Abstract
FAU, a pyrimidine nucleotide analog, is a prodrug bioactivated by intracellular thymidylate synthase to form FMAU that is incorporated into DNA causing cell death. This study presents a model-based approach to integrating dynamic positron emission tomography (PET) and conventional plasma pharmacokinetic studies to characterize the plasma and tissue pharmacokinetics of FAU and FMAU. Twelve cancer patients were enrolled into a phase I study, where conventional plasma pharmacokinetic evaluation of therapeutic FAU (50 – 1600 mg/m2) and dynamic PET assessment of 18F-FAU were performed. A parent-metabolite population pharmacokinetic model was developed to simultaneously fit PET-derived tissue data and conventional plasma pharmacokinetic data. The developed model enabled separation of PET-derived total tissue concentrations into the parent drug and metabolite components. The model provides quantitative, mechanistic insights into the bioactivation of FAU and retention of FMAU in normal and tumor tissues, and has potential utility to predict tumor responsiveness to FAU treatment.
Keywords: 1-(2'-deoxy-2'-fluoro-β-D-arabinofuranosyl) uracil (FAU), Parent-metabolite population pharmacokinetic model, Positron emission tomography (PET), Thymidylate synthase, Tumor pharmacokinetics
INTRODUCTION
The knowledge of drug exposure and kinetics in tumor and normal tissues can provide a mechanistic understanding of the target modulation and off-target effect and help predicting the efficacy and toxicity of a chemotherapeutic agent. However, conventional pharmacokinetic (PK) studies in early phase clinical trials are usually performed by measuring drug concentrations in the system (plasma) as a function of time after drug administration. While plasma PK is critical to the selection of the most appropriate route of administration and dosing regimen, it reveals little information on tumor and normal tissue PK. Tumor biopsies could be obtained in some cases, but the procedure is invasive and sparse biopsy samples are generally collected, which does not allow characterization of the drug tissue kinetics. Advances in modern imaging techniques have led to a noninvasive approach that can be used to obtain PK data from deep tissues1, 2.
Positron emission tomography (PET) is a noninvasive, quantitative, and highly sensitive dynamic imaging modality that provides an idea tool in evaluating tissue pharmacology of radiolabeled drugs1, 2. Given its high temporal and spatial resolution as well as tomographic imaging with excellent quantitative accuracy, dynamic PET imaging allows measurement of radioactivity – time course of a drug candidate radiolabeled with a positron emitting radionuclide in blood and different tissues. However, one major technical drawback associated with dynamic PET imaging is the short follow-up time, restricted by the radioactive half-life of the radionuclide. For example, in PET studies with 18F-labeled drugs, the 110 min radioactive half-life of 18F restricts the dynamic PET imaging times up to a few hours. Hence, although it may provide important information on the drug distribution and disposition in the early kinetic phase, dynamic PET study has limited value in characterizing late-phase kinetics for drugs with a biological half-life longer than the radioactive half-life of the tracer3. Therefore, combining conventional plasma PK study and dynamic PET assessment is a logical strategy that allows the acquirement of long-term plasma PK data along with tissue kinetic information from the same patients. When a tracer dose (< 100 µg) of radiolabeled drug is co-administered with a therapeutic dose of unlabeled drug, dynamic PET allows accurate determination of radioactivity concentrations in tumor and normal tissues. As the radiolabeled and unlabeled drugs are chemically identical, they share identical tissue distribution and disposition. Thus, tissue concentrations of the unlabeled drug can be derived from the tracer radioactivity concentrations. Nevertheless, PET measures total radioactivity concentrations in the region of interest regardless of the molecules the radiolabel belongs to. This limitation could be overcome by an appropriate PK model that makes it possible to distinguish the parent drug signal from signals of metabolites4.
Here, we present a model-based approach to integrating conventional plasma PK and dynamic PET studies to characterize the plasma and tumor PK of 1-(2'-deoxy-2'-fluoro-β-D-arabinofuranosyl) uracil (FAU), a first-in-class investigational anticancer agent. FAU is a pyrimidine nucleoside that acts as a suicide prodrug. FAU has been shown to be readily transported into cells and converted by intracellular thymidine kinase (TK) to its monophosphate, 1-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) uracil monophosphate that is further methylated by intracellular thymidylate synthase (TS) to form 1-(2-deoxy-2-fluoro- β-D-arabinofuranosyl) 5-methyluracil monophosphate (FMAU)5. Then, the methylated product (FMAU) is incorporated into DNA causing cell death5. Greater DNA incorporation of FAU metabolites in cell lines with high TS activity resulted in increased cytotoxicity compared to cell lines with lower TS activity5. In light of the essential role of TS in DNA biosynthesis, inhibition of this enzyme using chemotherapeutic agents such as 5-fluorouracil (5-FU) and capecitabine (orally bioavailable 5-FU prodrug) is an important antitumor strategy. However, tumors with high TS expression/activity can develop resistance to TS inhibitors6, 7. FAU, taking advantage of high TS activity in the tumor as a mechanism of activation, may provide an effective treatment for patients whose tumors overexpress TS and are resistant to TS inhibitor-based therapy. FAU has been evaluated for the safety, PK, and potential antitumor activity in a phase I clinical trial, where we combined dynamic PET assessment using 18F-FAU as the tracer and conventional plasma PK evaluation of therapeutic FAU to better understand the disposition and bioactivation of FAU in cancer patients.
In this study, we compiled FAU and FMAU plasma concentration data determined by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) in the conventional PK study as well as the total FAU plus FMAU tissue concentration data derived from the dynamic PET assessment. By simultaneously fitting all the data, we developed a parent-metabolite population model that simultaneously characterized the plasma, normal and tumor tissue PK of the parent drug (FAU) and its active metabolite (FMAU).
METHODS
Patients and Treatment
The protocol was approved by the Wayne State University Institutional Review Board (IRB #: 090807M1F). A written informed consent was obtained from each patient. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation or with the Helsinki Declaration of 1975 (as revised in 1983). Twelve patients with histologically or cytologically confirmed diagnosis of solid tumors that are metastatic or refractory to standard treatment were enrolled in the phase I study of FAU (NCI study #7916). Additional eligibility criteria included age above 18 years, an Eastern Cooperative Oncology Group performance status of 2 or less, adequate marrow function (leukocyte count ≥ 3×109/L, absolute neutrophil count ≥ 1.5×109/L, platelet count ≥ 100×109/L), adequate liver and kidney functions (total bilirubin ≤ 2 mg/dL, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 times the institution upper limit of normal, calculated creatinine clearance ≥ 60 mL/min/1.73 m2), and no chemotherapy within 4 weeks prior to the enrollment.
FAU was provided by the National Cancer Institute Division of Cancer Treatment and Diagnosis (Bethesda, MD) as injection solution (25 mg/mL FAU in 0.45% sodium chloride solution, USP). The first two patients (001 and 002) were treated with FAU at the dose of 800 and 1600 mg/m2/day, respectively, as a 1-hour intravenous infusion daily for 5 consecutive days of a 28-day cycle. These two patients experienced Grade 3 neuropathy. Because of the toxicity and based on the preliminary PK data, the FAU dosing regimen was adjusted for the rest of patients. Patients 003 – 012 were treated with FAU at 50 or 100 mg/m2 administered as a 1-hour intravenous infusion weekly for 4 weeks (on day 1, 8, 15, and 22) of a 28-day cycle.
Conventional Plasma PK Study
Plasma PK of FAU was evaluated in all 12 patients. Serial blood sampling was obtained at pre-infusion, 1, 1.25, 1.5, 2, 3, 5, 8 h, and 24 h after the start of infusion on day 1 (for all patients) and day 5 (for Patient 001 and 002 receiving FAU 800 or 1600 mg/m2 daily for 5 days) or day 22 (for Patients 003 – 012 receiving FAU 50 or 100 mg/m2 weekly for 4 weeks). Blood samples were collected in EDTA tubes, placed on ice, and processed within 1 hour of collection. Plasma was separated by centrifugation at 1500 g at 4°C for 10 minutes, and stored at −80 °C until analysis. The plasma concentrations of FAU and FMAU were determined using a validated liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method8.
PET Study
PET procedure
PET study was performed using 18F-FAU as the tracer. 18F-FAU was synthesized using 2-O-[(trifluoromethyl)sulfonyl]-1,3,5-tri-O-benzoyl-α-D-ribofuranose as the substrate for the initial kryptofix[2.2.2]-facilitated nucleophilic fluorination with fluoride-18, as described previously9. Radiopharmaceuticals were quality checked to ensure sterility and apyrogenicity. The chemical and radiochemical purity was > 95% with specific activity > 2.8 × 1013 Bq/mol.
