Antibody-secreting plasma cells play critical roles in protective immunity and antibody mediated autoimmune disease. During immune responses a small fraction of newly generated plasma cells enter either the bone marrow (BM) or the lamina propria of the small intestine (siLP) where they appear to survive indefinitely [1-3], thus maintaining antibody titers for extended periods. The factors that influence the generation and survival of plasma cells remain mysterious. Indeed, recent data suggest that the BM in mice and people also harbors considerable numbers of short-lived cells [4, 5], raising questions about how immature plasma cells achieve longevity after they enter the BM. Other recent work has also revealed that plasma cells can secrete immunoregulatory cytokines [6, 7], adding to the motivation to better define plasma cells with these novel functions. Yet the ability to characterize plasma cell subpopulations routinely has been hampered by their relatively paucity in complex immune tissues such as the spleen, siLP, and BM, coupled with the lack of standardized flow cytometric and related methods to identify such cells.
Perhaps the most reliable surface marker for identifying plasma cells in mice is CD138, also known as Syndecan-1. However it should be noted that CD138 is also expressed, albeit at lower surface densities, by many B cell precursors in the BM [8]. A major advance for resolving plasma cells from pre-B cells and other cell types was the generation of reporter mice for the plasma cell-requisite transcription factor Blimp1. Two such lines have been generated. Nutt and colleagues inserted a cDNA encoding GFP into the 3-prime UTR of the Blimp1 locus [9], resulting in C57BL/6 backcrossed B6.Blimp1+/GFP mice. Alternatively Meffre and colleagues generated a bacterial artificial chromosome in which YFP expression is controlled by the Blimp1 locus [10]. Both strategies appear to faithfully mark Blimp1-positive cells. However, because it is not always feasible or practical to breed mice of interest with Blimp1 reporter mice, we sought to establish a flow cytometric protocol for resolving immature and mature plasma cell subsets in standard inbred mice lacking a Blimp1 reporter. Our approach can be applied to any tissue provided that single cell suspensions of viable cells can be generated, but optimal results are seen in BM and spleen preparations. A key additional surface antigen for this approach is Stem cell antigen-1 (Sca-1), also known as Ly6A/E.
In considering how to verify our strategy, we reasoned that effective resolution of bona fide plasma cells would reveal cell populations that were nearly 100% GFP+ in B6.Blimp1+/GFP mice. To begin we stained BM cells from B6.Blimp1+/GFP mice with a cocktail of antibodies suitable for resolving immature B220+ and mature long-lived B220- plasma cells [4, 11]. These included a cocktail of “Dump” channel antibodies designed to exclude non-B-linage cells, antibodies to IgD to identify and exclude naive B cells, CD19 and B220 to resolve immature and mature plasma cells, and the pan plasma cell marker CD138. Regarding the “Dump” channel, because Blimp1 is expressed at low levels by CD4+ and CD8+ T cells [12], it is critical to include antibodies to CD4 and CD8. To further focus on rare plasma cells we also include “Dump” antibodies to the erythroid surface antigen TER-119, and the monocyte marker F4/80. Notably, in our experience BM plasma cells are rather heterogeneous for numerous surface antigens including CD11b and Gr-1, both of which are commonly associated with myeloid lineage cells. Therefore we do not include antibodies to these surface antigens in our “dump” channel.
