leukemic mice using multiple infusions of RNA-electroporated CAR T cells in ALL, we sought to assess the efficacy of this engineering platform in treating solid tumors. In the traditional flank tumor xenograft model, our RNA GD2 CAR T cells demonstrated rapid and potent anti-tumor activity when delivered locally. While this demonstrated cytolytic activity against neuroblastoma in vivo, this model is more difficult to translate clinically, as local tumor injections may not be feasible in some patients. Moreover, late-stage patients may have many sites of disease. To more closely mimic systemic disease, we developed a sensitive in vivo bioluminescent model that enabled establishment and monitoring of disseminated disease, primarily manifested in the liver and bone marrow. We found that a single small dose of permanently-modified second generation GD2/4-1BB/ζ CAR T cells could produce long-term disease control, while multiple infusions of RNA GD2 CAR T cells were able to delay disease progression and enhance animal survival, but not control disease long-term.
Based on the fundamental differences in tumor biology, we have hypothesized that the success observed using RNA CAR T cells for liquid tumors (20) might not directly translate to solid tumors. T cells targeting leukemic cells immediately traffic to sites of disease, as disease reservoirs are the natural sites of T cell residence and surveillance. This all but removes the necessity of antigen-driven T cell trafficking and retention. Trafficking in solid tumors is further complicated given the disorganized nature of tumor vasculature, another issue which is averted when targeting liquid tumors. Thus, while creating a proliferative niche and optimal dosing strategy were the primary hurdles in successful targeting of liquid tumors using RNA CAR T cells (19), solid tumors present the additional barriers of trafficking, penetration and efficient antigen engagement within a six day CAR expression period.
Our histologic data support these hypotheses, elucidating the dynamics of T cell and tumor interaction in vivo. Within 72 hours of infusion of lentiviral CAR T cells, there is a large burden of T cells within the tumor site, and by day 5 all tumor sites are heavily infiltrated with CAR T cells. The dynamics of RNA CAR T cell activity are quite different: 72 hours after the first cell infusion a few cells have penetrated into the tumor site, providing a potentially favorable effector:target ratio on day 3, but these cells are lost by day 6. After a second infusion, the T cells localize to site of these expanding tumors, but are unable to penetrate and are again quickly lost.
The third infusion results in similar kinetics of T cell localization to the periphery of enlarging tumor masses. These observations correlate well with our bioluminescent data, which show a transient decline in tumor burden after the first cell infusion, followed by modest disease stabilization, likely reflecting a short-lived anti-tumor response at the periphery after the second and third T cell infusions (Figure 3B). The diminishing returns seen with each additional T cell infusion reflect the fact that each infusion faces the same structural and vascular barriers as the first, in addition to an expanding tumor burden.
The rapid increase in number of permanently-modified GD2 CAR T cells present within the tumor seen over days 1–5 after T cell infusion raises the question of whether this occurs as a result of continued cell recruitment, in situ expansion of recruited cells, or both. If indeed this is continued cell recruitment of circulating CAR T cells to the site of tumor antigen over days 1–5, it would suggest that RNA CAR T cells do not have the same antigen-driven migratory capabilities, as this is within the window of RNA CAR expression. If the increased presence of T cell infiltration is a result of intra-tumoral proliferation in response to antigen, this suggests a difference in proliferative capacity of RNA and permanently-modified CAR T cells. While we cannot formally distinguish between these two possibilities, the latter seems likely to be the dominant mechanism, given the importance of antigen-driven proliferation to CAR T cell efficacy.
The observation that 3 infusions of RNA GD2 CAR T cells were not able to control systemic disease led us to assess the efficacy of repeated infusions. In another iteration of the multiple infusion strategy, we injected a total of 108 RNA CAR T cells over the course of 8 weeks and found that while 9 infusions further slowed disease progression as compared to three infusions, disease continued to progress (data not shown). This, along with the poor tumor infiltration of RNA CAR T cells, suggests that increasing cell dose will not increase efficacy of this therapy, as it did with ALL (20).
In order to translate this therapy, which shows greater efficacy with local delivery, into a clinically-viable treatment modality, solving the problem T cell recruitment and expansion in metastatic tumors is key. One likely option is the prolongation of RNA CAR expression. One such method under study is the biochemical stabilization of transferred RNA molecules (36). This strategy will produce CAR T cells with more sustained receptor expression that retain the self-inactivating qualities of RNA electroporation, prolonging the critical time period of T cell infiltration.
Combination immunotherapies also warrant investigation, and inclusion of supportive cytokines and chemokines presents a promising approach. Preliminary studies suggest that CAR T cells modified to secrete IL-12 are more effective, and perhaps the autocrine support provided by IL-12 or other cytokines may enhance T cell activity within the tumor microenvironment (37, 38). Modification of the tumor vasculature to enhance extravasation of lymphocytes on the first day after delivery would increase the number of cells with high CAR expression engaging tumor antigen.