Two PET scans were performed in each individual patient. The first PET scan was performed within 14 days before the treatment with therapeutic FAU, and the second was performed at a median time of 1.5 h after the intravenous infusion of FAU (50 – 1600 mg/m2) on Day 1 of Cycle 1. For each scan, a microdose of 18F-FAU was administered intravenously through a peripheral venous cannula over ~ 1 min, followed by dynamic PET scanning for 1 h and serial venous blood sampling. Dynamic PET scans were performed on a whole body PET scanner ECAT EXACT/HR (Siemens, Knoxville, TN). The emission scans were measured dynamically for a total acquisition time of 1 h. The measured sonograms were corrected for attenuation, detector efficiency (scatter, random, dead time), and detector nonuniformity, and reconstructed into tomographic images. PET imaging data were then calibrated to microcurie per milliliter (µCi/mL). Regions of interest (ROI) representing the liver and tumor were manually defined on the PET images with the aid of computed tomography films. The radioactivity per unit volume (µCi/mL) for each ROI was derived for each time frame to obtain radioactivity versus time curves. Throughout the scans, serial venous blood samples were collected from an intravenous catheter that was different from the one used for the tracer injection. Plasma was separated from blood samples by centrifugation (1500 g, 4°C, 10 minutes). The plasma radioactivity associated with the unchanged 18F-FAU and its potential metabolites were separated using a high-performance liquid chromatography (HPLC) method, and radioactivity concentrations (µCi/mL) were determined by a Packard Cobra II Auto-Gamma Counter Model D5005, as described previously10.
PET Data for PK Modeling
Given the fact that the radiolabeled and unlabeled drugs are chemically identical, the plasma/tissue concentrations of the unlabeled drug can be calculated from the tracer radioactivity concentrations when the tracer is administered concomitantly with a therapeutic dose of the unlabeled drug, using Eq. 111.
| (1) |
where the standardized uptake value (SUV) is defined as plasma/tissue radioactivity concentration (µCi/mL) at a particular time point decay corrected to the time of injection and normalized to the injected radioactivity dose per body weight (µCi/g).
Since PET measured the total concentration of 18F (consisting of 18F-FAU and 18F-FMAU) in a region of interest, the total tissue concentrations of FAU plus FMAU in the liver and tumor following concomitant intravenous injection of the therapeutic FAU (50, 100, 800, or 1600 mg/m2) and tracer 18F-FAU in individual patients could be constructed based on PET-measured radioactivity – time curves, using Eq. 1. Pooled data including LC-MS/MS-determined FAU and FMAU plasma concentrations following 1-h intravenous infusion of FAU (at 50, 100, 800, and 1600 mg/m2), PET-derived FAU plasma concentrations after intravenous injection of FAU (at 50, 100, 800, and 1600 mg/m2), as well as PET-derived total FAU plus FMAU liver and tumor concentrations after intravenous injection of FAU (at 50, 100, 800, and 1600 mg/m2), were simultaneously fitted to a parent-metabolite population PK model, as described in the next section.
Population PK Modeling and Simulation
Model structure
Both two- and three-compartment models with first-order elimination from the central compartment were tested to fit the plasma concentration – time profiles of FAU and 18F-FAU. Based on the model selection criteria as described below, three- and two-compartment models were selected for FAU and 18F-FAU, respectively, as their final models. For the comparison of PK profiles between FAU and 18F-FAU, the models were parameterized in both micro-constants (e.g., CL and V) and macro-constants (e.g., A, B, C, α, β, γ).
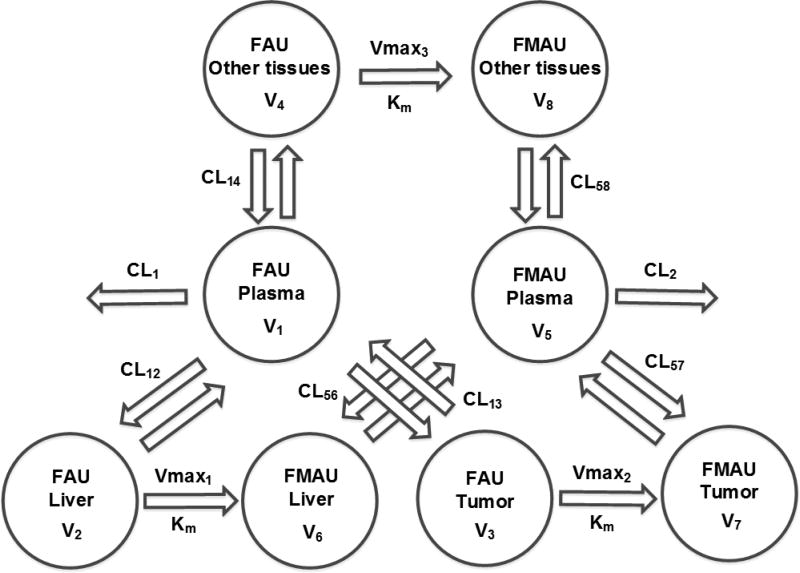
Several parent-metabolite models were tested to simultaneously describe FAU and FMAU plasma and tissue PK profiles. The initial model was a three-compartment model where the disposition of FAU and FMAU occurred in 3 compartments representing the plasma, liver, and tumor, and the conversion of FAU to FMAU was characterized by first-order kinetics. Then the complexity of the initial model was increased stepwise by replacing first-order kinetics with Michaelis-Menten kinetics to characterize the conversion of FAU to FMAU and by adding another compartment to describe the disposition of FAU and FMAU in other peripheral tissues. Based on the selection criteria outlined below, a complex parent-metabolite compartment model (as shown in Figure 2) was selected as the final model, where the disposition of FAU and FMAU occurred in 4 compartments representing the plasma, liver, tumor, and other peripheral tissues, and the conversion of FAU to FMAU was characterized by Michaelis-Menten kinetics (Eq. 2) in tissue compartments (including tumor, liver and other peripheral tissues).
| (2) |
where R is the metabolic conversion rate; Vmax is the maximum conversion rate; Km is the substrate (FAU) concentration at which half-maximum conversion rate is achieved; and C represents the substrate (FAU) concentration. It is assumed that the metabolism of FAU to FMAU in different tissues exhibits different Vmax (representing different enzyme abundance) but a same Km value (representing same enzyme affinity to FAU). As TK and TS are mainly expressed in tissues (tumor and normal tissues) but negligibly in blood, the conversion of FAU to FMAU is assumed to occur in tissue compartments only.
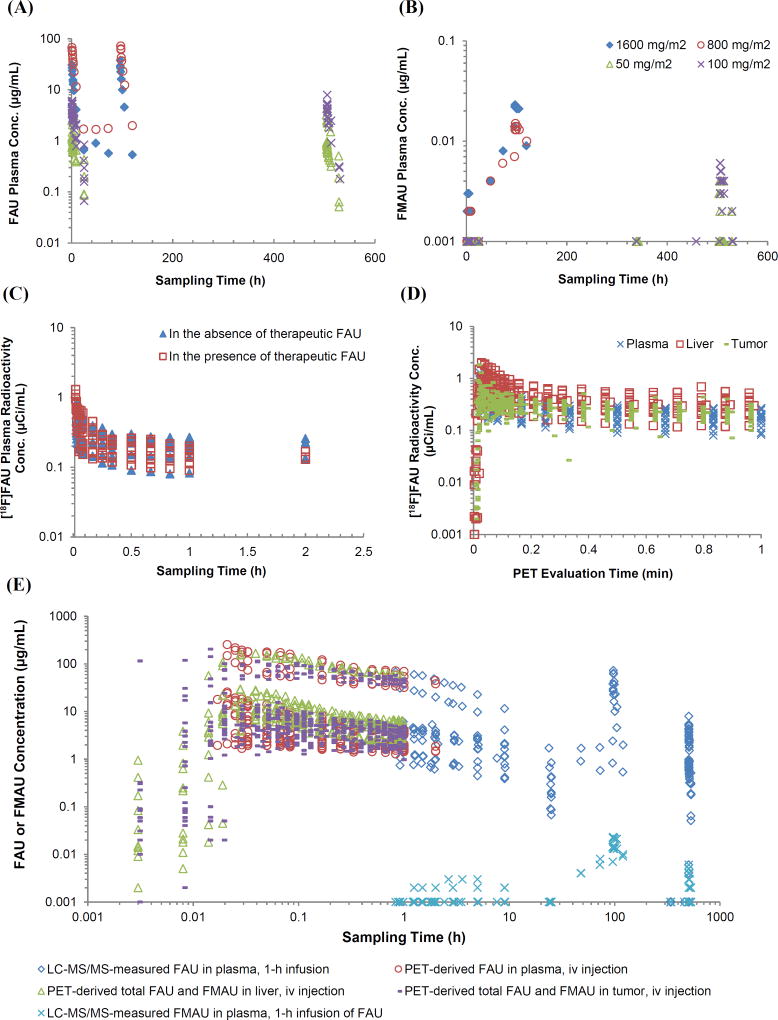
Figure 2.