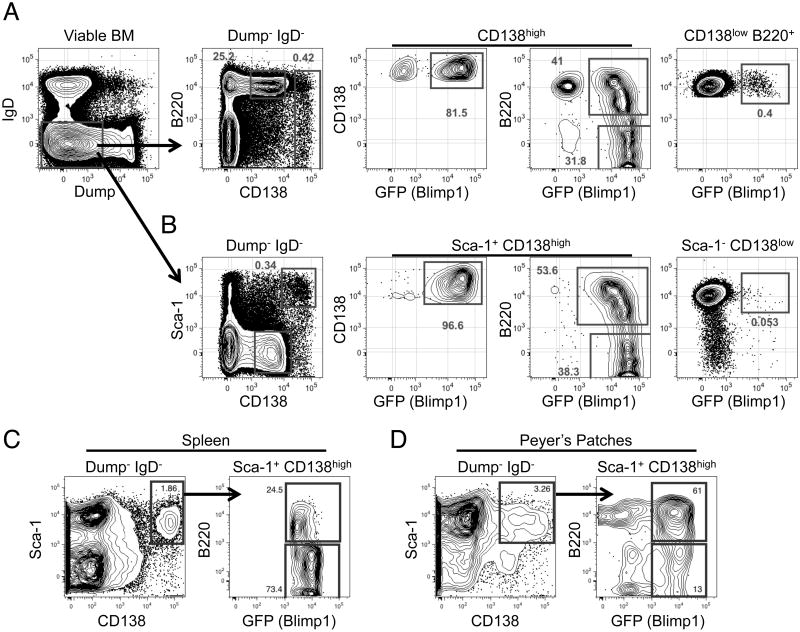
In Figure 1A we illustrate the ability of this basic strategy to resolve CD138high GFP+ cells among total BM cells from an adult B6.Blimp1+/GFP mouse. As shown, by gating on all Dump- IgD-CD138high cells, irrespective of B220+ expression, we achieved only 90% GFP+ cells. Moreover small numbers of GFP+ cells were also observed within a gate focused on CD138low B220+ events. Notably, in our experience these results varied considerably from experiment to experiment, due mainly to small changes in gate placement and slight but important differences in separation between the CD138low and CD138high populations. Consequently we sought additional markers to more effectively resolve all BM plasma cells. After screening a collection of antibodies targeting proteins expressed on a variety of BM cells, we discovered that BM plasma cells are considerably heterogeneous for a variety of markers expressed on B cells in various stages of differentiation. Consequently, staining panels that included antibodies such as AA4.1, CXCR4, BP-1, CD43, CD24, IL-7Rα, CD48, and CD34 did not yield a clear separation of CD138high from CD138low B220+ cells (not shown). By contrast the most promising candidate marker was Sca-1, which we found to be highly expressed by the vast majority of CD138high plasma cells. Indeed, addition of antibodies to Sca-1 to pre-existing panels resulted in nearly 100% Blimp1/GFP+ cells within the Sca-1+ CD138high gate, with few GFP+ cells among CD138low Sca-1- BM cells (Figure 1B). Moreover with this approach we readily resolved both immature B220+ and mature B220- BM plasma cells at the expected 50:50 ratio. When we tested this antibody in tissues outside of the BM we found Sca-1+ CD138high cells in the spleen were 100% Blimp1/GFP+ (Figure 1C). However, the use of Sca-1 to differentiate plasma cells in Peyer's patches is not as ideal, resulting in only 75% Blimp1/GFP+ cells (Figure 1D). It should also be noted, due to decreased Sca-1 staining intensity of Ly6.1 mouse strains such as BALB/c [13], this approach was not effective when applied to BM cells from BALB/c adults (not shown), and thus is only effective in Ly6.2 haplotype strains such as C57BL/6. Nonetheless, in C57BL/6 mice antibodies to Sca-1 improved resolution of immature and mature BM and splenic plasma cells.
Figure 1.
Flow cytometric analysis of mouse BM, splenic, and Peyer's patch plasma cells. (A) BM cells from an adult B6.BlimpGFP/+ were isolated and stained for analysis by flow cytometry. Cells were pre-gated based on size using FSC-A by SSC-A then doublets were excluded by gating on FSC-W by SSC-W. Live cells were analyzed by gating on Zombie Aqua negative cells. Gating on IgD- and Dump- cells enriched for cells that include plasma cells. Plasma cells make up the CD138high B220+/- population and developing B cells are found in the CD138low B220+ population. Both cell populations can be further analyzed for Blimp-GFP expression to confirm plasma cell identity. (B) Cells are pre-gated as in (A) to reveal a CD138high Sca-1+ population. Further analysis of these cells reveals Blimp-GFP expression and both immature B220+ and mature B220- plasma cells. (C) Splenic plasma cells were analyzed by pre-gating as in (A) then examining the CD138high Sca-1+ cells for Blimp-GFP expression. (D) Representative Peyer's patch cells gated as in (C). Flow cytometric graphs shown are representative of >5 independent experiments of 3 or more mice per group.