While the RNA CAR T cell approach will minimize toxicities that occur over days, such as seen with permanently-modified CD19 CARs (10), this may not be the case for adverse events reported in previous clinical trials (12, 13) that occur within hours of cell infusion. We can predict, however, that the toxicities observed would have been self-limited and thus potentially less severe, as antigen-driven cell division would result in restriction and temporally-restricted expression of CAR molecules. Our recent report of cytokine release syndrome in children treated with CD19 CAR T cells (10) is an example of toxicity that may have been self-limited had these T cells been modified with RNA CARs as opposed to permanently-modified with lentiviral CARs. Similarly, long-term B cell aplasia, which we have observed as a necessary complication of CD19 CAR T cell persistence, would not occur using this approach. Another approach to limit CAR T cell persistence is to utilize suicide gene systems that may eliminate 1–3 logs of cells. However, T-cells have robust proliferative capacity and are capable of repopulating from a single cell, and thus a guaranteed “off” system such as RNA electroporation remains an attractive approach.
In conclusion, we demonstrate here the efficacy of permanently-modified GD2 CAR T cells in controlling disseminated neuroblastoma, and that lack of longer-term control of metastatic disease by temporarily-modified GD2 CAR T cells results from reduced tumor infiltration in the setting of large tumor burdens.
Supplementary Material
Acknowledgments
Support: Supported by NIH R01CA120409 (C.H.J. and Y.Z), P01CA066726 (C.H.J. and Y.Z.), and grants from the Pennsylvania Department of Health, Cookies for Kids Cancer, Solving Kids Cancer, the WW Smith Charitable Trust and the Weinberg Funds (S.A.G.)
Footnotes
Author contributions:
N.S., C.H.J., S.A.G., D.M.B. and Y.Z. designed the research, interpreted the data and reviewed the manuscript
N.S., X.L., S.J. and J.H. performed the research
N.S. wrote and all authors revised the manuscript
Conflicts of Interest: C.H.J. and Y.Z. have patent applications in some of the technology described in this manuscript, and S.A.G, C.H.J. and Y.Z. receive research funding from Novartis. All other authors: no conflict of interest.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341(16):1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 4.Mehta J. Graft-versus-leukemia reactions in clinical bone marrow transplantation. Leukemia & lymphoma. 1993;10(6):427–32. doi: 10.3109/10428199309148199. [DOI] [PubMed] [Google Scholar]
- 5.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshhar Z. Tumor-specific T-bodies: towards clinical application. Cancer immunology, immunotherapy : CII. 1997;45(3–4):131–6. doi: 10.1007/s002620050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013 doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heslop HE. Safer CARS. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(4):661–2. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buning H, Uckert W, Cichutek K, Hawkins RE, Abken H. Do CARs need a driver's license? Adoptive cell therapy with chimeric antigen receptor-redirected T cells has caused serious adverse events. Human gene therapy. 2010;21(9):1039–42. doi: 10.1089/hum.2010.131. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151–9. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer research. 2010;70(22):9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaft N, Dorrie J, Muller I, Beck V, Baumann S, Schunder T, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer immunology, immunotherapy : CII. 2006;55(9):1132–41. doi: 10.1007/s00262-005-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011;22(12):1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-Specific Effects of RNA-Modified Chimeric Antigen Receptor T Cells in Mice with Advanced Leukemia. Hum Gene Ther. 2013;24(8):717–27. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kampgen E, et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene therapy. 2009;16(5):596–604. doi: 10.1038/gt.2008.189. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SH, Lee JM, Cho HI, Kim EK, Kim HS, Park MY, et al. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer gene therapy. 2009;16(6):489–97. doi: 10.1038/cgt.2008.98. [DOI] [PubMed] [Google Scholar]
- 23.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171(1):166–74. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 27.Barrett DM, Seif AE, Carpenito C, Teachey DT, Fish JD, June CH, et al. Noninvasive bioluminescent imaging of primary patient acute lymphoblastic leukemia: a strategy for preclinical modeling. Blood. 2011;118(15):e112–7. doi: 10.1182/blood-2011-04-346528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98(14):8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4–1BB) Cancer Res. 2011;71(13):4617–27. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–34. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvon E, Del Vecchio M, Savoldo B, Hoyos V, Dutour A, Anichini A, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009;15(18):5852–60. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gade TP, Hassen W, Santos E, Gunset G, Saudemont A, Gong MC, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65(19):9080–8. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, Kramer K, Modak S, Cheung NK. Successful multifold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: a phase I study. J Clin Oncol. 2011;29(9):1168–74. doi: 10.1200/JCO.2010.28.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–75. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–40. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–41. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780–8. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.