Observed concentration data obtained from conventional pharmacokinetic study and dynamic PET assessment. (A and B) Observed multiple-dose plasma concentration – time profiles of FAU and FMAU in individual patients treated with a 1-h intravenous infusion of FAU daily (800 or 1600 mg/m2) for 5 days or weekly (50 or 100 mg/m2) for 4 weekly. (C) Observed plasma radioactivity concentration – time profiles of 18F-FAU in individual patients receiving an intravenous injection of the tracer prior to and during intravenous infusion of the therapeutic FAU. (D) Observed plasma radioactivity concentration – time profiles of 18F-FAU as well as radioactivity concentration – time profiles of 18F (including 18F-FAU and 18F-FMAU) in the liver and tumor in individual patients receiving an intravenous injection of the tracer prior to and during intravenous infusion of the therapeutic FAU. (E) Pooled data acquired from conventional pharmacokinetic study and dynamic PET assessment, including 1) plasma concentrations of FAU and FMAU following 1-h intravenous infusion of FAU (at 50, 100, 800, and 1600 mg/m2) determined by LC-MS/MS; and 2) plasma concentrations of FAU as well as liver and tumor concentrations of total FAU plus FMAU after intravenous injection of FAU (at 50, 100, 800, and 1600 mg/m2) calculated based on the SUVs from dynamic PET study.
Parameter estimation
Population PK analyses were performed using the nonlinear mixed effects (population) modeling approach with the MONOLIX software Version 4.3.2 (http://software.monolix.org). The R software version 3.1.2 (www.R-project.org) was used for graphical visualization and diagnosis. All concentration data were log-transformed in the data file. Population PK parameters, variability, and residual error were assessed in the model. The variability (including interindividual and inter-occasional variability) on individual PK parameter was modeled as an exponential function. Notably, the interindividual and inter-occasional variabilities couldn’t be differentiated due to the relatively small sample size and the complexity of the model. Thus, the estimated variability represents the combined interindividual and inter-occasional variability. Residual error was modeled with a combination method, including an additive and a proportional error component. Parameter estimation was performed by using the Stochastic Approximation of the Expectation Maximization (SAEM) algorithm in combination with the Markov Chain Monte Carlo (MCMC) method, as implemented in MONOLIX, where parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model and standard error for the maximum likelihood estimator. Individual PK parameters were obtained by posterior Bayesian estimation. Below the lower limit of quantitation (BLQ) data were considered as left censored data and were taken into account in the MONOLIX estimation using the left censored lower limit of quantitation. The MONOLIX structure model code for the final parent-metabolite model is presented in Supplementary Appendix 1.
Model selection
Model selection aims at identifying a model that best fits the observed data with the smallest possible dimension. Model selection was guided by a number of model evaluation criteria, including Akaike Information Criterion (AIC), objective function value (OFV; −2log likelihood), precision of estimates (relative standard error of the estimate), and goodness-of-fit plots. The likelihood ratio test was used to compare between hierarchical models. Assuming that the OFV is χ2 distributed, a decrease in OFV of 3.84 between hierarchical models with one parameter differing is considered as statistically significant difference (P < 0.05). AIC was used to compare between non-hierarchical models. AIC is calculated as (−2LL) + 2 × p, where −2LL is the OFV and p is the number of parameters. Candidate models with lower AIC were selected over those with higher AIC. In addition, the following criteria were also considered when selecting a model: 1) better precision of estimates (reduction of the relative standard errors of estimates); 2) minimization of interindividual variability; and 3) no prediction bias as visualized from goodness-of-fit plots.
Model evaluation
The final parent-metabolite model was further assessed using a visual predictive check12, where a parametric bootstrap resampling was used to simulate 1,000 replicates of the observed dataset based on the original study design and final parameter estimates. In addition, identifiability of the model parameters was assessed using a previously reported method13. The diagnostic procedure (i.e., convergence assessment) available in MONOLIX was used. First, 20 intervals of individual parameters were constructed based on the final parameter estimates. Specifically, the lower and upper bounds of the intervals were set as multiplying the final estimate by 0.6 and 1.65, respectively, which are the same as subtracting and adding 0.5 to the final estimate in log-scale. Then, 50 runs were performed by using the set of initial values randomly selected from the 20 intervals. If the 95% confidence intervals of a parameter estimate from 50 runs are overlapped with the 95% confidence intervals of the final parameter, this parameter was considered as statistically identifiable.
Simulations
To illustrate the potential utility of the model for selecting optimal dosing regimen, FAU and FMAU concentration – time profiles in the plasma, tumor and normal tissues were simulated in a typical individual (with population PK parameters) following 1-h intravenous infusion of FAU (1600 mg/m2) with 4 different treatment schedules (i.e., daily for 5 days of a 28-day cycle, 3 day on/4 day off for 4 weeks, weekly for 4 weeks, and biweekly for 4 weeks). In addition, to demonstrate the potential utility of the model for predicting the clinical response to FAU, the plasma and tumor concentration – time profiles of FAU and FMAU were simulated in a typical individual whose tumor has varying TS catalytic activity, i.e., Vmax2/Km = 1.6 (population median value), 0.16, or 16 h−1, following daily FAU treatment (1600 mg/m2) for 5 days of a 28-day cycle.
RESULTS
Comparison of Plasma PK between therapeutic FAU and tracer 18F-FAU
Multiple-dose plasma concentration – time profiles of FAU following 1-h intravenous infusion daily for 5 days (at the dose of 800 or 1600 mg/m2) or weekly for 4 weeks (at the dose of 50 or 100 mg/m2) (Figure 2A) were well described by a linear three-compartment model, which was characterized by a fast and slow disposition phase (with a half-life of 0.9 and 6.9 h, respectively) and a terminal elimination phase (with a half-life of 7.7 h). Goodness-of-fit plots are shown in Supplementary Figure S-1A. Population plasma PK parameters of FAU estimated from the models parameterized in micro or macro constants are summarized in Table 1.
Table 1.
Plasma pharmacokinetic parameters of therapeutic FAU and tracer 18F-FAU in cancer patients
| FAUa | 18F-FAUb | |||
|---|---|---|---|---|
|
|
||||
| Parameter | Population estimate (%RSE) |
IIV (%) (%RSE) |
Population estimate (%RSE) |
IIV (%) (%RSE) |
| Macro Model | ||||
| α (h−1) | 0.81 (18) | 17 (42) | 24.07 (−) | 72 (14) |
| β (h−1) | 0.10 (1) | 33 (9) | 0.13 (5) | 108 (28) |
| γ (h−1) | 0.09 (54) | - | - | - |
| A (mg/L) | 0.008 (0.2) | 95 (25) | 0.07 (1) | 42 (12) |
| B (mg/L) | 0.01 (0.2) | 38 (9) | 0.02 (0) | 24 (5) |
| C (mg/L) | 0.0002 (0.02) | 111 (87) | - | - |
| Additive residual error | 0.18 (1) | 17 (42) | 0.12 (1) | |
| OFV | 276.1 | −613.7 | ||
| Micro Model | ||||
| V1 (L) | 9.3 (41) | 139.1 (21) | 10.3 (4) | 9.9 (39) |
| V2 (L) | 28.8 (6) | 13.0 (39) | 30.5 (8) | 31.5 (20) |
| V3 (L) | 20.5 (53) | 167.5 (23) | - | - |
| CL12 (L/h) | 223.4 (12) | 13.4 (82) | 173.1 (20) | 76.0 (19) |
| CL13 (L/h) | 5.0 (8) | 6.6 (219) | - | - |
| CL (L/h) | 5.6 (17) | 56.0 (21) | 5.8 (22) | 75.1 (23) |
| K12c (h−1) | 24.0 | 16.8 | ||
| K21c (h−1) | 7.8 | 5.7 | ||
| K13c (h−1) | 0.54 | - | ||
| K31c (h−1) | 0.24 | - | ||
| K10c (h−1) | 0.60 | 0.56 | ||
| Additive residual errord | 0.24 (7) | 0.20 (11) | ||
| Proportional residual errore | 0.05 (10) | 0.06 (22) | ||
| OFV | 9.3 | −112.3 | ||
Parameters of FAU were estimated by simultaneously fitting FAU multiple-dose plasma concentration – time profiles from 12 patients receiving 1-h intravenous infusion of FAU daily (800 or 1600 mg/m2) or weekly (50 or 100 mg/m2) to a three-compartment linear model.
Parameters of 18F-FAU were estimated by simultaneously fitting 18F-FAU plasma radioactivity concentration – time profiles from 12 patients receiving an intravenous injection of a tracer dose prior to and during FAU infusion to a two-compartment linear model.
Calculated from population estimates of CL and V, using equations: K12 = CL12/V1; K21 = CL12/V2; K13 = CL13/V1; K31 = CL13/V3; K10 = CL/V1
Additive component (a) in the residue error model: y = f + (a + b · f) · e.