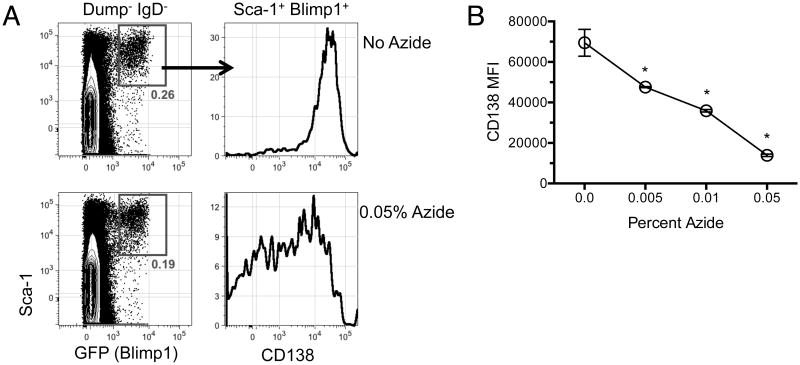
A second parameter concerns the conditions with which single cell suspensions are prepared for analysis. A common practice is to use buffers consisting of PBS with bovine serum albumin or fetal calf serum, often supplemented with sodium azide at a concentration of 0.01-1% for its anti-bacterial and fungal growth properties as well as its ability to decrease membrane internalization. We were surprised to notice that the inclusion of azide in our cell preparation and staining buffers appeared, at first glance, to result in lower frequencies of BM plasma cells (not shown). Further investigation however led us to conclude that azide results in decreased surface staining for CD138 without affecting plasma cell viability, as in these samples frequencies of Blimp1+ Sca-1+ BM cells remained unchanged (Figure 2A). Instead, the inclusion of azide caused a dose-dependent loss in CD138 staining intensity (Figure 2B). We conclude that it is important to use azide-free buffers when evaluating CD138+ plasma cell frequencies.
Figure 2.
Sodium azide decreases the intensity of CD138 staining of Blimp-GFP+ plasma cells. (A) BM cells were prepared in FACS buffer with 0, 0.005, 0.01, or 0.05% sodium azide. Cells were then stained for flow cytometry as in Figure 1 and gated on viable IgD- Dump- cells. Plasma cells were first gated on the Blimp-GFP+ Sca-1+ before examining CD138 staining intensity. Shown are representative plots from cells prepared with 0 versus 0.05% sodium azide. (B) Mean fluorescence intensity of CD138 derived from the Blimp-GFP+ Sca-1+ plasma cell population prepared with the indicated concentration of sodium azide. Results are representative of 3 independent experiments with 3 or more samples per group. Error bars represent the standard error of the mean. *, p<0.001 from control as determined by one way ANOVA with a Bonferroni post-test.
Our results illustrate key facets of approaches for evaluating immature and mature plasma cells in mouse BM by flow cytometry. Moreover, the approach detailed here allows cleaner separation of plasma cells from potentially contaminating cells, most notably CD138low B cell precursors, without necessarily evaluating genetic reporters for Blimp1 or other plasma cell-associated gene products. This approach should facilitate the study of plasma cells, and hence increase our understanding of these critical players in humoral immunity.
Supplementary Material
Acknowledgments
We gratefully thank the UPenn Flow Cytometry and Cell Sorting facility. This work was supported by National Institutes of Health (NIH) grants R01-AI097590 and R01-AI097590 to D. Allman, F32-AI114089 to J. Wilmore, and D. Jones by NIH training grant T32CA009140.
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflicts of interest.
References
- 1.Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol. 2016;9:83–97. doi: 10.1038/mi.2015.38. [DOI] [PubMed] [Google Scholar]
- 2.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 3.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 4.Chernova I, Jones DD, Wilmore JR, Bortnick A, Yucel M, Hershberg U, Allman D. Lasting antibody responses are mediated by a combination of newly formed and established bone marrow plasma cells drawn from clonally distinct precursors. J Immunol. 2014;193:4971–4979. doi: 10.4049/jimmunol.1401264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE. Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity. 2015;43:132–145. doi: 10.1016/j.immuni.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, Martin A, Casellas R, Philpott DJ, Girardin SE, McCoy KD, Macpherson AJ, Paige CJ, Gommerman JL. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tung JW, Mrazek MD, Yang Y, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci U SA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DD, Gaudette BT, Wilmore JR, Chernova I, Bortnick A, Weiss BM, Allman D. mTOR has distinct functions in generating versus sustaining humoral immunity. J Clin Invest. 2016;126:4250–4261. doi: 10.1172/JCI86504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 13.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.