Proportional component (b) in the residue error model: y = f + (a + b · f) · e
Abbreviations: V1, volume of distribution in the central compartment; V2 and V3, volume of distribution in the peripheral compartments; CL12 and CL13, inter-compartment clearance; CL, systemic clearance; K12 and K21, distribution rate constant from V1 to V2 and from V2 to V1, respectively; K13 and K31, distribution rate constant from V1 to V3 and from V3 to V1, respectively; K10, elimination rate constant from the central compartment; IIV, inter-individual variability; OFV, objective function value (−2 times log transformed likelihood); RSE, relative standard error of estimation.
Single-dose plasma radioactivity concentration – time profiles of 18F-FAU in individual patients following an intravenous injection of the tracer 18F-FAU concomitantly with the administration of therapeutic FAU were not significantly different from those following the injection of the tracer alone (Figure 2C), suggesting that the presence of therapeutic concentrations of FAU in the body did not influence the distribution and disposition of the tracer. These data confirmed the linear (dose-independent) PK of FAU, at least at the tested dose levels (50 – 1600 mg/m2). The plasma radioactivity – time profiles of 18F-FAU from all patients were adequately described by a linear two-compartment model, characterized by a rapid distribution and slow disposition phase (with a half-life of 0.03 and 5.3 h, respectively). Goodness-of-fit plots are shown in Supplementary Figure S-1B. Population PK parameters of 18F-FAU are summarized in Table 1.
The comparison of PK parameters between FAU and 18F-FAU revealed that the tracer kinetics well mirrored the early-phase kinetic profile of the therapeutic FAU, as indicated by an excellent concordance between FAU and 18F-FAU parameters (i.e., V1, V2, CL12, CL, K12, K21 and K10) that characterized the drug distribution and disposition in the central compartment (V1) and rapidly equilibrating compartment (V2) (Table 1). However, limited by the short radioactive half-life of 18F (110 min), the tracer 18F-FAU could not capture the distribution and disposition kinetics of FAU in the slowly equilibrating compartment (V3) and during the terminal elimination phase.
Parent-metabolite PK model simultaneously describing plasma and tissue PK of FAU and FMAU
The plasma levels of FMAU were approximately 3 orders of magnitude lower than those of FAU in individual patients following 1-h intravenous infusion of FAU (Figure 2A and 2B). Interestingly, FMAU was accumulated in the system (plasma) after either daily or weekly FAU treatment (Figure 2B). For example, in patients who received daily FAU infusion (800 or 1600 mg/m2) for 5 consecutive days, FMAU systemic exposure (i.e., Cmax, and AUC) increased by ~10 fold on day 5 compared to that on day 1, and FMAU trough plasma levels consistently increased from treatment days 2 to 5 (Figure 2B). The accumulation of FMAU in the system was also noted in the patients receiving weekly FAU infusion for 4 weeks at lower dose levels (50 and 100 mg/m2), as indicated by the observation that FMAU plasma concentrations were below the lower limit of quantitation (< 0.001 µg/mL) on day 1, while becoming measurable on day 22 (Figure 2B).
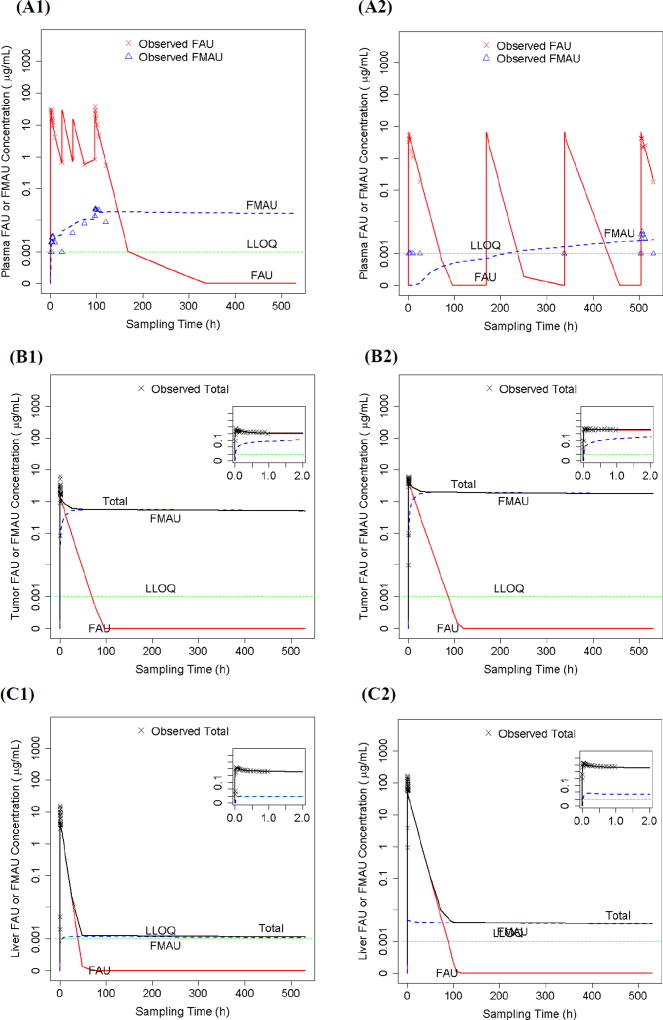
A parent-metabolite population PK model (Figure 1) was developed to simultaneously fit LC-MS/MS-determined FAU and FMAU plasma concentrations as well as PET-derived total FAU plus FMAU liver and tumor tissue concentrations (Figure 2E). The model adequately described the observed data, as suggested by goodness-of-fit plots (Supplementary Figure S-2) and visual predictive check (Supplementary Figure S-3). Model-predicted and LC-MS/MS-determined FAU and FMAU plasma concentration – time profiles in two representative individual patients receiving 5-day daily and 4-week weekly FAU treatment are shown in Figure 3A1 and 3A2, respectively. The model-predicted and PET-derived total FAU plus FMAU concentration – time profiles in the tumor and liver following a single-dose intravenous injection of FAU in these two patients are shown in Figures 3B1/2 and 3C1/2, respectively. Notably, the parent-metabolite model enabled the PET-derived total FAU plus FMAU tissue concentrations to be distinguished into the parent drug (FAU) and metabolite (FMAU) components (Figures 3B1/2 and 3C1/2). Thus, the model not only well described the observed FMAU accumulation in plasma (Figures 3A1 and 3A2), but also predicted accumulation of FMAU in the tumor (Figures 3B1 and 3B2), following chronic daily or weekly FAU treatment.
Figure 1.
Schematic illustration of the parent–metabolite pharmacokinetic model that simultaneously characterizes the plasma and tissue pharmacokinetics of FAU and its active metabolite FMAU.
Figure 3.
(A1 and A2) LC-MS/MS-determined and parent-metabolite model-predicted plasma concentration – time profiles of FAU and its metabolite FMAU in two representative patients treated with 1-h intravenous infusion of FAU either daily (1600 mg/m2) for 5 days of a 28-day cycle (A1) or weekly (100 mg/m2) for 4 weeks of a 28-day cycle (A2). (B1 and B2) PET-derived and parent – metabolite model-predicted tumor concentration – time profiles of FAU/FMAU in the same two representative patients receiving a single intravenous injection of FAU at the dose of 1600 mg/m2 (B1) or 100 mg/m2 (B2). (C1 and C2) PET-derived and parent-metabolite model-predicted liver concentration – time profiles of FAU/FMAU in the same two representative patients receiving a single intravenous injection of FAU at the dose of 1600 mg/m2 (C1) or 100 mg/m2 (C2).
Typical population PK parameters estimated from the parent-metabolite model are summarized in Table 2. Following intravenous administration, FAU rapidly distributed from the systemic circulation (Plasma; V1) to the liver (V2) and other peripheral tissues (V4) (at a distribution rate constant of 28.6 and 50.7 h−1, respectively), while relatively slowly distributing to the tumor (V3) (at a distribution rate constant of 12.0 h−1). FAU underwent metabolic conversion to FMAU in the tumor, liver, and other peripheral tissues, with an intrinsic clearance (Vmax/Km) of 1.6, 2.5×10−4, and 8.4×10−4 L/h, respectively (Table 2), suggesting that the tumor exhibited a significantly higher catalytic activity (Vmax/Km) for conversion of FAU to FMAU compared to normal tissues. Once it was formed, FMAU was released from the tumor, liver, and other peripheral tissues to the systemic circulation (plasma), at a rate constant of 3.6×10−4, 11.5, and 2.5×10−3 h−1, respectively (Table 2). Concurrently, FMAU was eliminated from the plasma at an elimination rate constant of 7.6 h−1.
Table 2.
Population pharmacokinetic parameters of FAU and FMAU, estimated from the parent-metabolite pharmacokinetic modela
| Population estimate (%RSE) |
Variability (%)b (%RSE) | |
|---|---|---|
| FAU | ||
| V1 (L) | 1.1 (45) | 300.0 (10) |
| V2 (L) | 2.7 (5) | 1.9 (665) |
| V3 (L) | 1.3 (37) | 209.0 (14) |
| V4 (L) | 26.6 (7) | 29.9 (20) |
| CL12 (L/h) | 31.5 (25) | 119.0 (16) |
| CL13 (L/h) | 13.2 (31) | 163.0 (16) |
| CL14 (L/h) | 55.8 (19) | 95.0 (15) |
| CL1 (L/h) | 3.8 (15) | 63.1 (18) |
| FMAU | ||
| V5 (L) | 1.6 (35) | 142.0 (19) |
| V6 (L) | 0.2 (39) | 77.2 (49) |
| V7 (L) | 27.6 (22) | 29.6 (81) |
| V8 (L) | 40.2 (100) | 102.0 (136) |
| CL56 (L/h) | 2.3 (22) | 37.0 (48) |
| CL57 (L/h) | 0.01 (25) | 39.5 (67) |
| CL58 (L/h) | 0.1 (65) | 70.9 (133) |
| CL2 (L/h) | 12.2 (24) | 23.5 (151) |
| Conversion of FAU to FMAU | ||
| Km (mg/L) | 119 (18) | 20.3 (149) |
| Vmax1 (mg/h) | 0.03 (29) | 36.3 (62) |
| Vmax2 (mg/h) | 191 (27) | 115.0 (−) |
| Vmax3 (mg/h) | 0.1 (70) | 151.0 (42) |
| Residual Errorsc | ||
| Additive (plasma) | 0.02 (33) | |
| Proportional (plasma) | 0.1 (5) | |
| Additive (liver) | 0.4 (6) | |
| Proportional (liver) | 0.3 (4) | |
| Additive (tumor) | 0.2 (33) | |
| Proportional (tumor) | 0.3 (8) | |
| Proportional (metabolite) | 0.5 (−) | |
| OFV | 4770.4 |
Parameters were estimated by simultaneously fitting LC-MS/MS-determined FAU and FMAU plasma concentrations as well as PET-derived liver and tumor concentrations of FAU plus FMAU in 12 patients.
Combined inter-individual variability and inter-occasion variability (i.e., variability between LC-MS/MS determined data and PET-derived data).
Residue error model: y = f + (a + b · f) · e, where f is the parametric function of the pharmacokinetic compartment model, a is an additive component, b is a proportional component, and e is the residual error.
Abbreviations: V, volume of distribution; CL12, CL13, CL14, CL56, CL57, and CL58, inter-compartment clearance; CL1 and CL2, systemic clearance of FAU and FMAU, respectively; OFV, objective function value (−2 times log transformed likelihood); RSE, relative standard error of estimation.
Model identifiability
There are two types of identifiability in nonlinear models in general, including mathematical identifiability and statistical identifiability. Mathematical identifiability also referred to as structural or deterministic identifiability is the identifiability of model parameters from noise-free data. Statistical identifiability, also termed as numerical identifiability, is the identifiability of parameters estimated from noise data14–16. The mathematical identifiability mostly is not a challenge in PK modeling because a PK model is developed by the use of the ordinary differential equations that have solid theoretical and mathematical foundations. The major challenge in identifiability of PK modeling predominantly occurs from the statistical identifiability because of the paucity of samples and high residual variability. Several methods have been published to diagnose the statistical identifiability of PK modeling13–19. In these methods, numerous runs with different initial values are performed, and then convergence to different solutions can indicate a lack of identifiability13–19. Using this approach, the statistical identifiability of the parent-metabolite model was assessed. As shown in Supplementary Figure S-4, the 95% confidence intervals of each individual parameter estimated from 50 runs with different initial values were overlapped with the 95% confidence intervals of the final parameter, indicating the identifiability of the model parameters.
It should be noted that the diagnostic method used here does not guarantee that all parameter estimates have unique solutions in the entire domain (i.e., globally identifiable), but it ensures that parameter estimates have unique solutions in the neighborhood of the final estimates (i.e., locally identifiable). Thus, there may be a possibility that some parameter estimates had a finite number of alternate solutions in the entire domain. Very large standard errors of estimation may indicate some issues in the parameterization. Given the sparse observed FMAU concentration data, it was not surprising that several PK parameters for describing FMAU kinetics (i.e., V8, CL58, and Vmax3) exhibited a relatively large standard error of estimation (> 50%) (Table 2). These parameters may not be reliably estimated. Nevertheless, it has been well recognized that a trade-off between model complexity and parameter identifiability may be needed based on available clinical data13. Despite the limitation noted here, the developed parent-metabolite model provided reasonable, clinical meaningful parameter estimates to describe the plasma and tissue PK profiles of FAU and FMAU.
Predictions from the parent-metabolite model
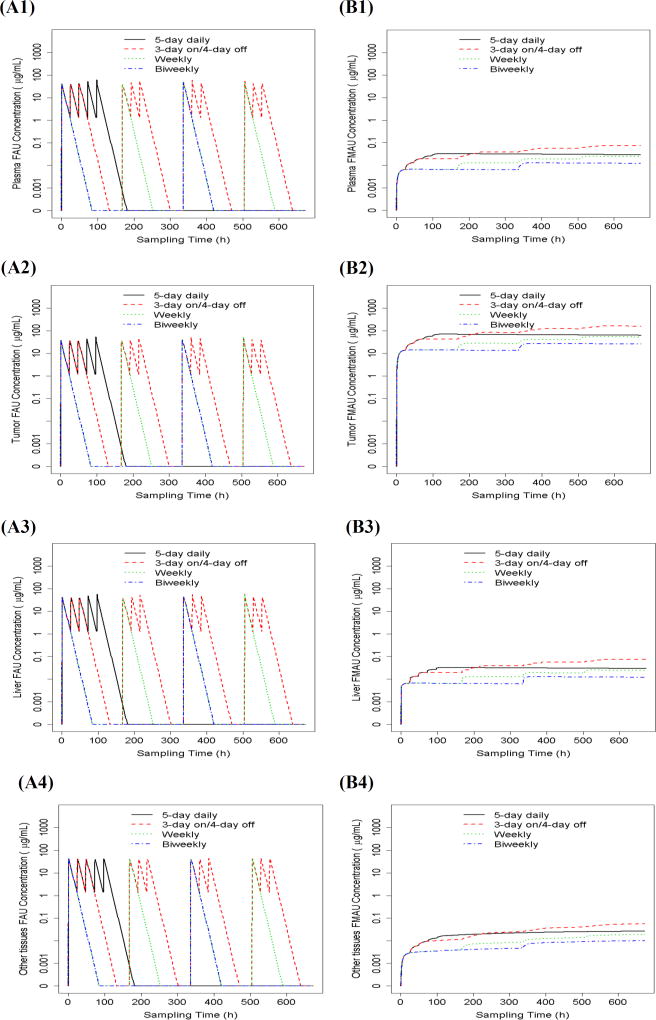
With the developed parent-metabolite model, the plasma and tumor/normal tissue concentration – time profiles of FAU and FMAU were predicted in a typical individual treated with 4 different FAU treatment schedules (i.e., 3 day on/4 day off for 4 weeks, daily for 5 days of a 28-day cycle, weekly for 4 weeks, and biweekly for 4 weeks) (Figure 4). Predicted drug exposure parameters, including steady state Css,max and Css,min and 28-day cumulative AUC0–672h, are summarized in Table 3. While there was no apparent difference in FAU plasma or tumor/normal tissue steady-state levels (i.e., Css,max and Css,min) following different treatment schedules, higher cumulative plasma and tissue exposure (i.e., AUC0–672h) to FAU were achieved following the more intensive dosing regimen than the less intensive regimen (Figure 4 and Table 3). For example, FAU plasma AUC0–672h was ~ 6-fold higher following 3 day on/4 day off schedule than following biweekly schedule (Table 3). Notably, 5-day daily and 4-week weekly schedules resulted in similar plasma/tissue exposure to FAU (including steady-state levels and cumulative AUC0–672h) (Table 3).
Figure 4.
Parent-metabolite model-predicted concentration – time profiles of FAU (A1–A4) and FMAU (B1–B4) in the plasma, tumor, liver, and other peripheral tissues in a typical individual treated with 1-h intravenous infusion of FAU (1600 mg/m2) at 4 different dosing regimens (i.e., daily for 5 days of a 28-day cycle, 3 day on/4 day off for 4 weeks, weekly for 4 weeks, and biweekly for 4 weeks).
Table 3.
Parent-metabolite model predicted plasma and tumor/normal tissue exposure to FAU and FMAU following 4 different treatment schedules with 1-h intravenous infusion of FAU (1600 mg/m2) in a typical individual with population pharmacokinetic parameters
| 4 day on/3 day off for 4 weeks |
Daily for 5 days of a 28-day cycle |
Weekly for 4 weeks |
Biweekly for 4 weeks |
||
|---|---|---|---|---|---|
| FAU | Plasma | ||||
| Css,max (µg/ml) | 59.51 | 61.42 | 52.83 | 48.72 | |
| Css,min (µg/ml) | BLQ | 1.28 | BLQ | BLQ | |
| AUC0–672h (µg/ml*h) | 3673 | 1538 | 1219 | 603 | |
| AUC0–2h (µg/ml*h) | 27 | 27 | 27 | 27 | |
|
|
|||||
| Tumor | |||||
| Css,max (µg/ml) | 53.52 | 53.33 | 53.52 | 41.18 | |
| Css,min (µg/ml) | BLQ | 1.16 | BLQ | BLQ | |
| AUC0–672h (µg/ml*h) | 3354 | 1398 | 1118 | 551 | |
| AUC0–2h (µg/ml*h) | 26 | 26 | 26 | 26 | |
|
|
|||||
| Liver | |||||
| Css,max (µg/ml) | 58.25 | 58.09 | 58.25 | 45.18 | |
| Css,min (µg/ml) | BLQ | 1.3 | BLQ | BLQ | |
| AUC0–672h (µg/ml*h) | 3698 | 1541 | 1233 | 607 | |
| AUC0–2h (µg/ml*h) | 28 | 28 | 28 | 28 | |
|
|
|||||
| Other Tissues | |||||
| Css,max (µg/ml) | 44.18 | 42.44 | 42.44 | 42.44 | |
| Css,min (µg/ml) | BLQ | 1.38 | BLQ | BLQ | |
| AUC0–672h (µg/ml*h) | 3753 | 1561 | 1250 | 623 | |
| AUC0–2h (µg/ml*h) | 29 | 29 | 29 | 29 | |
|
| |||||
| FMAU | Plasma | ||||
| Css,max (µg/ml) | 0.07 | 0.03 | 0.03 | 0.01 | |
| Css,min (µg/ml) | 0.06 | 0.02 | 0.02 | 0.01 | |
| AUC0–672h (µg/ml*h) | 30 | 19 | 10 | 6 | |
| AUC0–2h (µg/ml*h) | 0.001 | 0.001 | 0.001 | 0.001 | |
|
|
|||||
| Tumor | |||||
| Css,max (µg/ml) | 160.07 | 69.74 | 53.75 | 27.32 | |
| Css,min (µg/ml) | 119.98 | 40.3 | 39.34 | 12.12 | |
| AUC0–672h (µg/ml*h) | 63052 | 41057 | 22356 | 13377 | |
| AUC0–2h (µg/ml*h) | 1 | 1 | 1 | 1 | |
|
|
|||||
| Liver | |||||
| Css,max (µg/ml) | 0.08 | 0.03 | 0.03 | 0.01 | |
| Css,min (µg/ml) | 0.06 | 0.02 | 0.02 | 0.01 | |
| AUC0–672h (µg/ml*h) | 30 | 19 | 11 | 6 | |
| AUC0–2h (µg/ml*h) | 0.003 | 0.003 | 0.003 | 0.003 | |
|
|
|||||
| Other Tissues | |||||
| Css,max (µg/ml) | 0.06 | 0.03 | 0.02 | 0.01 | |
| Css,min (µg/ml) | 0.04 | 0.01 | 0.01 | 0 | |
| AUC0–672h (µg/ml*h) | 19 | 14 | 7 | 4 | |
| AUC0–2h (µg/ml*h) | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
BLQ, below the lower limit of quantitation (FAU concentration < 0.001 µg/mL)
Compared to FAU, the impact of treatment schedules on FMAU plasma and tissue exposure was more pronounced. In general, the more intensive dosing regimen resulted in higher FMAU steady-state level and higher cumulative exposure (AUC0–672h) in plasma and tumor/normal tissues than the less intensive regimen (Figure 4 and Table 3). FMAU cumulative tumor exposure (AUC0–672h) was increased by 83% following 5-day daily treatment schedule, compared to 4-week weekly treatment (Table 3). With further increasing dosing intensity (e.g., following 4-week 3 day on/4 day off schedule), FMAU AUC0–672h was increased moderately in the tumor (by 54%) as compared to that following 5-day daily schedule (Table 3). These data suggested that 5-day daily schedule may be the optimal dosing regimen to achieve adequate tumor exposure. However, the first two patients experienced grade 3 neuropathy when being treated with FAU at the dose of 800 mg and 1600 mg daily for 5 days. While the exact mechanism underlying neuropathy remained unknown, a more conservative dosing regimen (i.e., 4-week weekly treatment at 50 and 100 mg/m2) was applied to the subsequent patients. It should be noted that although the conservative regimen produced a favorable safety profile (data not shown here), it might result in sub-therapeutic FMAU concentrations in tumors. This could be a potential reason for the lack of clinical efficacy in the present phase I study.
In addition, the developed parent-metabolite model was used to predict the bioactivation of FAU and retention of FMAU in tumors with varying catalytic activity (i.e., Vmax2/Km) for the conversion of FAU to FMAU. Supplementary Figure S-5 shows the simulated FAU and FMAU concentration – time profiles in the plasma, tumor, liver, and other tissues following daily FAU infusion (1600 mg/m2) for 5 days of a 28-day cycle in a typical individual with the Vmax2/Km value varying 10 fold relative to the population median value. Table 4 summarizes the predicted plasma and tissue exposure parameters of FAU and FMAU, including AUC0–2h (as a measure of early retention), AUC0–24h, and AUC0–672h (as a measure of cumulative exposure). In general, with increasing tumor catalytic activity (Vmax2/Km), FMAU plasma and tumor/normal tissue exposure was increased, while FAU exposure was decreased (Table 4). The impact of Vmax2/Km on FMAU exposure was more significant than that on FAU exposure. These data collectively suggested that the tumoral catalytic activity towards the conversion of FAU to FMAU was an important determinant of the tumor exposure to FMAU.
Table 4.
Parent-metabolite model predicted plasma, tumor, liver, and other tissue exposure to FAU and FMAU in a typical individual whose tumor has low, median, and high catalytic activity (i.e., Vmax2/Km value of 0.16, 1.6, and 16 h-1, respectively) for the conversion of FAU to FMAU, following daily 1-h infusion of FAU (1600 mg/m2) for 5 days of a 28-day cycle.
| Vmax2/Km (h−1) |
AUC (mg/L*h) |
FAU | FMAU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma | Tumor | Liver | Other tissues | Plasma | Tumor | Liver | Other tissues | ||
| 0.16 | AUC0–2h | 28 | 29 | 29 | 29 | 0.001 | 0.2 | 0.003 | 0.0003 |
| AUC0–24h | 356 | 358 | 361 | 377 | 0.02 | 30 | 0.1 | 0.1 | |
| AUC0–672h | 1983 | 1968 | 1987 | 2007 | 3 | 5599 | 3 | 7 | |
| 1.6 | AUC0–2h | 27 | 26 | 28 | 29 | 0.001 | 1 | 0.003 | 0.0003 |
| AUC0–24h | 283 | 261 | 288 | 304 | 0.1 | 242 | 0.1 | 0.05 | |
| AUC0–672h | 1538 | 1398 | 1541 | 1561 | 19 | 41057 | 19 | 14 | |
| 16 | AUC0–2h | 21 | 10 | 22 | 24 | 0.004 | 8 | 0.01 | 0.0003 |
| AUC0–24h | 122 | 59 | 127 | 143 | 0.4 | 770 | 0.4 | 0.03 | |
| AUC0–672h | 694 | 329 | 697 | 714 | 51 | 109153 | 51 | 26 | |
DISCUSSION
The process of integrating dynamic PET assessment into early phase clinical trials to obtain information on drug distribution to normal and tumor tissues have been applied in the development of a number of anticancer drugs such as 5-FU, BCNU (an alkylating agent specific for brain tissue), DACA (a topoisomerase I/II inhibitor), and SMT487 (a somatostatin analogue)20–23. However, the major barriers to the utility of dynamic PET for plasma and tissue PK evaluation are the short follow up time of PET and its inability to distinguish the radiolabeled parent drug and metabolites. This study, for the first time to our best knowledge, presents a pharmacometric modeling approach to overcome these barriers by simultaneously modeling dynamic PET tissue data and long-term conventional plasma PK data. This modeling approach enables separation of the PET-derived total tissue concentrations into the parent drug (FAU) and metabolite (FMAU) components, thus allowing simultaneous characterization of the plasma, normal and tumor tissue PK of FUA and FMAU. The developed parent-metabolite model not only complements previous knowledge on the disposition of FAU and 18F-FAU, but also provides further quantitative, mechanistic insights into the bioactivation of FAU and retention of FMAU in normal and tumor tissues.
First, the parent-metabolite model suggests that the tumor exhibits > 100 fold higher catalytic activity (Vmax/Km) for the conversion of FAU to FMAU than normal tissues (Table 2), implying that the bioactivation of FAU occurs predominantly in the tumor, but is negligible in normal tissues. This could be attributable, for a large part, to the overexpression of intratumoral TS, a rate-limiting determinant for the conversion of FAU to FMAU. While FAU must undergo multiple steps, including transport across the cell membrane, phosphorylation by TK and methylation by TS, prior to its incorporation into DNA, TS activity has been proven to be the critical, rate-limiting determinant for incorporation of FAU into cellular DNA as FMAU5. TS is an E2F1-regulated enzyme that is essential for DNA synthesis and repair, with the physiological function of converting deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP)24. TS protein and mRNA levels are elevated in many human cancers, and high TS levels have been correlated with poor prognosis in patients with colorectal, breast, cervical, bladder, kidney, and non-small cell lung cancers24–30. It has been demonstrated that ectopic expression of catalytically active human TS is sufficient to induce a transformed phenotype in mammalian cells in vitro and in vivo, indicating an oncogene-like role of TS in tumorigenesis31. In addition, cells can acquire resistance to TS inhibitors (e.g., 5-FU) through TS gene amplification32–34. Tumors with high TS gene expression are generally non-responsive to 5-FU based therapies7, 35, 36. Although the data regarding the intratumoral TS mRNA/protein levels are not available in the present study, elevated intratumoral TS expression/activity would be expected in the majority, if not all, of the patients enrolled in this phase I trial, whose tumors are advanced and refractory to standard treatments (including 5-FU). The preferential activation of FAU in tumors as indicated by the parent-metabolite model provides quantitative evidence supporting the concept of using the high catalytic activity of intratumoral TS to activate deoxyuridine prodrugs (such as FAU) to form toxic metabolites that can be incorporated into DNA and act as suicide inhibitors of cell growth37.
Second, the parent-metabolite model suggests that the toxic metabolite FMAU is slowly released from the tumor to systemic circulation (with a half-life of 57 days) (Table 2) and it is accumulated in tumors after either 5-day daily or 4-week weekly FAU treatment (Figure 3), indicating that FMAU is trapped in tumors once it is formed. These findings are consistent with the observations from preclinical in vivo tissue distribution studies using radiolabeled tracers with a long radioactive half-life38,5. In the study with 14C-FAU (radioactivity half-life, 5730 y) in a human tumor xenograft mouse model, 14C-FAU rapidly distributed throughout the body in a generally nonspecific manner immediately after the injection (≤ 2 h), whereas differential and preferential accumulation of radioactivity in the tumor was magnified over time38. Specifically, at 2 h after the intravenous injection of 14C-FAU, similar amounts of radioactivity were detected in all tissues examined; whereas at 24 h after the injection, only tumor tissues contained appreciable amounts of radioactivity, and further examination of purified genomic DNA from tumor and all other tissues indicated that radioactivity was accumulated in tumor DNA only but not in all other tissues38. Similar pattern was also observed for tissue distributions of 3H-FAU (radioactive half-life, 12.3 y) in mice bearing colorectal cancer xenografts5. These results collectively suggest that the radioactivity recovered from tumor tissues at later times (e.g., 24 h) reflects the incorporation of FMAU (converted from FAU) into tumor DNA because the DNA-incorporated drug (FMAU) persists while the un-incorporated drug (FAU) is cleared out of tissues over time.
It should be mentioned that 18F-FAU has been evaluated for its potential as a PET probe in dogs and humans10, 39. 18F-FAU evenly distributed to most organs in dogs and humans, and in contrast to 18F labeled thymidine and its analogs, bone marrow had less retention of 18F-FAU than non-proliferating tissues10, 39. In patients with breast cancer, tumors had ~ 2-fold higher retention of 18F-FAU than normal tissues39. Based on these preliminary data, it has been speculated that PET measurement of 18F-FAU tumor retention may provide important predictive information to determine which patients are likely to respond to therapeutic FAU. However, findings from the modeling and simulations provide strong evidence against this speculation, as follows: First, the parent-metabolite model suggests that FAU is rapidly cleared from the tumor, but FMAU is trapped in the tumor. As a consequence, the observed tumor radioactivity (total 18F-FAU plus 18F-FMAU) is dominantly attributable to 18F-FAU at early times (e.g., ≤ 2 h), but to 18F-FMAU at later times (Figures 2B1 and 2B2). However, an extension of dynamic PET imaging of 18F to longer times (i.e., > 2 h) is hampered due to the short radioactive half-life (~ 110 min). Thus, PET-measured early-time tumor retention of 18F is not a reliable marker for the tumor exposure to FMAU, the cytotoxic metabolite exerting antitumor activity. Second, the parent-metabolite model enables separation of the PET-derived total tissue concentrations into the parent drug (FAU) and metabolite (FMAU) components, it is therefore possible to predict the tumor and normal tissue exposure to FMAU. Given the determinant role of TS catalytic activity for the conversion of FAU to FMAU, tumors with higher catalytic activity (i.e., Vmax2/Km) would be expected to retain more FMAU than those with lower activity. As expected, simulations demonstrate that individuals with higher tumor Vmax2/Km exhibit higher FMAU tumor exposure (including early exposure AUC0–2h, AUC0–24h, and cumulative AUC0–672h) than those with lower Vmax2/Km (Table 4 and Supplementary Figure S-5). On the contrary, the simulated AUC0–2h of total FAU plus FMAU in the tumor, which represents the PET-measured intratumoral radioactivity of 18F at early times (up to 2 h), is similar across tumors with varying Vmax2/Km (Table 4), suggesting that the PET-measured early time intratumoral radioactivity cannot truly reflect the differential incorporation of FAU into DNA as FMAU in tumors with varying catalytic activity. These data further indicate that the use of PET measurement of 18F-FAU tumor retention has little value in guiding selection of patients for FAU treatment.
Despite the lack of efficacy data to validate the model, the developed parent-metabolite model has a potential utility not only for predicting tumor responsiveness but also for guiding the selection of dosing regimen, as demonstrated by simulations. The apparent limitation for the model presented is the relatively small sample size used in its development. Although the model’s identifiability has been demonstrated (Supplementary Figure S-4), the relative standard error of estimation for several PK parameters related to FMAU kinetics and formation (e.g., V8, CL58, and Vmax3) are over 50% (Table 2), given the sparse observed FMAU data (with ~2/3 of observed FMAU plasma concentrations below the lower limit of quantitation). Another limitation is that the model doses not differentiate the inter-individual PK variability and inter-occasion variability (i.e., variability between LC-MS/MS determined data and PET-derived data). These limitations may hinder the clinical applicability of the model for accurate prediction of tumor responsiveness to FAU. Regardless, the developed parent-metabolite model provides clinical meaningful, quantitative insights into the bioactivation of FAU and accumulation of the active metabolite FMAU in the tumor.
Although the development of FAU was discontinued because of a patient death caused by potential drug-related delayed liver injury, our study may have important implications for drug development in general for two reasons. First, it demonstrates the feasibility and advantage of integrating long-term conventional plasma PK study and dynamic PET assessment into early-phase clinical trials. Second, it illustrates the necessity and utility of PK modeling approaches to separate PET-derived tissue concentrations into the parent drug and metabolite components, thus allowing quantitatively assess drug distribution and metabolism (in particular bioactivation) in tumor and normal tissues. This makes it possible to not only choose optimal treatment schedules but also select most appropriate patient population for further development.
Supplementary Material
Acknowledgments
This study was supported by the United States Public Health Service Cancer Center Support Grant P30 CA022453 and the National Institute of Health Grant U01 CA062487. We particularly thank the patients enrolled in the study.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.L. and S.K. wrote Manuscript; J.L., A.F.S, and P.M.L. designed Research; J.L., A.F.S, K.A.D., C.I.M., J.M.L, J.M., and P.M.L. performed research; J.L., S.K., A.F.S, and P.M.L. analyzed Data; T.J.M. contributed new reagents/Analytical Tools.
References
- 1.Klimas MT. Positron emission tomography and drug discovery: contributions to the understanding of pharmacokinetics, mechanism of action and disease state characterization. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2002;4(5):311–337. doi: 10.1016/s1536-1632(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 2.Boss DS, Olmos RV, Sinaasappel M, Beijnen JH, Schellens JH. Application of PET/CT in the development of novel anticancer drugs. The oncologist. 2008;13(1):25–38. doi: 10.1634/theoncologist.2007-0097. [DOI] [PubMed] [Google Scholar]
- 3.Saleem A, Aboagye EO, Matthews JC, Price PM. Plasma pharmacokinetic evaluation of cytotoxic agents radiolabelled with positron emitting radioisotopes. Cancer chemotherapy and pharmacology. 2008;61(5):865–873. doi: 10.1007/s00280-007-0552-2. [DOI] [PubMed] [Google Scholar]
- 4.Kissel J, Brix G, Bellemann ME, et al. Pharmacokinetic analysis of 5-[18F]fluorouracil tissue concentrations measured with positron emission tomography in patients with liver metastases from colorectal adenocarcinoma. Cancer research. 1997;57(16):3415–3423. [PubMed] [Google Scholar]
- 5.Eiseman JL, Brown-Proctor C, Kinahan PE, et al. Distribution of 1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) uracil in mice bearing colorectal cancer xenografts: rationale for therapeutic use and as a positron emission tomography probe for thymidylate synthase. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(19):6669–6676. doi: 10.1158/1078-0432.CCR-03-0686. [DOI] [PubMed] [Google Scholar]
- 6.Johnston PG, Drake JC, Trepel J, Allegra CJ. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992;52(16):4306–4312. [PubMed] [Google Scholar]
- 7.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6(4):1322–1327. [PubMed] [Google Scholar]
- 8.Wiegand R, Wu J, Shields AF, Lorusso P, Li J. Simultaneous determination of 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl) uracil (FAU) and 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl) 5-methyluracil (FMAU) in human plasma by liquid chromatography/tandem mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2012;891–892:64–70. doi: 10.1016/j.jchromb.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangner TJ, Klecker RW, Anderson L, Shields AF. Synthesis of 2'-deoxy-2'-[18F]fluoro-beta-D-arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential PET agents for imaging cellular proliferation. Synthesis of [18F]labelled FAU, FMAU, FBAU, FIAU. Nuclear medicine and biology. 2003;30(3):215–224. doi: 10.1016/s0969-8051(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Collins JM, Mangner TJ, Muzik O, Shields AF. Imaging [18F]FAU [1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl) uracil] in dogs. Nuclear medicine and biology. 2003;30(1):25–30. doi: 10.1016/s0969-8051(02)00376-1. [DOI] [PubMed] [Google Scholar]
- 11.Bergstrom M, Grahnen A, Langstrom B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. European journal of clinical pharmacology. 2003;59(5–6):357–366. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 12.Post TM, Freijer JI, Ploeger BA, Danhof M. Extensions to the visual predictive check to facilitate model performance evaluation. Journal of pharmacokinetics and pharmacodynamics. 2008;35(2):185–202. doi: 10.1007/s10928-007-9081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavielle M, Samson A, Karina Fermin A, Mentre F. Maximum likelihood estimation of long-term HIV dynamic models and antiviral response. Biometrics. 2011;67(1):250–259. doi: 10.1111/j.1541-0420.2010.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey KR, Fitch WR. The Deterministic Identifiability of Nonlinear Pharmacokinetic Models. J Pharmacokinet Biop. 1984;12(2):177–191. doi: 10.1007/BF01059277. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Li L. A Switching Markov Chain Monte Carlo Method for Statistical Identifiability of Nonlinear Pharmacokinetics Models. Stat Sinica. 2012;22(3):1199–1215. [Google Scholar]
- 16.Kim S, Li L. Statistical identifiability and convergence evaluation for nonlinear pharmacokinetic models with particle swarm optimization. Comput Meth Prog Bio. 2014;113(2):413–432. doi: 10.1016/j.cmpb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengl S, Kreutz C, Timmer J, Maiwald T. Data-based identifiability analysis of non-linear dynamical models. Bioinformatics. 2007;23(19):2612–2618. doi: 10.1093/bioinformatics/btm382. [DOI] [PubMed] [Google Scholar]
- 18.Miao HY, Dykes C, Demeter LM, Wu HL. Differential Equation Modeling of HIV Viral Fitness Experiments: Model Identification, Model Selection, and Multimodel Inference. Biometrics. 2009;65(1):292–300. doi: 10.1111/j.1541-0420.2008.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavielle M, Samson A, Fermin AK, Mentre F. Maximum Likelihood Estimation of Long-Term HIV Dynamic Models and Antiviral Response. Biometrics. 2011;67(1):250–259. doi: 10.1111/j.1541-0420.2010.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moehler M, Dimitrakopoulou-Strauss A, Gutzler F, Raeth U, Strauss LG, Stremmel W. 18F-labeled fluorouracil positron emission tomography and the prognoses of colorectal carcinoma patients with metastases to the liver treated with 5-fluorouracil. Cancer. 1998;83(2):245–253. [PubMed] [Google Scholar]
- 21.Tyler JL, Yamamoto YL, Diksic M, et al. Pharmacokinetics of superselective intra-arterial and intravenous [11C]BCNU evaluated by PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1986;27(6):775–780. [PubMed] [Google Scholar]
- 22.Saleem A, Harte RJ, Matthews JC, et al. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide in patients by positron emission tomography. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(5):1421–1429. doi: 10.1200/JCO.2001.19.5.1421. [DOI] [PubMed] [Google Scholar]
- 23.Propper DJ, de Bono J, Saleem A, et al. Use of positron emission tomography in pharmacokinetic studies to investigate therapeutic advantage in a phase I study of 120-hour intravenous infusion XR5000. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(2):203–210. doi: 10.1200/JCO.2003.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Johnston PG, Lenz HJ, Leichman CG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55(7):1407–1412. [PubMed] [Google Scholar]
- 25.Leichman CG. Predictive and prognostic markers in gastrointestinal cancers. Curr Opin Oncol. 2001;13(4):291–299. doi: 10.1097/00001622-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani Y, Wada H, Yoshida O, et al. Significance of thymidylate synthase activity in renal cell carcinoma. Clin Cancer Res. 2003;9(4):1453–1460. [PubMed] [Google Scholar]
- 27.Nomura T, Nakagawa M, Fujita Y, Hanada T, Mimata H, Nomura Y. Clinical significance of thymidylate synthase expression in bladder cancer. Int J Urol. 2002;9(7):368–376. doi: 10.1046/j.1442-2042.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 28.Pestalozzi BC, Peterson HF, Gelber RD, et al. Prognostic importance of thymidylate synthase expression in early breast cancer. J Clin Oncol. 1997;15(5):1923–1931. doi: 10.1200/JCO.1997.15.5.1923. [DOI] [PubMed] [Google Scholar]
- 29.Shintani Y, Ohta M, Hirabayashi H, et al. New prognostic indicator for non-small-cell lung cancer, quantitation of thymidylate synthase by real-time reverse transcription polymerase chain reaction. Int J Cancer. 2003;104(6):790–795. doi: 10.1002/ijc.11014. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Tsukagoshi S, Saga Y, Ohwada M, Sato I. Enhanced expression of thymidylate synthase may be of prognostic importance in advanced cervical cancer. Oncology. 1999;57(1):50–54. doi: 10.1159/000012000. [DOI] [PubMed] [Google Scholar]
- 31.Rahman L, Voeller D, Rahman M, et al. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004;5(4):341–351. doi: 10.1016/s1535-6108(04)00080-7. [DOI] [PubMed] [Google Scholar]
- 32.Berger SH, Jenh CH, Johnson LF, Berger FG. Thymidylate synthase overproduction and gene amplification in fluorodeoxyuridine-resistant human cells. Mol Pharmacol. 1985;28(5):461–467. [PubMed] [Google Scholar]
- 33.Danenberg KD, Danenberg PV. Activity of thymidylate synthetase and its inhibition by 5-fluorouracil in highly enzyme-overproducing cells resistant to 10-propargyl-5,8-dideazafolate. Mol Pharmacol. 1989;36(2):219–223. [PubMed] [Google Scholar]
- 34.Spears CP, Gustavsson BG, Berne M, Frosing R, Bernstein L, Hayes AA. Mechanisms of innate resistance to thymidylate synthase inhibition after 5-fluorouracil. Cancer Res. 1988;48(20):5894–5900. [PubMed] [Google Scholar]
- 35.Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15(10):3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 36.Leichman L, Lenz HJ, Leichman CG, et al. Quantitation of intratumoral thymidylate synthase expression predicts for resistance to protracted infusion of 5-fluorouracil and weekly leucovorin in disseminated colorectal cancers: preliminary report from an ongoing trial. Eur J Cancer. 1995;31A(7–8):1306–1310. doi: 10.1016/0959-8049(95)00326-e. [DOI] [PubMed] [Google Scholar]
- 37.Collins JM, Klecker RW, Katki AG. Suicide prodrugs activated by thymidylate synthase: rationale for treatment and noninvasive imaging of tumors with deoxyuridine analogues. Clin Cancer Res. 1999;5(8):1976–1981. [PubMed] [Google Scholar]
- 38.Wang H, Oliver P, Nan L, et al. Radiolabeled 2'-fluorodeoxyuracil-beta-D-arabinofuranoside (FAU) and 2'-fluoro-5-methyldeoxyuracil-beta -D-arabinofuranoside (FMAU) as tumor-imaging agents in mice. Cancer chemotherapy and pharmacology. 2002;49(5):419–424. doi: 10.1007/s00280-002-0433-7. [DOI] [PubMed] [Google Scholar]
- 39.Sun H, Collins JM, Mangner TJ, Muzik O, Shields AF. Imaging the pharmacokinetics of [F-18]FAU in patients with tumors: PET studies. Cancer chemotherapy and pharmacology. 2006;57(3):343–348. doi: 10.1007/s00280-005-0037-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.